Abstract
Objectives:
To compare physical activity, postural stability, and muscle strength in Saudi adolescents with normal and poor sleep quality.
Methods:
This cross-sectional study investigated 62 Saudi adolescents between December 2017 and April 2018 at Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. Participants were classified into 2 equal groups; one with normal sleep (NS) and the other with poor sleep (PS). TecnoBody balance system was used to measure postural stability, ActiGraph to assess physical activity, and hand dynamometer and pinch gauge to assess hand grip and key pinch strength respectively.
Results:
At low platform stability, PS group showed poorer postural stability indices than NS group either with eyes opened or closed (p<0.05). ActiGraph data revealed that the physical activity parameters including the total steps count, total activities count, activity rate, and the vigorous activity time were significantly lower in PS group (p<0.05). The PS group had significantly more total sedentary time than the NS group. Muscle strength parameters did not show any significant difference between groups (p>0.05).
Conclusion:
Poor sleep significantly impaired postural stability and physical activity in Saudi adolescents. However, poor sleep had no effect on their isometric muscle strength.
Keywords: poor sleep quality, partial sleep deprivation, postural stability, physical activity, muscle strength
Saudi Arabian adolescents have greater levels of poor sleep quality than adolescents from other countries. They also delay weekend sleep and rise times longer.1 Poor sleep quality can affect wakefulness, cognitive ability, motor performance, and postural control.2 Aguiar and Barela3 demonstrated that poor sleep quality affects driving through reaction time and eye-hand coordination, and increases body sway, thereby affecting postural stability. Bromley et al4 reported that people with insufficient sleep complained of daytime sleepiness and decreased physical activity levels. Few studies have investigated the effects of poor sleep quality on muscle strength and body performance, and the results are conflicting. One study reported that total sleep deprivation had no effect on the muscle strength of the right- and left-hand grips or the leg and back musculature. Conversely, another study showed that weight-lifting performance decreased in participants with partial sleep deprivation.5 Fullagar et al6 have reported that poor sleep quality negatively affects muscle strength, speed, and power.
Despite being a large field and important for human health and growth, sleep medicine studies are limited. Furthermore, the effects of poor sleep quality on the human body are unclear. The goal of our study was to compare physical activity level, postural stability, and isometric muscle strength in Saudi adolescents with normal and poor sleep quality.
Methods
A convenience sample of 62 Saudi adolescents (boys and girls) aged 12-15 years participated in this cross-sectional study after obtaining written informed consent. The participants were recruited from the primary and intermediate governmental and private schools in the Eastern Region of Saudi Arabia between December 2017 and April 2018.
We initially screened 200 adolescents for eligibility. Based on the Pittsburgh Sleep Quality Index (PSQI), 110 adolescents were eligible to participate. However, 48 were excluded because they either did not meet the inclusion criteria or refused to participate. The exclusion criteria included 1) a history of musculoskeletal, neural, visual, vestibular, mental, or psychological disorders; 2) diabetes mellitus; 3) obesity and underweight based on the Centers for Disease Control/World Health Organization (CDC/WHO) body mass index (BMI) classification. Based on the remaining eligible adolescents’ sleep status (PSQI results), they were divided into one group of 31 students with normal sleep quality (NS) and another equally homogenous group with poor sleep quality (PS). This study was approved by the Institutional Review Board (IRB) committee at Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
We assessed sleep quality and distinguished poor sleep quality from normal sleep quality using the PSQI, which is a self-reported questionnaire that assesses sleep quality over a one-month period. It consists of 24 questions covering 7 sleep areas: subjective sleep quality, sleep latency, sleep duration, regular sleep efficiency, sleep disturbances, the use of sleep medication, and daytime dysfunction. A PSQI score >5 indicates the participant has poor sleep quality. The PSQI has been validated and is reliable in its English and Arabic versions.7
Two well trained research assistants conducted all outcome measurements and were blinded to the groups’ allocation. Measurements were performed in strict sequence as follows: we obtained the height, weight, and BMI of all participants using a comfortable digital weight and height scale (Detecto PD300MHR, USA). We obtained the postural stability measurements using a ProKin 212 N (TecnoBody, Italy). This is a valid and reliable device8 that measures balance and postural stability under dynamic stress. On this device, the foot platform can tilt up to 15° from horizontal on all sides. There are 50 levels of stability, levels 1-10 on a dynamic platform and levels 10-50 on a static platform. Level 1 is considered the least stable, and level 50 is the most stable.8 We determined the anterior-posterior stability index (APSI), medial-lateral stability index (MLSI), and overall stability index (OSI) for all study participants with their eyes opened and closed. We considered the OSI to be the best measure of overall subject stability on the platform. A high score meant impaired postural stability, while a low score indicated normal postural stability.9 We tested the students’ postural stability at dynamic levels 1, 5, and 10, which was suitable for the abilities of our healthy adolescents. We based this testing protocol on the TecnoBody manual and procedures described by Ibrahim et al.9
Each level was composed of 2 trials with each trial lasting 30 seconds. The participant rested for 5 min between trial levels. We then used the mean of the 2 trials at each level to reduce the learning effects.
We measured hand grip and key pinch strength with a hydraulic hand dynamometer and pinch gauge (JAMAR Hand Evaluation Kit, Patterson Medical Holdings, Inc., Canada), valid and reliable instruments.10 The adolescents rested for 1 minute after the postural stability tests before we took the measurements, and the researcher explained the measurement procedure to the adolescents before the test. We conducted 3 trials and recorded the highest result, which was expressed in kilograms. There was a 30-seconds rest period between trials to decrease the influence of fatigue.
We measured physical activity parameters using a wrist ActiGraph (ActiGrapgh wGT3X-BT, Pensacola, FL, USA), which is a valid and reliable device for assessing physical activity and various sleep variables.11 All adolescents wore the ActiGraph on their wrist for 4 consecutive days. As recommended in the literature, we instructed the students to wear the accelerometer (ActiGraph) on their dominant hand 24 hours/day, removing it only during swimming, bathing, and ablutions.
The ActiGraph wear-time requirement is ≥2 days and ≥10 hours/day, and we excluded wear times less than that from the analysis. The parameters presented in Table 2 were recorded and analyzed for all participants. In addition, the total wear time (min); average wear time per day (min); total non-wear time (min); average non-wear time per day (min) were recorded and analyzed.
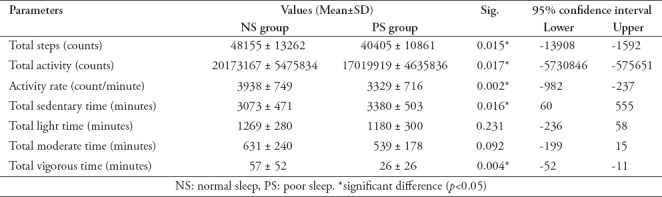
Table 2.
Comparing the ActiGraph physical activities in both groups (independent t-test).
Statistical analysis
We performed the statistical analyses using SPSS software version 20 (IBM Corp, Armonk, NY). Independent t-test was used to compare between the 2 groups for all continuous variables. Statistical significance was indicated by a probability of p<0.05.
Results
Sixty-two adolescents (25 males and 37 females) with a mean age of 13.24 ± 0.82 years participated in this study. The groups with normal and poor sleep showed no significant differences in their demographic or anthropometric characteristics.
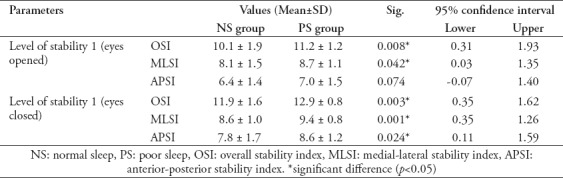
No significant differences in the postural stability parameters (eyes opened and closed) were recorded between the NS and PS groups (p>0.05) at stability levels 5 and 10. However, at stability level 1 (least stable), all postural stability indices (except eyes-opened APSI) were significantly higher (more impaired) in the PS group than in the NS group (p<0.05) (Table 1).
Table 1.
Comparing the postural stability indices in both groups at stability level 1 with eyes opened and closed (Independent t-test).
No significant differences were found between the NS and PS groups in the ActiGraph wear-time information (p>0.05). Except for the total light and moderate activity times, the physical activity parameters were significantly lower in the PS group compared to the NS group (p<0.05). The PS group had significantly more total sedentary time than the NS group in conjunction with significantly less total vigorous activity time than the NS group (Table 2).
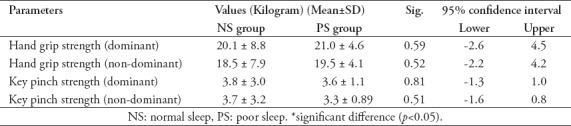
The isometric muscle strength parameters did not significantly differ between the NS and PS groups (p>0.05) (Table 3).
Table 3.
Comparing the isometric muscle strength in both groups (Independent t-test).
Discussion
The PS group had poorer postural stability indices than the NS group only at stability level 1 (lowest stability; both eyes opened and closed). This agrees with Karita et al12 who found significant balance and postural stability impairments in a group they examined that worked overtime and had insufficient sleep. Furtado et al2 have also reported that a group with poor sleep quality performed significantly worse on a balance test than a group with normal sleep quality, both with their eyes opened or closed. They suggested the poor performance of the PS group at the low stability level might be due to an increased demand for attention and alertness, which are reduced in people with poor sleep quality.1,13 Furtado et al1 further suggests that all stability indices are poorer when the eyes are closed due to the lack of visual input to the brain sensory integration regions. In addition, they proposed that these brain regions are directly affected in people who have not slept for 24 hours (acute sleep deprivation).
ActiGraph data revealed that all physical activity parameters including total steps, total activity counts, and activity rate were significantly lower in the PS group compared to the NS group. Furthermore, we found that the time spent on sedentary activities was significantly higher in the PS group than in the NS group, while the time spent on vigorous activities was significantly higher in the NS group than in the PS group. Bromley et al4 have reported similar findings. In this study, they compared physical activity parameters in children who slept <9 hours/day with those of children who slept ≥10 hours/day. The total physical activity was significantly lower in the children who slept <9 hours/day compared to those who slept ≥10 hours/day. In addition, the children who slept <9 hours/day spent more time in sedentary activity compared to those who slept more (p<0.01).
The decreased level of physical activity associated with poor sleep quality is believed to be due to a combination of multiple factors. Bromley et al4 proposed that poor sleep quality can increase overnight energy expenditure by 20% to 30%, causing a reduced metabolic rate in the early morning. The lowered physical activity level may be attributable to this reduced early morning metabolic rate. In addition, Chennaoui et al14 have reported that sleep deprivation can increase insulin resistance and reduce glucose tolerance, precipitating body weakness and a reduction in overall physical activity level. Chennaoui et al14 have also recorded a reduction in VO2 max in association with sleep deprivation.
Muscle strength data showed no significant differences between the PS and NS groups for any of the isometric muscle strength parameters. In agreement with our results, Bambaeichi et al15 found no significant effect on isometric muscle strength over the course of the day after a sleep duration of 2.5 hours per night. Contradicting our results, a review article by Fullagar et al6 reported that poor sleep quality negatively affected a weightlifting task, possible due to an associated impairment in mood state. They concluded that the effect of poor sleep quality on muscle strength was still not clear, and in general, the mechanisms behind muscle strength changes associated with poor sleep quality remain unexplained.
Study limitations
First, the sample was a convenience sample and limited to a small number of adolescents. The participants were only recruited from schools in the Eastern Province, which could affect the generalizability of the results to the entire Kingdom.
In conclusions, we can conclude that poor sleep quality significantly impairs postural stability only at the lowest platform stability level. Poor sleep quality also significantly impairs some physical activity parameters. However, it had no effect on isometric muscle strength.
Acknowledgements
We would like to thank the American manuscript editors (www.americanmanuscripteditors.com) for English language editing.
Footnotes
References
- 1.Merdad RA, Merdad LA, Nassif RA, El-Derwi D, Wali SO. Sleep habits in adolescents of Saudi Arabia;distinct patterns and extreme sleep schedules. Sleep Med. 2014;15:1370–1378. doi: 10.1016/j.sleep.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Furtado F, Goncalves BD, Abranches IL, Abrantes AF, Forner-Cordero A. Chronic Low Quality Sleep Impairs Postural Control in Healthy Adults. PloS One. 2016;11:e0163310. doi: 10.1371/journal.pone.0163310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguiar SA, Barela JA. Adaptation of sensorimotor coupling in postural control is impaired by sleep deprivation. PloS One. 2015;10:e0122340. doi: 10.1371/journal.pone.0122340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromley LE, Booth JN, 3rd, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35:977–984. doi: 10.5665/sleep.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bambaeichi E, Reilly T, Cable N, Giacomoni M. The influence of time of day and partial sleep loss on muscle strength in eumenorrheic females. Ergonomics. 2005;48:1499–1511. doi: 10.1080/00140130500101437. [DOI] [PubMed] [Google Scholar]
- 6.Fullagar HH, Skorski S, Duffield R, Hammes D, Coutts AJ, Meyer T. Sleep and athletic performance:the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015;45:161–186. doi: 10.1007/s40279-014-0260-0. [DOI] [PubMed] [Google Scholar]
- 7.De la Vega R, Tome-Pires C, Sole E, Racine M, Castarlenas E, Jensen MP, et al. The Pittsburgh Sleep Quality Index:Validity and factor structure in young people. Psychological Assessment. 2015;27:e22–e27. doi: 10.1037/pas0000128. [DOI] [PubMed] [Google Scholar]
- 8.Zawadzki J, Bober T, Siemienski A. Validity analysis of the Biodex System 3 dynamometer under static and isokinetic conditions. Acta Bioeng Biomech. 2010;12:25–32. [PubMed] [Google Scholar]
- 9.Ibrahim AI, Muaidi QI, Abdelsalam MS, Hawamdeh ZM, Alhusaini AA. Association of postural balance and isometric muscle strength in early-and middle-school-age boys. J Manipulative Physiol Ther. 2013;36:633–643. doi: 10.1016/j.jmpt.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 11.Lee PH, Suen LK. The convergent validity of Actiwatch 2 and ActiGraph Link accelerometers in measuring total sleeping period, wake after sleep onset, and sleep efficiency in free-living condition. Sleep Breath. 2017;21:209–215. doi: 10.1007/s11325-016-1406-0. [DOI] [PubMed] [Google Scholar]
- 12.Karita K, Nakao M, Nishikitani M, Iwata T, Murata K, Yano E. Effect of overtime work and insufficient sleep on postural sway in information-technology workers. J Occup Health. 2006;48:65–68. doi: 10.1539/joh.48.65. [DOI] [PubMed] [Google Scholar]
- 13.Quarck G, Ventre J, Etard O, Denise P. Total sleep deprivation can increase vestibulo-ocular responses. J Sleep Res. 2006;15:369–375. doi: 10.1111/j.1365-2869.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- 14.Chennaoui M, Arnal PJ, Sauvet F, Leger D. Sleep and exercise:a reciprocal issue?Sleep Med Rev . 2015;20:59–72. doi: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Bambaeichi E, Reilly T, Cable N, Giacomoni M. The influence of time of day and partial sleep loss on muscle strength in eumenorrheic females. Ergonomics. 2005;48:1499–1511. doi: 10.1080/00140130500101437. [DOI] [PubMed] [Google Scholar]


