Abstract
Limited data exist on characteristics of central nervous system viruses (CNS-V) in allogeneic hematopoietic stem cell transplant (HCT) recipients. Between 2007 and 2015, the Center for International Blood and Marrow Transplant Research (CIBMTR) received information on 27,532 patients undergoing HCT. Of these, centers reported 165 HCT recipients with CNS-V detected in cerebrospinal fluid within six months after HCT. CNS viruses predominantly included human herpes virus 6 (HHV-6) (73%), followed by Epstein-Barr Virus (10%), cytomegalovirus (3%), varicella zoster virus (3%), herpes simplex virus (3%) and Adenovirus (3%). Median time of viral detection in CNS was 31 days after HCT; and viral detection was earlier in patients with CNS HHV-6. Concurrent viremia occurred in 52% of patients. Cord blood transplant recipients (CBT) accounted for the majority (53%) of patients with CNS-V. Myeloablative conditioning (65%), use of fludarabine (63%), or use of anti-thymocyte globulin (61%) were also predominant. Overall survival from the time of detection of CNS-V was 50% at 6 months and 30% at 5 years. Infections were the leading cause of death (32%). In summary, CBT recipients predominated in the population with CNS-V. Outcomes after CNS-V were poor with significant mortality seen in the first 6 months.
Keywords: Viral infections, cerebrospinal fluid, stem cell transplant recipients
Introduction
Allogeneic hematopoietic cell transplant (HCT) is a potentially curative treatment option for a wide range of disorders such as fatal hematological malignancies and non-malignant disorders(1). HCT recipients are susceptible to infections with, or re-activation of, opportunistic pathogens. This is due to deficiencies in immune recovery post-HCT as well as graft versus host disease (GVHD), associated with aberrant immune response and requirement of immunosuppressive therapies for prevention and treatment.
Neurologic complications involving the central nervous system (CNS) reportedly occur in as many as 25% of HCT recipients (2, 3). Infections in HCT recipients are generally associated with high mortality but summary data do not detail type or site of infection (1). Many viruses including herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), human herpes virus 6 (HHV-6), Adenovirus (ADV), and others (JC virus or West Nile virus) may cause CNS infection. Limited data exist in the literature on CNS viruses (CNS-V) identified after HCT and most focus on HHV-6. Our aim was to conduct a large-scale study utilizing the Center for International Blood and Marrow Transplant Research (CIBMTR) database to define patient characteristics and survival outcomes in HCT recipients with identified virus in the CNS.
Methods
Data source and patients
The CIBMTR is a working group of more than 500 transplant centers worldwide that contribute detailed data on HCT patients to the statistical center at the Medical College of Wisconsin. Participating centers are required to report all consecutive transplants and follow patients longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. The CIBMTR performs observational studies in compliance with all applicable federal regulations pertaining to the protection of human research participants. The CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, gender, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. All CIBMTR centers contribute TED data. CRF data are collected on a subset of registered patients, selected by weighted randomization. CRF data include more detailed disease and pre- and post-transplant clinical information, including infection data. TED and CRF level data are collected pre-transplant, 100 days and six months post-HCT and annually thereafter or until death. This analysis includes only patients with CIBMTR CRF data. Infection data reported include date of infection, site of infection and organism identified. Information on symptoms, diagnostic criteria, and therapy of infection are not collected.
Subject eligibility
Eligible patients included all adult and pediatric patients undergoing first alloHCT for acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL) and non-malignant disorders reported to CIBMTR from 2007–2015. Those patients reported with a CNS-V in the first six months after HCT were included in this analysis.
Statistical analysis and definitions
Patient, disease, and transplant factors were reported as frequencies or medians and ranges as appropriate. The Chi-square test (categorical variables) or Mann-Whitney U test (continuous) were used for comparisons between the HHV-6 patients and non-HHV-6 CNS-V patients. Kaplan-Meier estimates for overall survival from the time of diagnosis of CNS-V until death or last follow-up were calculated. Early malignant disease included patients with AML/ALL in first complete remission (CR), refractory anemia or refractory cytopenias with/without ringed sideroblasts, 5q- MDS, CML in CR1 or first chronic phase, and Non-Hodgkin’s Lymphoma (NHL) in CR1. Advanced disease included AML/ALL that was primary refractory, never treated, or active relapse; Refractory anemia with excessive blasts (RAEB), CML in 3rd or more chronic or accelerated phase, or any blast phase; and NHL that is primary refractory, or untreated or resistant relapse. All other malignant diseases were categorized as intermediate. Acute GVHD was defined by consensus criteria(4). Neutrophil engraftment was defined as the first of three days with an absolute neutrophil count (ANC) >0.5 × 109/L. Concurrent viremia was defined as the presence of the reported CNS virus in the bloodstream within 14 days before or after the reported date of CNS-V.
Results
Patients Characteristics
Between 2007 and 2015, the CIBMTR received information on 27,532 patients undergoing HCT. Of these, 165 (0.6%) were reported to have a CNS-V six months after HCT. Patient, disease and transplant characteristics for the patients with CNS-V are presented in Table 1. Patients were primarily from US Centers (n= 147, 89%). The majority of patients received a transplant for treatment of a malignant disease (n =144, 87%), and advanced malignancy was present in 42 (29%) patients. Median time from diagnosis to HCT was 11 months (range, 1–253 months). Predominant stem cell source was umbilical cord blood with 88 patients receiving either single (n=35) or double (n=53) unit grafts. Three additional patients received umbilical cord in addition to another stem cell source from a different donor. Myeloablative conditioning was most common (65%) with fludarabine (63%) and/or total body irradiation (58%) being incorporated into the conditioning regimen. Anti-thymocyte globulin was used in 61% patients. Nearly all patients (n=150, 91%) received either acyclovir or valacyclovir prophylaxis at the time of transplant. Post-transplant use of colony-stimulating factors (n=115, 70%) and intravenous immunoglobulin (n=99, 60%) were reported frequently. Median follow-up of surviving patients was 49 months (range 3–100).
Table 1:
Characteristics of patients who underwent first allogeneic HCT with detection of CNS-V within 180 days as reported to the CIBMTR, 2007 to 2015
| Variable | HHV-6 CNS-V n=123 N(%) | Non-HHV-6 CNS-V n=42 N(%) | p-value |
|---|---|---|---|
| Gender, male | 77 (63) | 23 (55) | 0.369 |
| Age, median (range), years | 43 (2 – 76) | 42 (1 – 74) | 0.996 |
| Age at transplant, years | 0.676 | ||
| <1 – 20 | 40 (33) | 13 (31) | |
| 21–40 | 20 (16) | 6 (14) | |
| 41–60 | 38 (31) | 15 (36) | |
| >60 | 25 (20) | 8 (19) | |
| Disease | 0.712 | ||
| AML | 40 (330 | 13 (31) | |
| ALL | 24 (20) | 6 (14) | |
| CLL/PLL | 7 (6) | 2 (5) | |
| CML | 7 (6) | 2 (5) | |
| MDS | 16 (13) | 9 (21) | |
| MPS | 2 (2) | 0 | |
| Other acute leukemia | 3 (2) | 0 | |
| NHL | 9 (7) | 4 (10) | |
| Severe aplastic anemia | 2 (2) | 1 (2) | |
| Inherited abnormalities erythrocyte differentiation or function | 2 (2) | 1 (2) | |
| SCID and other immune system disorders | 3 (2) | 2 (5) | |
| Inherited abnormalities of platelets | 0 | 1 (2) | |
| Inherited disorders of metabolism | 6 (5) | 0 | |
| Histiocytic disorders | 1 (<1) | 1 (2) | |
| Autoimmune Diseases | 1 (<1) | 0 | |
| Time from diagnosis to HCT, median (range), months | 11 (1 – 155) | 12 (3 – 253) | 0.227 |
| Graft type | 0.050 | ||
| Bone Marrow | 11 (9) | 8 (19) | |
| Peripheral blood | 36 (29) | 19 (45) | |
| Cord blood | 73 (59) | 15 (36) | |
| UCB + other | 3 (2) | 0 | |
| Donor type | 0.099 | ||
| Cord | 73 (59) | 15 (36) | |
| HLA-matched related | 10 (8) | 7 (16) | |
| HLA-mismatched relative | 1 (<1) | 1 (2) | |
| 8/8 unrelated | 17 (14) | 13 (31) | |
| 7/8 unrelated | 15 (12) | 4 (10) | |
| <6/8 unrelated | 1 (<1) | 0 | |
| Multiple donors (UCB + Other donor) | 3 (2) | 0 | |
| Unrelated, HLA unknown | 3 (2) | 2 (5) | |
| Conditioning regimen intensity | 0.851 | ||
| Myeloablative | 80 (65) | 27 (64) | |
| RIC | 24 (20) | 10 (24) | |
| NMA | 18 (15) | 5 (12) | |
| Missing | 1 (<1) | 0 | |
| Received ATG | 40 (33) | 24 (57) | 0.005 |
| Received Alemtuzumab | 5 (4) | 6 (14) | 0.022 |
Abbreviations:
Abbreviations: AML=acute myelogenous leukemia; ALL=acute lymphoblastic leukemia; ATG=anti-thymocyte globulin; CIBMTR= Center for International Blood and Marrow Transplantation; CML= chronic myelogenous leukemia; CMV=cytomegalovirus; CNS-V,= central nervous system-virus; HCT= hematopoietic cell transplantation; HLA=human leukocyte antigen; MDS =myelodysplastic syndromes; MPS= myeloproliferative syndromes; N=number; NHL= non-Hodgkin lymphoma; NMA, nonmyeloablative; RIC,= reduced intensity conditioning; SCID=severe combined immunodeficiency; TAC = tacrolimus; TBI = total body irradiation; UCB= umbilical cord blood; RIC,=reduced intensity conditioning; NMA=nonmyeloablative.
Clinical factors related to identified CNS viruses
At the time of transplant, patients were lymphopenic with the median absolute lymphocyte count (ALC) 1×109/μL (range, 0–71.3). Post-transplant, neutrophil engraftment occurred at a median of 16 days (range, 6–87). Acute GVHD occurred in 96 (58%) patients prior to the diagnosis of CNS-V. Forty-seven (28%) patients reported a CMV reactivation within the first 100 days after HCT. For patients with available data, (n=46, 28%), CD4 + and CD8 + lymphopenia were generally present at day 100 [CD4 median 0.3 × 109/μL (range, 0–1420); CD8 median 0.2 × 109/μL (range, 0–637)].
CNS virus detection
Median onset of CNS-V was 31 days (range 12–180) in this cohort and 144 (87%) patients developed CNS-V by day 100. Time from HCT to detection of first CNS virus occurred earlier for HHV-6 [median 28 days (12–176)] than in non-HHV-6 CNS-V [median 80 days (13–180), <0.001]. Table 2 denotes the frequency of specific isolates by time period after HCT. By day 60 of HCT, a total of 123 CNS-V had been detected. Of these, HHV-6 was the predominant CNS-V reported by day 60 of HCT.
Table 2.
Frequency of CNS viral organisms by time period
| Time period Virus in CNS | Day 0 – 30 | Day 31 – 60 | Day 61 – 100 | Day 101 – 180 | Total Infections |
|---|---|---|---|---|---|
| HHV-6 | 77 | 32 | 8 | 6 | 122 |
| CMV | 0 | 3 | 2 | 3 | 8 |
| EBV | 3 | 3 | 8 | 4 | 18 |
| Adeno | 0 | 1 | 1 | 2 | 4 |
| VZV | 1 | 0 | 3 | 1 | 5 |
| HSV | 0 | 1 | 1 | 2 | 4 |
| Polyoma | 0 | 2 | 0 | 4 | 6 |
| Total infections | 81 | 42 | 23 | 23 | 168* |
3 patients had more than one virus type
Abbreviations: CNS=central nervous system; HHV-6= human herpes virus-6; CMV=cytomegalovirus; EBV=Epstein-Barr virus; Adeno=adenovirus; VZV=varicella zoster virus; HSV=herpes simplex virus.
Of the 165 patients with CNS-V detected six months after HCT, HHV-6 was the CNS-V in 123 patients (76%), with non-HHV-6 CNS-V accounting for the remainder. Two patients in the HHV-6 cohort did have additional CNS-V reported as well. Concurrent viremia (by same virus as identified in CNS) was reported in 75 (45%) of these total 165 patients.
In the HHV-6 CNS-V only cohort (excludes the 2 patients with multiple viruses detected), isolated HHV-6 CNS detection was reported in 63 patients (52%), whereas 56 patients were identified with HHV-6 in the CNS and had viremia reported within 14 days prior or after the date of CNS viral detection. An additional 2 patients had HHV-6 identified in the CNS as well as non-viremia, non-CNS detection of HHV-6 in broncho-alveolar fluid.
Amongst the patients with non-HHV-6 CNS-V (n=44), EBV was the leading causative virus in 18 patients (41%). Isolated EBV CNS involvement was seen in 10 patients, whereas 8 patients had concurrent EBV viremia and CNS involvement.
Few (n=3) patients experienced more than one CNS virus detectable, with 2 patients reported with both CMV and HHV-6 and 1 patient with CMV and HSV.
Overall Survival and Cause of Death
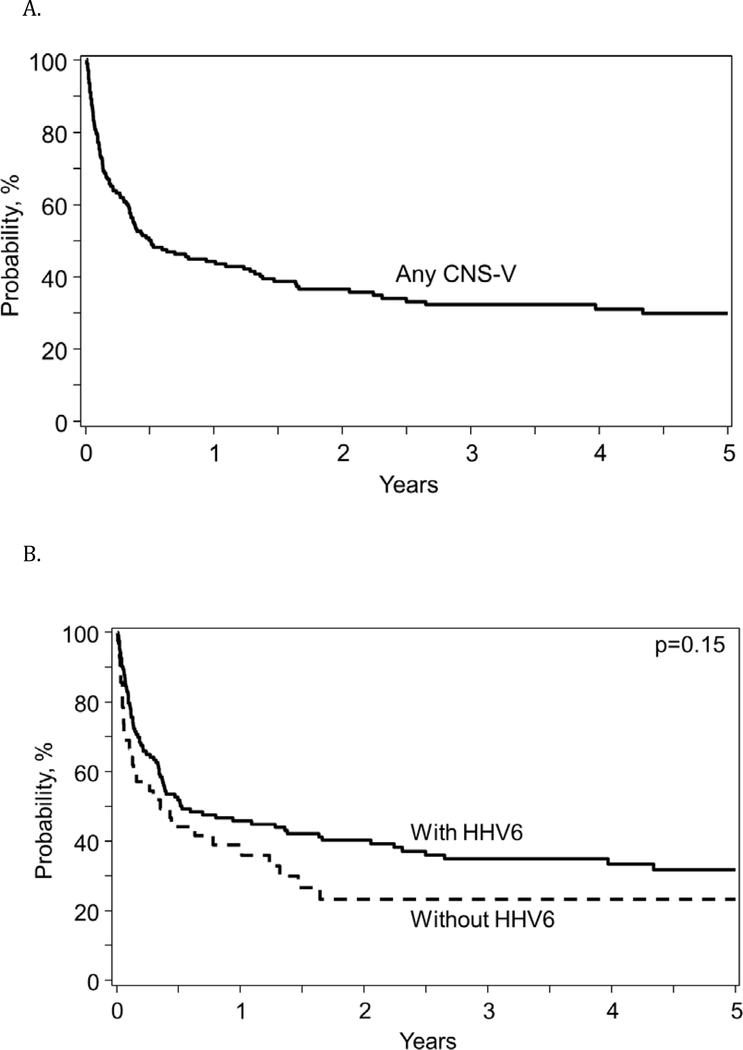
Overall survival from the diagnosis of CNS-V was 50% (95% Confidence Interval (CI): 42–57%) at 6 months and decreased to 30% (95% CI: 22–37%) by 5 years after the CNS-V (Figure 1A) and there was no difference in OS based upon HHV-6 vs non-HHV-6 CNS-V (Figure 1B). Infection was reported as the primary cause of death in 35 patients (32%) with viral infections reported as the cause in 21 of these patients, followed by recurrence of underlying malignancy in 27 patients (24%), organ failure in 25 patients (23%) and GVHD in 15 patients (14%). For patients with relapse, organ failure, and GVHD as the primary cause of death, infections were reported as a contributing factor in 6, 9, and 5 patients, respectively. Thus, infection was a primary or secondary cause of death in 55 (50%) patients.
Figure 1:
Overall survival for allogeneic HCT patients after diagnosis of any CNS-V (A) and comparing HHV-6 to non-HHV-6 CNS-V (B)
Discussion
Utilizing the CIBMTR database, we analyzed the characteristics and the outcomes of 165 HCT recipients with CNS-V. Consistent with other studies, we found HHV-6 to be the leading CNS-V in HCT recipients (5). Median time for viral detection in CNS occurred earlier for HHV-6 vs. non-HHV-6 viruses (28 vs. 80 days), with concurrent viremia reported in only 52% of the patients. In our cohort, patients with CNS-V detected were more commonly recipients of CBT [n = 88 (53%)] compared to other donor types [related donor = 19, unrelated adult donor = 55, or multiple non-CBT donors = 3]. Other predominate transplant characteristics in our cohort includes use of myeloablative-conditioning (65%), and receipt of fludarabine (63%) or anti-thymocyte globulin (61%). Overall prognosis was poor after detection of CNS-V, with only 50% survival at 6 months.
Schmidt-Hieber et al, conducted a retrospective, multi-center study that describes detection of CNS-V in 1.2% of HCT recipients during the first 3 years post-transplant (5). Similar to our findings, HHV-6 was the most frequently detected CNS-V. The authors noted a shorter time to detection of HHV-6 CNS-V compared with the non-HHV-6 CNS-V group. They also reported detection of more than one CNS-V in 16% of the patients, with 80% mortality rate for deaths attributed to viral encephalitis in this group. In contrast with the findings of this study, in our cohort we only found 3 patients with CNS-V due to more than one virus type. It is possible that the difference in the findings on CNS-V due to more than one virus type may reflect under-reporting or under diagnosis in our cases. In a prospective study on herpes virus CNS infections in HCT recipients conducted by Wu M et al, EBV was noted to be the most common herpes virus followed by HSV, CMV and VZV(6). The authors describe an overall earlier median onset time of 65 days, which co-relates with our study findings supporting an overall early onset time for CNS-V following HCT.
Previous studies have identified recipients of CBT to be at higher risk for HHV-6 reactivation and also for HHV-6 encephalitis (cumulative incidence at day 70, 7.9% in CBT recipients vs. 1.2% in other donor sources, p =.008) (7). Hill et al describe the incidence of HHV-6 encephalitis to be 9.9% after CBT and 0.7% after adult-donor HCT (8). Cord blood contains smaller numbers of T cells, the majority of which are immunologically naïve and thus lack a primed HHV-6 specific memory T cells. In our study, CBT recipients accounted for 53% of the cases with CNS-V. Multiple retrospective studies have analyzed an association between high levels of HHV-6 reactivation and CNS dysfunction (7, 9, 10). In a large prospective study, Zerr et al identified HHV-6 reactivation as a predictor of CNS dysfunction, however, most patients did not undergo CSF examination (11). Ogata et al conducted a prospective, multi-center study in which they showed high levels of plasma HHV-6 DNA at day 70 after HCT to be associated with a high risk of HHV-6 encephalitis (7).
The challenge remains regarding on-going monitoring for risk of CNS viral reactivation. Olson et al, reported a high frequency of HHV-6 viremia but low incidence of encephalitis in CBT recipients transplanted without ATG (12). In this registry analysis, data regarding the frequency and indication of plasma testing for viremia is unknown and non-testing may account for the seemingly low occurrence of concurrent viremia. We identified a concurrent HHV-6 viremia in 34% of our cases with HHV-6 CNS-V; however, correlation with viral loads was not possible due to non-collection of viral load data within CIBMTR. Zerr et al described detection of HHV-6 in plasma of HCT recipients with HHV-6 encephalitis to be short-lived, whereas HHV-6 DNA can be detected longer in CSF (13). This may explain why 63 (52%) patients with HHV-6 CNS-V in our study presented with only CNS involvement without reported viremia. Alternatively, under-reporting of viremia and/or lack of serum testing for HHV-6 may contribute to our findings.
There are several limitations to this study including its retrospective nature. CIBMTR forms do not collect detailed information on clinical presentation, indication for evaluation of CSF, CSF and whole blood viral loads, or neuroimaging. However, given the invasive nature of lumbar puncture for CSF analysis, it is unlikely that patients reported with CNS-V were identified without clinical symptoms. More importantly, without data capture of negative CSF evaluations in patients with presumably similar symptoms, our dataset is unable to determine the likelihood of finding a CNS-V in patients with clinical symptoms prompting lumbar puncture and CSF analysis. This also limits a risk factor analysis for CNS-V as the appropriate control population, (HCT recipients receiving a lumbar puncture without a virus identified) is unavailable for comparative analysis. We do not have information on how many subjects had biopsy or autopsy proven encephalitis or had false negative cultures. There is also no means of determining chromosomal integration for HHV-6 (14, 15). Furthermore, some viral infections of the CNS may be rapidly fatal and the patient may have expired before CSF could be obtained. Finally, response to treatment and neurologic sequelae of CNS-V is unable to be ascertained with the current CIBMTR data capture.
In conclusion, this study demonstrates HHV-6 as the predominant CNS-V. HHV-6 CNS-V occurred early (median 28 days) after transplant and recipients of CBT were predominant in our cohort. Mortality following detection of CNS-V remains high. The results of our large-scale analysis serve to improve our understanding of the mortality impact of HHV-6 and non-HHV-6 CNS-V following HCT. Prospective studies are needed to better define the incidence and clinical outcomes of HHV-6 and non-HHV-6 related viral encephalitis as well as to identify improved diagnostic approaches and early treatment options in the HCT recipients.
Acknowledgements
Financial disclosure: The authors declare no financial disclosures relevant to the work presented in this report.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report
References
- 1.D’Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2017. Available at: http://www.cibmtr.org.
- 2.Denier C, Bourhis JH, Lacroix C, Koscielny S, Bosq J, Sigal R, et al. Spectrum and prognosis of neurologic complications after hematopoietic transplantation. Neurology. 2006;67(11):1990–7. [DOI] [PubMed] [Google Scholar]
- 3.Sostak P, Padovan CS, Yousry TA, Ledderose G, Kolb HJ, Straube A. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology. 2003;60(5):842–8. [DOI] [PubMed] [Google Scholar]
- 4.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 5.Schmidt-Hieber M, Schwender J, Heinz WJ, Zabelina T, Kuhl JS, Mousset S, et al. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica. 2011;96(1):142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, Huang F, Jiang X, Fan Z, Zhou H, Liu C, et al. Herpesvirus-associated central nervous system diseases after allogeneic hematopoietic stem cell transplantation. PLoS One. 2013;8(10):e77805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013;57(5):671–81. [DOI] [PubMed] [Google Scholar]
- 8.Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18(11):1638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ljungman P, Wang FZ, Clark DA, Emery VC, Remberger M, Ringden O, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111(3):774–81. [PubMed] [Google Scholar]
- 10.Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193(1):68–79. [DOI] [PubMed] [Google Scholar]
- 11.Zerr DM, Gooley TA, Yeung L, Huang ML, Carpenter P, Wade JC, et al. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;33(6):763–71. [DOI] [PubMed] [Google Scholar]
- 12.Olson AL, Dahi PB, Zheng J, Devlin SM, Lubin M, Gonzales AM, et al. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transplant. 2014;20(6):787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerr DM, Gupta D, Huang ML, Carter R, Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(3):309–17. [DOI] [PubMed] [Google Scholar]
- 14.Hill JA, Magaret AS, Hall-Sedlak R, Mikhaylova A, Huang ML, Sandmaier BM, et al. Outcomes of hematopoietic cell transplantation using donors or recipients with inherited chromosomally integrated HHV-6. Blood. 2017;130(8):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedlak RH, Hill JA, Nguyen T, Cho M, Levin G, Cook L, et al. Detection of Human Herpesvirus 6B (HHV-6B) Reactivation in Hematopoietic Cell Transplant Recipients with Inherited Chromosomally Integrated HHV-6A by Droplet Digital PCR. J Clin Microbiol. 2016;54(5):1223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]