Abstract
Background
Seizures commonly occur in patients with cryptococcal meningitis, yet risk factors and outcomes related to seizures are not well described.
Methods
We performed post hoc analyses on participants prospectively enrolled in 3 separate human immunodeficiency virus (HIV)-associated cryptococcal meningitis clinical trials during 2010–2017. Documentation of seizures at presentation or during hospitalization and antiseizure medication receipt identified participants with seizures. We summarized participant characteristics by seizure status via Kruskal-Wallis and χ 2 tests. Cox proportional hazards models analyzed the relationship between seizures and mortality. We compared mean quantitative neurocognitive performance Z (QNPZ-8) scores, and individual domain z-scores, at 3-months using independent t tests.
Results
Among 821 HIV-infected cryptococcal meningitis participants, 28% (231 of 821) experienced seizures: 15.5% (127 of 821) experienced seizures at presentation, and 12.7% (104 of 821) experienced incident seizures. Participants with seizures at presentation had a significantly lower Glasgow coma scale ([GCS] <15; P < .001), CD4 count (<50 cells/mcL; P = .02), and higher cerebrospinal fluid (CSF) opening pressure (>25 cm H2O; P = .004) when compared with participants who never experienced seizures. Cerebrospinal fluid fungal burden was higher among those with seizures at presentation (125 000 Cryptococcus colony-forming units [CFU]/mL CSF) and with seizures during follow-up (92 000 CFU/mL) compared with those who never experienced seizures (36 000 CFU/mL, P < .001). Seizures were associated with increased 10-week mortality (adjusted hazard ratio = 1.45; 95% confidence interval, 1.11–1.89). Participants with seizures had lower neurocognitive function at 3 months (QNPZ-8 = −1.87) compared with those without seizures (QNPZ-8 = −1.36; P < .001).
Conclusions
Seizures were common in this HIV-associated cryptococcal meningitis cohort and were associated with decreased survival and neurocognitive function.
Keywords: cohort studies, cryptococcal, cryptococcus, HIV, meningitis, seizures
Seizures are common in cryptococcal meningitis. We identified several clinical risk factors related to seizures and found that seizures were associated with an increased risk in mortality and poor neurocognitive outcomes in HIV-infected persons with cryptococcal meningitis.
Cryptococcal meningitis is one of the most common human immunodeficiency virus (HIV)-related opportunistic infections in sub-Saharan Africa with mortality rates upward of 70% in routine care [1]. Patients with cryptococcal meningitis often present with nonspecific complaints such as headache, fever, altered mental status, nausea, and vomiting. However, some patients with cryptococcosis present with more severe neurological complications, including a variety of neuro-ophthalmologic symptoms and seizures [2–5]. These complications are presumptively due to high intracranial pressure and/or brain parenchymal involvement (ie, meningoencephalitis) [6, 7].
Seizures can be a presenting symptom or sequela of central nervous system infections. Approximately 16%–34% of patients recently diagnosed with various forms of meningitis will experience seizures during disease progression [5, 8–10]. In cryptococcal meningitis, seizures are present in 8%–29% of HIV-negative and 6%–35% of HIV-infected patients [5, 11–17]. At this time, there are no criteria to predict which cryptococcal meningitis patients will develop seizures. Furthermore, the use of available antiseizure medications in resource-limited settings is often complicated by drug-drug interactions with HIV antiretroviral or tuberculosis (TB) medicines, hindering the safe and effective use of anticonvulsants in controlling seizures [18–20].
In HIV-negative cryptococcal, bacterial, and TB meningitis (TBM) cohorts, seizures have generally been associated with significant morbidity and mortality, longer hospital stays, and poorer outcomes [5, 10, 21]. To our knowledge, these findings have not been described in HIV-associated cryptococcal meningitis patients. In this analysis of 3 HIV-associated cryptococcal meningitis cohorts, we report the observed prevalence of seizures, evaluate associations between clinical characteristics in relation to seizure risk, characterize all-cause 10-week mortality and 3-month neurocognitive outcomes related to seizures, and discuss commonly used antiseizure medications and associated drug-drug interactions.
METHODS
Patient Population
Participants with HIV-associated cryptococcal meningitis were prospectively enrolled in 3 separate clinical trials in Uganda and South Africa from November 22, 2010 to May 31, 2017. The first trial enrolled antiretroviral therapy (ART)-naive, HIV-infected participants with no previous history of cryptococcal meningitis (ClinicalTrials.gov identifier: NCT01075152, Cryptococcal Optimal ART Timing [COAT]) [22]; the latter 2 trials enrolled ART-naive and ART-experienced HIV-infected participants, and followed those with a previous history of cryptococcosis (ClinicalTrials.gov identifier: NCT01802385, ASTRO-CM) [23, 24].
Participants in all 3 cohorts were ≥18 years of age and had documented HIV-1 infection. Pregnant or breastfeeding women were excluded from all studies. Cryptococcal meningitis was initially determined by a positive cerebrospinal fluid (CSF) cryptococcal antigen assay (CrAg) (LFA; Immy, Norman, OK) and confirmed with CSF culture. Participants in all 3 trials received combination induction therapy with 0.7–1.0 mg/kg amphotericin B deoxycholate and 800 mg/day fluconazole. Participants enrolled in the ASTRO-CM pilot study additionally received 100–400 mg/day of open-label sertraline [23]. Participants enrolled in the ASTRO-CM double-blind, randomized clinical trial received either 400 mg sertraline or placebo for 2 weeks, followed by 200 mg/day sertraline or placebo for 12 weeks, and a tapered sertraline or placebo dose over 3 weeks thereafter [24]. Follow-up time for participants was 12, 18, or 46 weeks depending on study cohort. For purposes of this post hoc analysis, participants with first-episode cryptococcal meningitis, who were eligible for randomization or inclusion into the parent trials, were included in these analyses, because long-term follow-up data existed.
In all 3 cohorts, “seizure” was defined by motor seizures, because electroencephalograms (EEGs) were not available. “Baseline seizure” was defined as any seizure event between symptom onset and study enrollment among all study participants. “Incident seizure” was defined as any seizure event that occurred during hospitalization or during the outpatient follow-up period among participants who did not experience baseline seizures. “Any seizure” was defined as baseline or incident seizures during follow-up among all participants.
Data capturing seizure events were collected at presentation and prospectively on case report forms. Recorded seizure events by clinicians on case report forms were predominantly subjective generalized seizures, but study staff did occasionally report events of impaired awareness suspicious for nonmotor seizures. Administration of antiseizure medication(s) (carbamazepine, levetiracetam, phenobarbital, phenytoin, and valproate) was also used as a proxy for seizure events.
Statistical Analysis
Demographic and clinical characteristics at cryptococcal meningitis disease presentation were summarized and compared by seizure status via Kruskal-Wallis tests for continuous variables and χ 2 tests for categorical variables. Among those who did not experience a baseline seizure, a Cox proportional hazards model estimated hazard of incident seizure and evaluated potential seizure predictors from among the following baseline covariates: study cohort, age, sex, Glasgow Coma Scale (GCS) <15, lumbar puncture opening pressure >25 cm H2O, CSF fungal burden (quantitative cryptococcal culture), and CD4 <100 cells/μL.
We evaluated 2- and 10-week mortality between participants who had and had not experienced baseline seizures via a log-rank test depicted with Kaplan-Meier curve. Cox proportional hazards models were used to estimate the hazard of mortality by baseline seizure and considering seizures as a time-dependent covariate. Final models were adjusted for study cohort, baseline GCS, CSF opening pressure, CSF fungal burden, and CD4 <100 cells/μL.
The relationship between any seizure before 3 months and neurocognitive function was assessed by comparing mean quantitative neurocognitive performance Z (QNPZ-8) scores at 3 months (previously described [25, 26]) using an independent t test. The QNPZ-8 score was calculated as the mean of domain-specific raw scores, including Symbol Digit Modality, WHO-UCLA Auditory Verbal Learning, Verbal Fluency, Color Trails 1 and 2, Groove Pegboard (mean of dominant and nondominant), and Finger Tapping. Scores were stratified on age and education level and standardized based on the sample mean and standard deviation of an HIV-negative cohort [27]. The domain-specific tests were similarly compared between baseline seizure status. Patients who were scheduled to perform the tests and were too ill to complete a test were assigned a value for that missed test equal to 2 standard deviations less than the mean z-score for the cohort. Scores without imputation were also assessed. Neurocognitive testing in the COAT trial was only performed in Kampala, because there was an HIV-negative language-specific control group, which existed to establish population norms. Participants elsewhere were therefore not scheduled for neurocognitive testing and subsequently were not included in this part of the analysis. All neurocognitive assessments were conducted by a local study nurse who received in-depth training related to the specific battery of tests.
P < .05 were considered statistically significant. No adjustments were made for multiple testing. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
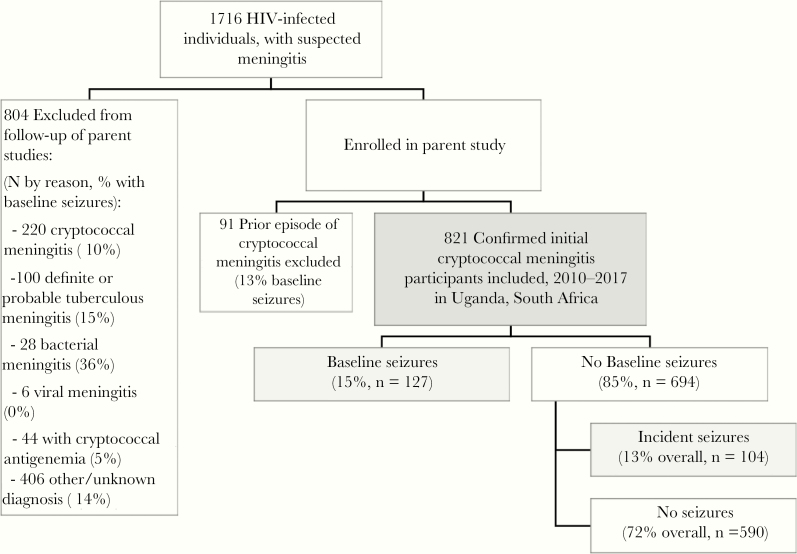
From 1716 potentially eligible HIV-infected participants with suspected meningitis, 66% (1132 of 1716) had confirmed cryptococcal meningitis with positive CSF CrAg. Based on individual parent study inclusion criteria, 821 participants with first episode cryptococcal meningitis were included in this cohort analysis. Of the 895 excluded, 220 had first-episode cryptococcal meningitis and were screened but not enrolled into the parent trials based on eligibility criteria (10% with baseline seizures), 91 had a prior history of cryptococcal meningitis (13% with baseline seizures), and 44 were found to have positive serum CrAg but had a negative CSF CrAg (5% with baseline seizures). An additional 100 participants had definite/probable TBM (15% with baseline seizures), 28 had bacterial meningitis (36% with baseline seizures), 6 had confirmed viral meningitis (0% with baseline seizures), and 406 had unknown/other diagnosis (14% with baseline seizures) (Figure 1).
Figure 1.
Cohort diagram. Depiction of participants enrolled in the parent studies and included in this analysis. HIV, human immunodeficiency virus.
Overall, 15.5% (127 of 821) of participants with first episode cryptococcal meningitis included in this study presented with seizures either before or at the time of cryptococcal meningitis diagnosis (“baseline” seizure). An additional 12.7% (104 of 821) of participants who did not present with baseline seizures experienced incident seizures during follow-up.
Higher baseline CSF quantitative culture fungal burdens occurred among participants who experienced either baseline seizures (median 125 000 Cryptococcus CFU/mL; IQR, 12 400–567 000) or incidence seizures (92 000 CFU/mL; IQR, 6883–360 000) compared with those who did not experience seizures (36 000 CFU/mL; IQR, 1011–230 000; P < .001). Participants who had seizures at baseline were more likely to have a baseline GCS <15 (61%) compared with participants who later experienced incident seizures (36%) or did not experience seizures at all (36%; P < .001). Participants who experienced baseline seizures were also more likely to have CD4 counts <50 cells/μL (84%) compared with those who later experienced incident seizures (74%) or had no seizures (72%; P = .02). Those who experienced seizures at baseline more frequently had elevated baseline CSF opening pressures of >25 cm H2O (68%) compared with those who later experienced incident seizures (54%) or had no seizures (51%; P = .004). Age, sex, ART status, CSF white cell count, and CSF protein did not differ between those with baseline seizures, those that later experienced incident seizures, or those without seizures in this cohort (Table 1). Severe hyponatremia (sodium <120 mEq/L) was not significantly different between those with or without seizures at baseline (baseline seizure 7%; incident seizure 2%; no seizure 5%; P = .41), nor was it significantly different between those with baseline seizures and those with incident seizures (P = .17).
Table 1.
Demographic and Clinical Parameters by Baseline Seizure
| Baseline Seizure | Incident Seizure | No Seizure | ||
|---|---|---|---|---|
| Median [IQR] or N (%)a | Median [IQR] or N (%)a | Median [IQR] or N (%)a | P Valueb | |
| N | 127 (15%) | 104 (13%) | 590 (72%) | |
| Cohort | .13 | |||
| 2010–2012 COAT | 30 (14%) | 21 (10%) | 163 (76%) | |
| 2013–2014 ASTRO Pilot | 16 (11%) | 22 (15%) | 111 (74%) | |
| 2015–2017 ASTRO-CM | 81 (18%) | 61 (13%) | 316 (69%) | |
| Demographics | ||||
| Age, years | 34 [29–40] | 36 [29–42] | 35 [30–40] | .92 |
| Women | 55 (43%) | 46 (44%) | 233 (39%) | .53 |
| Glasgow Coma Score <15 | 78 (61 %) | 37 (36%) | 209 (36%) | <.001 |
| ART status | .65 | |||
| ART naive | 88 (69%) | 63 (61%) | 383 (65%) | |
| On ART <4 months | 11 (9%) | 11 (11%) | 59 (10%) | |
| On ART >4 months | 27 (21%) | 29 (28%) | 147 (25%) | |
| Unknown ART status | 1 (1%) | 1 (1%) | 1 (0%) | |
| Symptom duration, days | 21 [10–30] | 14 [12–30] | 14 [10–30] | .84 |
| Blood Results | ||||
| Absolute CD4 cells/μL | 14 [6–35] | 17 [6–58] | 17 [7–55] | .07 |
| CD4 <50 cells/μL | 102 (84%) | 73 (74%) | 397 (72%) | .02 |
| Sodium, mEq/L | 128 [124–133] | 129 [126–133] | 130 [126–134] | .19 |
| Glucose, mg/dL | 110 [94–119] | 104 [86–110] | 100 [85–115] | .29 |
| CSF Results | ||||
| White cells/μL | <5 [<5–45] | <5 [<5–45] | <5 [<5–56] | .76 |
| White cells <5 cells/μL | 71 (58%) | 59 (60%) | 323 (57%) | .88 |
| Protein, mg/dL | 73 [36–120] | 62 [27–124] | 60 [24–125] | .28 |
| Opening pressure, cm H2O | 33 [21–47] | 27 [19–45] | 26 [17–37] | .002 |
| Opening pressure >25 cm H2O | 77 (68%) | 49 (54%) | 261 (51%) | .004 |
| Cryptococcus CFU/mL CSF | 125 000 [12 400–567 000] | 92 000 [6883–360 000] | 36 000 [1011–230 000] | <.001 |
Abbreviations: ART, antiretroviral therapy; CFU, colony-forming units; COAT, Cryptococcal Optimal ART Timing; CSF, cerebrospinal fluid; IQR, interquartile range.
aPercentages of each seizure group are displayed for categorical variables.
b P values from Kruskal-Wallis tests for continuous variables and χ 2 tests for categorical variables.
Of those who experienced incident seizures, the median time to seizure event from meningitis diagnosis was 9 days (IQR, 4–36 days). In a fully adjusted model, sex, higher baseline fungal burdens, and enrollment into later study cohorts (2013–2017) were found to be significantly associated with incident seizures (Table 2). Women had a 62% higher hazard of incident seizure, but the confidence interval (CI) was wide (adjusted hazard ratio [aHR] = 1.62; 95% CI, 1.05–2.49; P = .03). For every unit increase in log10 CFU/mL of CSF fungal burden, the risk of incident seizure was 16% higher (aHR = 1.20; 95% CI, 1.03–1.40; P = .01). The risk of incident seizure in the ASTRO-CM study was 2.3 times that of participants in COAT (aHR = 2.28; 95% CI, 1.23–4.23; P < .01). Because of the large difference in incident seizure risk between study cohorts, we conducted analyses adjusting for sertraline administration in ASTRO-CM clinical trial. Sertraline was not statistically significant between seizure groups.
Table 2.
Hazard of Incident Seizure, Among Those Without a Baseline Seizure
| Baseline Characteristica | HR (95% CI) | P Value |
|---|---|---|
| Cohort | ||
| 2010–2012 (COAT Trial) | Reference | |
| 2013–2017 (ASTRO Trials) | 2.28 (1.23–4.23) | <.01 |
| Age, years | 1.00 (0.98–1.03) | .45 |
| Women | 1.62 (1.05–2.49) | .03 |
| Glasgow Coma Score <15 | 1.05 (0.65–1.67) | .84 |
| CSF opening pressure, cm H20 | 1.00 (0.99–1.02) | .27 |
| Quantitative culture, log10 CFU/mL CSF | 1.20 (1.03–1.40) | .01 |
| CD4 <100 cells/μL | 1.02 (0.43–2.38) | .96 |
Abbreviations: CFU, colony-forming units; COAT, Cryptococcal Optimal ART Timing; CSF, cerebrospinal fluid; HR, hazard ratio.
aFull model adjusted for study cohort, age, sex, baseline Glasgow Coma Score <15, CSF opening pressure, CSF fungal burden, and CD4 <100 cells/μL.
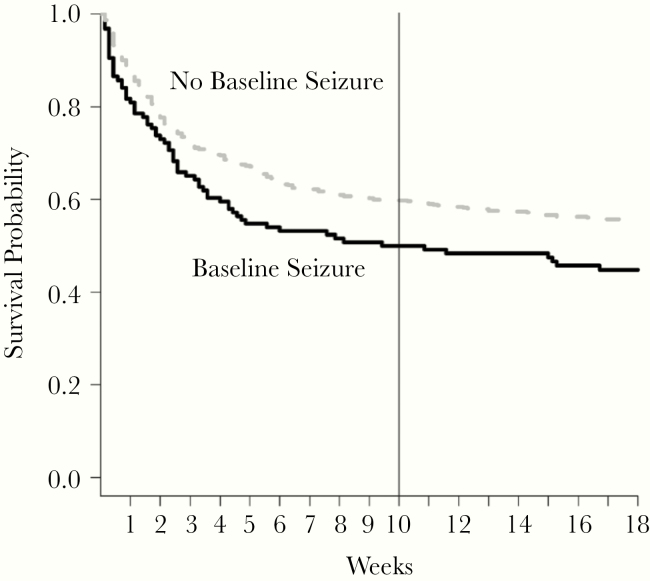
There was no significant difference in 2-week mortality for participants who ever experienced a seizure during follow-up compared with those who did not experience a seizure (aHR = 1.06; 95% CI, 0.77–1.45). Mortality was high among all participants within the first 2 weeks (Figure 2). More participants with baseline seizures died by 10 weeks (50%, 63 of 126) than those without baseline seizures (40%, 271 of 682) (log-rank P = .02). Participants who experienced any seizure had a significantly higher risk of 10-week mortality than participants who had never experienced a seizure at any point during follow-up in the time-dependent analysis (aHR = 1.40; 95% CI, 1.06–1.84) (Table 3, Figure 2).
Figure 2.
Ten-week survival outcomes by presence of seizure at hospital presentation among human immunodeficiency virus-infected persons with cryptococcal meningitis. More participants with baseline seizures died by 10 weeks (50%, 63 of 126) than those without baseline seizures (40%, 271 of 682) (log-rank P = .02). Participants who experienced any seizure had a significantly higher risk of 10-week mortality than participants who had never experienced a seizure at any point during follow-up in the time-dependent analysis (adjusted hazard ratio = 1.45; 95% confidence interval, 1.11–1.89).
Table 3.
Hazard of Mortality Related to Seizure Statusa
| 2-Week Mortality | 10-Week Mortality | |
|---|---|---|
| Model | HR (95% CI) | HR (95% CI) |
| Baseline Seizure vs No Baseline Seizure | ||
| Adjusted for study | 1.25 (0.86–1.82) | 1.34 (1.02–1.77) |
| Adjusted for study and baseline opening pressure, GCS, CSF fungal burden, CD4 <100 cells/μL | 0.92 (0.60–1.42) | 1.06 (0.77–1.45) |
| Seizure as a Time-Dependent Covariate | ||
| Adjusted for study | 1.43 (1.01–2.02) | 1.81 (1.42–2.30) |
| Adjusted for study and baseline opening pressure, GCS, CSF fungal burden, CD4 <100 cells/μL | 0.99 (0.66–1.49) | 1.40 (1.06–1.84) |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; GCS, Glasgow Coma Score; HR, hazard ratio.
aResults were similar when adjusted for antiretroviral therapy (ART) status: on ART at diagnosis or ART naive. ART status was not included in the final model.
Antiseizure medications were prescribed at or within 1 week of the first seizure event for 54% (125 of 231) of participants. Of those who received antiseizure medications, 62% (77 of 125) received valproic acid, 31% (39 of 125) received phenytoin, 6% (8 of 231) received carbamazepine, and <1% (1 of 231) received phenytoin and valproic acid at their first seizure event. The median time on any antiseizure medication during follow-up was 13 days (IQR, 6–26; maximum 185 days). Antiseizure medications were not routinely prescribed in cases in which participants had isolated seizure events that were self-reported and/or not witnessed by medical staff nor when seizures were controlled with the initial benzodiazepine medications. A subset of participants who experienced seizures may have died before the initiation of antiseizure medication, contributing to the low percentage.
Overall, 292 participants completed at least a portion of the neurocognitive assessment at 3 months. Cryptococcal meningitis survivors’ neurocognitive QNPZ-8 scores were 1.27 standard deviations below the average for an HIV-negative person with the same age and education level. Participants who had experienced any seizure by 3 months had significantly lower QNPZ-8 scores compared with participants who had not experienced a seizure (P < .001). This difference remained statistically significant when adjusted for study cohort, baseline GCS, CSF opening pressure, CSF fungal burden, and CD4 <100 cells/μL. Persons who had experienced any seizure had lower neurocognitive performances in executive function, language fluency, verbal learning and memory, fine motor, motor speed, and gross motor function. See Supplemental Table 1 for additional information.
DISCUSSION
In this post hoc analysis of 3 prospective studies, seizures occurred in 28% (231 of 821) of participants with HIV-associated cryptococcal meningitis, and seizures were associated with increased 10-week mortality. Previous studies in HIV-negative cryptococcal meningitis cohorts have reported a seizure incidence between 8% and 29% [5, 12, 13]. In our cohort, a similar proportion of participants experienced baseline and incident seizures (15.5% vs 12.7%). Baseline seizures additionally occurred at a similar frequency to screened TBM patients (15%) and participants with a prior history of cryptococcal meningitis (13%), but less than that of bacterial meningitis participants (36%).
Among cryptococcal meningitis patients, altered mental status (GCS <15) is a known independent predictor of mortality and is associated with increased intracranial pressure [28, 29]. We found that persons presenting with seizures at baseline were also more likely to present with a GCS <15 and increased intracranial pressure. Increased CSF fungal burden was another risk factor for seizures; we postulate that high CSF fungal burden is also likely associated with greater extension of cryptococcal infection throughout the cerebral parenchyma, predisposing the presence of convulsions [6, 7].
In our cohort, mortality was high amongst all participants with cryptococcal meningitis within the first 2 weeks of treatment. This likely explains why we did not observe an increased risk of mortality at 2 weeks when comparing those who experienced baseline seizures with those who did not experience baseline seizures in the adjusted model. When treating seizures as a time-dependent covariate, thereby including participants who experienced incident seizures during follow-up, we saw an increased 10-week mortality risk, even after adjusting for study, baseline opening pressure, GCS <15, CSF fungal burden, and CD4 <100 cells/μL. Incident seizures after the first 2 weeks of cryptococcal meningitis induction therapy thereby pose a continual threat to patients in their recovery.
Survivors who experienced any seizures had worse neurocognitive performance at 3 months when compared with survivors without any seizures. Although QNPZ-8 scores tend to improve with appropriate cryptococcal meningitis treatment at 6 months [25], it is unknown whether seizures impact neurocognitive recovery. Future work assessing long-term neurocognitive outcomes is warranted to determine whether there are any residual neurocognitive impairments related to seizures during cryptococcal meningitis.
In our cohort of cryptococcal meningitis participants with seizures, carbamazepine, phenytoin, and valproic acid were the most commonly prescribed antiseizure medications at time of first seizure event. Phenytoin, valproate, and carbamazepine all undergo hepatic metabolism by the cytochrome P450 (CYP450) system and are therefore susceptible to drug-drug interactions when coadministered with CYP450 inhibitors or inducers [30]. This includes many antiretrovirals, antifungals, antimycobacterials, and antimalarials commonly used in this population (see Supplemental Table 2) [18, 30–33]. Of the more commonly used antiseizure medications in resource-limited settings, levetiracetam has the lowest risk of potential drug-drug interactions, yet, availability and cost may be prohibitive [19]. Valproate is less affected by drug-drug interactions when compared with carbamazepine and phenytoin, and it may be a suitable alterative if applicable. In the treatment of cryptococcal meningitis, amphotericin B deoxycholate-related nephrotoxicity is common and may result in the impaired renal clearance of phenobarbital, levetiracetam, and valproate [30]. Special consideration of antiseizure medications should therefore be made in cryptococcal meningitis patients with amphotericin-related renal dysfunction [34].
Several important limitations exist within this cohort analysis. First, the prevalence of seizures was likely underreported. Observed seizures were limited mainly to motor seizures as opposed to nonmotor seizures, and a greater proportion of participants may have experienced nonmotor seizures that were not observed or detected because EEGs were not available. Second, seizure events were not always directly observed, and events that were not in fact seizures may have been classified as seizures, resulting in possible misclassification. Third, ongoing seizures and subsequent interictal periods may have influenced neurocognitive testing. Although neurocognitive testing was performed 3 months after acute hospitalization and most seizures occurred during the initial hospitalization, multiple factors could additionally influence outcomes, including possible subclinical seizures or poor adherence to or subtherapeutic levels of antiseizure medication. Finally, although no participant had a known history of prior epilepsy, the possibility remains.
When examining risk factors for incident seizures, later study cohorts were significantly associated with increased risk. This likely was due to increased awareness of seizures in the later study cohorts, in addition to improved detection and reporting by more experienced study staff. Inflammatory immune reconstitution syndrome has not been evaluated in this study, so its contribution to incident seizures is unknown.
Although it is believed that approximately 2%–20% of HIV-seropositive persons will experience seizures post-HIV diagnosis, these seizures are thought to be largely attributable to opportunistic central nervous system infections, as opposed to primary HIV infection [35, 36]. Acute onset seizures in this cohort were presumed to be secondary to cryptococcal infection because of diagnostic testing and other meningeal symptoms. However, due to the high prevalence of HIV-related opportunistic infections, other secondary opportunistic infections may have contributed to seizures [37–41]. The short median time (9 days, IQR: 4–36) to incident seizures from cryptococcal meningitis diagnosis dramatically reduces this possibility, but the lack of diagnostics and imaging studies likely contributed to under diagnosis of other clinical causes of seizures.
CONCLUSIONS
Overall, seizures were common in this cohort of HIV-infected cryptococcal meningitis participants and were associated with an increased risk of 10-week mortality and poor neurocognitive outcomes at 3 months. Fungal burden was identified as a potential clinical risk factor that may be valuable in the identification of patients at risk for the development of seizures during treatment for cryptococcal meningitis. Future work related to the detection and the causes and pathophysiology of seizures in this population is warranted given the high risk of mortality. Antiseizure medications should be prescribed with care among this patient population due to existing drug-drug interactions and possible impaired renal clearance of such medications due to amphotericin B toxicity.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This research was funded by the National Institute of Neurologic Diseases and Stroke and the Fogarty International Center (R01NS086312, R25TW009345), Grand Challenges Canada (S4-0296-01), and National Institute of Allergy and Infectious Diseases (U01AI089244, T32AI055433). This work was supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. A. G. F. was a Doris Duke International Clinical Research Fellow. K. A. P. is a 2018 HIVMA Medical Students Program Awardee.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atherton RR, Ellis J, Cresswell FV, et al. Ophthalmic signs in Ugandan adults with HIV-associated cryptococcal meningitis: a nested analysis of the ASTRO-CM cohort. Wellcome Open Res 2018; 3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krishnamoorthy A, Joel A, Abhilash KP. Cryptococcal meningitis with multiple cranial nerves palsies: a review of literature. J Glob Infect Dis 2015; 7:123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duggan J, Walls HM. Ocular complications of cryptococcal meningitis in patients with HIV: report of two cases and review of the literature. J Int Assoc Physicians AIDS Care (Chic) 2012; 11:283–8. [DOI] [PubMed] [Google Scholar]
- 5. Hung CW, Chang WN, Kung CT, et al. Predictors and long-term outcome of seizures in human immuno-deficiency virus (HIV)-negative cryptococcal meningitis. BMC Neurol 2014; 14:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klock C, Cerski M, Goldani LZ. Histopathological aspects of neurocryptococcosis in HIV-infected patients: autopsy report of 45 patients. Int J Surg Pathol 2009; 17:444–8. [DOI] [PubMed] [Google Scholar]
- 7. Colombo AC, Rodrigues ML. Fungal colonization of the brain: anatomopathological aspects of neurological cryptococcosis. An Acad Bras Cienc 2015; 87:1293–309. [DOI] [PubMed] [Google Scholar]
- 8. Rosman NP, Peterson DB, Kaye EM, Colton T. Seizures in bacterial meningitis: prevalence, patterns, pathogenesis, and prognosis. Pediatr Neurol 1985; 1:278–85. [DOI] [PubMed] [Google Scholar]
- 9. van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351:1849–59. [DOI] [PubMed] [Google Scholar]
- 10. Misra UK, Kumar M, Kalita J. Seizures in tuberculous meningitis. Epilepsy Res 2018; 148:90–5. [DOI] [PubMed] [Google Scholar]
- 11. Satishchandra P, Mathew T, Gadre G, et al. Cryptococcal meningitis: clinical, diagnostic and therapeutic overviews. Neurol India 2007; 55:226–32. [DOI] [PubMed] [Google Scholar]
- 12. Tiamkao S, Sawanyawisuth K, Chotmongkol V. Seizure in non-HIV cryptococcal meningitis. J Med Assoc Thai 2007; 90:1298–302. [PubMed] [Google Scholar]
- 13. Zhu LP, Wu JQ, Xu B, et al. Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997-2007. Med Mycol 2010; 48:570–9. [DOI] [PubMed] [Google Scholar]
- 14. Kendi C, Penner J, Koech J, et al. Predictors of outcome in routine care for Cryptococcal meningitis in Western Kenya: lessons for HIV outpatient care in resource-limited settings. Postgrad Med J 2013; 89:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naik KR, Saroja AO, Doshi DK. Hospital-based retrospective study of cryptococcal meningitis in a large cohort from india. Ann Indian Acad Neurol 2017; 20:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kisenge PR, Hawkins AT, Maro VP, et al. Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis 2007; 7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antinori S. New insights into HIV/AIDS-associated cryptococcosis. ISRN AIDS 2013; 2013:471363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romanelli F, Jennings HR, Nath A, et al. Therapeutic dilemma: the use of anticonvulsants in HIV-positive individuals. Neurology 2000; 54:1404–7. [DOI] [PubMed] [Google Scholar]
- 19. Siddiqi O, Birbeck GL. Safe treatment of seizures in the setting of HIV/AIDS. Curr Treat Options Neurol 2013; 15:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirmani BF, Mungall-Robinson D. Role of anticonvulsants in the management of AIDS related seizures. Front Neurol 2014; 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg NM, Meert K, Marino D, De Baker K. Seizures associated with meningitis. Pediatr Emerg Care 1992; 8:67–9. [DOI] [PubMed] [Google Scholar]
- 22. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhein J, Morawski BM, Hullsiek KH, et al. ; ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhein J, Huppler Hullsiek K, Tugume L, et al. ; ASTRO-CM team Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 2019; 19:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carlson RD, Rolfes MA, Birkenkamp KE, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis 2014; 29:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montgomery MP, Nakasujja N, Morawski BM, et al. ; COAT and ORCAS Trial Teams Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: a comparison of three prospective cohorts. BMC Neurol 2017; 17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robertson KR, Nakasujja N, Wong M, et al. Pattern of neuropsychological performance among HIV positive patients in Uganda. BMC Neurol 2007; 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lofgren S, Hullsiek KH, Morawski BM, et al. ; COAT and ASTRO-CM Trial Teams Differences in immunologic factors among patients presenting with altered mental status during cryptococcal meningitis. J Infect Dis 2017; 215:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006; 61:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin D, Tucker MJ, Rieder MJ. Increased adverse drug reactions to antimicrobials and anticonvulsants in patients with HIV infection. Ann Pharmacother 2006; 40:1594–601. [DOI] [PubMed] [Google Scholar]
- 32. IBM Micromedex® DRUGDEX [electronic version ]. Greenwood Village, CO: Truven Health Analytics. Accessed 1 June 2019. [Google Scholar]
- 33. Liverpool HIV Pharmacology Group [electronic version] Cheshire. United Kingdom: The University of Liverpool and eMed- Fusion; https://www.hiv-druginteractions.org/. Accessed 1 July 2019. [Google Scholar]
- 34. Schutz C, Boulware DR, Huppler-Hullsiek K, et al. Acute kidney injury and urinary biomarkers in human immunodeficiency virus-associated cryptococcal meningitis. Open Forum Infect Dis 2017; 4:ofx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Satishchandra P, Sinha S. Seizures in HIV-seropositive individuals: NIMHANS experience and review. Epilepsia 2008; 49(Suppl 6):33–41. [DOI] [PubMed] [Google Scholar]
- 36. Singhi P. Infectious causes of seizures and epilepsy in the developing world. Dev Med Child Neurol 2011; 53:600–9. [DOI] [PubMed] [Google Scholar]
- 37. Renold C, Sugar A, Chave JP, et al. Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Medicine (Baltimore) 1992; 71:224–39. [DOI] [PubMed] [Google Scholar]
- 38. Satishchandra P, Nalini A, Gourie-Devi M, et al. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989-96). Indian J Med Res 2000; 111:14–23. [PubMed] [Google Scholar]
- 39. Subsai K, Kanoksri S, Siwaporn C, Helen L. Neurological complications in AIDS patients: the 1-year retrospective study in Chiang Mai University, Thailand. Eur J Neurol 2004; 11:755–9. [DOI] [PubMed] [Google Scholar]
- 40. Siddiqi OK, Ghebremichael M, Dang X, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis 2014; 58:1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang R, Zhang H, Xiong Y, et al. Molecular diagnosis of central nervous system opportunistic infections and mortality in HIV-infected adults in Central China. AIDS Res Ther 2017; 14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.