Abstract
Diabetic retinopathy (DR) is a common microvascular complication in young individuals with type 1 diabetes. It is recommended to implement structured screening programs and adopt an appropriate referral mechanism at all levels of the health system to prevent vision loss in this disease. We developed and pilot-tested the feasibility of a comprehensive DR screening model at a tertiary care diabetes clinic in India. The model comprised an affordable DR screening facility at the diabetes clinic, structured education sessions, and annual inhospital diabetes complication screening camps. Over the span of 2 years, we screened 413 eligible patients with type 1 diabetes and 17.4% (n = 72) had any form of DR in at least one eye. Half of the retinopathy positive patients had mild DR. However, only one-third of newly diagnosed patients reported to the eye care facility for DR management. Based on this study, it is feasible to screen all patients with type 1 diabetes for DR by increasing awareness and providing opportunities for DR screening at a tertiary care diabetes clinic. Our model combined with formal referral and follow-up systems would be a potentially scalable approach for DR prevention and management at diabetes care facilities in India.
Keywords: Diabetes complications, diabetic retinopathy, screening, type 1 diabetes
Type 1 diabetes mellitus (T1DM) is one of the most common pediatric endocrine illnesses. It is estimated that globally there are 1.1 million children and adolescents with T1DM below the age of 20 years.[1] Of these, more than 50% are from developing countries. In India, according to the recent International Diabetes Federation estimates, 128,500 children and adolescents are living with T1DM.[1] However, there is a paucity of population-level data on burden as well as the natural history of T1DM from developing countries.
Studies have reported that patients with T1DM are at a higher risk for developing long-term vascular complications due to their younger age at onset and longer duration of the disease.[2,3] Diabetic retinopathy (DR) is one of the common complications among people with T1DM and is responsible for 86% of the blindness among the younger age groups.[4] Longer diabetes duration, poorer glycemic and blood pressure control are strongly associated with DR. After 20 years of diabetes, nearly all patients with T1DM develop some degree of DR.[4] Most patients who develop DR have no symptoms until it progresses to an advanced stage. Further, the treatment for DR can be beneficial for both symptom amelioration and reduction in disease progression. It is highly recommended to have an annual screening for DR among patients with T1DM as it provides an opportunity to detect and manage vision-threatening stages of the disease to reduce the risk of loss of vision.
In India, T1DM patients experience many socioeconomic, health systems, and cultural barriers to seek long-term continuous care.[5] The majority of patients are unaware of the requirement for regular screening and therefore are not aware of their DR status.[6] Further, there is an acute shortage of manpower and infrastructure for DR screening and management in India. A recent facility assessment survey across 11 cities in India indicated that more than 40% of eye departments/hospitals lacked the infrastructure necessary for diagnosis and treatment of DR. More than half the eye care facilities would like further training for their ophthalmologist in the retina. There was a shortage of low-vision therapists, counselors, and optometrist across all types of facilities. Nearly half the hospitals did not possess a system to track patients needing treatment or for follow-up.[7]
Lack of DR screening facilities at the primary and secondary level and absence of referral mechanisms often results in overcrowding of patients at the tertiary health care facilities. This would significantly increase the waiting time and hinder timely access to care for patients especially those with vision-threatening DR. Despite the availability of infrastructure and trained manpower, tertiary care settings in India including the All India Institute of Medical Sciences (AIIMS, New Delhi) are unable to ensure annual screening for DR for all diabetes patients attending the outpatient department. Our analysis indicated that only 61.5% of the patients who attended the Diabetes of Young (DOY) Clinic at AIIMS during 2013–2015 had undergone DR screening. In order to address these challenges, we developed a structured screening and diabetes education program at the tertiary care level for the prevention of visual impairment and blindness due to DR among T1DM patients in India. The program envisages the establishment of cost-effective screening facility, referral and follow-up services for DR management at the diabetes clinic. The major objective of the program was to test the feasibility of a comprehensive screening model for DR among patients with T1DM attending tertiary care settings.
Methods
The program was pilot tested in the DOY Clinic at the AIIMS, New Delhi. The DOY clinic is a dedicated facility for the management of youth-onset diabetes patients including T1DM under the Department of Endocrinology and Metabolism at the AIIMS. This weekly outpatient clinic functions on every Saturday from 8.30 am to 1 pm. All T1DM patients who attended the DOY clinic from 9th April 2016 to 18th June 2018, with the age of 10 years and above or with at least 5 years of diabetes duration at registration were eligible to participate in the program. Informed consent was obtained from all participating patients. The Institutional Ethics Committee of AIIMS, New Delhi approved the program.
Diabetic retinopathy (DR) screening approaches
Clinic-based opportunistic screening
Our DR screening model had two main components: 1) establishment of a DR screening facility within the AIIMS-DOY clinic; 2) a diabetes education component to increase the awareness for DR screening among T1DM patients. We recruited a trained optometrist under the program to facilitate DR screening at the diabetes clinic and also to coordinate with the ophthalmologists for grading, referral, and management.
Visual acuity of the patients was measured using the ETDRS chart placed at 3 m distance under standard lighting conditions. A comprehensive eye examination was not done in the DOY clinic. An optometrist captured the retinal photographs using a digital fundus imaging system (non-mydriatic digital camera—initially by Bosch-handheld fundus camera, Stuttgart, Germany and later by Remidio-fundus on phone camera, Bangalore, India). The fundus photographs were manually transferred and graded by an ophthalmologist at the Dr. R. P. Centre for Ophthalmic Sciences at AIIMS, New Delhi. We used a structured questionnaire to obtain demographic and clinical details of the registered patients.
During the screening visit, patients with T1DM were educated about the need for screening and treatment for DR. They were counseled by a diabetes educator on diet, calorie counting, regular physical activity, insulin injection technique, sick-day management, and risk factors such as hypertension, dyslipidemia, etc. The education materials (posters, pamphlets, and brochures) were prepared in local vernacular and English and distributed among the patients. The DR screening reports were distributed to the patients in the subsequent follow-up visits. Those with any form of DR were referred to the Dr. R.P. Centre for Ophthalmic Sciences at AIIMS for further assessment. Patients with sight-threatening DR were contacted telephonically and referred immediately to the above tertiary eye care facility.
In-hospital diabetes complication screening camps
During the initial few months of the screening program, we realized that it was not possible to screen all the patients in the clinic-based opportunistic screening approach alone. This was due to several factors including large patient volumes, varying follow-up frequencies of the patients, the efficiency of the optometrist, etc. In order to increase the coverage of our DR screening model, we organized two inhospital diabetes complication screening camps during the project period. In the camps, we ensured a comprehensive evaluation of patients for all components of an annual metabolic and vascular complication assessment. Fig. 1 depicts the activities and patient flow in diabetes screening camps. Registered patients were sequentially directed to each counter to undergo anthropometric measurement, biochemical assessments, diabetes complication screening, and diabetes education. Apart from DR screening, patients were screened for other microvascular complications (diabetic neuropathy and diabetic nephropathy) and cardiovascular disease risk factors.
Figure 1.
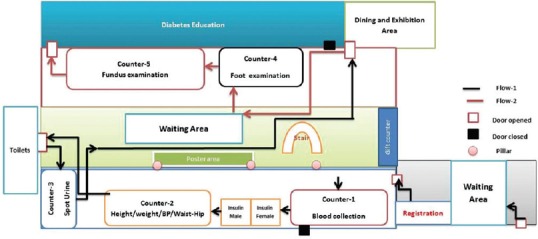
Schematic flow diagram of inhospital diabetes complication and education camps
Results
The screening details are provided in Table 1. During the project period, we screened 413 (53.7%, n = 221 males) TIDM patients for DR. 17.4% (n = 72) had any DR in one of the eyes, which includes nine patients who had received laser therapy for retinopathy earlier [Fig. 2]. 9.7% (n = 40) had any DR in both eyes. In 63 people, DR was diagnosed for the first time and half of them (55.6%, n = 35) had mild DR and nearly one-third (30.2%; n = 20) had macular edema [Fig. 3]. One-third (20 of 63 newly diagnosed DR—8 of 35 with mild DR, 4 of 6 with moderate DR, and 8 of 22 with severe DR/macular edema) people have since undergone treatment at the Dr. R. P. Centre for Ophthalmic Sciences at AIIMS. Details of the inhospital diabetes complications camps are shown in Table 2. We also organized two diet-awareness exhibitions and structured diabetes education sessions as part of the annual diabetes complication screening camps.
Table 1.
Details of diabetic retinopathy (DR) screening
| Number of patients | |
|---|---|
| Number of registered patients | 482 |
| Excluded from DR screening (age <10 years at registration or with cataract) | 69 |
| Undergone DR screening at least once during the project period | 413 |
| Undergone DR screening twice during the project period | 144 |
| Undergone DR screening three or more times during the project period | 138 |
Figure 2.
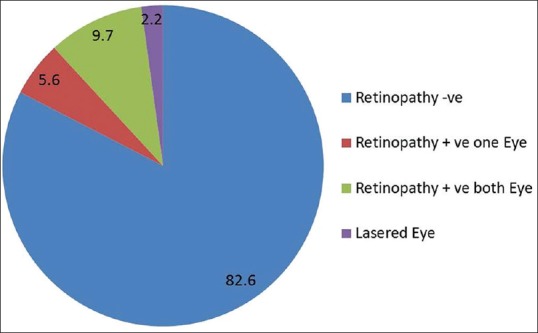
Prevalence (%) of diabetic retinopathy (n = 413)
Figure 3.
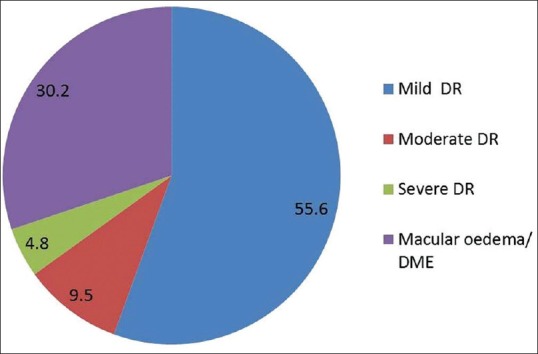
Results—grading of fundus photographs of newly diagnosed patients (n = 63)
Table 2.
Details of inhospital diabetes complication and education camps
| Date of camp | Camp-1* | Camp-2Ɨ |
|---|---|---|
| No. of patients attended | 356 | 374 |
| Number of patients | Number of patients | |
| Anthropometric measurements | ||
| Height | 351 | 372 |
| Weight | 350 | 372 |
| Waist and hip circumference | 324 | 359 |
| Blood pressure | 345 | 366 |
| Body fat composition (BIA) | 311 | 343 |
| Biochemical measurements | ||
| Fasting blood glucose | 345 | - |
| HbA1C | 346 | 374 |
| Lipids | 346 | 374 |
| Blood urea | 344 | 374 |
| Serum creatinine | 345 | 374 |
| Spot urine | 344 | 341 |
| Foot examination | 347 | 370 |
*24th Dec. 2016; Ɨ23rd Dec. 2017
Discussion
We have learned several lessons during the implementation of our DR screening model at AIIMS. First, it is feasible to establish a DR screening facility at a busy diabetes clinic at a tertiary care center. Second, our DR screening model is potentially scalable and can be implemented in similar tertiary care hospitals providing care for T1DM patients. Third, patient compliance with the screening was excellent as we could screen all the regular patients (with at least one follow-up during the program period) attending the DOY clinic and inhospital diabetes complication screening camps.
We also had challenges in various stages of the program. One, the handheld fundus camera used for DR screening was operator dependent and resulted in sub-optimal image quality in certain circumstances. We employed several measures to rectify the errors [Fig. 4]. In our experience, it is convenient and efficient to use a tabletop fundus imaging system in a clinic setting for DR screening. However, both these systems require skilled manpower for execution. With the improvement in technology and availability of non-mydriatic wide-angle fundus cameras, the screening may be more sensitive. It is necessary to develop more user-friendly fundus imaging systems that could be operated by minimally trained manpower. We encountered an unexpected delay in the grading of fundus photographs because these were manually transferred to the ophthalmologist for grading and diagnosis. Since the program did not support a trained ophthalmologist exclusively for diagnosis and management of these patients, we had to request the service of an ophthalmologist from the Dr. R.P. Centre for Ophthalmic Sciences at AIIMS. We used to transfer a set of photographs on a monthly basis. However, it added to the burden of the ophthalmologist and resulted in a delay in the grading of images. Given a large number of images taken, the waiting period was much longer to receive the grading of the camp patients. A possible solution to address this problem could be a robust automated fundus grading facility at the diabetes clinic and a web-based platform for the transfer of fundus images to finalize the grading. This would allow swift grading of fundus images at the diabetes clinic and instant referral of those with sight-threatening DR.
Figure 4.
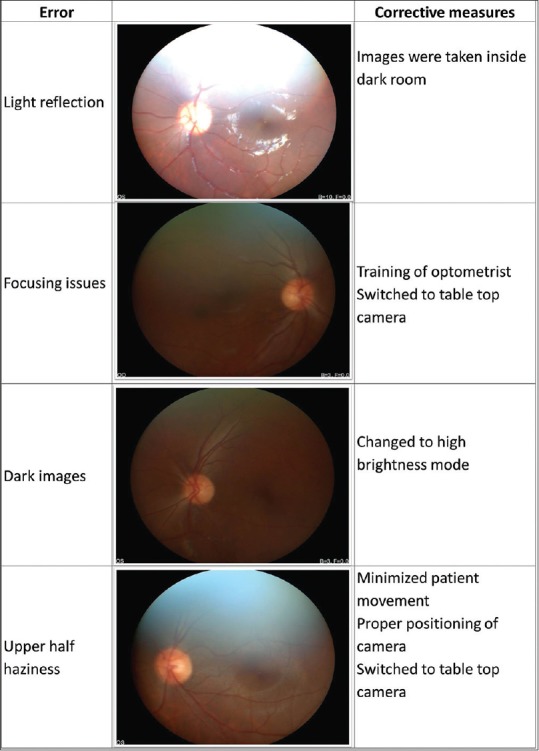
Handheld camera image error corrections
As mentioned earlier only one-third of the patients with any form of DR have attended the ophthalmic center for further assessment and management. Despite our education efforts and telephonic contact, we were unable to achieve an acceptable level of patient compliance on DR management. This underscores the need to establish formal linkages between the diabetes clinics and eye care facilities at the tertiary care level and follow-up system at the eye care facility. Moreover, the primary and secondary health care facilities should be equipped with DR screening models to minimize the inadvertent patient inflow to tertiary care settings.
Conclusion
With minimal infrastructure and manpower, it is feasible to establish a DR-screening facility at T1DM clinics at tertiary care centers. Structured diabetes education and inhospital diabetes complication camps have contributed to the coverage and acceptability of our DR screening model. However, the compliance of screened patients to DR treatment and follow-up needs further improvement. Innovative strategies for grading of fundus photographs and a formal referral/follow-up mechanism at the screening facility are highly desirable. These are vital not only to optimize the benefits of any DR screening model but also to ensure swift management of the patients with sight-threatening DR.
Financial support and sponsorship
The Queen Elizabeth Diamond Jubilee Trust, London, UK.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Last accessed on 2019 Sep 02]. Available from: http://www.diabetesatlas.org . [Google Scholar]
- 2.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–24. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 3.Walsh MG, Zgibor J, Borch-Johnsen K, Orchard TJ. A multinational comparison of complications assessment in type 1 diabetes: The DiaMond substudy of complications (DiaComp) level 2. Diabetes Care. 2004;27:1610–7. doi: 10.2337/diacare.27.7.1610. [DOI] [PubMed] [Google Scholar]
- 4.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):s84–7. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 5.Prasanna Kumar K, Saboo B, Rao P, Sarda A, Viswanathan V, Kalra S, et al. Type 1 diabetes: Awareness, management and challenges: Current scenario in India. Indian J Endocrinol Metab. 2015;19:6–8. doi: 10.4103/2230-8210.155339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy K, Raman R, Tandon M. Current state of care for diabetic retinopathy in India. Curr Diab Rep. 2013;13:460–8. doi: 10.1007/s11892-013-0388-6. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert C, Babu Rg, Gudlavalleti AV, Anchala R, Shukla R, Ballabh P, et al. Eye care infrastructure and human resources for managing diabetic retinopathy in India: The India 11-city 9-state study. Indian J Endocrinol Metab. 2016;20:s3–10. doi: 10.4103/2230-8210.179768. [DOI] [PMC free article] [PubMed] [Google Scholar]


