Abstract
The prevalence of youth-onset diabetes, both type 1 diabetes (T1D) and young-onset type 2 diabetes (YT2D) are gradually increasing in India. Early and repetitive screening for diabetic retinopathy (DR) is essential to provide timely management, and thereby prevent visual impairment due to the silent sight-threatening microvascular complication of diabetes. A study was undertaken at a diabetes care center in Chennai, south India, to assess the feasibility of screening for DR in T1D in a diabetes clinic and determine the burden of sight-threatening DR (STDR) in individuals with T1D. 315 people with T1D were screened for DR (mean age at onset of diabetes 12.3 ± 6.4 years) by digital retinal color photography, at the urban diabetes center, in a semi-urban and rural diabetes clinic. Counseling about diabetes and the importance of annual screening for retinopathy was provided by diabetes educators. Participants were reviewed after 6 months/1 year based on ophthalmologist's advice. DR was detected in 37.1% (n = 117), 42 (13%) of whom had STDR.Three-quarter participants were compliant with the annual follow-up retinal examination. The peer support group was established for participants with T1D and their families to foster interactions with service providers. The peer group meetings helped to increase the awareness of retinopathy among the parents and individuals with T1D. This narrative provides details of the study that shows that screening for DR among individuals with T1D in a diabetes clinic is a feasible model, irrespective of its location.
Keywords: Diabetic retinopathy, sight-threatening diabetic retinopathy, type 1 diabetes
Diabetic retinopathy (DR) is the most common sight-threatening microvascular complication of diabetes, occurring in both types 1 and 2 diabetes and is secondary to prolonged uncontrolled hyperglycemia and other risk factors.[1,2] In adolescents and young adults with type 1 diabetes (T1D), over 80% have some form of DR when the duration of diabetes is over 15 years.[3] It is estimated that 90% of the blindness due to DR is preventable.[4,5]
As the clinical course of DR has a long asymptomatic stage, the importance of screening is well established for early detection and calls for timely intervention in order to reduce visual impairment due to the sight-threatening DR (STDR).[6] The American Diabetes Association (ADA) recommends that people with T1D undergo the first retinal examination within 5 years of diagnosis of diabetes with life-long annual retinal examinations thereafter.[7] Fundus photography has been accepted as a mode of screening for DR and it is equivalent or superior to ophthalmoscopic examination for diagnosing DR.[8,9] As children/adolescents or young adults with T1D may not have visual symptoms related to DR, except transient blurring of vision due to severe hyper or hypoglycemia, they may not visit the ophthalmologist for regular retinal screening. Their regular point of care for type 1 diabetes management is the endocrinology or diabetology clinic.
The primary objective of this study was to assess the feasibility of screening for retinopathy in type 1 diabetes in a diabetes care clinic and determine the burden of STDR in people with T1D. The secondary objective was the establishment of peer support and family support groups for people with T1D and their families to foster interactions with service providers and thus increase awareness about DR.
Methods
The DRAFT (Diabetic Retinopathy Assessment For Type 1 diabetes) study was started at a tertiary care diabetes center in Chennai, south India after obtaining Institutional Ethical Committee approval in November 2015. The duration of the study was 2 years.
Individuals with confirmed T1D visiting a tertiary care diabetes center at Gopalapuram, Chennai (urban diabetes center), and a semi-urban diabetes clinic at Tambaram, Chennai, and the rural diabetes clinic, Chunampet village, Kanchipuram and willing to participate in the study were recruited in this study. Written informed consent was obtained from the study participants and assent from participants who were less than 18 years of age in addition to parental consent.
T1D was diagnosed as diabetes (onset usually below the age of 25 years) associated with abrupt onset of symptoms such as polyuria, polydipsia, or unexplained weight loss, diabetic ketoacidosis (DKA), absent insulin reserve as shown by fasting and stimulated C-peptide (<0.3 pmol/mL), and requirement for insulin from the time of diagnosis for control of hyperglycemia. For this study, the criteria for inclusion were a diagnosis of T1D (on insulin and absent insulin reserve, confirmed by fasting and stimulated C peptide assessment), current age above 10 years, a minimum duration of diabetes of 3 years, and willing to undergo retinal color photography. A pretested study questionnaire was administered to collect the general demographic details, family history of diabetes, treatment history, diet, lifestyle, and socioeconomic status. Clinical assessment (anthropometric measurement and blood pressure measurement), biochemical testing (fasting plasma glucose, glycated hemoglobin [HbA1C], urine microalbumin), and a complete ophthalmic assessment were performed. Retinal color photography was performed using a digital retinal camera (using Carl Zeiss FF450 fundus camera in the urban center, and Remidio Fundus on phone camera[9] in the semi-urban and rural diabetic clinics). The grading of DR of both eyes was done using the “International Clinical classification of DR (ICDR)” grading system.[10] STDR was defined as the presence of severe nonproliferative DR (NPDR), proliferative DR (PDR), and/or diabetic macular edema (DME). The images were graded on the same day and the findings and management decisions were communicated to patients. One-to-one consultation with a retina specialist was also provided face-to-face or remotely. Free glucometers and strips were provided and insulin and syringes were provided at no cost to a subset of the patients who could not afford them.
Dieticians and the diabetes educators/counselors provided counseling on all weekdays on topics such as administration of insulin, identifying symptoms of hypoglycemia and managing it, self-monitoring of blood glucose (SMBG), diet and lifestyle, and the importance of regular retinal screening. Participants were given a pamphlet on DR and another on frequently asked questions.
Peer-to-peer support group meetings for people with T1D took place on the second Saturday of every alternate month at the main diabetic center, to create awareness about diabetes and DR, answer queries, and to provide support by means of group therapy. Some of the participants screened at the semi-urban and rural diabetic clinics came to the urban diabetic center to participate in the peer-group meetings [Fig. 1].
Figure 1.
(a) Fundus photography for participants with type 1 diabetes. (b) Peer group support meets people with type 1 diabetes
Patients recruited in the study were advised follow-up retinal examination after 1 year. Those with the evidence of DR were advised more frequent follow-ups based on the severity of retinopathy. Patients with STDR were advised a quarterly review.
Results
A total of 315 (182 [58%] males) individuals with T1D (mean age 24.5 ± 9.8 years, mean age at onset of diabetes12.3 ± 6.4 years) were recruited. There are about 4,500 registered individuals with T1D in the tertiary care diabetes center. About 7% of the T1D patients participated in this study. 277 individuals with T1D (159 [63%] males) were screened at the urban tertiary care diabetes center, 30 (17 [57%] males) at the semi-urban clinic, and eight individuals (6 [75%] males) at the rural diabetes clinic. Even though the onset age of diabetes did not differ significantly between those with DR and without DR, the age, duration of diabetes, body mass index, systolic and diastolic blood pressure, HbA1c, microalbuminuria, and serum creatinine were significantly different between the two groups (P < 0.05) [Table 1]. The majority of participants (79%) were school or college students.
Table 1.
Baseline characteristics of people with type 1 diabetes by retinopathy status
| Variables | Without diabetic retinopathy (n=198) | With diabetic retinopathy (n=117) | P |
|---|---|---|---|
| Age (years) | 20.2±7.8 | 29.7±9.5 | <0.001 |
| Age at onset (years) | 11.7±6.2 | 12.9±6.6 | 0.110 |
| Duration of diabetes (years) | 7.8±4.6 | 16.4±8.0 | <0.001 |
| Gender (Males) n (%) | 113 (57.1) | 69 (58.9) | 0.73 |
| Occupation (%) | |||
| Business | 7 (3.5) | 16 (13.7) | |
| Clerical | 10 (5.1) | 17 (14.5) | |
| Professional | 18 (9.1) | 26 (22.2) | |
| Housewives | 6 (3.0) | 4 (3.4) | |
| Students | 157 (79.3) | 54 (46.2) | |
| Body mass index (kg/m2) | 20.3±4.2 | 24.6±3.8 | <0.001 |
| Systolic blood pressure (mm Hg) | 111±12 | 117±13 | <0.001 |
| Diastolic blood pressure (mm Hg) | 73±7 | 75±8 | 0.004 |
| Fasting plasma glucose (mg/dL) | 194±100 | 195±101 | 0.762 |
| Glycated hemoglobin (%) | 9.1±1.9 | 9.3±1.6 | <0.001 |
| Microalbuminuria(µg/mg of creatinine) | 20±52 | 47±92 | 0.002 |
| Serum creatinine (mg/dL) | 0.6±0.2 | 0.8±0.8 | 0.005 |
Values are mean±SD
About 75% of the participants developed diabetes between 6 and 20 years of age [Fig. 2]. Any DR was present in 117 (37.1%) and STDR was present in 42 (13.3%) participants [Table 2]. People with T1D who had DR were older, had a longer duration of diabetes, had higher glycated hemoglobin (HbA1C), blood pressure and had microalbuminuria than those without DR. Twenty-one patients received laser photocoagulation for DME/PDR and one patient received intravitreal anti-vascular endothelial growth factor (VEGF) injection for severe DME. The remaining patients with STDR (severe NPDR and non-center involving DME) did not require any intervention except glycemic control and more frequent and regular follow up.
Figure 2.
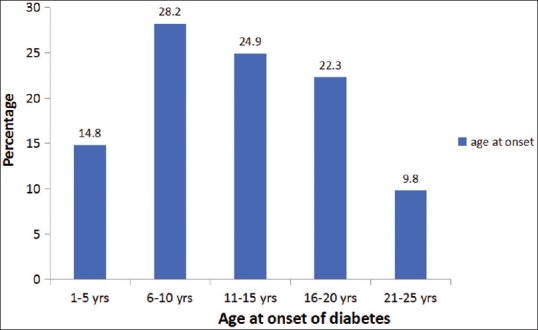
Distribution of study participants based on age at onset of type 1 diabetes
Table 2.
Diabetic retinopathy (DR) assessment in type 1 diabetes at Chennai
| Ocular findings | TOTAL (n=315) n (%) |
|---|---|
| Any DR | 117 (37.1%) |
| Mild nonproliferative DR (NPDR) | 68 (21.6%) |
| Moderate NPDR | 29 (9.2%) |
| Severe NPDR | 9 (2.9%) |
| PDR | 11 (3.4%) |
| Diabetic macular edema (DME) (present in NPDR or PDR stage) | 26 (8.3%) |
| Sight-threatening DR (STDR) | 42 (13.3%) |
| Laser photocoagulation/intravitreal injection for STDR | 22 (7.0%) |
| Other eye disorders-congenital cataract, optic atrophy, glaucoma, retinitis pigmentosa | 7 (2.2%) |
| 1-year annual retinal examination follow-up completed | 74.5% |
The youngest child detected with retinopathy (mild NPDR) was 10 years old. 158 (74.5%) of the 212 T1D study participants examined in 2016 completed the 1-year follow-up retinal examination and fundus photography. Some patients with STDR who underwent laser photocoagulation treatment returned for regular quarterly/half-yearly follow-up. Eleven peer group support meetings were completed on the second Saturdays of alternate months during the study period. Around 35–40 study participants took part in the peer group meetings every alternate month. The peer group meets were moderated by a diabetes educator and counselor. The parents of children with T1D and the individuals with T1D who participated in the peer group support meets benefitted from group therapy sessions conducted by the counselor. Interactions between older and younger T1D participants during the peer group meet helped to discuss diet, insulin administration, lifestyle, alleviate fears, and get answers to various queries related to diabetes, DR, and its management.
Discussion
The study has shown that a holistic approach to screening for DR and its complications integrated into the clinical care of people with T1D diabetes, with counseling at a diabetes clinic irrespective of its location is a feasible and accessible model to ensure regular and repetitive screening for DR and timely treatment of STDR. In this study, 37% of people with T1D had DR. Younger age of onset of diabetes and greater lifespan result in longer lifetime exposure to hyperglycemia and consequently a greater risk of developing DR in people with T1D.[1,3,11] Table 3 shows the comparison of the prevalence of DR in type 1 diabetes in different clinic-based and population-based studies done in India and elsewhere at different times.[1,3,12,13,14] People with T1D develop DR during their prime productive years of young adulthood. Hence, life-long repetitive retinal assessment is essential. We could achieve reasonably good adherence to the annual follow-up retinal photography advice by repeated counseling.
Table 3.
Various studies on DR in type 1 diabetes including the current study
| Name of the study | Type of study | Place and year | Number of T1D (n) | DR diagnosis by | DR % | PDR % |
|---|---|---|---|---|---|---|
| WESDR, Klein et al.[1] | Population based | Wisconsin, USA, 1996 | 996 | Fundus photography | 17% in <5 years of diabetes, 97.5% in >15 years of diabetes | 1.2% in <10 years diabetes, 67% in >35 years of diabetes |
| Eppens et al.[12] | Clinic based | Australia, 2006 | 1433 | Fundus photography | 20 | - |
| SEARCH, Pilot Mayer Davis et al.[13] | Population based | USA, 2012 | 222 | Fundus photography | 17 | - |
| YDR, Rajalakshmi et al.[3] | Diabetic Clinic based | Chennai, India, 2013 | 150 | Fundus photography | 53.3 | 7.3 |
| Jansson et al.[14] | Population based | Norway, 2018 | 237 | Fundus photography | 61 | 13 |
| Current DRAFT study | Diabetic Clinic based | Chennai, India, 2019 | 315 | Fundus photography | 37.1 | 3.4 |
WESDR=Wisconsin Epidemiologic Study of Diabetic Retinopathy, YDR=Young Diabetes Registry, DRAFT=Diabetic retinopathy assessment for type 1 diabetes
Challenges and solutions
Despite the need for regular follow-up being emphasized, patient compliance with annual follow-up retinal photography was the main challenge as some parents of T1D children and some young adults with T1D did not understand the importance of regular screening as their vision was normal and they were asymptomatic. Reminder SMS, counseling by dieticians and diabetes educators, and encouragement to take part in the peer group meetings helped to improve the compliance of the study participants. Doubts regarding laser photocoagulation treatment for DR, and managing certain lifestyle issues related to diabetes care were clarified during the peer group interactive sessions.
Conclusion
Routine screening for DR in a diabetes clinic has enabled the detection of DR in one-third of the people with T1D. All those with STDR requiring treatment underwent treatment, so reducing the risk of visual impairment. Three quarters of the participants with T1D were compliant with the annual follow-up retinal examination. The peer group meetings provided good support in increasing awareness among individuals with T1D and their parents. Further steps to improve compliance with regular retinal screening are essential for early detection and treatment of STDR in individuals with type 1 diabetes.[15]
Financial support and sponsorship
The Queen Elizabeth Diamond Jubilee Trust, London, UK and the Leona M. and Harry B. Helmsley Charitable Trust.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge the help of all the optometrists and eye technicians of DMDSC for performing the digital retinal color photography and Ms. Uma Shankari, Ms. Thangamani and Ms. Meenakshi for recruitment of the individuals with type 1 diabetes and ensuring follow-up in the study.
References
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy II Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmo. 1984;102:520–26. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 2.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: The Chennai urban rural epidemiology study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 3.Rajalakshmi R, Amutha A, Ranjani H, Ali MK, Unnikrishnan R, Anjana RM, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 2014;28:291–97. doi: 10.1016/j.jdiacomp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Vision loss expert group of the global burden of disease study. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 5.Fong DS, Ferris FL, Davis MD, Chew EY ETDRS Research Group. Causes of severe visual loss in the early treatment diabetic retinopathy study ETDRS report No 24. Am J Ophthalmol. 1999;127:137–41. doi: 10.1016/s0002-9394(98)00309-2. [DOI] [PubMed] [Google Scholar]
- 6.Namperumalswamy P, Nirmalan PK, Ramaswamy K. Developing a screening program to detect sight-threatening retinopathy in south India. Diabetes Care. 2003;26:1831–35. doi: 10.2337/diacare.26.6.1831. [DOI] [PubMed] [Google Scholar]
- 7.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27:2540–53. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed J, Ward TP, Bursell SE, Aiello LM, Cavallerano JD, Vigersky RA. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205–9. doi: 10.2337/dc06-0295. [DOI] [PubMed] [Google Scholar]
- 9.Rajalakshmi R, Arulmalar S, Usha M, Prathiba V, Kareemuddin KS, Anjana RM, et al. Validation of smartphone based retinal photography for diabetic retinopathy screening? PLoS One. 2015;10:e0138285. doi: 10.1371/journal.pone.0138285. doi: 10.1371/journal.pone. 0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 11.Rajalakshmi R, Prathiba V, Mohan V. Does tight control of systemic factors help in the management of diabetic retinopathy? Indian J Ophthalmol. 2016;64:62–68. doi: 10.4103/0301-4738.178146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–6. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 13.Mayer-Davis EJ, Davis C, Saadine J, D'Agostino RB, Jr, Dabelea D, Dolan L, et al. SEARCH for diabetes in youth study group Diabetic retinopathy in the SEARCH for Diabetes in Youth cohort: A pilot study. Diabetic Med. 2012;29:1148–52. doi: 10.1111/j.1464-5491.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansson RW, Hufthammer KO, Krohn J. Diabetic retinopathy in type 1 diabetes patients in Western Norway. Acta Ophthalmol. 2018;96:465–74. doi: 10.1111/aos.13654. [DOI] [PubMed] [Google Scholar]
- 15.Kannuri NK, Anchala R, Murthy GV, Gilbert CE. Strengthening diabetes retinopathy services in India: Qualitative insights into providers' perspectives: The India 11-city 9-state study. Indian J Endocrinol Metab. 2016;20(Suppl 1):S59–66. doi: 10.4103/2230-8210.179775. [DOI] [PMC free article] [PubMed] [Google Scholar]


