Abstract
Local endometrial stem cells play an important role in regulating endometrial thickness, which is an essential factor for successful embryo implantation and pregnancy outcomes. Importantly, defects in endometrial stem cell function can be responsible for thin endometrium and subsequent recurrent pregnancy losses. Therefore, many researchers have directed their efforts toward finding a novel stimulatory factor that can enhance the regenerative capacity of endometrial stem cells. Sonic hedgehog (SHH) is a morphogen that plays a key role in regulating pattern formation throughout embryonic limb development. In addition to this canonical function, we identified for the first time that SHH is actively secreted as a stem cell-activating factor in response to tissue injury and subsequently stimulates tissue regeneration by promoting various beneficial functions of endometrial stem cells. Our results also showed that SHH exerts stimulatory effects on endometrial stem cells via the FAK/ERK1/2 and/or phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways. More importantly, we also observed that endometrial stem cells stimulated with SHH showed markedly enhanced differentiation and migratory capacities and subsequent in vivo therapeutic effects in an endometrial ablation animal model.
Keywords: sonic hedgehog; endometrial stem cells; migration, growth; differentiation; FAK/ERK1/2; PI3K/Akt
Graphical Abstract
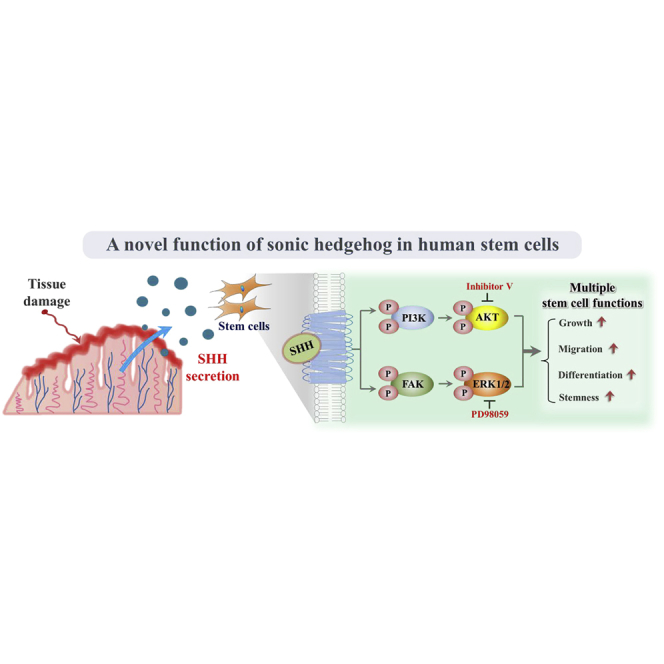
In addition to its previously known canonical functions, SHH is actively secreted in response to tissue damage as a stem cell-activating factor and subsequently stimulates the therapeutic potential of endometrial stem cells by enhancing their proliferative, migratory, and transdifferentiation capacities through the FAK/ERK1/2 and/or phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways. Our present study also provides novel insights into the mechanisms that regulate the therapeutic potential of endometrial stem cells with relevance to clinical applications.
Introduction
Reciprocal interactions between the endometrium (the inner layer of the uterine cavity) and embryo is a pivotal step in embryo implantation and a subsequent successful pregnancy.1 The human endometrium is one of the most dynamic mucosal tissues that undergoes cyclic growth of up to 7 mm within 1 week during the normal menstrual cycle for potential embryo implantation.2 The destruction and regeneration of the upper functional endometrium layer is repeated for approximately 500 cycles at approximately monthly intervals in fertile females.3 Similar to other human tissues, local endometrial stem cells are responsible for dynamic cyclic growth and regeneration of endometrial stratum functionalis.4,5 Successful embryo implantation also requires the constant recruitment and activation of resident endometrial stem cells that can differentiate into specialized uterine cell types, including endometrial epithelial and stromal cells.6 Recent work has also shown that accelerated senescence of local endometrial stem cells limits the regenerative potential of the inner lining of the uterus and subsequently decreases pregnancy rates.6 Therefore, many researchers have directed their efforts toward investigating novel regulatory molecules that can improve the regenerative capacity of endometrial stem cells.
Sonic hedgehog (SHH) signaling was first identified as a morphogen that plays a key role in regulating pattern formation throughout embryonic limb development.7,8 Interestingly, in addition to this canonical function, particular attention has been devoted to the noncanonical functions of SHH as a regulatory signaling molecule in various human uterine diseases, such as endometriosis9 and endometrial hyperplasia,10 because of its abnormal activation during the initiation and development of these diseases. Moreover, Palma et al.11 showed that SHH regulates self-renewal and lineage differentiation of stem cells in post-natal and adult brains.12 Therefore, we hypothesized that SHH is actively released as a stem cell-activating factor in response to tissue injury and subsequently stimulates tissue regeneration of damaged tissues by promoting multiple beneficial functions of endometrial stem cells. However, the molecular mechanisms underlying the noncanonical stem cell-activating effects of SHH signaling remain poorly understood. Interestingly, we showed for the first time that SHH is actively secreted from the site of injury in response to various damage signals both in vitro and in vivo and then acts as a potent promoting factor that stimulates the differentiation, self-renewal, and migratory capacities of endometrial stem cells to repair damaged tissues. Moreover, we investigated the underlying mechanism of the promoting effects of SHH on various functions of endometrial stem cells. Interestingly, SHH was shown to activate multifunctional signaling pathways, such as FAK/ERK1/2 and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways, which are involved in diverse physiological roles, including self-renewal,13,14 transdifferentiation,13,15,16 and migratory capacities of stem cells.13,17 Importantly, suppression of these signaling activities with specific inhibitors significantly decreased the SHH-induced promoting effects of endometrial stem cells. These results suggest that SHH promotes the self-renewal, differentiation, and homing potential of endometrial stem cells via the FAK/ERK1/2 and/or PI3K/Akt signaling pathways. Another key result from our study is that the in vivo therapeutic effects of endometrial stem cells were markedly enhanced upon stimulation with exogenous SHH in an endometrial ablation animal model. Overall, these results suggest that, in addition to its well-known canonical functions, SHH is actively secreted in response to tissue damage as a stem cell-activating factor and subsequently promotes the therapeutic effects of endometrial stem cells by activating various beneficial functions via the FAK/ERK1/2 and/or PI3K/Akt signaling pathways.
Results
SHH Is Actively Secreted in Response to Various Injury Signals from Endometrial Stem Cells In Vitro and In Vivo
To isolate human endometrial stem cells, we minced endometrial tissue into small pieces, and then the small pieces were digested with type I collagenase. The digestion mixture was then filtered through a 40-μm cell strainer to separate spindle-shaped endometrial stem cells from epithelial gland fragments and undigested tissue (Figure S1A); then their biological properties were characterized using various stem cell surface markers, including CD34, CD44, CD45, CD73, CD105, CD140b, CD146, and W5C5 (Figure S1B). Four positive surface markers were mostly expressed (CD44, CD73, CD105, and CD140b), whereas a small percentage of some positive marker (CD146 and W5C5) positive cells was detected in the whole cell population. Therefore, it is possible that our endometrial stem cells are a mixed-cell population consisting of at least two types of cells. The transdifferentiation capacity of endometrial stem cells into different cell types was evaluated by inducing adipogenesis and osteogenesis in vitro (Figure S1C). These results suggested that isolated endometrial stem cells may be a heterogeneous population but have clear stem cell characteristics. A schematic of the main hypothesis regarding the noncanonical stem cell-activating effects of SHH is shown in Figure 1A. To investigate whether SHH is secreted from endometrial stem cells in response to various injury signals, endometrial stem cells were exposed to multiple cell-damaging conditions, such as radiation (4 Gy), serum depletion, and oxidative stress (H2O2). Interestingly, endometrial stem cells actively secreted SHH into the surrounding culture medium in response to various types of cellular stress or damage in vitro (Figures 1B–1D). Additionally, to determine whether SHH is secreted in response to various injury signals from other non-stem cell types, such as fibroblasts and vascular endothelial cells, these cells were exposed to multiple cell-damaging conditions. Consistent with stem cells, these non-stem cells also actively secreted SHH into the surrounding culture medium in response to various types of cellular stress or damage in vitro (Figures S2A–S2C). These results suggest the autocrine and/or paracrine effects of SHH in response to various injury signals. To further determine whether local tissue damage can promote SHH secretion into the blood circulation in vivo, we analyzed systemic SHH levels in mice peripheral blood samples after acid solution-induced endometrial injury. Histological examination of uterine lesions showed that acidic TCA (trichloroacetic acid) solutions significantly decreased the thickness of the functional layer of endometrium and increased degenerative vacuoles and apoptosis (Figure 1E). The endometrial damage resulted in increased SHH secretion into the peripheral circulation compared with the uninjured control group (Figure 1F). These results indicate that SHH is actively secreted from endometrial stem cells in response to multiple injury signals both in vitro and in vivo.
Figure 1.
SHH Actively Secreted in Response to Various Injury Signals
SHH is considered a morphogen that regulates pattern formation during embryonic limb development. In addition to this canonical function, we hypothesized that SHH is selectively secreted under specific cell-damaging conditions as a stem cell-activating factor (A). Endometrial stem cells were incubated in standard culture medium with or without H2O2 (10 mM) for 30 min, after which the medium was replaced with serum-free medium, and the cells were cultured for 48 h (B). Endometrial stem cells were exposed to acute X-ray radiation at a dose of 4 Gy, after which the medium was replaced with serum-free medium, and the cells were cultured for 48 h (C). Endometrial stem cells were cultured with or without serum for 48 h (D). The 2% TCA treatment (150 μL, administered directly into uterine horn) caused significant histological uterine endometrial ablation. The acute TCA treatment caused significant histological endometrial damage with increased degenerative vacuoles and apoptosis (E). After albumin/immunoglobulin depletion, the proteins in the serum samples were precipitated with 10% TCA and subjected to SDS-PAGE. To prove that media samples are not contaminated with cytosolic or nuclear content during the TCA precipitation procedure, the levels of actin in the culture medium were analyzed. Compared with the uninjured control mice, TCA-induced acute endometrial ablation resulted in a significant increase in SHH secretion into the peripheral circulation (F). β-Actin was used as the internal control. The results are presented as the mean ± SEM from three independent experiments.
SHH Significantly Stimulates Multiple Beneficial Functions of Endometrial Stem Cells In Vitro
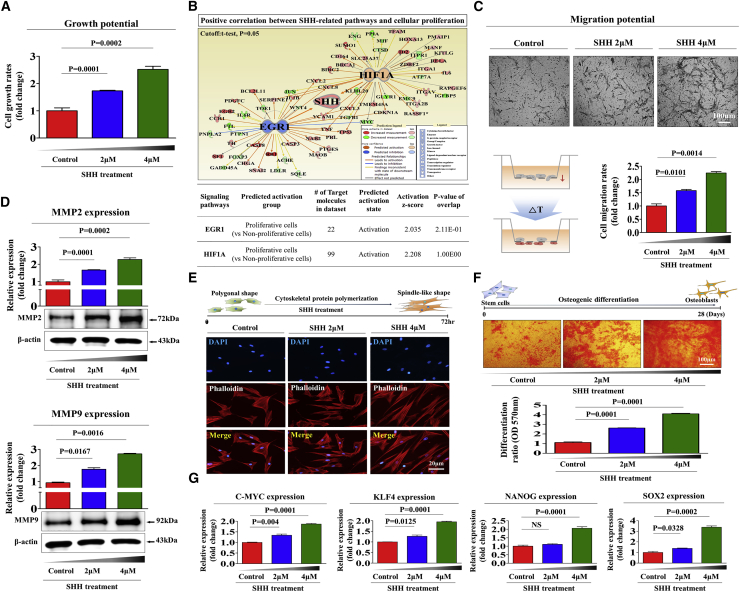
Because SHH is actively secreted from endometrial cells in response to tissue damage, we investigated whether exogenous SHH can stimulate various beneficial functions of endometrial stem cells as a stem cell-activating factor. First, we evaluated the effect of SHH treatment on the in vitro self-renewal capacity of endometrial stem cells. The treatment concentration of SHH was assessed based on our two previous articles that revealed the stimulating effects of SHH signaling on the various stem cell functions.18,19 We observed steadily increased proliferation rates in endometrial stem cells treated with SHH compared with the nontreated control cells (Figure 2A). To further confirm whether enhanced SHH signaling integrity is positively correlated with stem cell self-renewal capacity, we investigated the gene expression profiles of a large clinical database using Ingenuity Pathway Analysis (IPA) software. Positive regulators of SHH, such as early growth response protein 1 (EGR1) (Z score = 2.035, p = 2.11E−1) and hypoxia-inducible factor 1-alpha (HIF1A) (Z score = 2.208, p = 1.00E00), were activated in proliferative stem cells (Figure 2B). We also analyzed the GEO database to further verify the correlation between SHH signaling and stemness. Consistently, the expression levels of SHH were markedly decreased in differentiated cells compared with undifferentiated stem cells (Figures S3A and S3B). More strikingly, SHH significantly increased the migratory capacity of endometrial stem cells (Figure 2C). To further confirm the stimulatory effect of SHH on the migratory capacity of endometrial stem cells, we conducted western blot analysis to measure the expression levels of matrix metalloproteinase-2 (MMP-2) and MMP-9, which play important roles in regulating cell migration and invasion (Figure 2D). Previous studies have suggested that branched actin-filament networks regulate cell migration by pulling or pushing on the leading edge of the plasma membrane.20 Interestingly, phalloidin staining for actin filaments showed a clear correlation between SHH exposure and increased actin filament disorganization (Figure 2E), indicating that the significantly enhanced migratory capability of SHH-treated endometrial stem cells could be related to the disorganization of actin filament networks. Moreover, SHH treatment significantly enhanced the multilineage differentiation capacity of endometrial stem cells toward osteoblasts in vitro (Figure 2F). Consistent with these results, the expression levels of transcription factors associated with stemness and pluripotency, such as C-MYC, KLF4, NANOG, and SOX2, were markedly increased by SHH treatment (Figure 2G). In addition, SHH also significantly increased the proliferation (Figure S4A) and migration (Figure S4B) potential of other stem cell types, such as human umbilical cord blood-derived stem cells and adipose tissue-derived stem cells. Overall, these results indicate that SHH significantly increases various beneficial functions of endometrial stem cells, such as the self-renewal, migratory, and multilineage differentiation capacities in vitro.
Figure 2.
SHH Promotes Various Functions of Endometrial Stem Cells In Vitro
The stimulation of endometrial stem cell viability by SHH (4 μM) treatment for 72 h was determined by an MTT assay. Endometrial stem cell viability (%) was calculated as a percent of the vehicle control (A). Differentially expressed genes from nonproliferative cells and proliferative cells (GEO: GSE63074) were analyzed using Ingenuity Pathway Analysis (IPA) software (https://www.ingenuity.com/) to predict the activation state (either activated or inhibited) of the SHH (B). Endometrial stem cells were treated with SHH for 24 h, and the effect of SHH on the migration ability was then evaluated using a Transwell migration assay. The SHH treatment significantly increased stem cell migration across the membrane compared with the negative control (C). The relative expression levels of key positive regulators of cell migration (MMP-2/-9) were analyzed using western blotting (D). SHH-induced actin filament disorganization and the morphological transition of endometrial stem cells were observed by staining the actin filaments with phalloidin (E). The effects of SHH on osteoblast differentiation were determined by alizarin red staining. The relative quantification of calcium mineral content was determined by measuring the absorbance at a wavelength of 570 nm (F). The real-time PCR results showed changes in the expression of the stem cell markers C-MYC, KLF4, NANOG, and SOX2 after SHH treatment for 24 h (G). DAPI staining was used to label the nuclei. β-Actin was used as the internal control. The results are presented as the mean ± SEM from three independent experiments.
The FAK/ERK1/2 and/or PI3K/Akt Signaling Cascade Mediates SHH-Induced Stimulating Effects on Various Beneficial Functions of Endometrial Stem Cells
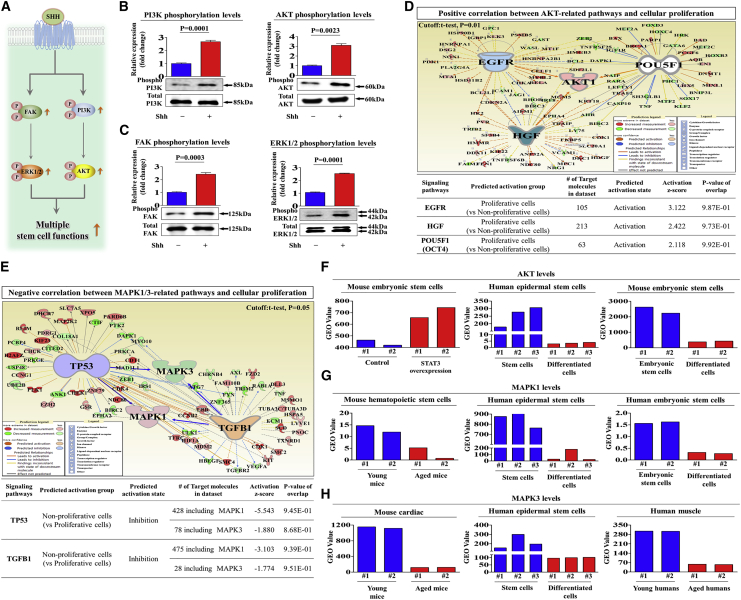
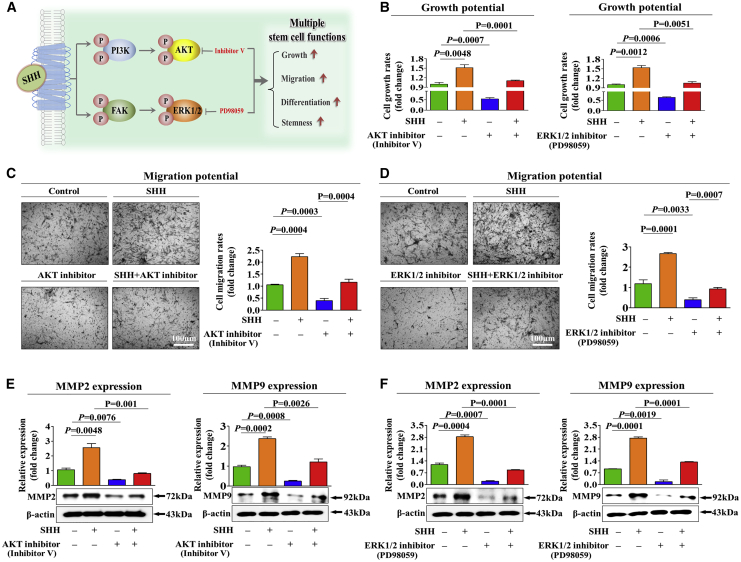
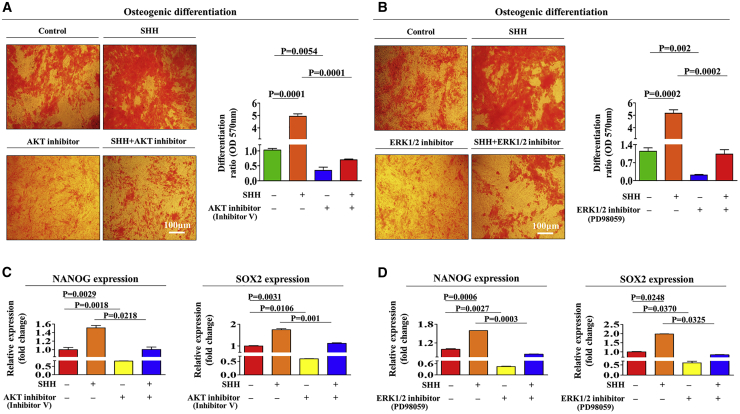
To investigate the underlying molecular mechanisms of the promoting effects of SHH on the multiple functions of endometrial stem cells, we evaluated the effects of SHH on the FAK/ERK1/2 and/or PI3K/Akt signaling components, which have been associated with the multilineage differentiation,21 self-renewal,22 and migratory23 capacities of multiple types of stem cells. A schematic of the main hypothesis is shown in Figure 3A. Using western blot analysis, we evaluated whether the FAK/ERK1/2 or PI3K/Akt signaling components are upregulated in SHH-treated endometrial stem cells. Importantly, the activation states of these signaling cascades were significantly upregulated in response to SHH treatment (Figures 3B and 3C). To further confirm whether enhanced Akt and ERK1/2 signaling integrity is positively correlated with stem cell self-renewal capacity, we investigated the gene expression profiles of a large clinical database using IPA software. The IPA results showed that positive regulators of Akt, such as epidermal growth factor (EGF) receptor (EGFR; Z score = 3.122, p value = 9.87E−1), hepatocyte growth factor (HGF; Z score = 2.422, p = 9.73E−1), and OCT4 (Z score = 2.118, p = 9.92E−1), were activated in proliferative stem cells (Figure 3D). Negative regulators of ERK1/2, such as TTP53 (Z score = −5.543/−1.880, p = 9.45E−1/8.68E−1) and transforming growth factor β1 (TGF-β1; Z score = −3.103/−1.774, p = 9.39E−1/9.51E−1), were inhibited in proliferative stem cells (Figure 3E). We also analyzed the GEO database to further verify the correlation between Akt or ERK1/2 signaling and stemness. Consistently, the expression levels of Akt and ERK1/2 were markedly decreased in differentiated and aged cells (Figures 3F–3H). Next, to confirm whether the inhibition of these signaling components downregulated the stimulating effects of SHH on various beneficial functions of endometrial stem cells, we evaluated the attenuating effects of the ERK1/2 inhibitor PD98059 or the Akt inhibitor V on the proliferation, migratory, and differentiation capacities of endometrial stem cells with or without SHH treatment. A schematic of the main hypothesis is shown in Figure 4A. Importantly, SHH-induced stimulating effects on self-renewal capacity were significantly attenuated by PD98059 or Akt inhibitor V treatment (Figure 4B). The stimulating effects of SHH on migratory capacity (Figures 4C and 4D) and MMP-2/-9 expression (Figures 4E and 4F) were also markedly disrupted by PD98059 or Akt inhibitor V treatment. Consistently, the SHH-mediated multilineage differentiation capacity toward osteoblasts (Figures 5A and 5B) and expression levels of the transcription factors associated with stemness and pluripotency, such as NANOG and SOX2 (Figures 5C and 5D), were significantly attenuated by PD98059 or Akt inhibitor V pretreatment. In addition, to evaluate whether Akt or ERK1/2 inhibitor treatment induces cell-cycle arrest or growth inhibition, we cultured endometrial stem cells with or without Akt or ERK1/2 inhibitor treatment. Interestingly, Akt or ERK1/2 inhibitor treatment suppressed cell growth (Figures S5A and S5B) but barely affected the cell-cycle stages in endometrial stem cells (Figures S5C and S5D). These results suggest that the FAK/ERK1/2 and/or PI3K/Akt signaling cascade may be involved in the stimulating effects of SHH on multiple beneficial functions of endometrial stem cells, such as self-renewal, migratory, and differentiation capacities.
Figure 3.
SHH-Induced Promoting Effects on Endometrial Stem Cells Are Mediated through the FAK/ERK1/2 and/or PI3K/Akt Signaling Pathways
A schematic showing an overview of the molecular mechanisms of SHH-mediated effects on endometrial stem cells (A). Endometrial stem cells were stimulated for 10 min with or without SHH (4 μM) treatment. The endometrial stem cells were then lysed, and the protein expression was analyzed by western blotting using antibodies targeting the phosphorylated forms of FAK, ERK1/2, PI3K, and Akt. The phosphorylation levels of these signaling molecules were significantly increased in cells treated with SHH (B and C). Differentially expressed genes from nonproliferative cells and proliferative cells (GEO: GSE62564 and GSE85047) were analyzed using Ingenuity Pathway Analysis (IPA) software (https://www.ingenuity.com/) to predict the activation state (either activated or inhibited) of the Akt (D) and ERK1/2 (E) signaling pathways. The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to further verify the significant correlation between stemness and the activation state of Akt and ERK1/2 signaling pathways (F–H). β-Actin was used as the internal control. The data represent the mean ± SEM from three independent experiments.
Figure 4.
Inhibition of the Akt or the ERK1/2 Pathway with Specific Inhibitors Significantly Attenuated SHH-Mediated Stimulating Effects on the Growth and Migration of Endometrial Stem Cells
A schematic showing an overview of the molecular mechanisms of SHH-mediated effects on endometrial stem cells (A). Endometrial stem cells were pretreated with inhibitor V (10 μM) or PD98059 (20 μM) for 1 h prior to an additional 48-h treatment with 4 μM SHH, and subsequent changes in cell viability were analyzed by an MTT assay (B). The attenuating effects of Akt (C) or ERK1/2 (D) inhibition on SHH-induced changes in migratory capacity were analyzed by a Transwell assay (C and D) and western blotting for MMP-2 (E) and MMP-9 (F). β-Actin was used as the internal control. The data represent the mean ± SEM from three independent experiments.
Figure 5.
Inhibition of the Akt or the ERK1/2 Pathway with Specific Inhibitors Significantly Attenuated SHH-Mediated Stimulating Effects on the Differentiation Capacity and Stemness of Endometrial Stem Cells
The downregulating effects of Akt or ERK1/2 signaling inhibition on SHH-induced stimulating effects on the differentiation potential (A and B) and the expression of the pluripotency-associated factors NANOG and SOX2 (C and D) were analyzed by alizarin red staining and real-time PCR, respectively. The data represent the mean ± SEM from three independent experiments.
Proteomic Analysis of SHH-Mediated Protein Secretion and Their Interconnected Signaling Networks Responsible for the Promoting Effects of SHH
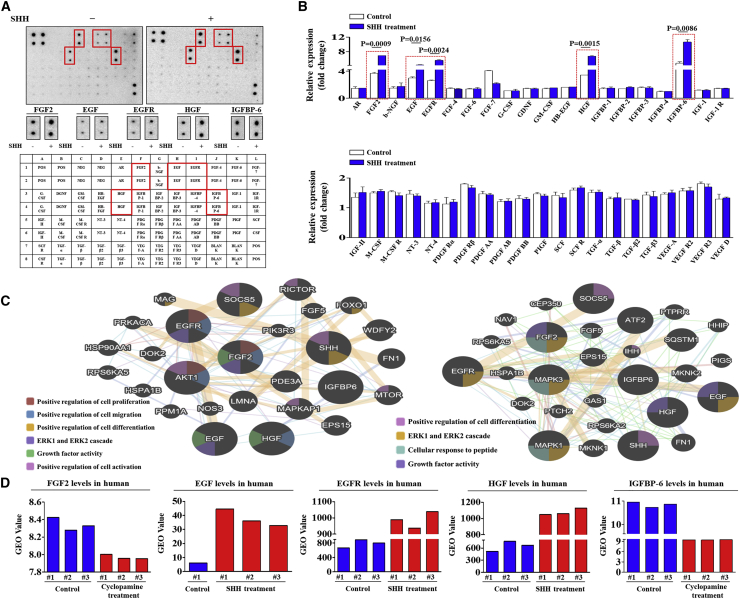
To identify the key secretory proteins and their interconnected signaling networks responsible for the promoting effects of SHH on endometrial stem cells, we analyzed the SHH-induced secretion of multiple growth factors or cytokines using antibody array kits with or without SHH treatments. In the present study, we observed some expression changes in SHH-treated endometrial stem cells. The expression levels of five growth factors, including FGF-2 (fibroblast growth factor-2),24 EGF,25 EGFR,26 HGF,27 and IGFBP-6 (insulin-like growth factor-binding protein-6),28 were substantially increased by SHH treatment, whereas the expression levels of other proteins showed limited changes or only slight increases (Figures 6A and 6B). Interestingly, these SHH-induced proteins can function as potent upstream signaling regulators of the FAK/ERK1/2 and PI3K/Akt signaling pathways in endometrial stem cells. Therefore, we further evaluated the correlation between the activation states of the FAK/ERK1/2 and PI3K/Akt signaling pathways and the expression levels of FGF-2, EGF, EGFR, HGF, and IGFBP-6 using GeneMANIA (http://genemania.org/) to confirm that the interconnected signaling networks regulate self-renewal, migratory, and differentiation capacities (Figure 6C). These results suggest that five SHH-induced proteins could be at least partly responsible for SHH-mediated FAK/ERK1/2 and PI3K/Akt signaling pathways and their subsequent beneficial effects on endometrial stem cells. We also analyzed the GEO database to further verify the correlation between SHH signaling and five SHH-induced proteins. Both bioinformatics analyses consistently showed that the expression levels of five SHH-induced proteins were markedly increased by SHH treatment (Figure 6D).
Figure 6.
SHH Treatment Induces the Secretion of Multiple Growth Factors or Cytokines, which Are Associated with the FAK/ERK1/2 and/or PI3K/Akt Signaling Pathways
Human growth factor antibody array analysis was performed using SHH-treated or nontreated control samples. The membrane was printed with antibodies for 40 growth factors, cytokines, and receptors, with four positive and four negative controls in the upper and lower left corners. Five growth factors or related proteins (FGF-2, EGF, EGFR, HGF, and IGFBP-6) were markedly enriched in the SHH-treated groups compared with the control groups (A and B). Signaling network analysis was performed using GeneMANIA (http://genemania.org/) to predict the connections between five markedly upregulated growth factors and the FAK/ERK1/2 and/or PI3K/Akt signaling pathway. The results revealed a positive relationship between each of the six prominent factors (FGF-2, EGF, EGFR, HGF, and IGFBP-6) and the FAK/ERK1/2 and/or PI3K/Akt signaling pathway (C). The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to further verify the significant correlation between the five upregulated growth factors and the activation state of Akt and ERK1/2 signaling pathways (D). The data represent the mean ± SEM from three independent experiments.
SHH Significantly Increases the Therapeutic Potential of Endometrial Stem Cells In Vivo by Promoting Migratory and Differentiation Capacities
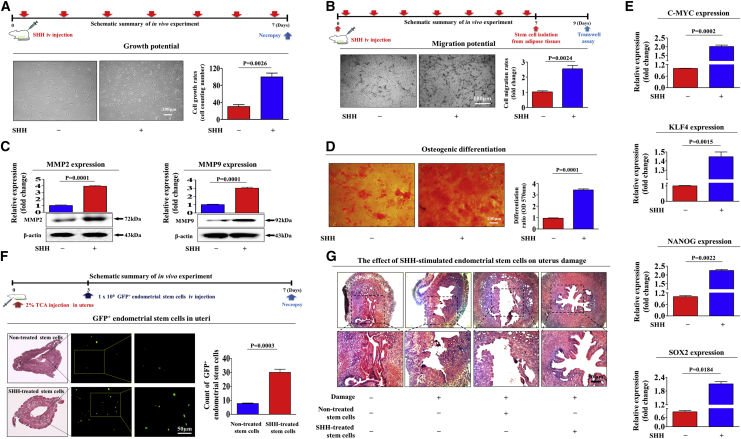
Our in vitro results suggested that SHH may act as an activating factor that stimulates various beneficial functions of endometrial stem cells, such as their self-renewal, migratory, and multilineage differentiation potential. Therefore, we further evaluated whether SHH stimulates multiple beneficial functions of endometrial stem cells in vivo and their subsequent therapeutic potential in animal models of human disease. After 7 consecutive days of intravenous SHH (1 mg/kg) injection, stem cells were isolated from mouse adipose tissues (Figure S6A), and their multilineage differentiation capacity was evaluated by inducing adipogenesis (Figure S6B) and osteogenic (Figure S6C) differentiation. Consistent with our in vitro data, the in vivo results indicated that SHH significantly increased the self-renewal capacity of stem cells (Figure 7A). Moreover, a Transwell-based cell migration assay (Figure 7B) and western blot analysis of the cell migration regulators MMP-2/-9 (Figure 7C) also indicated the significant promoting effect of SHH on the migratory capacity of stem cells in vivo. Strikingly, SHH markedly increased the multilineage differentiation capacity of stem cells toward osteoblasts in vivo (Figure 7D). The expression levels of transcription factors associated with stemness and pluripotency, such as C-MYC, KLF4, NANOG, and SOX2, were significantly increased by exogenous SHH administration in vivo (Figure 7E). Furthermore, we also evaluated whether SHH can promote not only the in vivo homing potential of exogenous stem cells to sites of injury but also their subsequent therapeutic potential in an animal model of endometrial ablation. First, to evaluate the stimulating effect of SHH on the homing potential of exogenous endometrial stem cells to the site of endometrial damage in vivo, we labeled endometrial stem cells with a GFP (green fluorescent protein)-expressing vector to monitor the transplanted cells in the animals. Endometrial stem cells were stably transfected with a GFP-expressing vector (Figure S7). GFP-labeled and SHH-prestimulated endometrial stem cells were intravenously injected into NOD/SCID/IL-2Rynull (NSG)-immunodeficient mice, and the in vivo homing potential of the endometrial stem cells to the injured site of the endometrium was monitored. GFP-labeled fluorescent endometrial stem cells were counterstained with hematoxylin and eosin (H&E) staining to determine their precise homing location within the injured site of mouse endometrium. The number of GFP-labeled cells on 5-μm-thick endometrial sections was counted and quantified using fluorescent microscope. Importantly, compared with nonstimulated endometrial stem cells, we observed markedly enhanced recruitment of SHH-prestimulated endometrial stem cells toward the site of endometrial injury (Figure 7F), indicating that SHH treatment significantly stimulated the in vivo migratory capacity of exogenous endometrial stem cells to the injured site. Consistent with these results, the loss of the endometrial functional layer with severe degenerative changes was markedly relieved by intravenous administration of SHH-prestimulated endometrial stem cells compared with unstimulated endometrial stem cells (Figure 7G). Although we did not confirm the direct transdifferentiation capacity of our endometrial stem cells into various endometrial cell types in vitro, the significant restoration of damaged endometrial functional layer by intravenously administrated endometrial stem cells indirectly suggests the endometrial reconstitution abilities of our endometrial stem cells. Overall, these results suggest that SHH significantly promotes the therapeutic potential of endometrial stem cells in vivo by increasing their migratory and differentiation capacities.
Figure 7.
SHH Stimulates the Therapeutic Potential of Endometrial Stem Cells by Promoting Migratory and Differentiation Capacities In Vivo in an Endometrial Ablation Animal Model
Mice were treated daily for 7 days with SHH (1 mg/kg, intravenously) or vehicle (PBS). Stem cells were isolated from mouse adipose tissue, and the changes in cell viability were analyzed by an MTT assay (A). The in vivo effects of SHH on the migratory capacity of the stem cells were analyzed by a Transwell assay (B) and western blotting for MMP-2 and MMP-9 (C). The changes in osteoblast differentiation were analyzed by alizarin red staining. The relative quantification of calcium mineral content was performed by measuring the absorbance at a wavelength of 570 nm (D). The real-time PCR results showed changes in the expression of the mouse stem cell markers C-MYC, KLF4, NANOG, and SOX2 after SHH treatment in vivo (E). The 2% TCA treatment (150 μL, administered directly into the uterine horn) caused significant histological uterine endometrial ablation compared with the vehicle (PBS) control. SHH-stimulated or nonstimulated endometrial stem cells (1 × 106 cells) were labeled with GFP and injected intravenously into the tail veins of 7-week-old immunodeficient NSG mice with acute TCA-induced endometrial ablation. The mice were sacrificed 7 days after the GFP-labeled endometrial stem cells were injected. Green fluorescent images of consecutive sections showed the presence of GFP-labeled cells (F). Uterine endometrial tissue was collected and subjected to hematoxylin and eosin (H&E) staining. TCA-induced loss of the endometrial functional layer with degenerative changes was significantly more alleviated by the transplantation of SHH prestimulated endometrial stem cells (G). β-Actin was used as the internal control. The data represent the mean ± SEM from eight independent experiments.
Discussion
Despite recent advances in in vitro fertilization (IVF), the successful implantation of embryos and subsequent pregnancy rates remain relatively low. Sufficient growth and appropriate endometrial thickness are essential factors for successful embryo implantation and pregnancy outcomes,29,30 and previous studies have shown that low pregnancy rates can be associated with a thin endometrial functional layer.31,32 However, in fertility treatment, increasing the endometrial thickness in patients with thin endometrium is very challenging. Although various therapeutic approaches, such as growth hormones,33 chemokines,34 tamoxifen,35 and granulocyte colony-stimulating factor,36 have been investigated to increase the thickness of the endometrium, these approaches have not been able to sufficiently increase endometrial thickness and subsequent clinical pregnancy rate. Previous studies have shown that endometrial stem cells are present in the basal layers of the endometrium, and these local stem cells play an important role in regulating endometrial thickness and function throughout the menstrual cycle in humans.4 Similarly, defects in endometrial stem cell function can be responsible for thin endometrium (<7 mm)37 and recurrent pregnancy losses.6 Therefore, increased efforts have been directed toward regenerating the endometrium by administering diverse exogenous stem cells in various endometrial-deficient animal models. Kilic et al.38 showed that the effects of adipose tissue-derived mesenchymal stem cells (MSCs) stimulate angiogenesis and endometrial proliferation in animal models of endometrial ablation. Similarly, Zhao et al.39 effectively improved endometrial thickness in a rat model of thin endometrium by administering bone marrow-derived MSCs. Furthermore, Zhang et al.40 also demonstrated that the transplantation of umbilical cord-derived MSCs can improve endometrial regeneration in animal models of thin endometrium. There are few clinical cases regarding human endometrial regeneration using autologous adult stem cells in patients with severe endometrial deficiency.41, 42, 43 Despite some promising initial results, these stem cell-based therapies did not sufficiently improve embryo implantation rates and subsequent pregnancy outcomes in most cases. The limited therapeutic effects of these stem cell-based therapies can be attributed to the following reasons: (1) transplanted stem cells were not cognate endometrial stem cells, which are optimal stem cells for endometrium repair or regeneration; and (2) the therapeutic effects of the transplanted stem cells were not sufficient for the proper regeneration of the endometrium. Recently, Tersigni et al.44 found higher levels of pro-inflammatory cytokines in endometrial tissues of recurrent pregnancy loss patients than normal women. These results suggested that the increased levels of circulating pro-inflammatory cytokines may be able to induce endometrial inflammation, which in turn may lead to miscarriage. Therefore, further investigation is required to uncover the detailed mechanisms underlying how SHH regulates inflammatory response in endometrial tissues.
The key challenges of many stem cell therapies are the low differentiation capacity of stem cells into specialized cell types and/or the limited migratory potential of stem cells to the site of injury.45 Therefore, many researchers have recently been investigating novel activating factors that can significantly stimulate the homing efficiency or differentiation potential of various stem cells. Therefore, we aimed to investigate the noncanonical function of SHH signaling as a stem cell-activating factor that can stimulate tissue regeneration by promoting various beneficial functions of endometrial stem cells in accordance with previous results reported by Palma et al.,11 who revealed that SHH regulates the self-renewal ability of neural stem cells in post-natal and adult brains. Recently, Pyczek et al.12 also showed that upregulated SHH stimulates neural stem cell growth and hormone release in the adult pituitary gland. In the current study, we demonstrated for the first time that SHH is actively secreted as an endogenous damage signal in response to various types of injury signals in vitro (Figures 1B–1D) and in vivo (Figure 1F). Importantly, we also observed that SHH markedly stimulates the therapeutic potential of endometrial stem cells by increasing their migratory (Figures 2C and 2D) and multilineage differentiation (Figure 2F) capabilities in vitro. In addition, SHH-stimulated endometrial stem cells actively secreted many growth factors and cytokines, such as FGF-2,24 EGF,25 EGFR,26 HGF,27 and IGFBP-6,28 to stimulate tissue regeneration (Figures 6A and 6B). These results suggest that the SHH-mediated beneficial effects on endometrial stem cells for tissue regeneration may partly be because of the multiple secretory proteins that promote diverse biological functions, including self-renewal ability, multilineage differentiation, and wound healing. In addition, the loss of the endometrial functional layer with severe degenerative changes was markedly relieved by intravenous administration of human endometrial stem cells in immunodeficient mice (Figure 7G). This result also revealed the therapeutic potential of intravenously administrated human endometrial stem cells in vivo. However, at this stage, it is unclear whether these therapeutic effects were mediated, at least partly, by various indirect paracrine factors secreted by stem cells or direct cell replacement of damaged cells. Therefore, further studies are needed to confirm the detailed therapeutic mechanism of endometrial stem cells.
The precise molecular mechanisms involved in noncanonical SHH functions have not yet been completely elucidated. It has been shown that ERK1/246 and PI3K/Akt47 signaling is frequently upregulated in response to various survival-associated cytokines or growth factors. Indeed, SHH markedly upregulated the FAK/ERK1/2 and PI3K/Akt signaling pathways in endometrial stem cells (Figures 3B and 3C). The effect of SHH on upregulating these two signaling pathways is not surprising, considering previous results in muscle satellite cells, which showed that SHH promotes proliferation and differentiation through ERK1/2 and PI3K/Akt signaling. Inhibition of these signaling pathways with selective inhibitors significantly diminished the SHH-mediated stimulating effects on the proliferative (Figure 4B), migratory (Figures 4C and 4F), and transdifferentiation capacities (Figures 5A and 5B) of endometrial stem cells, suggesting that SHH exerts promoting effects on endometrial stem cells via the FAK/ERK1/2 and/or PI3K/Akt signaling pathways.
Overall, these results suggest that, in addition to its previously known canonical functions, SHH is actively secreted in response to tissue damage as a stem cell-activating factor and subsequently stimulates the therapeutic potential of endometrial stem cells by enhancing their proliferative, migratory, and transdifferentiation capacities through the FAK/ERK1/2 and/or PI3K/Akt signaling pathways. This study provides novel insights into the underlying molecular mechanisms regulating the regenerative potential of endometrial stem cells with relevance to potential clinical applications. This raises clinically important roles of SHH in protecting endometrium from age-associated degeneration during IVF treatment and subsequently increased pregnancy rates.
Materials and Methods
Isolation and Culture of Human Endometrial Stem Cells
Human endometrial stem cells were obtained from endometrial tissues of uterine fibroid patients with written informed consent from the patients and approval of the Gachon University Institutional Review Board (IRB No: GAIRB2018-134). Endometrial tissue was minced into small pieces; then the small pieces were digested in DMEM containing 10% fetal bovine serum (FBS) and 250 U/mL type I collagenase for 5 h at 37°C in a rotating shaker. The digestion mixture was then filtered through a 40-μm cell strainer to separate stromal-like stem cells from epithelial gland fragments and undigested tissue. Isolated cells were then cultured in endothelial basal medium-2 (EBM-2) medium (Lonza) with endothelial basal medium (EGM-2) supplements at 37°C and 5% CO2. At least three different human endometrial stem cells were obtained from endometrial tissues and also used for all experiments.
Isolation and Culture of Mouse Adipose Tissue-Derived Stem Cells
The isolation of mouse adipose tissue-derived stem cells was approved and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) (LCDI-2018-0015) of the Lee Gil Ya Cancer and Diabetes Institute of Gachon University. Adipose tissue was minced into small pieces; then the small pieces were digested in DMEM containing 10% FBS and 250 U/mL type I collagenase for 5 h at 37°C. The digestion mixture was then filtered through a 40-μm cell strainer. Isolated cells were then cultured in EBM-2 medium (Lonza) with EGM-2 supplements at 37°C and 5% CO2.
Cell Proliferation Assay
The MTT assay was used to determine the anti-proliferative capacity of SHH (ab120933; Abcam), according to the manufacturer’s protocol (Cat No: M5655; Sigma). Cells (1 × 104 cells/well) were seeded in 96-well plates. After 24 h of incubation, the cells were treated with SHH or vehicle for 72 h. The viable cells were measured at 570 nm using a Versa Max microplate reader.
In Vitro Cell Migration Assay
Cells were plated at 1 × 105 cells/well in 200 μL of culture medium in the upper chambers of Transwell permeable supports (Corning, Corning, NY, USA) to track the migration of cells. The Transwell chambers had 8.0-μm pores in 6.5-mm-diameter polycarbonate membranes and used a 24-well plate format. Non-invading cells on the upper surface of each membrane were removed by scrubbing with laboratory paper. Migrated cells on the lower surface of each membrane were fixed with 4% paraformaldehyde for 5 min and stained with hematoxylin for 15 min. Later, the number of migrated cells was counted in three randomly selected fields of the wells under a light microscope at ×50 magnification. To calculate the chemotactic index, the number of cells that migrated in response to the treatment of SHH was divided by the number of spontaneously migrating cells.
Protein Isolation and Western Blot Analysis
The protein expression levels were determined by western blot analysis as previously described.48 Cells were lysed in a buffer containing 50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, and 0.2 mM PMSF. The protein concentrations of the total cell lysates were measured by using bovine serum albumin as a standard. Samples containing equal amounts of protein were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked with 5% skim milk in Tris-buffered saline containing Tween 20 at room temperature. Then the membranes were incubated with primary antibodies against β-actin (ab189073; Abcam), MMP-2 (#4022; Cell Signaling), MMP-9 (#13667; Cell Signaling), SERPINB2 (ab47742; Abcam), SHH (SC-373779; Santa Cruz), total PI3K (#4292; Cell Signaling), phospho-PI3K (#4228; Cell Signaling), total Akt (#4491; Cell Signaling), phospho-Akt (#4060; Cell Signaling), total-ERK1/2 (#9012; Cell Signaling), phospho-ERK1/2 (#9101; Cell Signaling), total FAK (sc-558; Santa Cruz), or phospho-FAK (sc-11765; Santa Cruz) overnight at 4°C and then with HRP-conjugated goat anti-rabbit immunoglobulin G (IgG; 554021; BD PharMingen) or goat anti-mouse IgG (554002; BD PharMingen) secondary antibodies for 60 min at room temperature. Antibody-bound proteins were detected using enhanced chemiluminescence (ECL) reagents.
Adipogenic Differentiation
Endometrial stem cells were incubated in DMEM low-glucose medium supplemented with 500 μM methylxanthine, 5 μg/mL insulin, and 10% FBS. Endometrial stem cells were grown for 3 weeks, with medium replacement twice a week. Lipid droplet formation was confirmed by oil red O staining. Relative quantification of lipid droplet formation was determined by absorbance measurement at 500 nm.
Osteogenic Differentiation
Endometrial stem cells were incubated in DMEM high-glucose medium supplemented with 0.1 μM dexamethasone, 10 mM β-glycerophosphate, 50 μM ascorbate, and 10% FBS. Endometrial stem cells were grown for 3 weeks, with medium replacement twice a week. Differentiated cells were stained with alizarin red S to detect de novo formation of bone matrix. Alizarin red S in samples was quantified by measuring the optical density (OD) of the solution at 570 nm.
Flow Cytometry
Fluorescence-activated cell sorting (FACS) analysis and cell sorting were performed using FACSCalibur and FACSAria machines (Becton Dickinson, Palo Alto, CA, USA), respectively. FACS data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Antibodies to the following proteins were used: allophycocyanin (APC)-conjugated CD44 (Cat. No: 559942, dilution 1/40; BD Biosciences), phycoerythrin (PE)-conjugated CD133 (MACS; 130-080-081, dilution 1/40; Miltenyi Biotec), CD34 (MACS; 30-081-002; Miltenyi Biotec), CD44 (MACS; 130-095-180; Miltenyi Biotec), CD45 (MACS; 130-080-201; Miltenyi Biotec), CD73 (MACS; 130-095-182; Miltenyi Biotec) and CD105 (MACS; 130-094-941; Miltenyi Biotec), CD140b (MACS; 130-105-279; Miltenyi Biotec), CD146 (MACS; 130-111-322; Miltenyi Biotec), and W5C5 (MACS; 130-111-641; Miltenyi Biotec). The FACS gates were established by staining with an isotype antibody or secondary antibody.
Real-Time PCR
Total RNA from skin cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Real-time PCR was performed using a Rotor-Gene Q (QIAGEN). The reaction was subjected to amplification cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 25 s. The relative mRNA expression of the selected genes was normalized to that of peptidylprolyl isomerase A (PPIA) and quantified using the ΔΔCT (threshold cycle) method. The sequences of the PCR primers are listed in Table 1.
Table 1.
Primer Sequences for Quantitative RT-PCR
| Gene | GenBank No. | Direction | Primer Sequence (5′–3′) |
|---|---|---|---|
| Human PPIA | NM_021130 | F | TGCCATCGCCAAGGAGTAG |
| R | TGCACAGACGGTCACTCAAA | ||
| Human C-MYC | NM_002467 | F | AAAGGCCCCCAAGGTAGTTA |
| R | GCACAAGAGTTCCGTAGCTG | ||
| Human KLF4 | NM_001314052 | F | GAACTGACCAGGCACTACCG |
| R | TTCTGGCAGTGTGGGTCATA | ||
| Human NANOG | NM_024865 | F | TGGGATTTACAGGCGTGAGC |
| R | AAGCAAAGCCTCCCAATCCC | ||
| Human SOX2 | NM_003106 | F | AAATGGGAGGGGTGCAAAAGAGGAG |
| R | CAGCTGTCATTTGCTGTGGGTGATG | ||
| Mouse HPRT | NM_013556 | F | GCCTAAGATGAGCGCAAGTTG |
| R | TACTAGGCAGATGGCCACAGG | ||
| Mouse C-MYC | NM_010849 | F | CGCACACACAACGTCTTGGA |
| R | AGGATGTAGGCGGTGGCTTT | ||
| Mouse KLF4 | NM_010637 | F | GGTGCAGCTTGCAGCAGTAA |
| R | AAAGTCTAGGTCCAGGAGGT | ||
| Mouse NANOG | NM_028016 | F | GCCTTACGTACAGTTGCAGC |
| R | TCACCTGGTGGAGTCACAGA | ||
| Mouse SOX2 | NM_011443 | F | GAAGCGTGTACTTATCCTTCTTCAT |
| R | GAGTGGAAACTTTTGTCCGAGA |
Immunofluorescent Staining
Samples were fixed with 4% paraformaldehyde for fluorescent staining. Samples were permeabilized with 0.4 M glycine and 0.3% Triton X-100, and nonspecific binding was blocked with 2% normal swine serum (DAKO, Glostrup, Denmark). Staining was performed as described previously,49 using the primary anti-phalloidin (Cat. No: PHDH1; Cytoskeleton) or anti-GFP (Cat. No: V820-20; Invitrogen) antibody. Samples were examined by fluorescence microscopy (Zeiss LSM 510 Meta). The calculation of expression was based on green fluorescence area and density divided by cell number, as determined from the number of DAPI-stained nuclei, in three randomly selected fields for each sample from a total of three independent experiments.
IPA
An SHH, Akr, or ERK1/2-related genes analysis was performed with IPA version 2.0 software (Ingenuity Systems, Redwood City, CA, USA). Differentially expressed genes (t test, p < 0.005) between non-proliferative cells and proliferative cells were subjected to SHH, Akt, or ERK1/2-related genes analysis (GEO: GSE63074, GSE62564, and GSE85047). The significance of each molecule was measured by Fisher’s exact test (p value), which was used to identify differentially expressed genes from the microarray data that overlapped with genes known to be regulated by a molecule. The activation score (Z score) was used to show the status of predicted molecules by comparing the observed differential regulation of genes (“up” or “down”) in the microarray data relative to the literature-derived regulation direction, which can be either activating or inhibiting.
R2 Database Analysis
We used the R2: Genomics Analysis and Visualization Platform (https://hgserver1.amc.nl:443/cgi-bin/r2/main.cgi/) to analyze the expression levels of SHH, Akt, or ERK1/2 between non-proliferative cells and proliferative cells (GEO: GSE63074, GSE62564, and GSE85047 cohort dataset). The SHH or SERPINB2 value was log2 transformed and median centered. All of the graphics and statistic values were analyzed by GraphPad Prism 5.0, and p values were calculated by a two-tailed Student’s t test (p < 0.05).
Growth Factor Antibody Array
The assay was performed following the manufacturer’s protocol (Abnova AA0089). In brief, SHH or vehicle-treated protein samples were incubated with antibody membranes overnight at 4°C. After washing three times with wash buffer, the membranes were incubated with biotin-conjugated anti-cytokine antibodies overnight at 4°C. The membranes were then washed three times and incubated with HRP-conjugated streptavidin. Chemiluminescence was used to detect signals of the growth factors spotted on the nitrocellulose membrane.
GeneMANIA Algorithm-Based Bioinformatics Analysis
To further analyze genes that interact with or directly regulate SHH, Akt, or ERK1/2 signaling, we imported all identified genes and their corresponding accession numbers into GeneMANIA (http://genemania.org/). To find gene interactions, we considered several factors including co-expression, co-localization, and genetic interactions.
Evaluation of SHH Effects on Normal and TCA-Induced Endometrial Ablation Animal Models
All of the animal experiments were approved and conducted in accordance with the IACUC (LCDI-2018-0015) of the Lee Gil Ya Cancer and Diabetes Institute of Gachon University. The mice were randomly divided into control (vehicle) and SHH treatment groups. Institute of Cancer Research (ICR) mice were exposed to SHH (1 mg/kg) or vehicle (PBS) through intraperitoneal injection for 10 consecutive days. The mice were anesthetized and exsanguinated by cardiac puncture, and then stem cells were isolated from adipose tissues. In addition, 7-week-old immunodeficient NSG mice were subjected to treatment with 2% TCA treatment (150 μL, administered directly into uterine horn), to induce uterine endometrial ablation, or with sterilized PBS vehicle as control. SHH-stimulated or nonstimulated endometrial stem cells (1 × 106 cells) were labeled with GFP and injected intravenously into the tail veins of 7-week-old NSG mice with acute TCA-induced endometrial ablation. The mice were sacrificed 7 days after the GFP-labeled endometrial stem cells were injected. Mice of each group were sacrificed by cervical dislocation. Uterine endometrial tissue was collected and subjected to H&E staining.
Statistical Analysis
All the statistical data were analyzed in GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and evaluated using two-tailed Student’s t tests. The p values <0.05 were considered to indicate statistical significance.
Author Contributions
S.-R.P., S.-R.K., C.H.P., S.L., and S.Y.H. conducted the experiments. H.-Y.L. and I.-S.H. designed the experiments and wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1A2A3074613). This work was also supported by the Technology Innovation Program funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) (no. 20005221).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.11.024.
Contributor Information
In-Sun Hong, Email: hongstem@gachon.ac.kr.
Hwa-Yong Lee, Email: hylee@jwu.ac.kr.
Supplemental Information
References
- 1.Lucas E.S., Salker M.S., Brosens J.J. Uterine plasticity and reproductive fitness. Reprod. Biomed. Online. 2013;27:506–514. doi: 10.1016/j.rbmo.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 2.McLennan C.E., Rydell A.H. Extent of endometrial shedding during normal menstruation. Obstet. Gynecol. 1965;26:605–621. [PubMed] [Google Scholar]
- 3.Omidvar S., Begum K. Menstrual pattern among unmarried women from south India. J. Nat. Sci. Biol. Med. 2011;2:174–179. doi: 10.4103/0976-9668.92329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gargett C.E., Nguyen H.P., Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012;13:235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 5.Gargett C.E., Ye L. Endometrial reconstruction from stem cells. Fertil. Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Lucas E.S., Dyer N.P., Murakami K., Lee Y.H., Chan Y.W., Grimaldi G., Muter J., Brighton P.J., Moore J.D., Patel G. Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells. 2016;34:346–356. doi: 10.1002/stem.2222. [DOI] [PubMed] [Google Scholar]
- 7.Briscoe J., Thérond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 8.Young B., Minugh-Purvis N., Shimo T., St-Jacques B., Iwamoto M., Enomoto-Iwamoto M., Koyama E., Pacifici M. Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev. Biol. 2006;299:272–282. doi: 10.1016/j.ydbio.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 9.He Y., Guo Q., Cheng Y., Qu Y., Sun L., Kong C., Lei L., Zhang G. Abnormal activation of the sonic hedgehog signaling pathway in endometriosis and its diagnostic potency. Fertil. Steril. 2018;110:128–136.e122. doi: 10.1016/j.fertnstert.2018.02.138. [DOI] [PubMed] [Google Scholar]
- 10.Kim K.H., Kim J.M., Choi Y.L., Shin Y.K., Lee H.C., Seong I.O., Kim B.K., Chae S.W., Chung Y.S., Kim S.H. Expression of sonic hedgehog signaling molecules in normal, hyperplastic and carcinomatous endometrium. Pathol. Int. 2009;59:279–287. doi: 10.1111/j.1440-1827.2009.02366.x. [DOI] [PubMed] [Google Scholar]
- 11.Palma V., Lim D.A., Dahmane N., Sánchez P., Brionne T.C., Herzberg C.D., Gitton Y., Carleton A., Alvarez-Buylla A., Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyczek J., Buslei R., Schult D., Hölsken A., Buchfelder M., Heß I., Hahn H., Uhmann A. Hedgehog signaling activation induces stem cell proliferation and hormone release in the adult pituitary gland. Sci. Rep. 2016;6:24928. doi: 10.1038/srep24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forte G., Minieri M., Cossa P., Antenucci D., Sala M., Gnocchi V., Fiaccavento R., Carotenuto F., De Vito P., Baldini P.M. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 14.Gharibi B., Ghuman M.S., Hughes F.J. Akt- and Erk-mediated regulation of proliferation and differentiation during PDGFRβ-induced MSC self-renewal. J. Cell. Mol. Med. 2012;16:2789–2801. doi: 10.1111/j.1582-4934.2012.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng B., Wang C., He L., Xu X., Qu J., Hu J., Zhang H. Neural differentiation of mesenchymal stem cells influences chemotactic responses to HGF. J. Cell. Physiol. 2013;228:149–162. doi: 10.1002/jcp.24114. [DOI] [PubMed] [Google Scholar]
- 16.Song B.Q., Chi Y., Li X., Du W.J., Han Z.B., Tian J.J., Li J.J., Chen F., Wu H.H., Han L.X. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell. Physiol. Biochem. 2015;36:1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 17.Tang J.M., Yuan J., Li Q., Wang J.N., Kong X., Zheng F., Zhang L., Chen L., Guo L.Y., Huang Y.H. Acetylcholine induces mesenchymal stem cell migration via Ca2+ /PKC/ERK1/2 signal pathway. J. Cell. Biochem. 2012;113:2704–2713. doi: 10.1002/jcb.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong I.S., Kang K.S. The effects of Hedgehog on the RNA-binding protein Msi1 in the proliferation and apoptosis of mesenchymal stem cells. PLoS ONE. 2013;8:e56496. doi: 10.1371/journal.pone.0056496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong I.S., Lee H.Y., Choi S.W., Kim H.S., Yu K.R., Seo Y., Jung J.W., Kang K.S. The effects of hedgehog on RNA binding protein Msi1 during the osteogenic differentiation of human cord blood-derived mesenchymal stem cells. Bone. 2013;56:416–425. doi: 10.1016/j.bone.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi H., Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller P., Langenbach A., Kaminski A., Rychly J. Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PLoS ONE. 2013;8:e71283. doi: 10.1371/journal.pone.0071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong L., Hughes O., Yung S., Hyslop L., Stewart R., Wappler I., Peters H., Walter T., Stojkovic P., Evans J. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 23.Gao F., Hu X., Xie X., Liu X., Wang J. Heat shock protein 90 stimulates rat mesenchymal stem cell migration via PI3K/Akt and ERK1/2 pathways. Cell Biochem. Biophys. 2015;71:481–489. doi: 10.1007/s12013-014-0228-6. [DOI] [PubMed] [Google Scholar]
- 24.Eom Y.W., Oh J.E., Lee J.I., Baik S.K., Rhee K.J., Shin H.C., Kim Y.M., Ahn C.M., Kong J.H., Kim H.S., Shim K.Y. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014;445:16–22. doi: 10.1016/j.bbrc.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Q., Zhou C., Bi Z., Wan Y. EGF-induced cell migration is mediated by ERK and PI3K/AKT pathways in cultured human lens epithelial cells. J. Ocul. Pharmacol. Ther. 2006;22:93–102. doi: 10.1089/jop.2006.22.93. [DOI] [PubMed] [Google Scholar]
- 26.Gan Y., Shi C., Inge L., Hibner M., Balducci J., Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29:4947–4958. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 27.Ye M., Hu D., Tu L., Zhou X., Lu F., Wen B., Wu W., Lin Y., Zhou Z., Qu J. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Invest. Ophthalmol. Vis. Sci. 2008;49:497–504. doi: 10.1167/iovs.07-0975. [DOI] [PubMed] [Google Scholar]
- 28.Jeon H.J., Park J., Shin J.H., Chang M.S. Insulin-like growth factor binding protein-6 released from human mesenchymal stem cells confers neuronal protection through IGF-1R-mediated signaling. Int. J. Mol. Med. 2017;40:1860–1868. doi: 10.3892/ijmm.2017.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Toukhy T., Coomarasamy A., Khairy M., Sunkara K., Seed P., Khalaf Y., Braude P. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil. Steril. 2008;89:832–839. doi: 10.1016/j.fertnstert.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs P., Matyas S., Boda K., Kaali S.G. The effect of endometrial thickness on IVF/ICSI outcome. Hum. Reprod. 2003;18:2337–2341. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 31.De Geyter C., Schmitter M., De Geyter M., Nieschlag E., Holzgreve W., Schneider H.P. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil. Steril. 2000;73:106–113. doi: 10.1016/s0015-0282(99)00484-7. [DOI] [PubMed] [Google Scholar]
- 32.Evans J., Hannan N.J., Hincks C., Rombauts L.J., Salamonsen L.A. Defective soil for a fertile seed? Altered endometrial development is detrimental to pregnancy success. PLoS ONE. 2012;7:e53098. doi: 10.1371/journal.pone.0053098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui N., Li A.M., Luo Z.Y., Zhao Z.M., Xu Y.M., Zhang J., Yang A.M., Wang L.L., Hao G.M., Gao B.L. Effects of growth hormone on pregnancy rates of patients with thin endometrium. J. Endocrinol. Invest. 2019;42:27–35. doi: 10.1007/s40618-018-0877-1. [DOI] [PubMed] [Google Scholar]
- 34.Yi K.W., Mamillapalli R., Sahin C., Song J., Tal R., Taylor H.S. Bone marrow-derived cells or C-X-C motif chemokine 12 (CXCL12) treatment improve thin endometrium in a mouse model. Biol. Reprod. 2019;100:61–70. doi: 10.1093/biolre/ioy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S., Rani G., Bose G., Saha I., Bathwal S., Chakravarty B.N. Tamoxifen is Better than Low-Dose Clomiphene or Gonadotropins in Women with Thin Endometrium (<7 mm) after Clomiphene in Intrauterine Insemination Cycles: A Prospective Study. J. Hum. Reprod. Sci. 2018;11:34–39. doi: 10.4103/jhrs.JHRS_9_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L., Xu W.H., Fu X.H., Huang Q.X., Guo X.Y., Zhang L., Li S.S., Zhu J., Shu J. Therapeutic role of granulocyte colony-stimulating factor (G-CSF) for infertile women under in vitro fertilization and embryo transfer (IVF-ET) treatment: a meta-analysis. Arch. Gynecol. Obstet. 2018;298:861–871. doi: 10.1007/s00404-018-4892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deane J.A., Gualano R.C., Gargett C.E. Regenerating endometrium from stem/progenitor cells: is it abnormal in endometriosis, Asherman’s syndrome and infertility? Curr. Opin. Obstet. Gynecol. 2013;25:193–200. doi: 10.1097/GCO.0b013e32836024e7. [DOI] [PubMed] [Google Scholar]
- 38.Kilic S., Yuksel B., Pinarli F., Albayrak A., Boztok B., Delibasi T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J. Assist. Reprod. Genet. 2014;31:975–982. doi: 10.1007/s10815-014-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J., Zhang Q., Wang Y., Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod. Sci. 2015;22:181–188. doi: 10.1177/1933719114537715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L., Li Y., Guan C.Y., Tian S., Lv X.D., Li J.H., Ma X., Xia H.F. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell. Res. Ther. 2018;9:36. doi: 10.1186/s13287-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagori C.B., Panchal S.Y., Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J. Hum. Reprod. Sci. 2011;4:43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh N., Mohanty S., Seth T., Shankar M., Bhaskaran S., Dharmendra S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J. Hum. Reprod. Sci. 2014;7:93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santamaria X., Cabanillas S., Cervelló I., Arbona C., Raga F., Ferro J., Palmero J., Remohí J., Pellicer A., Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum. Reprod. 2016;31:1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 44.Tersigni C., D’Ippolito S., Di Nicuolo F., Marana R., Valenza V., Masciullo V., Scaldaferri F., Malatacca F., de Waure C., Gasbarrini A. Recurrent pregnancy loss is associated to leaky gut: a novel pathogenic model of endometrium inflammation? J. Transl. Med. 2018;16:102. doi: 10.1186/s12967-018-1482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mezey E., Chandross K.J., Harta G., Maki R.A., McKercher S.R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 46.Huang C., Jacobson K., Schaller M.D. MAP kinases and cell migration. J. Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Crawford R., Chen C., Xiao Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng. Part B Rev. 2013;19:516–528. doi: 10.1089/ten.TEB.2012.0672. [DOI] [PubMed] [Google Scholar]
- 48.Choi E.S., Jung J.Y., Lee J.S., Park J.H., Cho N.P., Cho S.D. Myeloid cell leukemia-1 is a key molecular target for mithramycin A-induced apoptosis in androgen-independent prostate cancer cells and a tumor xenograft animal model. Cancer Lett. 2013;328:65–72. doi: 10.1016/j.canlet.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Dong H.J., Jang G.B., Lee H.Y., Park S.R., Kim J.Y., Nam J.S., Hong I.S. The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci. Rep. 2016;6:22966. doi: 10.1038/srep22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.