Abstract
Background
Proline–glycine–proline (PGP) is a bioactive fragment of collagen generated by the action of matrix metalloproteinase-9 (MMP-9) and prolylendopeptidase (PE), and capable of eliciting neutrophil chemotaxis and epithelial remodelling. PGP is normally then degraded by leukotriene A4 hydrolase (LTA4H) to limit inflammation and remodelling. This study hypothesized that early and persistent airway neutrophilia in Cystic Fibrosis (CF) may relate to abnormalities in the PGP pathway and sought to understand underlying mechanisms.
Methods
Broncho-alveolar lavage (BAL) fluid was obtained from 38 CF (9 newborns and 29 older children) and 24 non-CF children. BAL cell differentials and levels of PGP, MMP-9, PE and LTA4H were assessed.
Results
Whilst PGP was present in all but one of the older CF children tested, it was absent in non-CF controls and the vast majority of CF newborns. BAL levels of MMP-9 and PE were elevated in older children with CF relative to CF newborns and non-CF controls, correlating with airway neutrophilia and supportive of PGP generation. Furthermore, despite extracellular LTA4H commonly being greatly elevated concomitantly with inflammation to promote PGP degradation, this was not the case in CF children, potentially owing to degradation by neutrophil elastase.
Conclusions
A striking imbalance between PGP-generating and -degrading enzymes enables PGP accumulation in CF children from early life and potentially supports airway neutrophilia.
Keywords: Neutrophil, Protease, Matrikine, Cystic fibrosis
Graphical abstract
Highlights
-
•
PGP is a collagen-derived pro-neutrophilic mediator.
-
•
PGP accumulates in the airways of children with cystic fibrosis.
-
•
Imbalance of PGP generation (MMP/PE) vs degradation (LTA4H) enables PGP build-up.
-
•
LTA4H is destroyed by neutrophil elastase to prevent PGP degradation.
-
•
Infections are key to spiking PGP generation in setting it cannot be degraded.
1. Introduction
Neutrophils are critical components of the body's immune response to infection, but owing to the indiscriminate nature of their products an over-exuberant or persistent neutrophilic inflammation is implicated in the pathologies of chronic airways diseases such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF). A protease-antiprotease imbalance is a hallmark of these diseases, whereby several matrix metalloproteinases (MMPs) and neutrophil elastase (NE) correlate with pathological changes. Proteases degrade components of the extracellular matrix (ECM), disrupting tissue architecture and releasing ECM-derived chemoattractant signals, termed ‘matrikines’, that perpetuate inflammation.
The tripeptide Proline-Glycine-Proline (PGP) is one such matrikine [1,2], being a neutrophil chemoattractant derived from ECM collagen via the sequential enzymatic activity of specific matrix metalloproteinases (MMPs) and prolylendopeptidase (PE) [3]. PGP can subsequently be chemically acetylated on its N-terminus (Ac-PGP), which functions to enhance its chemotactic potential [4,5]. Since neutrophils are a source of the proteases that generate PGP it is anticipated that this pathway can drive a self-sustaining circle of neutrophilic inflammation [6]. Accordingly, PGP/AcPGP is found in patients with chronic airways diseases, correlating with disease severity and supporting neutrophilic persistence [1,3,7,8]. More recently, we have demonstrated PGP/AcPGP is also capable of directly promoting proliferation and radial spreading of bronchial epithelial cells, independent of its action on neutrophils [9]. PGP/AcPGP persistence was subsequently demonstrated to drive pathological epithelial remodelling by augmenting epithelial hypertrophy and mucus hypersecretion [9], which are again hallmark feature of specific chronic lung diseases.
To limit PGP persistence, we identified an anti-inflammatory pathway whereby PGP is degraded during episodes of acute neutrophilic inflammation by the extracellular activity of the enzyme leukotriene A4 hydrolase (LTA4H) [4]. We demonstrated that this degradation was critical to limit inflammation [10] and to prevent pathological epithelial remodelling [9]. LTA4H classically functions intracellularly to catalyse the generation of pro-inflammatory lipid mediator leukotriene B4 (LTB4) [11,12]. LTB4 can drive the recruitment and activation of an array of cells including neutrophils and is implicated in protection against micro-organisms but also in the pathology of multiple diseases [13]. Thus LTA4H represents a highly unusual enzyme with opposing pro- and anti-inflammatory activities that dictate the amplitude and persistence of neutrophilic inflammation [14].
CF is a lethal genetic disorder caused by defective function of the Cystic Fibrosis Transmembrane Conductance Regulator, which predispose patients to infection, chronic airway inflammation and airway remodelling. The airways of CF patients are thought to lack inflammation at birth, but become inflamed and infected with characteristic pathogens early in life [15]. Neutrophils are a prominent feature of CF airway inflammation, with numbers and activity exhibiting an inverse relationship with lung function [[16], [17], [18]]. We hypothesized that PGP could support neutrophilic inflammation in early life CF as a consequence of an aberrant LTA4H-PGP pathway. We subsequently demonstrate that BAL fluid in children with established CF contains PGP, correlating with neutrophilic inflammation, as a consequence of a striking imbalance between PGP-generating and –degrading enzymes, with the latter targeted for proteolytic degradation in the CF lung.
2. Methods
2.1. Subjects
Samples were obtained from patients undergoing clinically-indicated fibreoptic bronchoscopy, as previously described [19], in the Pediatric department of the Royal Brompton Hospital. BAL fluid was processed as described previously [20] and stored for research once all clinical tests had been undertaken. CF had been diagnosed on conventional criteria [21]. CFTR genotyping had been undertaken as part of clinical diagnosis by the local screening genetics centres. Those in the ‘routine screened infant surveillance programme’ (RSISP)-CF group had all been diagnosed as part of the UK Newborn Screening programme and were undergoing the Centre's routine surveillance investigations scheduled for around 4 months of life; the ‘clinical concern’ (CC)-CF group comprised of patients older than 4 months who were having a bronchoscopy because of clinical deterioration; this was a mix of medium-term concerns and acute (symptomatic) exacerbation, but as there was substantial overlap between these groups, further subdivision has not been undertaken. Indications for bronchoscopy within the non-CF controls were as defined in Table 1. Microbiological culture status was prospectively defined as positive if any airway sample grew bacteria or fungi at the time of the bronchoscopy or in the previous two weeks. Data were not available for viral detection. Spirometric data were collected, where available, from the CF annual assessments for the 5 years following bronchoscopy; in our centre, these measurements are only made on children from around 5 years of age. The research project was approved by the Royal Brompton & Harefield Research Ethics Committee and the institution's Respiratory Biomedical Research Unit under project 10/H0504/9. All parents gave informed consent; age-appropriate assent was also sought.
Table 1.
Demographics of CF and control children undergoing bronchoscopy.
| Non-CF control (n = 24) | RSISP-CF (n = 9) | CC-CF (n = 29) | |
|---|---|---|---|
| Male: Female (%) | 33: 67 | 50: 50 | 45: 55 |
| Age, years | 6.5 (10.6) | 0.3 (0.2) | 11.9 (5.8) |
| CFTR genotype (n) | ND | ΔF508 / ΔF508 = 5 ΔF508 / Other = 3 Other / Other = 1 | ΔF508 / ΔF508 = 16 ΔF508 / Other = 10 Other / Other = 1 Unknown = 2 |
| Indication for bronchoscopy (n) | Stridor (n = 4); Recurrent croup (n = 3); Broncho/Laryngomalacia (n = 6); Haemoptysis (n = 4); Persistent dry cough (n = 2); Barking cough (n = 1); Vascular ring (n = 1); Recurrent chest infections (n = 1); Breathing difficulties (n = 2) | Routine surveillance as part of newborn screening programme | Clinical deterioration or loss of lung function |
| Culture positive (%) | 33% | 11% | 79% |
| Pathogens in BAL fluid | Bacteria P. aeruginosa (n = 1) S. aureus (n = 5) Other (n = 3) |
Bacteria P.aeruginosa (n = 1) |
Bacteria P. aeruginosa (n = 12) S. aureus (n = 5) Other (n = 4) Fungi A. fumigatus (n = 12) C. albicans (n = 4) |
Data presented as median value (IQR) unless otherwise stated.
BAL, bronchoalveolar lavage; RSISP, routine screened infant surveillance program; CC, bronchoscopy for clinical concern; ND, not determined.
2.2. Protease quantification and activity
The concentration of MMP-9 (R&D Systems, Minneapolis, MN), PE (MyBiosource, San Diego, CA, USA) and LTA4H (USCN Life Science, Hubei, PRC) in BAL fluid was measured using an ELISA, according to the manufacturer's directions. BAL fluid MMP-9 activity was determined by Fluorokine E kit (R&D Systems, Minneapolis, MN), and NE activity by a Fluorometric assay kit (Abcam, Cambridge, UK), according to the manufacturer's directions.
2.3. Interleukin-8 (IL-8) quantification
The concentration of IL-8 in BAL fluid was measured using an ELISA, according to the manufacturer's directions (R&D Systems, Minneapolis, MN).
2.4. LTB4 quantification
The concentration of LTB4 in BAL fluid was assayed using an ELISA, according to manufacturer's directions (R&D systems, Minneapolis, MN).
2.5. PGP interrogation
BAL fluid concentrations of PGP/AcPGP were determined by liquid chromatography-electrospray ionization-tandem mass spectrometry (ESI-LC/MS/MS), as previously described [10]. To ascertain the PGP-degrading activity of BAL fluid, it was incubated with 0.4 mM PGP at 37 °C in 5% CO2 for varying periods of time. Degradation was subsequently calculated my enumeration of loss of peptide by ESI-LC/MS/MS and release of free proline and its ensuing reaction with Ninhydrin, as previously described [10] (additional details provided in an online data supplement).
2.6. LTA4H degradation by NE
Recombinant human LTA4H (final concentration 20-100 g/ml; Cayman Chemical, Ann Arbor, USA) and NE (final concentration 20-100 g/ml; Abcam, Cambridge, UK) were incubated alone or in combination in a final volume of 100 l PBS at 37 °C in 5% CO2 for 2 h and LTA4H protein levels determined via Coomassie staining of gels and Western blot. Ensuing LTA4H PGP-degrading activity was assessed by ESI-LC/MS/MS and generation of free proline as described above (additional details provided in an online data supplement).
2.7. Statistical analysis
There were no previous data to inform a power calculation, so group size is opportunistic based on availability of samples. For nonparametric data, shown as median (IQR), between-group comparisons were performed with a Kruskal-Wallis test followed by a Dunns post-test. Associations were tested by Spearman rank correlation.
3. Results
3.1. Patient demographics
BAL fluid was analysed from 62 children: 24 non-CF controls, 29 in the CC-CF group and 9 in the RSISP-CF group (Table 1). Indications for bronchoscopy within the non-CF controls were as defined in Table 1. Of the non-CF controls, 8 were culture positive (P. aeruginosa (n = 1); M. catarrhails (n = 1); coliforms (n = 1); S. aureus (n = 4); H. influenzae and S. pneumoniae (n = 1)). The majority of the CC-CF group (23/29, 79% patients) were culture positive. The RSISP-CF group were culture negative (n = 8, 89%) with the exception of one child who was culture positive for P. aeruginosa. (Table 1). Accordingly, this cohort of patients provided an excellent opportunity to retrospectively assess the effect of infection and CF status, alone and in combination, upon the PGP pathway. 18 (62%) CC-CF patients had spirometry available from the annual assessment within the year following bronchoscopy (mean (SD) % predicted FEV1 = 73.4 (17.2); mean (SD) % predicted FVC = 83.5 (16.1)). 21 (72%) CC-CF patients had at least 3 annual spirometry values during the 5 years following bronchoscopy, enabling annual change in FEV1 to be calculated.
3.2. Elevated neutrophilic inflammation in the BAL fluid of CC-CF children
BAL absolute cell counts and differentials were available respectively in 19 (79%) and 23 (96%) of the non-CF controls, 20 (69%) of CC-CF patients and 8 (89%) of the RSISP-CF patients (Table 2). As expected, BAL fluid from the CC-CF group contained more neutrophils than non-CF controls and RSISP-CF patients (Table 2). Whilst macrophages were prominent component of the infiltrate in the BAL fluid of all children, total numbers were not significantly different between groups. Numbers of lymphocytes and eosinophils were modest but a greater number of eosinophils were present in the CC-CF group versus non-CF controls. No statistically significant differences were observed between non-CF controls and RSISP-CF patients in total numbers of any cell population.
Table 2.
Total and differential inflammatory cell counts in bronchoalveolar lavage (BAL) fluid.
| Non-CF control (n = 24) | RSISP-CF (n = 9) | CC-CF (n = 29) | |
|---|---|---|---|
| Absolute cells x103 (/ml) | 270 (235) | 386 (453.8) | 976.5 (2516.3)** |
| Macrophages (%) | 87 (20) | 82.5 (16.1) | 49.7 (60)***^ |
| Macrophages x103 (/ml) | 158.4 (145.7) | 428.6 (337.4) | 191.1 (387.7) |
| Neutrophils (%) | 1 (5.7) | 11.2 (23.7) | 44.7 (56.4)**^ |
| Neutrophils x103 (/ml) | 3.3 (29.8) | 57.7 (101.1) | 528 (1755.1)***^ |
| Eosinophils (%) | 0.3 (0.3) | 0.3 (0.7) | 0.9 (4)* |
| Eosinophils x103 (/ml) | 0.2 (1) | 1.9 (3.1) | 17.9 (31.2)** |
| Lymphocytes (%) | 3.3 (8.7) | 6 (7.2) | 1.7 (5.3) |
| Lymphocytes x103 (/ml) | 7.3 (17.5) | 33 (43.4) | 30.8 (49.6) |
Data presented as median value (IQR) unless otherwise stated.
Statistical significance between groups was tested using a Kruskal-Wallis test followed by a Dunns post-test. * = between non-CF controls vs CC children (* ≤0.05; ** ≤0.01; *** ≤0.001); ^ = between RSISP vs CC children (^ ≤0.05).
3.3. PGP is elevated in the BAL fluid of CC-CF children
BAL fluid PGP levels were below the lower limit of detection in 23 of the 24 (96%) non-CF control patients with the remaining patient having very low levels of the peptide (0.27 ng/ml; Fig. 1A). This patient was culture-positive for P. aeruginosa (though it is noteworthy that the 7 other non-CF control patients who were culture-positive for other organisms lacked PGP). Conversely, all but one of the CC-CF patients tested contained PGP in their BAL fluid above our threshold of detection with a median value (IQR) of 2.15 ng/ml (2.57 ng/ml) (Fig. 1A). Within this group, culture status had no significant impact on the levels of PGP, and no significant difference was detected in PGP levels in CC-CF children that were culture positive for P. aeruginosa versus those culture positive for other bacteria. PGP levels were below the lower limit of detection in the majority of RSISP-CF patients (89%) with the exception being the single culture-positive infant (P. aeruginosa) in whom substantial quantities of the peptide were detected (3.0 ng/ml). For the cohort overall, a positive correlation was observed between PGP concentration and neutrophil numbers (Fig. 1B). Whilst the concentration of IL-8 was significantly elevated in the BALF of the CC-CF group relative to the RSISP-CF and non-CF control patients (Fig. 1C), the separation was not as striking as observed with PGP, highlighting the potential of PGP as a potential severity biomarker for the former group. From the limited spirometric data available, PGP concentrations did not show a significant relationship with % predicted FEV1 in the year following bronchoscopy. Additionally, no significant correlation was observed between PGP concentration and slope of FEV1 decline during 5 years post bronchoscopy. No AcPGP was detectable in any of these pediatric samples.
Fig. 1.
The neutrophil matrikine PGP is elevated in the BAL fluid of older children with CF. (A) Levels of PGP peptide in the BAL fluid of non-CF controls (n = 24), RSISP-CF children (n = 9) and CC-CF children (n = 25), as determined by LC-MS/MS. (B) Correlation between levels of BAL fluid PGP and airway neutrophils (n = 37: 16 non-CF, 7 RSISP-CF, 14 CC-CF). (C) Levels of IL-8 in the BAL fluid of non-CF controls (n = 23), RSISP-CF children (n = 9) and CC-CF children (n = 28), as determined by ELISA. Closed symbols represent patients that were culture negative and open symbols patients that were culture positive. The horizontal bar depicts the median of each group. Statistical significance between groups was tested using a Kruskal-Wallis test followed by a Dunns post-test and correlation analysis was performed using Spearman rank test. * = P < .05; ** = P < .01; *** = P < .001. RSISP, routine screened infant surveillance program; CC, bronchoscopy for clinical concern; CF = Cystic Fibrosis.
3.4. Elevated PGP-generating enzymes in the BAL fluid of CC-CF children
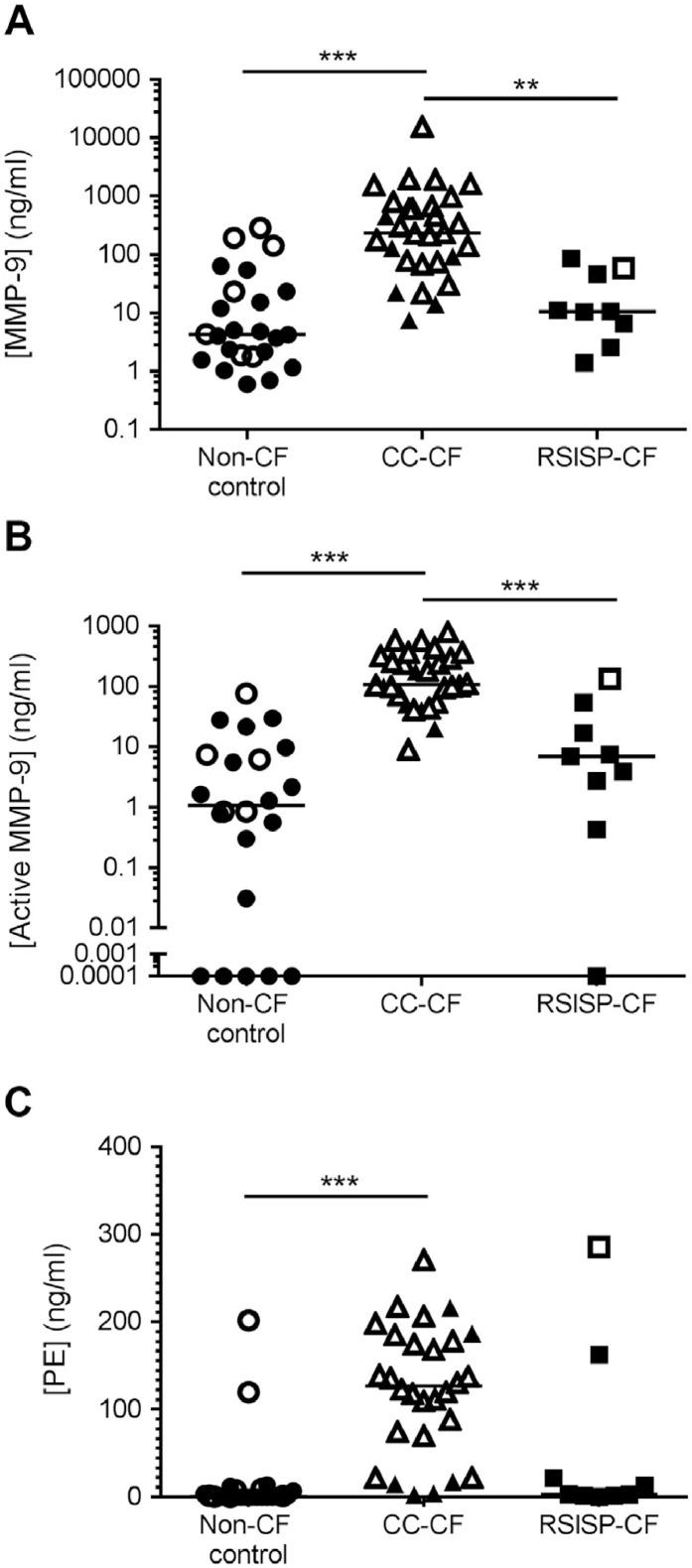
Levels of total (Fig. 2A) and active (Fig. 2B) MMP-9 were significantly elevated in CC-CF group compared to RSISP-CF patients and non-CF controls. In this CC-CF group, MMP-9 levels were also significantly higher in the culture positive than culture negative patients. In the entire cohort, there was a strong correlation between MMP-9 and airway neutrophilia (Supplementary Fig. 1A). Levels of PE showed a similar pattern to MMP-9, being significantly raised in CC-CF patients versus non-CF controls (Fig. 2C), demonstrating trends towards higher levels in the presence of infection and a relationship with neutrophilia (Supplementary Fig. 1B). It is pertinent that the one RSISP-CF patient that presented with BAL fluid PGP also had particularly pronounced PE levels (Fig. 2C). Whilst MMP-9 and PE levels correlated with PGP levels (Supplementary Fig. 1C and D), there were some non-CF control and RSISP-CF children that lacked PGP whilst possessing greater levels of MMP-9/PE than some CC-CF children. This suggests that elevated MMP-9/PE alone is insufficient to account for the striking augmentation in PGP levels observed in CC-CF children.
Fig. 2.
Elevated PGP-generating enzymes in the BAL fluid of older children with CF. Levels of total MMP-9 (A; n = 24 non-CF, 9 RSISP-CF, 29 CC-CF) and active MMP-9 (B; n = 22 non-CF, 9 RSISP-CF, 28 CC-CF) and PE (C; n = 24 non-CF, 9 RSISP-CF, 29 CC-CF) in the BAL fluid. Closed symbols represent patients that were culture negative and open symbols patients that were culture positive. The horizontal bar depicts the median of each group. Statistical significance between groups was tested using a Kruskal-Wallis test followed by a Dunns post-test. * = P < .05; ** = P < .01; *** = P < .001. RSISP, routine screened infant surveillance program; CC, bronchoscopy for clinical concern; CF = Cystic Fibrosis.
3.5. Reduced levels of extracellular LTA4H in the BAL fluid of CC-CF children
PGP persistence is a function of the relative activities of MMP-9/PE versus LTA4H. Extracellular LTA4H levels are substantially elevated during inflammatory episodes concomitant with MMP-9 and PE to ensure that PGP is efficiently degraded [4,9,10]. However, counter to expectations, CC-CF patients displayed comparable or even reduced levels of LTA4H in their BAL fluid relative to the non-CF controls (Fig. 3A). It is therefore feasible that the relative loss of LTA4H contributes to the PGP accumulation in older CF children. NE is a prominent protease implicated in the pathology of CF lung disease, and analysis of the LTA4H protein sequence revealed multiple potential NE cleavage sites. Accordingly, NE activity was significantly elevated in the CC-CF children (Fig. 3B). Incubation of recombinant LTA4H with recombinant active NE resulted in loss of LTA4H protein, as adjudged by Coomassie stained gel (Fig. 3C) and Western blot (Fig. 3D), and enzymatic PGP-degrading activity (Fig. 3E). It has recently been demonstrated that NE can activate MMP-9 [22] and could thus further perpetuate the PGP cycle of inflammation. Accordingly, levels of active MMP-9 correlated with NE activity (Fig. 3F). Thus NE activity, a surrogate marker for lung disease severity in CF children, can activate PGP-generating MMP-9 and destroy PGP-degrading LTA4H. Consequently, a significant correlation is observed between NE activity and PGP levels (Fig. 3G).
Fig. 3.
Neutrophil elastase can cleave LTA4H, abrogating its PGP-degrading activity. (A) Levels of LTA4H in the BAL fluid of non-CF controls (n = 24), RSISP-CF patients (n = 9) and CC-CF patients (n = 29), as determined by ELISA. (B) NE activity in the BAL fluid of non-CF controls (n = 23), RSISP-CF patients (n = 7) CC-CF patients (n = 20), as determined by fluorometric assay. (C-E) Recombinant LTA4H was co-incubated with recombinant neutrophil elastase (NE) for 2 h at 37 °C and cleavage of LTA4H assessed by Coomassie stained gel (C) and Western blot (D). (E) Recombinant LTA4H was co-incubated with recombinant NE for 2 h at 37 °C and capacity of LTA4H to degrade PGP subsequently determined by incubation with the peptide for 2 h and assessing PGP degradation by LC-MS/MS. (F) Correlation between levels of active MMP-9 and NE activity in the BAL fluid (n = 46: 22 non-CF, 7 RSISP-CF, 17 CC-CF). (G) Correlation between levels of PGP and NE activity in the BAL fluid (n = 50: 23 non-CF, 7 RSISP-CF, 20 CC-CF). Closed symbols represent patients that were culture negative and open symbols patients that were culture positive. For (A and B) the horizontal bar depicts the median of each group. Statistical significance between groups was tested using a Kruskal-Wallis test followed by a Dunns post-test and correlation analysis was performed using Spearman rank test. * = P < .05; ** = P < .01; *** = P < .001. RSISP, routine screened infant surveillance program; CC, bronchoscopy for clinical concern; CF = Cystic Fibrosis.
Whilst PGP degradation is mediated by the aminopeptidase activity of LTA4H in an extracellular environment, LTB4 is generated intracellularly by the epoxide hydrolase activity of LTA4H. Clearly, only extracellular LTA4H would be liable to neutrophil elastase degradation as seen in the CC-CF children, with the intracellular enzyme still capable of generating LTB4. Accordingly, we report elevated LTB4 levels in the BAL fluid of CC-CF children (Supplementary Fig. 2A).
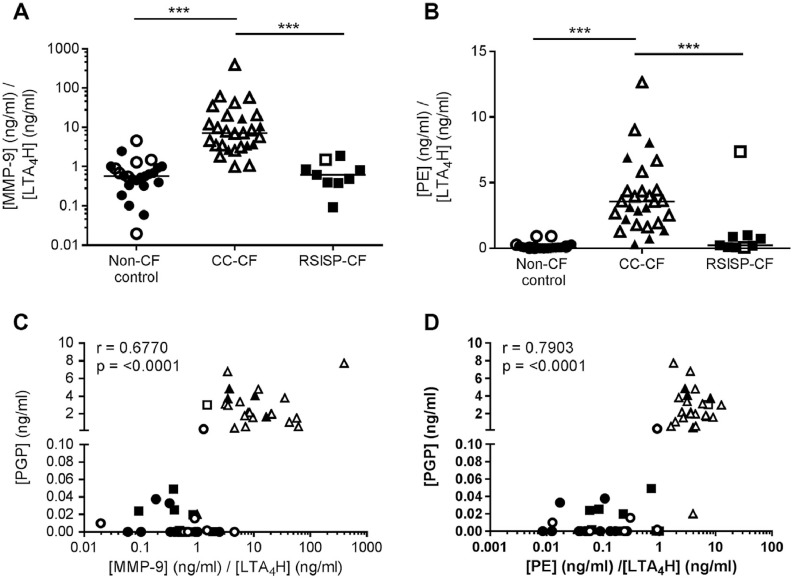
3.6. An imbalance between PGP-generating and -degrading enzymes permits PGP accumulation in children with CF
We rationalized that the relative amounts of PGP-generating and –degrading enzymes in each child ultimately defined the level of PGP able to persist. When the ratio of total or active MMP-9 to extracellular LTA4H levels/activity was determined, there was a clear and striking augmentation in the CC-CF children relative to non-CF controls and RSISP-CF patients (Fig. 4A; Supplementary Fig. 3A and B), rationalizing the clear separation in levels of PGP between the groups of children. When expressing the ratio of PE to LTA4H levels/activity there was once again the same clear distinction between groups (Fig. 4B; Supplementary Fig. 3C). It is noteworthy that the one RSISP-CF patient that was culture positive (P. aeruginosa) and possessed comparable levels of PGP relative to the older CC-CF children, also presented with a comparable PE: LTA4H ratio. Thus children that displayed a marked imbalance in the relative levels of PGP-generating to PGP-degrading enzymes were those that possessed PGP. Indeed, a strong positive correlation is observed when comparing the MMP-9: LTA4H ratio to PGP (Fig. 4C) or the PE: LTA4H ratio to PGP (Fig. 4D).
Fig. 4.
An imbalance between PGP-generating and -degrading enzymes permits PGP accumulation in CF children. Ratio of [MMP-9]/[LTA4H] (A) or [PE]/[LTA4H] (B) in the BAL fluid of non-CF controls (n = 24), RSISP-CF patients (n = 9) and CC-CF patients (n = 27), as determined by ELISA. (C) Correlation between the ratio of [MMP-9]/[LTA4H] versus [PGP] or (D) between the ratio of [PE]/[LTA4H] versus PGP (n = 56: 24 non-CF, 9 RSISP-CF, 23 CC-CF). Closed symbols represent patients that were culture negative and open symbols patients that were culture positive. For (A and B) the horizontal bar depicts the median of each group. Statistical significance between groups was tested using a Kruskal-Wallis test followed by a Dunns post-test and correlation analysis was performed using Spearman rank test. * = P < .05; ** = P < .01; *** = P < .001. RSISP, routine screened infant surveillance program; CC, bronchoscopy for clinical concern; CF = Cystic Fibrosis.
4. Discussion
Characteristic features of CF airways disease are chronic infection and the predominance of neutrophils in the airway lumen, which ultimately drive tissue damage and disease progression [[23], [24], [25], [26]]. Within weeks of birth some CF infants have been reported to present with infection and inflammation [25,[27], [28], [29]] and by adolescence inflammation and infection are uniformly present even in the absence of symptoms [30]. Debate continues as to whether the inflammation arises in response to infection or whether the CF airway is intrinsically pro-inflammatory. In this study, we questioned whether PGP could support neutrophilic inflammation in children with CF, including newborn infants without current or prior detectable infection.
We report that the vast majority of older children with CF (CC-CF) have substantial quantities of PGP in their BAL fluid, which correlated with the elevated neutrophil numbers in this population. Conversely, non-CF controls were largely devoid of BAL PGP, even though some were culture positive and presented with neutrophilia - supportive of the notion that PGP is normally efficiently degraded to limit neutrophilic inflammation and only accumulates in chronic diseases when this system fails [4,10]. PGP was below the limit of detection in the vast majority of newborn children with CF (RSISP-CF). Thus it would appear that the augmented PGP levels observed in the CC-CF group are not intrinsic to CF status but are instead acquired with disease progression. Intriguingly, the one RSISP-CF patient that was culture-positive displayed substantial quantities of the peptide. Despite small sample sizes, it is appealing to speculate that a combination of CF status and infectious insult is required to initiate PGP accumulation. In older children with CF, current culture status may be only partially relevant since they will have likely previously experienced multiple infective “triggers” leading to PGP accumulation and ensuing self-propagating cycle of neutrophilic inflammation.
A pertinent question is how PGP is able to accumulate in CC-CF children. We report that levels of PGP-generating enzymes MMP-9 and PE are elevated in the CC-CF group relative to non-CF controls and RSISP-CF patients. Levels of these enzymes were generally higher in children that were culture positive, potentially indicative of the greater neutrophilic infiltrate [4,10]. It is noteworthy, however, that release of PE from airway epithelia has also been reported in response to TLR4 stimulation via exosomes and that CF subjects colonized with P. aeruginosa demonstrate elevated exosome PE content [31] – potentially rationalizing the elevated levels of this enzyme with infection. Whilst MMP-9/PE levels were elevated in CC-CF patients, there are some non-CF control children that lacked PGP whilst possessing greater neutrophil numbers and levels of MMP-9/PE than some in the CC-CF group. This suggests that elevated MMP-9/PE alone was insufficient to drive PGP accumulation in CF children.
Extracellular LTA4H levels are normally augmented with neutrophilic inflammation concomitantly with MMP-9/PE to ensure PGP is efficiently degraded [4,9,10]. Whilst one would envisage that extracellular LTA4H levels should therefore be substantially higher in CC-CF children, this was not the case and levels were generally very low. NE is a protease that is most prominently implicated in the pathology of CF lung disease and a surrogate marker of disease severity in children with CF [[32], [33], [34], [35], [36]]. We demonstrate that NE can degrade LTA4H, abrogating its activity. Accordingly NE activity was augmented in CC-CF children, supportive of the notion that it may contribute to the loss of LTA4H in these patients. We have previously demonstrated that PGP not only drives neutrophil recruitment but also promotes neutrophil NE release [6,10]. In light of current findings, this would result in degradation of extracellular LTA4H, prevention of PGP degradation and would re-enforce the whole vicious cycle of protease/matrikine driven inflammation. Furthermore, it has recently been demonstrated that NE can activate MMP-9 [22] thus further perpetuating this PGP cycle.
It would seem that PGP is able to accumulate in older children with CF as a consequence of elevated PGP-generating enzymes and a synchronized reduction in PGP-degrading LTA4H to in essence create the perfect storm. Accordingly, MMP-9: LTA4H or PE: LTA4H ratios were substantially greater in this CC-CF group than any other children and correlated strongly with PGP levels. The one exception being the one RSISP-CF patient that was culture positive and presented with substantial quantities of PGP – this infant showed a spike in PGP- displayed MMP-9: LTA4H and PE: LTA4H ratios more in keeping with the older CF children that also possessed PGP. Thus we would speculate that CFTR dysfunction is not directly driving the PGP accumulation observed in the CC-CF children, but rather that the persistent inflammation and infectious insults in the CF airways creates an environment that subverts the PGP pathway. Specifically, elevated NE levels in the CF airways destroys extracellular LTA4H, and thus when infections spike neutrophilic inflammation and release of PGP-generating enzymes, the liberated PGP cannot be efficiently degraded. Accumulated PGP would subsequently function to exacerbate inflammation by perpetuating a vicious circle of neutrophilic inflammation. We have previously demonstrated that a failure of LTA4H to degrade PGP in response to a normally self-limiting pulmonary bacterial infection, resulted in a greater neutrophilic exacerbation [4,10]. We speculate that this is essentially what is occurring in the CF lung whereby the LTA4H system is defective and thus upon infectious challenge, PGP generation spikes but the peptide is unable to be efficiently degraded. Recently, we have demonstrated in a mouse model of allergic airways disease that a failure to degrade PGP directly, and independently of its action on neutrophils, elicited pathological epithelial remodelling and mucus hypersecretion [9]. Given that structural airway wall changes are a hallmark feature of CF that begin in early life [37], then it is tempting to speculate that PGP could potentially be an instigator of this remodelling.
Whilst PGP degradation is mediated by the aminopeptidase activity of LTA4H in an extracellular environment, LTB4 is generated intracellularly by the epoxide hydrolase activity of LTA4H. Clearly, only extracellular LTA4H would be liable to neutrophil elastase degradation as seen in the CC-CF children, with the intracellular enzyme still capable of generating LTB4. Accordingly, we report elevated LTB4 levels in the BAL fluid of CC-CF children. Thus within this CC-CF group, the pro-inflammatory activity of LTA4H is functional but its anti-inflammatory activity is not, enabling accumulation of both LTB4 and PGP. Our findings are in agreement with previous studies describing increased LTB4 levels in CF airways [38]. Furthermore, the levels of pro-resolving eicosanoid lipoxin A4 (LXA4) are reduced in CF lung disease owing to reduced expression of the enzyme 15-lipoxygenase, giving rise to a LTB4 /LXA4 ratio that favours inflammation and impedes resolution [39,40]. Celtaxsys have looked to ameliorate LTB4-driven inflammation in CF through administration of their LTA4H inhibitor, Acebilustat. Encouragingly, this resulted in a modest reduction in CF exacerbations in a recent phase IIb trial. It could be argued that Acebilustat would inadvertently lead to PGP accumulation, which in turn could mask some of the benefits of ameliorating LTB4-driven inflammation [41]. However, our current study demonstrates that PGP degradation by LTA4H is already aberrant in CC-CF children, potentially owing to degradation of LTA4H by neutrophil elastase, and thus it is feasible that any potential adverse effects of Acebilustat in inhibiting PGP degradation by LTA4H would be negligible in CF.
Whilst IL-8 concentrations were also significantly elevated in older children with CF relative to newborn children with CF and non-CF controls, the separation in IL-8 levels between these patient groups was not as clearly defined or absolute as observed with PGP. This highlights the potential of PGP as a biomarker, superior to other neutrophil surrogate markers, for CF lung disease progression. Clearly, there are challenges with utilization of LC-MS/MS for biomarker assessment, including the high initial cost of the equipment, the high complexity of the instrumentation's operation and maintenance and the relatively low sample throughput. Nonetheless, LC-MS/MS has now become a widespread technology and routine analysis within clinical laboratories, and it is realistic to consider use of PGP as a biomarker for evaluation of CF disease progression.
There are some inevitable limitations associated with this study. The number of newborn children within the RSISP-CF group is relatively small, whilst the CC-CF group encompassed a relatively diverse range of clinical situations combining any patients older than 4 months who were having a bronchoscopy because of clinical deterioration. An associated potential caveat to these findings is that patients within the older CC-CF group were unstable and bronchoscopy was performed due to clinical concern, thus potentially limiting comparison to stable CF patients. This is an unavoidable limitation of our study given that routine surveillance bronchoscopy beyond 4 months of age has not been part of CF care at our centre. The variability of PGP with change in clinical stability in older children with CF is therefore an important question that requires further exploration. It is also feasible that medications differentially prescribed to patients could impact on the PGP pathway. Previous studies in the context of COPD have suggested that treatment of patients with macrolides [42] or Roflumilast [43] can reduce sputum PGP levels, particularly with increased duration of therapy. A proportion of the CC-CF children included in our study had received azithromycin, however this had no significant impact on PGP levels in the BAL fluid. Finally, our analysis has focused upon host-derived enzymes central to the PGP pathway, but it is feasible that bacteria express related enzymes with primitive PGP-generating or –degrading activities. Whilst beyond the scope of this study, it would be interesting in future to ascertain whether any bacterial species prevalent within the CF lung produce enzymes with the capacity to generate or degrade PGP and consequently the physiological relevance of these putative enzymes in defining protection and pathology.
PGP and its acetylated variant, AcPGP, have been reported in adult CF patients at baseline and during exacerbations, with levels of the peptides depreciating with inpatient therapy [3]. We didn't observe a significant relationship between PGP concentration and % predicted FEV1 in the year following bronchoscopy, or between PGP and rate of decline in FEV1 in the 5 years following bronchoscopy, in the patients for whom this measurement was available. The group is likely underpowered for analyses of this highly variable outcome measure, and also included children of variable ages, who may not be directly comparable. Furthermore, assessment of FEV1 was not close enough to the bronchoscopy to reflect acute changes in lung function. A final limitation is that at our centre, an annual assessment is not cancelled if the patient is clinically unwell, so values may not reflect their optimal clinical status. Unfortunately, the use of Lung Clearance Index was not part of routine clinical assessment at the time when the majority of these patient samples were collected, and so this data isn't available. It was surprising, given the abundance of AcPGP previously reported in adult CF patients [3], that none was present in CF children. Reactive aldehydes, such as acrolein, can acetylate PGP to generate AcPGP [5]. Acrolein can be generated during severe inflammation [44,45] and one could rationalize that with increasing age and recurrent and persistent inflammation in the CF lung, acrolein could accumulate and exacerbate inflammation via conversion of PGP to AcPGP.
5. Conclusions
In conclusion, this study has demonstrated that PGP accumulates in the CF lung during early life, as a consequence of a striking imbalance between PGP-generating (MMP-9 and PE) and –degrading (LTA4H) enzymes; with the latter being targeted for proteolytic degradation in the CF lung. We speculate that infectious insult is a key event by augmenting PGP generation in a context where it cannot be efficiently degraded, and in so doing elicit a self-sustaining vicious cycle of inflammation. The accumulated PGP can subsequently promote persistent airway neutrophilia and protease imbalance that are characteristic features of CF.
Author contributions
RJS designed, executed (along with CJP, DFP and MMF) and interpreted the experiments and prepared the manuscript; JCD, AB and EWFWA led the clinical fellows (TH, NR, HT, SB, RT) acquiring the samples and clinical data; the fellows also performed cytospin analysis of BAL. JCD assisted with interpretation of the experiments and preparation of the manuscript. AT acquired and interpreted data within the manuscript and contributed discussions throughout the work. PLJ aided with mass spectrometry, and PLJ, AG, JEB and CML contributed discussions throughout the work.
Conflict of interests
ART, PLJ, TNH, NR, HLT, SB, RT, EWFWA, AG, JEB, AB and RJS have no competing interests. JCD has financial relationships with Vertex, Proteostasis, PTC, Pulmocide, Bayer, Boehringer Ingelheim, Novartis and Enterprise Therapeutics, all independent from submitted work. CML has received funding from Janssen Biotech Inc. independent from the submitted work.
Acknowledgements
We would like to thank Dr. Anna Caldwell and the Centre of Excellence for Mass Spectrometry at King's College London for the use of equipment and technical assistance. RJS is a Wellcome Trust Senior Research Fellow in Basic Biomedical Sciences (209458/Z/17/Z). CML is a Wellcome Trust Senior Fellow in Basic Biomedical Sciences (086718/Z/08/Z). The National Heart, Lung and Blood Institute funds JEB (HL077783, HL110950, HL114439 and HL126596) and AG (HL102371). AG is also funded through the Veterans Administration (1I01BX001756). This project was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The UAB Lung Health Center Pulmonary Proteomics Laboratory is funded through the UAB Health Service Foundation General Endowment Fund.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcf.2019.05.017.
Appendix A. Supplementary data
Supplementary material
References
- 1.Weathington N.M., van Houwelingen A.H., Noerager B.D., Jackson P.L., Kraneveld A.D., Galin F.S. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 2.Patel D.F., Snelgrove R.J. The multifaceted roles of the matrikine pro-gly-pro in pulmonary health and disease. Eur Res Rev. 2018;27(148) doi: 10.1183/16000617.0017-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaggar A., Jackson P.L., Noerager B.D., O'Reilly P.J., McQuaid D.B., Rowe S.M. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snelgrove R.J., Jackson P.L., Hardison M.T., Noerager B.D., Kinloch A., Gaggar A. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardison M.T., Brown M.D., Snelgrove R.J., Blalock J.E., Jackson P. Cigarette smoke enhances chemotaxis via acetylation of proline-glycine-proline. Front Biosci. 2012;4:2402–2409. doi: 10.2741/e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Jackson P.L., Tanner S., Hardison M.T., Abdul Roda M., Blalock J.E. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardison M.T., Galin F.S., Calderon C.E., Djekic U.V., Parker S.B., Wille K.M. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J Immunol. 2009;182(7):4423–4431. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Reilly P., Jackson P.L., Noerager B., Parker S., Dransfield M., Gaggar A. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel D.F., Peiro T., Shoemark A., Akthar S., Walker S.A., Grabiec A.M. An extracellular matrix fragment drives epithelial remodeling and airway hyperresponsiveness. Sci Transl Med. 2018;10(455) doi: 10.1126/scitranslmed.aaq0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akthar S., Patel D.F., Beale R.C., Peiro T., Xu X., Gaggar A. Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat Commun. 2015;6:8423. doi: 10.1038/ncomms9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haeggstrom J.Z., Tholander F., Wetterholm A. Structure and catalytic mechanisms of leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat. 2007;83(3):198–202. doi: 10.1016/j.prostaglandins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 13.Di Gennaro A., Haeggstrom J.Z. The leukotrienes: immune-modulating lipid mediators of disease. Adv Immunol. 2012;116:51–92. doi: 10.1016/B978-0-12-394300-2.00002-8. [DOI] [PubMed] [Google Scholar]
- 14.Snelgrove R.J. Leukotriene A4 hydrolase: an anti-inflammatory role for a proinflammatory enzyme. Thorax. 2011;66(6):550–551. doi: 10.1136/thoraxjnl-2011-200234. [DOI] [PubMed] [Google Scholar]
- 15.Konstan M.W., Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24(2):137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [discussion 59-61] [DOI] [PubMed] [Google Scholar]
- 16.Muhlebach M.S., Stewart P.W., Leigh M.W., Noah T.L. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160(1):186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 17.Ordonez C.L., Henig N.R., Mayer-Hamblett N., Accurso F.J., Burns J.L., Chmiel J.F. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(12):1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 18.Sagel S.D., Kapsner R., Osberg I., Sontag M.K., Accurso F.J. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164(8):1425–1431. doi: 10.1164/ajrccm.164.8.2104075. Pt 1. [DOI] [PubMed] [Google Scholar]
- 19.Regamey N., Hilliard T.N., Saglani S., Zhu J., Scallan M., Balfour-Lynn I.M. Quality, size, and composition of pediatric endobronchial biopsies in cystic fibrosis. Chest. 2007;131(6):1710–1717. doi: 10.1378/chest.06-2666. [DOI] [PubMed] [Google Scholar]
- 20.Hilliard T.N., Regamey N., Shute J.K., Nicholson A.G., Alton E.W., Bush A. Airway remodelling in children with cystic fibrosis. Thorax. 2007;62(12):1074–1080. doi: 10.1136/thx.2006.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 22.Garratt L.W., Sutanto E.N., Ling K.M., Looi K., Iosifidis T., Martinovich K.M. Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur Respir J. 2015;46(2):384–394. doi: 10.1183/09031936.00212114. [DOI] [PubMed] [Google Scholar]
- 23.Regamey N., Tsartsali L., Hilliard T.N., Fuchs O., Tan H.L., Zhu J. Distinct patterns of inflammation in the airway lumen and bronchial mucosa of children with cystic fibrosis. Thorax. 2012;67(2):164–170. doi: 10.1136/thoraxjnl-2011-200585. [DOI] [PubMed] [Google Scholar]
- 24.Hamutcu R., Rowland J.M., Horn M.V., Kaminsky C., MacLaughlin E.F., Starnes V.A. Clinical findings and lung pathology in children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(8):1172–1175. doi: 10.1164/ajrccm.165.8.2104090. [DOI] [PubMed] [Google Scholar]
- 25.Sly P.D., Brennan S., Gangell C., de Klerk N., Murray C., Mott L. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 26.Bedrossian C.W., Greenberg S.D., Singer D.B., Hansen J.J., Rosenberg H.S. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976;7(2):195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong D.S., Grimwood K., Carzino R., Carlin J.B., Olinsky A., Phelan P.D. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. Bmj. 1995;310(6994):1571–1572. doi: 10.1136/bmj.310.6994.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan T.Z., Wagener J.S., Bost T., Martinez J., Accurso F.J., Riches D.W. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong D.S., Grimwood K., Carlin J.B., Carzino R., Gutierrez J.P., Hull J. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156(4):1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. Pt 1. [DOI] [PubMed] [Google Scholar]
- 30.Konstan M.W., Hilliard K.A., Norvell T.M., Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150(2):448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 31.Szul T., Bratcher P.E., Fraser K.B., Kong M., Tirouvanziam R., Ingersoll S. Toll-like receptor 4 engagement mediates prolyl endopeptidase release from airway epithelia via exosomes. Am J Respir Cell Mol Biol. 2016;54(3):359–369. doi: 10.1165/rcmb.2015-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twigg M.S., Brockbank S., Lowry P., FitzGerald S.P., Taggart C., Weldon S. The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/293053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taggart C., Coakley R.J., Greally P., Canny G., O'Neill S.J., McElvaney N.G. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am J Physiol Lung Cell Mol Physiol. 2000;278(1):L33–L41. doi: 10.1152/ajplung.2000.278.1.L33. [DOI] [PubMed] [Google Scholar]
- 34.Devaney J.M., Greene C.M., Taggart C.C., Carroll T.P., O'Neill S.J., McElvaney N.G. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003;544(1–3):129–132. doi: 10.1016/s0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 35.Jackson P.L., Xu X., Wilson L., Weathington N.M., Clancy J.P., Blalock J.E. Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction. Mol Med. 2010;16(5–6):159–166. doi: 10.2119/molmed.2009.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sly P.D., Gangell C.L., Chen L., Ware R.S., Ranganathan S., Mott L.S. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 37.Regamey N., Jeffery P.K., Alton E.W., Bush A., Davies J.C. Airway remodelling and its relationship to inflammation in cystic fibrosis. Thorax. 2011;66(7):624–629. doi: 10.1136/thx.2009.134106. [DOI] [PubMed] [Google Scholar]
- 38.Carpagnano G.E., Barnes P.J., Geddes D.M., Hodson M.E., Kharitonov S.A. Increased leukotriene B4 and interleukin-6 in exhaled breath condensate in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(8):1109–1112. doi: 10.1164/rccm.200203-179OC. [DOI] [PubMed] [Google Scholar]
- 39.Ringholz F.C., Buchanan P.J., Clarke D.T., Millar R.G., McDermott M., Linnane B. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur Respir J. 2014;44(2):394–404. doi: 10.1183/09031936.00106013. [DOI] [PubMed] [Google Scholar]
- 40.Higgins G., Ringholz F., Buchanan P., McNally P., Urbach V. Physiological impact of abnormal lipoxin a(4) production on cystic fibrosis airway epithelium and therapeutic potential. Biomed Res Int. 2015;2015 doi: 10.1155/2015/781087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Low C.M., Akthar S., Patel D.F., Loser S., Wong C.T., Jackson P.L. The development of novel LTA4H modulators to selectively target LTB4 generation. Sci Rep. 2017;7 doi: 10.1038/srep44449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Reilly P.J., Jackson P.L., Wells J.M., Dransfield M.T., Scanlon P.D., Blalock J.E. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open. 2013;3(12) doi: 10.1136/bmjopen-2013-004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells J.M., Jackson P.L., Viera L., Bhatt S.P., Gautney J., Handley G. A randomized, placebo-controlled trial of roflumilast effect on proline-glycine-proline and neutrophilic inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):934–942. doi: 10.1164/rccm.201503-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida K., Kanematsu M., Morimitsu Y., Osawa T., Noguchi N., Niki E. Acrolein is a product of lipid peroxidation reaction. formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273(26):16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 45.Anderson M.M., Hazen S.L., Hsu F.F., Heinecke J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99(3):424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material