Abstract
Purpose:
To assess the proportion of people with type 2 diabetes mellitus (T2DM) with diabetic retinopathy (DR) and sight-threatening DR (STDR) and associated risk factors in select eye-care facilities across India.
Methods:
In this observational study, data of people with T2DM presenting for the first time at the retina clinic of eye-care facilities across India was recorded. Data collected in 2016 over 6 months included information on systemic, clinical, and ocular parameters. International Clinical Diabetic Retinopathy (ICDR) classification scale was used to grade DR. STDR was defined as presence of severe nonproliferative (NPDR), proliferative diabetic retinopathy (PDR), and/or diabetic macular edema (DME).
Results:
The analysis included 11,182 people with T2DM from 14 eye-care facilities (mean age 58.2 ± 10.6 years; mean duration of diabetes 9.1 ± 7.6 years; 59.2% male). The age-standardized proportion of DR was 32.3% (95%Confidence Interval, CI: 31.4-33.2) and STDR was 19.1% (95%CI: 18.4-19.8). DME was diagnosed in 9.1% (95%CI: 8.5-9.6) and 10.7% (95%CI: 10.1-11.3) people had PDR. Statistically significant factors associated with increased risk of DR (by multivariate logistic regression analysis) were: male gender (Odds ratio[OR] 1.57, 95%CI: 1.16-2.15); poor glycemic control–glycated hemoglobin (HbA1c >10%)(OR 2.39, 95% CI: 1.1-5.22); requirement of insulin (OR 2.55, 95%CI: 1.8-3.6);history of hypertension (OR 1.42, 95%CI: 1.06-1.88) and duration of diabetes >15 years (OR 5.25, 95%CI: 3.01-9.15).
Conclusion:
Diabetic retinopathy was prevalent in 1/3rd and sight-threatening DR in 1/5th of people with T2DM presenting at eye-care facilities in this pan-India facility-based study. The duration of diabetes was the strongest predictor for retinopathy.
Keywords: Diabetic retinopathy, eye hospitals, India, risk factors, sight threatening diabetic retinopathy
The global burden of diabetes has resulted in an increase in the prevalence of both microvascular and macrovascular complications of diabetes.[1] Diabetic retinopathy (DR), a morbid microvascular complication of diabetes, affects 1 in 3people with diabetes and is one of the main causes of visual loss in them.[2] More than 75% of people who have diabetes for 20 years or longer would have some form of DR despite all the advances in diabetes care.[3,4] The World Health Organization (WHO) has declared DR as the 5th leading cause of blindness, and as one of the important causes of preventable blindness.[5] With proper management more than 90% of visual loss due to DR could be prevented.
India is home to over 72 million people with diabetes.[6,7] With the prevalence of type 2 diabetes mellitus (T2DM) now reaching pandemic proportions, there is a concomitant increase in the prevalence of DR.[7] Though epidemiological studies have been done in South India to assess the prevalence of DR,[8,9,10,11,12,13] there has been no study yet that has documented the complete spectrum of eye disorders, including the sight-threatening DR (STDR) in people with diabetes in India. The main aim of this study was to assess complete spectrum of eye diseases in people with known diabetes in India. The Report 2 of the study provides estimates of the proportion of people with T2DM presenting at eye facilities diagnosed with DR and STDR in India and the associated risk factors.
Methods
The SPEED (Spectrum of Eye Disease in Diabetes) study was a multi-centric, -cross-sectional, observational clinic-based study designed to collect data from major eye care facilities in India to determine the spectrum of eye disorders in people with known diabetes. The study included only people with confirmed diabetes visiting the eye-care facility for the first time. The study excluded review patients visiting the eye center or people without known diabetes. A common data capturing protocol software was designed by Indian Institute of Public Health (IIPH), Hyderabad, India. The study was approved by the Institutional Ethical Committee of each eye care facility participating in the SPEED study. Institutional Ethical Committee (IEC) Approval was obtained by all 14 eye care facilities who participated in the study at their respective IEC in May- June 2016. All the Institutional Ethical Committee approval letters were submitted to IIPH. The data collection software and app base using Java was supplied to all participating centers online. The collected data was entered by the health care personnel in a prescribed pro forma on-line under the supervision of each facility's principal investigator. The patient data from the participating 14 eye care facilities was deposited into the server of IIPH. The name of the eye care facilities that participated in the study are provided in Report 1 of the SPEED study.
The common data collected included basic demographics, the type and duration of diabetes, details of the systemic and all ocular parameters. The study and data entry were performed over a period of 6 months in 2016. The data entered was based on patient examination carried out in the respective eye hospitals. Questions for eliciting history were administered in English or in the local language. Systemic history included age, gender, type of diabetes, treatment history for diabetes (diet and exercise/use of oral hypoglycemic agents (OHA) only, OHA and Insulin and Insulin only), control of diabetes and latest glycated hemoglobin (HbA1c). History and details of the systemic co-morbidities such as hypertension, nephropathy, stroke, neuropathy etc., if available were also entered.
Ocular history details and ocular examination findings were entered into the pro forma. The recorded ocular examination included Snellen's presenting and best corrected visual acuity, intraocular pressure, and slit lamp examination of the anterior segment of the eye. Fundus examination/photography were performed and the retinal diagnosis and the optic disc evaluation of each eye were entered separately in the pro forma. The grading of DR was performed by ophthalmologists using the International Clinical Diabetic Retinopathy (ICDR) severity scale.[14] Sight threatening DR (STDR) was defined by the presence of severe nonproliferative diabetic retinopathy (NPDR), proliferative diabetic retinopathy (PDR), and/or diabetic macular edema (DME)/clinically significant macular edema (CSME).[15] Each eye was assigned a retinopathy level. For patient wise analysis, the final diagnosis for each patient was determined from the level of DR of the worse eye using ICDR severity scale.
Statistical analysis
Stata14SE for Windows (StataCorp., TX, USA) was used for data and statistical analysis. Continuous data were expressed as mean ± standard deviation while categorical data were presented as proportions. Student's t test was used to compare means of continuous variables between subjects with and without retinopathy. Chi square test was used to compare proportions. To assess independent risk factors for diabetic retinopathy, we used logistic regression models with DR as the dependent variable and reported odds ratios (OR) with 95% confidence interval (CI). For all statistical tests, P value < 0.05 was considered significant.
Results
The study collected data from 11,390 people with diabetes. After excluding 208 people with known type 1 diabetes, the data of 11,182 people with known type 2 diabetes (T2DM) from 14 eye care facilities, across India were analyzed. The mean age of the people who participated in the study was 58.2 ± 10.6 years, their mean duration of diabetes was 9.1 ± 7.6 years and 59.2% of them were male. In this study, 3611 people with T2DM had any DR and 66.9% of them were male. Table 1 shows the baseline general clinical and biochemical characteristics of the study participants with and without DR. People with T2DM who had DR had a longer duration of confirmed diabetes, had reported younger age of onset of diabetes, had higher glycated hemoglobin (HbA1c), had associated hypertension and significantly higher number of them required insulin for the management of diabetes [Table 1]. The prevalence of retinopathy increased significantly with increase in age (Pearson Chi2 = 119.97 P < 0.001); 80% of people with DR were above 50 years of age. The age-standardized proportion of DR was 32.3% (95% Confidence Interval, CI: 31.4-33.2); NPDR was diagnosed in 2416 (21.6%) people and 1195 (10.7%) people had PDR. STDR was present in 2133 (19.1%) people with T2DM. Table 2 shows the varying severity of retinopathy in the study population.
Table 1.
Baseline characteristics of the study population with and without retinopathy (n=11182)
| Variable | No DR (n=7571) Mean±SD | DR present (n=3611) Mean±SD | P |
|---|---|---|---|
| Age (years) | 58.5±11.1 | 57.5±9.5 | |
| Male (%) | 55.6% | 66.9% | <0.001 |
| Reported age of onset of diabetes (years) | 50.9±11.3 | 45.2±10.6 | <0.001 |
| Reported duration of diabetes (years) | 7.6±7.3 | 12.3±7.8 | <0.001 |
| Glycated hemoglobin (HbA1c) (%) (Mean±SD) | 8.0±1.8 | 8.5±2.1 | <0.001 |
| Reported history of Systemic hypertension | 46.1% | 53.4% | <0.001 |
| Treatment for diabetes | |||
| OHA n (%) | 6660 (87.9) | 2509 (69.5) | <0.001 |
| OHA+ Insulin n (%) | 431 (5.7) | 579 (16.0) | |
| Only Insulin n (%) | 354 (4.7) | 511 (14.2) | |
| Others | 126 (1.7) | 12 (0.3) |
Table 2.
Severity of Diabetic retinopathy (DR) in the SPEED study
| Retinopathy Severity | Total Population (n=11182) n (%) [95% CI] |
|---|---|
| Overall Diabetic Retinopathy (DR) | 3611 (32.3) [95% CI 31.4-33.2] |
| Sight threatening Diabetic Retinopathy (STDR) | 2133 (19.1) [95%CI -18.4-19.8] |
| Non-proliferative Diabetic Retinopathy (NPDR) | 2416 (21.6) [95% CI 20.8-22.4] |
| Mild NPDR | 846 (7.6) |
| Moderate NPDR | 1124 (10.1) |
| Severe NPDR | 446 (3.9) |
| Proliferative Diabetic Retinopathy (PDR) | 1195 (10.7) [95%CI-10.1-11.3] |
| Diabetic macular edema (DME) | 1013 (9.1) [95%CI- 8.5-9.6] |
Fig. 1 shows the frequency of distribution of DR and STDR based on the duration of diabetes. Diabetic retinopathy and STDR increased with longer duration of diabetes; it was highest in people with >15 years of diabetes. The increase in DR with diabetes age had a linear trend (Trend Chi2 = 1100, P < 0.001). The proportion of STDR increased significantly in people with duration of diabetes >10 years (Trend Chi2 = 760.2, P < 0.001) [Fig. 1].
Figure 1.
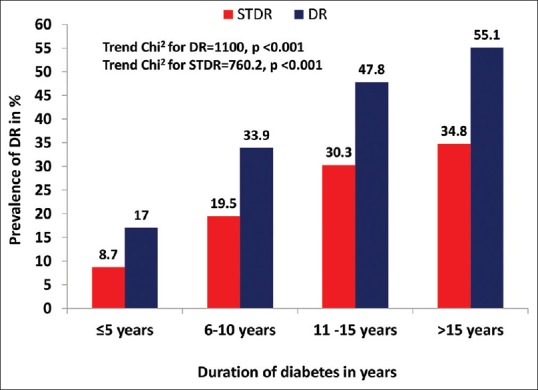
Frequency of any diabetic retinopathy (DR) and sight-threatening retinopathy (STDR), based on duration of diabetes
Multivariate logistic regression analysis was done with DR as dependent variable to assess the associated risk factors that increased the risk for retinopathy [Table 3]. Statistically significant factors associated with increased risk of DR (by multivariate logistic regression analysis) were: male gender (Odds ratio[OR] 1.51, 95%CI: 1.15-1.99); poor glycemic control–glycated hemoglobin (HbA1c >10%) (OR 2.39; 95% CI: 1.10-5.22); requirement of insulin in people on oral hypoglycemic agents (OR 2.55, 95%CI: 1.8-3.6); history of hypertension (OR1.42, 95%CI: 1.06-1.88) and duration of diabetes >15 years (OR 5.25, 95%CI: 3.01-9.15). The duration of diabetes remained the strongest predictor of DR, the odds for retinopathy was over 2 times higher in the 6-10 years of duration of diabetes, increasing to 3.4 times for a disease duration of 11–15 years and further increased to above 5 times when the duration of diabetes was >15 years. Poor glycemic control and requirement of insulin in people with T2DM were the additional significant risk factors associated with increased odds for retinopathy.
Table 3.
Logistic Regression Analysis with any DR as the Dependent Variable
| Risk factors for DR | Odds ratio (95% CI) | P |
|---|---|---|
| Male | 1.57 (1.16-2.15) | 0.0036 |
| Increase in Glycated hemoglobin (HbA1c) (%) (Reference <5.99%) | ||
| 6.0-7.99 | 1.09 (0.62-1.93) | 0.7605 |
| 8.0-9.99 | 1.50 (0.71-3.16) | 0.2817 |
| >10% | 2.39 (1.10-5.22) | 0.0237 |
| Reported Duration of diabetes (reference: ≤5 years) | ||
| 6-10 years | 2.67 (1.73-4.11) | <0.001 |
| 11-15 years | 3.41 (2.17-5.36) | <0.001 |
| >15 years | 5.25 (3.02-9.15) | <0.001 |
| Reported Hypertension | ||
| YES | 1.41 (1.06-1.88) | 0.0164 |
| Reported Treatment for Diabetes (reference: OHA) | ||
| OHA+ insulin | 2.55 (1.80-3.60) | <0.001 |
| Insulin | 2.17 (1.3-3.62) | 0.0024 |
Discussion
The proportion of people with T2DM with a confirmed diagnosis of DR and STDR from the eye care facility-based participants in this study was 32.3% and 19.1%, respectively. The risk factors identified in our study (such as male gender, higher HbA1c, people with type 2 diabetes who were also on insulin, associated hypertension, and longer duration of diabetes) are similar to the risk factors traditionally reported from earlier population-based epidemiological studies on DR.[8,9,10,11,12,13]
Prevalence of DR varies considerably in different populations and at different times.[2] It also varies with the settings of the study, a clinic based to a population-based study. Table 4 provides the comparison of prevalence of DR in different clinic based and population-based studies done in different parts of India at different times in the urban and rural areas. Irrespective of the methods used to detect DR, the prevalence of DR in India in various earlier epidemiological studies varied from 10.5% to 26.2%.[8,9,10,11,12,13,16,17] Five of 6 population-based studies in India on the DR prevalence were performed in the South India[8,9,10,11,12,16,17] and one study was performed in the West India.[13] The proportion of DR detected in this SPEED study across various tertiary eye care facilities is similar to DR detected in T2DM in the clinic-based study in Chennai, India.[15] The All India Ophthalmological Society (AIOS) Diabetic Retinopathy Eye Screening Study done in 2014 across India recruited 6218 people with known diabetes, was also a pan-India targeted study.[18] This study reported a DR prevalence of 21.7%,[18] but did not report DR severity. The DR (32.3%) reported in the current eye-care facility in 2016 is obviously higher because the SPEED study was a tertiary eye-care facility-based study.
Table 4.
Various studies on diabetic retinopathy in type 2 diabetes in India including the current SPEED study
| Name of the study | Type of Study | Place and year | Number of participants with type 2 diabetes | DR diagnosis by | DR% |
|---|---|---|---|---|---|
| Rema et al.[15] | Diabetic Clinic based | Chennai, 1996 | 6792 | Ophthalmoscopy | 34.1 |
| Andhra Pradesh eye disease survey (APEDS)[8] | Epidemiological (Urban) | Hyderabad 1999 | 124 | Ophthalmoscopy | 22.4 |
| Palakkad Eye Disease Survey, (PEDS)[9] | Epidemiological (Urban) | Kerala 2002 | 260 | Ophthalmoscopy | 26.8 |
| Chennai Urban Rural Epidemiology Study (CURES) - Eye Study[10] | Epidemiological (Urban) | Chennai 2003-2004 | 1715 | Fundus photography | 17.6 |
| Sankara Nethralaya Diabetic retinopathy epidemiology and molecular genetics study (SN DREAMS) Report 1[11] | Epidemiological (Urban) | Chennai 2009 | 1414 | Fundus photography | 18 |
| Aravind Theni Eye Study[12] | Epidemiological (Rural) | Theni 2009 | 2802 | Ophthalmoscopy | 12.2 |
| Sankara Nethralaya Diabetic retinopathy epidemiology and molecular genetics study (SN DREAMS) Report 2[16] | Epidemiological (Rural) | Kanchipuram, 2014 | 1360 | Fundus photography | 10.3 |
| The Chunampet Rural Diabetes Prevention Project Model (CRDPP)[17] | Epidemiological (Rural) | Chunampet, 2014 | 1001 | Fundus photography | 18.2 |
| AdityaJyot Diabetic Retinopathy Urban Mumbai Slums Study AJ-DRUMSS[13] | Epidemiological (Urban) | Mumbai, 2014 | 2415 | Fundus photography | 14.5 |
| All India Ophthalmological Society Diabetic Retinopathy Eye Screening Study[18] | Camp based | Across India, 2014 | 6218 | Ophthalmoscopy | 21.7 |
| Spectrum of eye disorders in diabetes (SPEED) Study. Report 2 | Eye care facility Based | Across India, 2016 | 11182 | Ophthalmoscopy/Fundus photography | 32.3 |
In our study about 1 in 3 Indians with T2DM presenting at an eye facility had DR. The proportion of STDR in our study was 19.1%, i.e., about 1/5th of people with T2DM had STDR and more than half of the people with DR had STDR possibly because this was data from tertiary eye care hospitals. This study analyzed pooled data of patients with T2DM presenting to the eye-care facilities for the first time and it is presumed that only those with more severe levels of retinopathy would have visited or were referred to these facilities. The male preponderance in this study is possibly because more males visited the tertiary eye care facilities for treatment.
STDR is a major concern due to visual impairment and the loss of productivity during the prime productive years of life. The vision loss could be minimized with the early detection, tight glycemic control right from the time of diagnosis of diabetes and timely treatment of STDR even before the patient is symptomatic.[19]
The risk factors associated with DR identified in this study are similar to the ones reported in earlier epidemiological studies.[10,11] Poor blood glucose management and duration of diabetes are the commonly reported risk factors associated with increased risk for DR.[20,21,22] The age at diabetes of T2DM is also an important risk factor as there is a trend of earlier age of onset of T2DM in the recent years.[21] Raman et al. (SN-DREAMS) has shown that people who develop T2DM early in their life (before 40 years age) have 2-time risk of DR and STDR.[23] In our study, the reported age of onset of T2DM was significantly lower in those with DR. In our series poor glycemic control and hence the need/requirement of insulin in T2DM (along with oral hypoglycemic agents/OHA) were associated with a 2-fold increased risk for retinopathy. Studies have shown that strict metabolic control and early detection reduces the progression and severity of DR.[19] Emphasis on glycemic control (HbA1c < 7%) and maintaining a near-normal blood pressure are important to reduce the risk of development and progression of retinopathy.[19,22]
Strengths of the study
This is possibly first eye care facility-based study from India that has looked at the complete spectrum of diabetic eye disorders. This report has estimated both the magnitude of DR and STDR among hospital attendances across the country.
Limitations of the study
It is cross-sectional hospital-based observational data-based study and hence causal directions are not clear. Our data was from patients with self-reported diabetes visiting eye-care facilities, and therefore findings may not directly apply to the entire population. Snellen visual acuity was recorded across the eye care facilities in the data collection app. Some of the eye care facilities didn't have access to complete systemic evaluation of the patients and hence there were some missing data of the systemic co-morbidities. Heterogeneity in the data collection across the various centers was a limitation. The possible inter-observer variation in retinopathy grading and assessment between the various eye care facilities, despite using the ICDR classification, was an additional limitation of this study.
Conclusion
Diabetic retinopathy was observed in one-third and sight-threatening diabetic retinopathy in one-fifth of the people with type 2 diabetes mellitus in the clinic population across India. Regular repetitive dilated fundus examination at shorter intervals would aid early detection of diabetic retinopathy and timely management of sight threatening diabetic retinopathy.[24,25]
SPEED study participating clinical facility organizations and investigators
Aravind Eye Hospital, Madurai (Dr. Kim Ramasamy, MD)
Divyajyoti Trust, Surat, India (Dr. Rohan Chariwala, MD; Dr. Uday Gajiwala, MD)
Dr Mohan's Diabetes Specialities Centre and Madras Diabetes Research Foundation, Chennai, India (Dr. R Rajalakshmi, MD)
Dr. Rajendra Prasad Center for Ophthalmic Sciences, New Delhi (Dr. Rohan Chawla, MD; Dr Atul Kumar, MD)
Dr. Shroff's Charity Eye Hospital, Delhi, India (Dr. Manisha Agarwal, MD)
H V Desai Eye Hospital (Dr. Kuldeep Dole, MD; Dr. Madan Despande, MD)
Little Flower Eye Hospital, Angamaly, India (Dr. Thomas Cherian, MD)
L V Prasad Eye Institute, Bhubaneswar, India (Dr. Umesh C Behera, MD)
L V Prasad Eye Institute, Hyderabad, India (Dr. Rajeev Reddy, MD; Dr. Taraprasad Das, MD)
Netra Nirmay Niketan, Purba Midnapur, Bengal (Dr Asim Sil, MD)
Post Graduate institute of Medical Education and Research, Chandigarh, India (Dr. Ramandeep Singh, MD; Dr. Mangat Dogra, MD)
Pushpagiri Eye Institute, Hyderabad, India (Dr. K Viswanath, MD)
Sankara Nethralaya, Chennai, India (Dr. Muna Bhende, MD)
Sri Sankaradeva Netralaya, Guwahati, India (Dr. Harsha Bhattacharjee, MS)
Financial support and sponsorship
The Queen Elizabeth Diamond Jubilee Trust, London, UK.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Meta-analysis for eye disease (META-EYE) study group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy III Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 4.Fong DS, Ferris FL, Davis MD, Chew EY. ETDRS research group: Causes of severe visual loss in the early treatment diabetic retinopathy study ETDRS Report No 24. Am J Ophthalmol. 1999;127:137–41. doi: 10.1016/s0002-9394(98)00309-2. [DOI] [PubMed] [Google Scholar]
- 5.Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 7.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian council of medical research-India diabetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 8.Dandona L, Dandona R, Naduvilath TJ, McCarty CA, Rao GN. Population based assessment of diabetic retinopathy in an urban population in Southern India. Br J Ophthalmol. 1999;83:937–40. doi: 10.1136/bjo.83.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendran V, John RK, Raghuram A, Ravindran RD, Nirmalan PK, Thulasiraj RD. Diabetic retinopathy among self reported diabetics in Southern India: A population based assessment. Br J Ophthalmol. 2002;86:1014–8. doi: 10.1136/bjo.86.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: The Chennai urban rural epidemiology study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 11.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Namperumalsamy P, Kim R, Vignesh TP, Nithya N, Royes J, Gijo T, et al. Prevalence and risk factors for diabetic retinopathy: A population-based assessment from Theni District, South India. Postgrad Med J. 2009;85:643–8. doi: 10.1136/bjo.2008.147934. [DOI] [PubMed] [Google Scholar]
- 13.Sunita M, Desai S, Vinay P, Moolani S, Rai N, Deepen S, et al. Aditya Jyot-diabetic retinopathy in urban Mumbai slums study (AJ-DRUMSS): Study design and methodology - report 1. Ophthalmic Epidemiol. 2014;21:51–60. doi: 10.3109/09286586.2013.867509. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 15.Rema M, Ponnaiya M, Mohan V. Prevalence of retinopathy in non-insulin dependent diabetes mellitus at a diabetes centre in southern India. Diabetes Res Clin Pract. 1996;34:29–36. doi: 10.1016/s0168-8227(96)01327-7. [DOI] [PubMed] [Google Scholar]
- 16.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan V, Prathiba V, Pradeepa R. Tele-diabetology to screen for diabetes and associated complications in rural India: The Chunampet rural diabetes prevention project model. J Diabetes Sci Technol. 2014;8:256–61. doi: 10.1177/1932296814525029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy India: The all India ophthalmological society diabetic retinopathy eye screening study 2014. Ind J Ophthalmol. 2016;64:38–44. doi: 10.4103/0301-4738.178144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed Q, Gilles MC, Wong TY. Management of diabetic retinopathy: A systemic review. JAMA. 2007;298:902–16. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 20.Pradeepa R, Anitha B, Mohan V, Ganesan A, Rema M. Risk factors for diabetic retinopathy in a South Indian type 2 diabetic population--the Chennai urban rural epidemiology study (CURES) eye study 4. Diabet Med. 2008;25:536–42. doi: 10.1111/j.1464-5491.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajalakshmi R, Amutha A, Ranjani H, Ali MK, Unnikrishnan R, Anjana RM, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 2014;28:291–7. doi: 10.1016/j.jdiacomp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Rajalakshmi R, Prathiba V, Mohan V. Does tight control of systemic factors help in the management of diabetic retinopathy? Indian J Ophthalmol. 2016;64:62–8. doi: 10.4103/0301-4738.178146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raman R, Vaitheeswaran K, Vinita K, Sharma T. Is prevalence of retinopathy related to the age of onset of diabetes. Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic report no 5? Ophthalmic Res. 2011;45:36–41. doi: 10.1159/000314720. [DOI] [PubMed] [Google Scholar]
- 24.Namperumalswamy P, Nirmalan PK, Ramaswamy KM. Developing a screening program to detect sight threatening retinopathy in south India. Diabetes Care. 2003;26:1831–5. doi: 10.2337/diacare.26.6.1831. [DOI] [PubMed] [Google Scholar]
- 25.Kannuri NK, Anchala R, Murthy GVS, Gilbert CE. Strengthening diabetes retinopathy services in India: Qualitative insights into providers' perspectives: The India 11-city 9-state study. Indian J Endocrinol Metab. 2016;20(Suppl 1):S59–66. doi: 10.4103/2230-8210.179775. [DOI] [PMC free article] [PubMed] [Google Scholar]


