Abstract
Integration of new DNA into a cellular chromosome can alter the activity of nearby genes, sometimes affecting subsequent cell growth. A potent form of insertional mutagenesis involves integration of retroviral DNA produced by reverse transcription, a required step in the replication of retroviruses. In recent years retroviral replication has been adapted to allow new gene addition by retroviral vectors. Early in the history of retrovirus research, analysis of insertional mutagenesis in laboratory animals was found at times to result in transformation, leading to the discovery of cellular proto-oncogenes. In-depth analysis of the genetic consequences showed that integration of retroviral DNA could alter the gene activity in a variety of ways. Mechanisms of retroviral insertional mutagenesis in humans are much less well documented. However, recent work from the gene therapy and HIV fields now specify four genetic mechanisms of retroviral insertional mutagenesis in humans: (1) gene activation by integration of an enhancer sequence encoded in a retroviral vector (enhancer insertion), (2) gene activation by promoter insertion, (3) gene inactivation by insertional disruption, and (4) gene activation by mRNA 3′ end substitution. In each example, integration in patients was associated with clonal expansion or frank transformation.
Introduction
Gene mutation can be caused by base substitution, deletion, translocation, or insertion of new DNA. New DNA insertion by retroviral integration is a particularly potent mechanism with a rich history. One of the first detections of viruses of any kind was the discovery by Ellermann and Bang1 of a filterable agent that caused leukemias in chickens. Extensive follow-up studies identified the agents, later named avian leukosis viruses (ALVs), and linked integration of ALV DNA near specific genes to cell transformation. Further studies showed that retroviruses could be found that infected several species of laboratory animals and caused cancer, specifying multiple genes—“proto-oncogenes”—involved in growth control and transformation.2, 3, 4, 5
Researchers went on to investigate the effects of integration on host cell genes in transformed cells with great care. Retroviral genomes encode potent signals for transcriptional control required for their replication, often including highly active enhancers and promoters. In some cases, integrated proviruses could be found installed near cellular genes, which follow-up studies specified as important for control of cell growth, thereby boosting their expression.6,7 In other cases the results were more complex—integration of retroviral sequences could result in truncation of the encoded protein at the N or C terminus, or in altering the structure of an mRNA to yield more or less of the encoded protein. A vast catalog of these molecular outcomes was assembled, which today specifies components of numerous regulatory pathways central to growth control and cancer.3
Studies of insertional mutagenesis by retroviral integration in humans have moved much more slowly. Research of course can only proceed by taking advantage of naturally occurring examples, so there are few settings where there is any opportunity to investigate insertional mutagenesis. For example, although HIV infection is unfortunately not rare, and there is increased risk of multiple cancers associated with HIV infection, the transformed cells do not contain integrated HIV genomes (“proviruses” in the jargon of the field). Thus, insertional mutagenesis does not directly cause HIV-associated malignancy.
Today retroviruses are used widely as vectors for gene addition in human gene therapy, and in a few cases there is clear evidence for insertional mutagenesis. Vector systems are constructed by removing the retroviral genes and replacing them with the gene of interest, for example the gene mutated in Wiskott-Aldrich syndrome (WAS). Vector sequences include only the viral sequences required for function in cis. The viral proteins can then be supplied in the same cell together with the vector in trans, allowing formation of viral particles that can in turn be used to infect target cells. Entry, reverse transcription, and integration of the vector genome can then install an intact copy of the mutant gene in patient cells, in favorable cases with therapeutic benefit.
This same process, integration of a new gene in a chromosome of a patient, alters the integration acceptor site, on occasion modifying a local gene and consequently the properties of the recipient cell. As is described below, there are now clear examples of mutagenesis by integration of gene therapy vectors in humans. In HIV biology, although there has not been insertional mutagenesis associated with transformation, there does seem to be mutagenesis linked to detectable expansion of T cell clones.
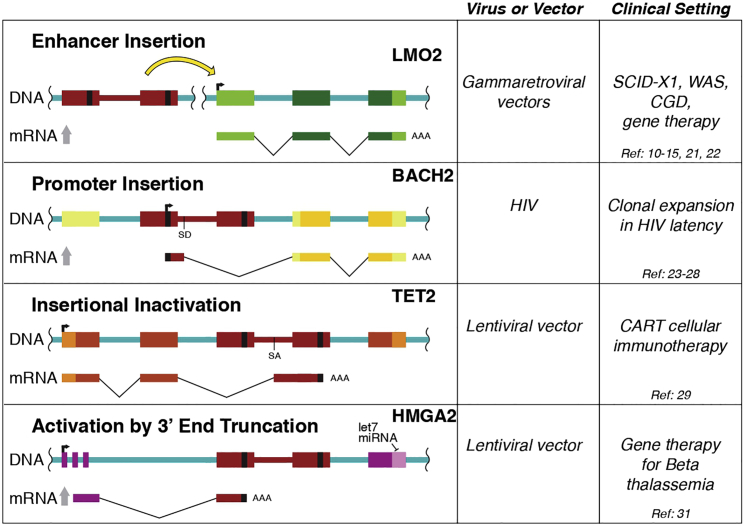
This article reviews examples of retroviral insertional mutagenesis in humans, revealing that there is actually support for four different mechanisms. Each of the four was reported previously in experimental animals but documented in humans relatively recently (Figure 1). In each case, insertional mutagenesis was associated with cell proliferation.
Figure 1.
Diagram of the Four Genetic Mechanisms of Insertional Mutagenesis by Retroviral DNA Integration Documented in Humans
Mutagenesis by Enhancer Insertion
Examples of mutagenesis by enhancer insertion come from human gene therapy trials with early gammaretroviral vectors. At the time, in the early 1990s, these were the only vectors available. Severely immunodeficient children with no good matches for bone marrow donors had extremely limited options, justifying aggressive new therapeutic approaches. Gammaretroviruses are known to harbor strong enhancers at each edge of the proviral DNA, in the long terminal repeats (“LTRs” in the retrovirology jargon). Early work in experimental animals implicated these enhancers in activating cancer-associated genes.8 The first gammaretroviral vectors preserved these enhancers.
The first successful treatment of a human immunodeficiency using gene therapy involved correction of severe combined immunodeficiency (SCID)-X1, in which an intact copy of the mutant IL2RG gene was introduced on a gammaretroviral vector.9 This resulted in dramatic correction of the immunodeficiency in many patients. However, in multiple treated subjects, subsequent sampling of gene-modified cells from blood showed that integration of the corrective vector near genes involved in cell proliferation (LMO2, CCND2, BMI1) was associated with clonal proliferation and transformation.10, 11, 12, 13, 14 Later transformation was seen in patients with integration near LMO2 in a WAS gene therapy trial with gammaretroviral vectors as well.15 The observation that integration events at LMO2 were associated with transformation in multiple different patients in two trials suggests direct involvement.
What is the evidence that these events involved enhancer insertion, and not some other mechanism? The integrated vectors were found in either orientation relative to LMO2. A defining characteristic of transcriptional enhancers is their orientation independence—they can be flipped around relative to the target gene, and they still work to boost transcription. Enhancers also can work at various distances from their targets, up to hundreds of kilobases in extreme cases. The gammaretroviral vectors integrated at LMO2 were found at various distances from the promoter, both upstream and downstream, distributed over about 50 kb around the start point of transcription. Follow-up studies showed that mRNA and protein levels were increased in patient cells harboring some of the LMO2 insertions,16,17 implicating mechanisms at the level of transcription.
Strong evidence for enhancer insertion comes from a second trial to treat SCID-X1, which was repeated in roughly the same way, but with the enhancers deleted from the gammaretroviral vector.18 A new cohort of SCID-X1 patients was treated, with similarly successful gene correction as in the first trial. In the SCID2 trial, however, to date there have been no adverse events associated with vector integration. A careful comparison of the cells that persisted long-term showed that cells with integration sites near genes such as LMO2 and CCND2 were much less prominent in samples from the SCID2 subjects, suggesting that the boosting effect of the gammaretrovirial enhancer had originally been responsible for promoting cell growth but was lacking in SCID2. To be fair, there were several other changes in the vector used, but the deletion of the enhancer was likely the most influential.
Altogether, these data make a strong case that enhancer insertion near LMO2 due to retroviral integration was responsible for the increased transcription observed. Several further studies support a link between higher levels of LMO2 and increased cell proliferation,16,17,19,20 all adding up to strong support for the enhancer insertion model. Another trial using early gammaretroviral vectors, which treated chronic granulomatous disease, also resulted in severe adverse events likely involving enhancer insertion.21,22 Thus, the data suggest that mutagenesis by enhancer insertion is a property of the early gammaretroviral vectors containing strong enhancer sequences.
Mutagenesis by Promoter Insertion
Promoters differ from enhancers in that promoters include the sequences required for transcription initiation, whereas enhancers are transcriptional boosting signals that act on promoters from a distance. Early studies in model organisms indicated that retroviral integration could boost oncogene activity by promoter insertion, and now this mechanism is documented in humans as well.
Data supporting insertional mutagenesis by promoter insertion come from studies of HIV latency.23 HIV-infected people, when their HIV is well suppressed by antiretroviral therapy, are nevertheless not cured. If antiretroviral therapy is discontinued, the virus rebounds and active infection ensues. Careful studies of the origin of persistent virus suggest that infected cell clones may proliferate, and that these can provide at least one potential source of the rebounding virus.23, 24, 25, 26, 27
Extensive studies of HIV-infected cells in patients suggest a possible role for insertional mutagenesis. In at least some cases, analysis of genes at sites of HIV DNA integration showed that some integration acceptor sites were clustered, and these were found more commonly among patients than expected by chance. For example, integrated HIV sequences at the BACH2, STAT5B, and MKL2 genes have been seen in expanded clones from multiple patients in independent studies,23, 24, 25, 26,28 suggesting a causal link.
What specifies promoter insertion as the mechanism by which HIV integration alters host gene activity? One piece of evidence involves orientation bias. As mentioned above, a defining feature of enhancers is that they are functional in either orientation relative to their target promoters. In contrast, promoters are directionally defined sequences, and so only work in the correct orientation relative to a host transcription unit. For the BACH2 insertions, the integrated HIV in expanded clones is almost always in the sense orientation, so that the promoter signals would promote transcription of the BACH2 gene. This is also seen for STAT5B in some though not all datasets. For MKL2, integration events are found in either orientation, suggesting a different mechanism.
Another piece of evidence comes from analysis of mRNA structures. If transcription initiates at an HIV promoter and extends into the body of the BACH2 or STAT5B gene, then the message should contain HIV RNA sequences appended to the mRNA 5′ end. Message structures were mapped by Cesana et al.23 in samples from HIV+ subjects with BACH2 or STAT5B expanded clones, and such 5′-chimeric messages were indeed isolated. Messages initiated in the promoter in the HIV LTR were extended into the HIV genome to the major splice donor, then spliced to an acceptor at the first cellular coding exon. The chimeric STAT5B and BACH2 messages thus encoded the correct full-length protein from the chimeric transcript.
Thus these data together make a strong case for expression of BACH2 and STAT5B at least in part from transcription initiated at the HIV promoter. The consequences are less clear—it seems likely that that increased transcription of BACH2 or STAT5B is responsible at least in part for proliferation of T cells containing the inserted HIV promoter, but the mechanism is not yet fully clarified. Cesana et al.23 did show that overexpression of BACH2 or STAT5B in T cells promoted survival, thus supporting a direct role, but the detailed mechanisms remain to be elucidated.
Mutagenesis by Gene Inactivation
A remarkable success in immunotherapy for cancer provides strong evidence for mutagenesis by gene inactivation in humans.29 A patient with chronic lymphocytic leukemia (CLL), designated patient 10, underwent a long series of treatments, then was selected for treatment with T cells modified to produce a chimeric antigen receptor (CAR) targeting his cancer cells (CAR T cell therapy). Today many centers are treating cancer by removing T cells from cancer patients, adding new CARs, and then reinfusing cells into patients. In favorable cases, the engineered T cells expand and kill the cancer cells, resulting in remission. The genes encoding the engineered receptors are typically introduced into T cells by integrating retroviral vectors, potentially disrupting the genome in T cells by insertional mutagenesis. For patient 10, therapeutic cells were generated by transducing his T cells with a lentiviral vector encoding the CAR.
After two infusions, the CAR T cells proliferated, and his CLL went into remission. Sequencing of sites of integration of the lentiviral vector showed, surprisingly, that the descendants of a single cell had proliferated at the time of tumor eradication to comprise most of the CAR-positive population. These cells harbored the lentiviral vector integrated within the gene TET2. The TET2 protein initiates a set of reactions that result in reversing DNA CpG methylation and altering cell transcription and proliferation. The integrated vector was in an intron, and mapping of messages showed that the TET2 RNA was spliced into the lentiviral vector and terminated. This had the effect of truncating the encoded protein to remove the catalytic domain.
However, TET2 is on one of the human autosomes, so there is another copy of the gene. Astoundingly, it turned out that the other copy of TET2 in patient 10 was already mutant. Mapping studies revealed a mutation in the other copy predicted to alter the encoded protein, and follow-up studies showed diminished function of the allele. Consistent with this, studies of CAR+ cells from patient 10 at the time of maximum clonal expansion showed a reduction in DNA modifications catalyzed by TET2. Follow-up studies modeled the action of the TET2 insertion in cell models of CART function, confirming the expected phenotype of TET2 mutants. Later studies of additional patients again associated TET2 with cell expansion, although clones expanded to a lesser degree than in patient 10—evidently this mechanism can operate widely, evidently not strictly requiring a pre-existing mutation in the other TET2 allelle.30 Thus, the case for loss of function by vector insertion at TET2 is quite tight, apparently promoting CAR T cell proliferation and effective therapy.
Mutagenesis by mRNA 3′ End Substitution
A fourth mechanism of insertional mutagenesis appeared in a study of new gene addition to treat beta-thalassemia.31 Point mutations in the beta-globin gene are common in the human population, likely representing evolved responses to infection with the malaria parasite Plasmodium. Although protective against malaria in the heterozygous state, these substitutions, when homozygous, can alter red blood cell function and cause thalassemia. Disease can be mitigated by addition of wild-type copies of the beta-globin gene in bone marrow stem cells, allowing production of red blood cells with partially wild-type hemoglobin.
A dramatic study from Cavazzana-Calvo et al.31 reported the first successful gene correction of beta-thalassemia. They used a lentiviral vector to introduce a wild-type copy of the beta-globin gene into bone marrow stem cells, followed by reinfusion into a subject. Surprisingly, subsequent monitoring of cells from blood showed that descendants of a single cell dominated the transduced cell population a year and a half later. These cells contained an integrated vector in the cellular HMGA2 gene, which is well known to be involved in a variety of cancers. The vector was integrated in the long third intron of the HMGA2 gene, a region often involved in chromosomal rearrangements in benign tumors.
Extensive studies of mechanisms of the chromosomal translocations have revealed the mechanisms of activation. The HMGA2 mRNA contains a 3′ untranslated region that is targeted by the cellular let7 microRNA. Binding of let7 programs RNA degradation. Removal of these sequence from the message stabilizes the message and allows production of more HMGA2 protein, which in turn promotes cell proliferation.32
In the case of the gene-corrected patient, mapping of transcript structure showed that the HMGA2 message was spliced into the integrated lentiviral vector and terminated, forming a chimeric message with vector sequences at the 3′ end and removing the let7 target site. The message accumulated to abnormally high levels in patient cells, and this was matched by accumulation of the HMGA2 protein. Thus, together with mechanistic studies from the cancer genetics field, this supports the idea that insertional mutagenesis resulting in 3′ end substitution was activating in this case. Happily, the HMGA2 clone slowly declined in abundance in the gene-modified subject over time, and the subject continues to benefit from the gene therapy.
Inferences from the Biology of Insertional Mutagenesis in Humans
In summary, the data above provide evidence for four mechanisms by which retroviral DNA integration can altered gene activity in humans: (1) enhancer insertion, (2) promoter insertion, (3) gene inactivation, and (4) gene activation by mRNA 3′ end substitution. All cases were associated with cell proliferation. All of these mechanisms have been anticipated by studies in model organisms. Examples include enhancer insertion to activate c-myc in some murine lymphomas,8 promoter insertion by ALVs in avian bursal tumors,6,7 insertional inactivation of the hairless gene in mice, resulting in the hairless phenotype,33 and activation by 3′ end truncation in murine pim1 in some murine lymphomas.34,35
Although the evidence for involvement of insertional mutagenesis in cell proliferation in humans is strong, there is also strong evidence for contributions from additional mechanisms. In the cases of LMO2 activation by enhancer insertion, follow-up studies revealed numerous additional mutations in the transformed cells, many of which are well known to be cancer-associated in other settings.14,15 For the case of gene inactivation by insertion in TET2, the gene-modified cells dropped in abundance as the antigen-positive target cells were cleared from patient 10, suggesting that stimulation through the CAR was also important for cell proliferation in this case.29 For the promoter insertions in HIV latency, it has been widely speculated that signaling through the T cell receptor may also contribute to cell outgrowth—intensive studies are underway to investigate this in more detail. It is unknown for the case of mRNA 3′ end substitution at HMGA2 whether there were any additional pro-proliferative signals.
Cataloging the mechanisms of insertional activation provides useful background for interrogating new data from human gene therapy trials or studies of HIV replication. Careful studies of human cells following retroviral integration commonly show examples of differential growth of gene-modified cell clones, and knowledge of established mechanisms provides a background for interpreting data. The fact that we are up to four mechanisms of insertional mutagenesis in humans—and also have evidence for additional pro-proliferative signals—does not simplify the picture, but it does bring us closer to understanding the full biology of gene modification and cell growth.
Author Contributions
F.D.B. wrote the article.
Conflicts of Interest
The author declares no competing interests.
Acknowledgments
I thank Joe Fraietta and Scott Sherrill-Mix for comments on the manuscript, and Laurie Zimmerman for help with art and manuscript preparation. This work was supported by NIH grants R01 AI082020, R61 HL137063, and R01 HL113252, the Penn Center for AIDS Research (P30 AI 045008), and the PennCHOP Microbiome Program.
References
- 1.Ellermann V., Bang O. Experimentelle Leukämie bei Hühnern. Zentralbl. Bakteriol. Parasitenkd. Infectionskr. Hyg. Abt. I. 1908;46:595–609. [Google Scholar]
- 2.Coffin J.M., Hughes S.H., Varmus H.E. The interactions of retroviruses and their hosts. In: Coffin J.M., Hughes S.H., Varmus H.E., editors. Retroviruses. 1997. pp. 335–342. Cold Spring Harbor. [Google Scholar]
- 3.Akagi K., Suzuki T., Stephens R.M., Jenkins N.A., Copeland N.G. RTCGD: Retroviral Tagged Cancer Gene Database. Nucleic Acids Res. 2004;32:D523–D527. doi: 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seggewiss R., Pittaluga S., Adler R.L., Guenaga F.J., Ferguson C., Pilz I.H., Ryu B., Sorrentino B.P., Young W.S., 3rd, Donahue R.E. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood. 2006;107:3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biasco L., Rothe M., Büning H., Schambach A. Analyzing the genotoxicity of retroviral vectors in hematopoietic cell gene therapy. Mol. Ther. Methods Clin. Dev. 2017;8:21–30. doi: 10.1016/j.omtm.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung Y.K., Fadly A.M., Crittenden L.B., Kung H.J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc. Natl. Acad. Sci. USA. 1981;78:3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne G.S., Courtneidge S.A., Crittenden L.B., Fadly A.M., Bishop J.M., Varmus H.E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981;23:311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- 8.Corcoran L.M., Adams J.M., Dunn A.R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984;37:113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- 9.Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.L. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 10.Cavazzana M., Bushman F.D., Miccio A., André-Schmutz I., Six E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat. Rev. Drug Discov. 2019;18:447–462. doi: 10.1038/s41573-019-0020-9. [DOI] [PubMed] [Google Scholar]
- 11.Deichmann A., Hacein-Bey-Abina S., Schmidt M., Garrigue A., Brugman M.H., Hu J., Glimm H., Gyapay G., Prum B., Fraser C.C. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J. Clin. Invest. 2007;117:2225–2232. doi: 10.1172/JCI31659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacein-Bey-Abina S., Hauer J., Lim A., Picard C., Wang G.P., Berry C.C., Martinache C., Rieux-Laucat F., Latour S., Belohradsky B.H. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D. Gene therapy for Wiskott-Aldrich syndrome—long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 16.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 18.Hacein-Bey-Abina S., Pai S.Y., Gaspar H.B., Armant M., Berry C.C., Blanche S., Bleesing J., Blondeau J., de Boer H., Buckland K.F. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014;371:1407–1417. doi: 10.1056/NEJMoa1404588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davé U.P., Jenkins N.A., Copeland N.G. Gene therapy insertional mutagenesis insights. Science. 2004;303:333. doi: 10.1126/science.1091667. [DOI] [PubMed] [Google Scholar]
- 20.Davé U.P., Akagi K., Tripathi R., Cleveland S.M., Thompson M.A., Yi M., Stephens R., Downing J.R., Jenkins N.A., Copeland N.G. Murine leukemias with retroviral insertions at Lmo2 are predictive of the leukemias induced in SCID-X1 patients following retroviral gene therapy. PLoS Genet. 2009;5:e1000491. doi: 10.1371/journal.pgen.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein S., Ott M.G., Schultze-Strasser S., Jauch A., Burwinkel B., Kinner A., Schmidt M., Krämer A., Schwäble J., Glimm H. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 22.Siler U., Paruzynski A., Holtgreve-Grez H., Kuzmenko E., Koehl U., Renner E.D., Alhan C., de Loosdrecht A.A., Schwäble J., Pfluger T. Successful combination of sequential gene therapy and rescue allo-HSCT in two children with X-CGD—importance of timing. Curr. Gene Ther. 2015;15:416–427. doi: 10.2174/1566523215666150515145255. [DOI] [PubMed] [Google Scholar]
- 23.Cesana D., Santoni de Sio F.R., Rudilosso L., Gallina P., Calabria A., Beretta S., Merelli I., Bruzzesi E., Passerini L., Nozza S. HIV-1-mediated insertional activation of STAT5B and BACH2 trigger viral reservoir in T regulatory cells. Nat. Commun. 2017;8:498. doi: 10.1038/s41467-017-00609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldarelli F., Wu X., Su L., Simonetti F.R., Shao W., Hill S., Spindler J., Ferris A.L., Mellors J.W., Kearney M.F. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner T.A., McLaughlin S., Garg K., Cheung C.Y., Larsen B.B., Styrchak S., Huang H.C., Edlefsen P.T., Mullins J.I., Frenkel L.M. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohn L.B., Silva I.T., Oliveira T.Y., Rosales R.A., Parrish E.H., Learn G.H., Hahn B.H., Czartoski J.L., McElrath M.J., Lehmann C. HIV-1 integration landscape during latent and active infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruner K.M., Wang Z., Simonetti F.R., Bender A.M., Kwon K.J., Sengupta S., Fray E.J., Beg S.A., Antar A.A.R., Jenike K.M. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566:120–125. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda T., Shibata J., Yoshimura K., Koito A., Matsushita S. Recurrent HIV-1 integration at the BACH2 locus in resting CD4+ T cell populations during effective highly active antiretroviral therapy. J. Infect. Dis. 2007;195:716–725. doi: 10.1086/510915. [DOI] [PubMed] [Google Scholar]
- 29.Fraietta J.A., Nobles C.L., Sammons M.A., Lundh S., Carty S.A., Reich T.J., Cogdill A.P., Morrissette J.J.D., DeNizio J.E., Reddy S. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobles C.L., Sherrill-Mix S., Everett J.K., Reddy S., Fraietta J.A., Porter D.L., Frey N., Gill S.I., Grupp S.A., Maude S.L. CD19-targeting CAR T cell immunotherapy outcomes correlate with genomic modification by vector integration. J. Clin. Invest. 2020 doi: 10.1172/JCI130144. Published online December 17, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng Y., Laser J., Shi G., Mittal K., Melamed J., Lee P., Wei J.J. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol. Cancer Res. 2008;6:663–673. doi: 10.1158/1541-7786.MCR-07-0370. [DOI] [PubMed] [Google Scholar]
- 33.Stoye J.P., Fenner S., Greenoak G.E., Moran C., Coffin J.M. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988;54:383–391. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- 34.Selten G., Cuypers H.T., Berns A. Proviral activation of the putative oncogene Pim-1 in MuLV induced T-cell lymphomas. EMBO J. 1985;4:1793–1798. doi: 10.1002/j.1460-2075.1985.tb03852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selten G., Cuypers H.T., Boelens W., Robanus-Maandag E., Verbeek J., Domen J., van Beveren C., Berns A. The primary structure of the putative oncogene pim-1 shows extensive homology with protein kinases. Cell. 1986;46:603–611. doi: 10.1016/0092-8674(86)90886-x. [DOI] [PubMed] [Google Scholar]