Abstract
In order to integrate and improve eye care in noncommunicable disease (NCD) clinics, screening for diabetic retinopathy (DR) in people with diabetes mellitus (DM) was introduced in primary and secondary-level government health facilities. Initially, the project was carried out at the fixed health facilities at one district hospital (DH), two sub-district hospitals (SDH) and two community health centers (CHCs). This was combined with training of existing health care personnel, information-education-communication (IEC) campaign among patients and service providers along with the provision of essential equipment required for screening. In the revised strategy, NCD nurses were also trained for screening. Of 12,788 DM patients registered in NCD clinics, 63.8% (n = 8159) were screened for DR by trained paramedical ophthalmic assistants and the four trained NCD nurses using non-mydriatic fundus camera and teleophthalmology supported remote grading of retinopathy. DR was detected in 9.45% (n = 771) patients and sight-threatening DR (STDR) was detected in 2.35% (n = 192) in one or both eyes. Of 8,159 people screened, 55% (n = 4481) and 45% (n = 3678) were screened at CHC and mobile screening at primary health centers (PHC), respectively. DR screening in a fixed facility at CHC combined with the mobile screening at PHC level and fixed-day screening strategy provides effective coverage.
Keywords: Diabetic retinopathy, Maharashtra model, screening
The need for screening for diabetic retinopathy (DR) emerges from an estimated 8.4% prevalence of diabetes mellitus (DM) in Maharashtra.[1] Longer duration of DM and poor control are the main risk factors for developing DR. Early detection and timely treatment preserves vision.[2] Nonadherence to medication and lack of awareness about eye complications of DM contributes to severe visual impairment and even blindness.[3,4] The pilot project was implemented from January 2017 to June 2019 in the Wardha district of Maharashtra, India where noncommunicable disease (NCD) clinics were functional in the government health facilities. These facilities have been in existence since 2014 with 18,510 registered people with DM.
The objectives of the project were to create a district model for reducing blindness from DR in people with DM registered in government-run NCD clinics through effective screening, referral, and management of sight-threatening DR (STDR).
Methods
A tripartite agreement was drawn between the Indian Institute of Public Health (IIPH), Hyderabad, Mahatma Gandhi Institute of Medical Sciences (MGIMS) Sewagram, Wardha and the Government of Maharashtra to implement the project on reducing blindness from DR in the district. Entire program coursed through two different phases, from pilot to a refined program planning after the lessons learnt in the pilot phase
Pilot strategy
Initially, the project activities were carried out at the fixed health facilities at one district hospital (DH), two sub-district hospitals (SDH), and two community health centers (CHCs) where NCD clinics and eye clinics were functional. In each government health facility orientation training of NCD and eye clinic staff including medical officers, nurses and paramedical staff was undertaken by staff from MGIMS to create awareness about magnitude of DM, DR, need for screening and their role in project activities. The eye clinics were equipped with a non-mydriatic fundus camera (Forus 3-Nethra, Bangalore, India), the early treatment diabetic retinopathy study (ETDRS) vision charts, tablets with DRROP (DR Retinopathy of Prematurity) software (developed by IIPH, Hyderabad), and internet facilities. The training institute MGIMS was equipped with green retinal LASER, B-scan ultrasonography, fundus fluorescein angiography (FFA), indirect ophthalmoscopes, and computers with internet facilities.
The available paramedical ophthalmic assistant (PMOA) were trained for obtaining history, measuring visual acuity on ETDRS charts, capturing and uploading the fundus photos, and managing the register. Audio-visual aids and flex banners were displayed in all health facilities in the patients waiting area of NCD clinic, and all people with diabetes were provided handouts in local language emphasizing the need for regular medication and annual eye screening.
Demographic details and medical history of people with known diabetes attending NCD clinics were recorded and entered in the software linked to unique ID (Aadhar, Government of India). In an eye clinic, visual acuity was tested and retinal imaging was done. Fundus photographs were uploaded on the cloud and were remotely graded by a trained ophthalmologist at the base hospital. Report/advice for referral of STDR patients including those with ungradable photographs was generated and was shared in real-time. Patients were counseled for repeat annual screening or referral for management. Patients with evidence of DR in one or both eyes, ungradable fundus images and patients with best-corrected visual acuity < 6/60 in either eye were referred to the base hospital. At the base hospital, the referred patients were further investigated and appropriate treatment (LASER and/or anti-vascular endothelial growth factor, anti -VEGF, injections) was instituted.
The main learning from the pilot project was the lack of awareness in people, inadequate staffing (the PMOAs were available in CHCs and PHCs only once or twice a week), and transport-related constraints. Based on these lessons, a revised program was drawn.
Revised strategy
Four NCD nurses were trained for measuring vision and fundus photography so that they could work on the days when the PMOAs were not available at the CHCs. 610 accredited social health activists (ASHA) were trained and provided with the information-education-communication (IEC) materials (posters, pamphlets, and flipcharts). Aligned with the government norms, an incentive was provided to the ASHA workers to communicate with people with known diabetes in their village and bring them to the nearest PHC/subcenters on the day fixed for mobile screening and also motivate referred patients for the uptake of services for DR management. Ophthalmology residents were introduced to the program for skill transfer, support, and troubleshooting initially. A screening van equipped with necessary eye screening devices including a fundus camera was introduced. Mobile screening at 22 PHCs following a fixed-day approach was added to fixed facility screening. Ophthalmology residents from the base hospital monitored the screening. Complimentary transportation was provided to all referred patients from fixed facilities and from screening sites at PHC to base hospitals.
The base hospital also organized education programs on DM and DR for physicians, including enrolling 41 physicians in the certificate course on evidence management of DR (CCDR) designed by the IIPH, Hyderabad. Peer group initiative was started in four PHC areas and pharmacists were nudged to create awareness of DR among people with DM.
DR was graded as per international clinical DR and diabetic macular edema (DME) disease severity scales.[5] Any grade worse than moderate NPDR or DME in one or both eyes was labeled as STDR. Data obtained were entered into pretested and validated computer-friendly pro forma. Data analysis was done using EPI Info™ Software.
Results
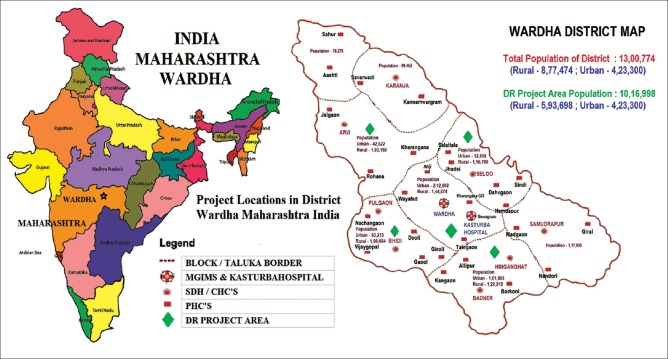
The project covered a population of 1,016,998 people spread over five blocks in four CHCs and 22 PHCs in Wardha district [Fig. 1]. Trained PMOAs/NCD nurses screened 63.8% (n = 8159) of registered 12,788 people for DR at government health facilities using a non-mydriatic fundus camera. This included 29.6% (n = 2413) people screened at fixed facility, and 70.4% (n = 5746) people screened by combined fixed and mobile facilities. In the final analysis 45% (n= 3,678 were screened by mobile screening at PHCs [Table 1]. The results showed that mobile screening at PHC covered a higher proportion of people with advanced age, longer duration of DM, uncontrolled blood sugar, and systemic comorbidities. Visual acuity of people screened at PHC was poorer compared to the people screened at the CHC, 9.8% versus 5.2% respectively [Table 2]. Of the people with DM screened, 35.7% (n = 2914) returned for annual review examination.
Figure 1.
Project Locations in District Wardha Maharashtra India
Table 1.
Coverage of DR Screening during Pilot and Revised Strategy (N=12788 Diabetics Registered in NCD Clinics)
| Fixed Facility screening (GH/SDH/CHC ) no of persons (%) | Mobile Screening (PHC/Sub Centre) No of Persons (%) | Total DR Screening No of Persons (%) | Significance | |
|---|---|---|---|---|
| Pilot Strategy | 2413 (18.87) | 0 | 2413 (18.87) | |
| Revised Strategy | 2068 (35.99) (16.17) | 3678 (64.01) (28.76) | 5746 (100.00) (44.93) | P<0.001 (Significant) |
| Total New Screening | 4481 (54.92) (35.04) | 3678 (45.08) (28.76) | 8159 (100.00) (63.80) | P<0.001 (Significant) |
| Repeat annual screening | 1512 (51.89) (18.53) | 1402 (48.11) (17.18) | 2914 (35.71) |
Table 2.
Profile of DM patients Screened for DR at CHC and PHC
| Fixed Facility Screening (GH/SDH/CHC) No. of Patients (%) (n=4481) | Mobile Screening (PHC/Sub Centre) No. of Patients (%) (n=3678) | Total Screening No. of Patients (%) (n=8159) | Significance | |
|---|---|---|---|---|
| Gender | ||||
| Male | 2669 (59.56) | 1602 (43.56) | 4271 (52.35) | P<0.001 (Significant) |
| Female | 1812 (40.44) | 2076 (56.44) | 3888 (47.65) | |
| Age in Years | ||||
| Mean±SD | 47.11±12.02 | 56.02±11.62 | 58.13±11.92 | P<0.001 (Significant) |
| Type of DM | ||||
| Type I | 71 (1.58) | 44 (1.20) | 115 (1.42) | P>0.05 Not Significant |
| Type II | 4410 (98.42) | 3634 (98.80) | 8044 (98.58) | |
| Duration of DM (in Years) | ||||
| Mean±SD | 4.14±4.53 | 5.51±4.93 | 4.76±4.73 | P<0.001 (Significant) |
| > 10 Years | 218 (4.86) | 402 (10.93) | 620 (7.60) | |
| Nature of Treatment | ||||
| Oral Drugs | 3981 (88.84) | 3374 (91.73) | 7355 (90.15) | P>0.05 Not Significant |
| Insulin | 121 (2.70) | 102 (2.77) | 223 (2.73) | |
| Diet Exercises | 211 (4.71) | 112 (3.05) | 323 (3.96) | |
| AYUSH System | 168 (3.75) | 90 (2.45) | 258 (3.16) | |
| Control of Blood Sugar | ||||
| Controlled | 2638 (58.87) | 1176 (31.97) | 3814 (46.75) | P<0.001 (Significant) |
| Uncontrolled | 1843 (41.13) | 2502 (68.03) | 4345 (53.25) | |
| Systemic Comorbidities | ||||
| Nephropathy | 28 (0.62) | 44 (1.20) | 72 (0.88) | P>0.05 (Not Significant) |
| H/o Stroke/MI | 36 (0.80) | 94 (2.56) | 132 (1.62) | |
| Visual Status as per (WHO) classification | ||||
| Near Normal | 2950 (65.83) | 2389 (64.95) | 5339 (65.44) | P<0.001 (Significant) |
| Visual impairment | 1034 (23.08) | 671 (18.24) | 1705 (20.90) | |
| Severe visual impairment | 294 (6.56) | 231 (6.28) | 525 (6.43) | |
| Blind | 231 (5.16) | 359 (9.80) | 590 (7.23) |
In 1146 (7.01%), eyes images could not be obtained/were not gradable. Out of remaining 15,212 eyes with gradable images 8.6% (n = 1316) eyes of 9.4% (n = 771) patients were detected to having DR in one or both eyes. DME was identified in 1.9% (n = 288) eyes of 1.9% (n = 157) patients. STDR was identified in 2.2% (n = 331) eyes of 2.3% (n = 192) patients. Significantly, a higher proportion of people with DM examined in mobile DR screening at PHC had any DR, severe nonproliferative DR (NPDR), proliferative DR (PDR), DME, and STDR. Combined screening at CHC and PHC increased the yield of detection of DR by 84% and STDR by two-fold [Table 3].
Table 3.
Prevalence of DR and STDR in DM patients Screened at CHC and PHC
| Screening at CHC (n=4481 Persons) (n=8519 Eyes) | Screening at PHC (n=3678 Persons) (n=6693 Eyes) | Total Screening (n=8159 Persons) (n=15212Eyes) | Significance | |
|---|---|---|---|---|
| Prevalence of DR | ||||
| Persons (in one or both eyes) | 354 (7.90) | 417 (11.34) | 771 (9.45) | P<0.001 (Significant) |
| Eyes (in Gradable images) | 717 (8.42) | 599 (8.95) | 1316 (8.65) | |
| Grade of DR (eyes) | ||||
| Mild NPDR | 374 (4.39) | 298 (4.45) | 672 (4.42) | P<0.001 (Significant) |
| Moderate NPDR | 277 (3.25) | 191 (2.85) | 468 (3.08) | |
| Severe NPDR | 42 (0.49) | 67 (1.00) | 109 (0.72) | |
| PDR | 24 (0.28) | 43 (0.64) | 67 (0.44) | |
| Prevalence of DME | ||||
| Persons (in one or both eyes) | 81 (1.81) | 76 (2.07) | 157 (1.92) | P<0.001 (Significant) |
| Eyes (Gradable images) | 147 (1.73) | 141 (2.11) | 288 (1.89) | |
| Prevalence of STDR | ||||
| Persons (in one or both eyes) | 87 (1.94) | 105 (2.85) | 192 (2.35) | P<0.001 (Significant) |
| Eyes (Gradable images) | 150 (1.76) | 181 (2.70) | 331 (2.18) |
Following the screening, 1,821 people with diabetes were referred and 68.2% (n = 1242) who attended base the hospital were investigated—771 for DR and 471 for other ocular comorbidities. There was better compliance with referral advice when the transport provision was available and the ASHA workers counseled the people. Treatment was offered to 249 people with STDR at the base hospital at no cost to them. This consisted of retinal laser to 128 people, intravitreal anti-VEGF to 98 people and combined retinal laser and anti-VEGF to 23 people [Table 4].
Table 4.
Compliance to Referral and Management of DR Patients Screened at CHC and PHC
| Screening at CHC No. of Persons (%) (n=4481) | Screening at PHC No. of Persons (%) (n=3678) | Total Screening No. of Persons (%) (n=8159) | Significance | |
|---|---|---|---|---|
| Referred | 997 (22.25) | 828 (22.51) | 1821 (22.32) | |
| Attended Base Hospital | 618 (13.79) | 624 (16.97) | 1242 (15.22) | |
| Compliance | 61.98% | 75.36% | 68.20% | |
| Investigated at Base Hospital | ||||
| For DR | 370 (8.26) | 401 (10.90) | 771 (9.45) | |
| For Ocular co-morbidity | 179 (3.99) | 292 (7.94) | 471 (5.77) | |
| Treatment for DR | 106 (29.94) | 143 (34.29) | 249 (32.30) | |
| LASER (PRP/Grid) | 52 (14.69) | 76 (18.23) | 128 (51.41) | P>0.05 (Not Significant) |
| Anti-VEGF Inj | 42 (11.86) | 56 (13.43) | 98 (39.36) | |
| LASER + anti - VEGF Inj | 12 (3.39) | 11 (2.64) | 23 (9.24) |
Discussion
The majority of the rural population is dependent on public health facilities for the provision of health care services because of socioeconomic constraints. Glycemic control that depends on regular availability and intake of anti-diabetes medication is not satisfactory in this population, which increases the risk for DR. Undiagnosed, uncontrolled diabetes with increasing duration leads to undetected DR. Screening services are practically nonexistent in primary and secondary government health facilities in Maharashtra. Under the project, health facilities in the district were equipped and manpower trained.
An integrated approach between eye care and diabetes care is required to reduce the risk of blindness from DR.[6] Orientation training, IEC campaign among the service providers helped in creating awareness and building bridges between the staff of NCD and the eye clinics. This program helped to create better coordination between district-level staff under the National Programme for Control of Blindness and Visual Impairment (NPCB and VI) and National Program for Prevention and Control of Cancer, Diabetes, CVD, and Stroke (NPCDCS). The implementation of the district model of screening for DR in people with known diabetes involving the existing staff of eye and NCD clinic in government health facilities helped in the detection of DR in 9.45% of screened and controlling blindness due to STDR. Combining fixed facility screening at CHC with the mobile screening at PHC improved the access to the services to people who needed them most, with 63.8% coverage of DR screening of the target group.
Lack of awareness of eye complications, asymptomatic nature of DR and nonadherence to eye care contributes to poor uptake of screening services. The burden of DR can be tackled by increasing interaction between the people with diabetes and their care providers.[7] ASHA workers empowered with knowledge could play an important role in conducting awareness campaigns on diabetes and its complications.[8] The role of a social worker as a link between community and service providers is vital for effective implementation of the DR screening model.[9]
People detected with any DR need a referral to the next level of health care for diagnosis and treatment if required.[10] Facilities for management of DR are available in few medical colleges in government, NGO and private sector tertiary care facilities but most are located in urban areas; but accessibility and affordability are common barriers. Counseling of patients by ASHA workers, referral tracking by use of software based on a unique ID (Aadhar in this case), provision of complementary transport from health facilities to the base hospital ensured accessibility and compliance. The management of referred patients was undertaken at no cost to them which meant that more people accepted treatment. The timely provision of services to the people at risk of visual loss helped reduce avoidable blindness due to DR.
Despite good knowledge and attitude, insufficient motivation for evaluation and follow-up are potential barriers to improve the practice pattern for which community empowerment is recommended.[11] Services for annual screening were availed by 35.7% of those screened but sustainability continues to be challenging.
Our model does not address the people with undiagnosed diabetes in the community. With improved detection by screening and reduction of blindness and visual impairment with timely and appropriate treatment might be an incentive for more people opting for screening.
Conclusion
Implementation of district model of screening for DR in people with known diabetes integrated in primary and secondary level government health facilities by trained paramedical staff of NCD and eye clinic by non-mydriatic fundus camera using teleophthalmology supported remote grading by ophthalmologist could help in early diagnosis and controlling blindness due to DR. Combining fixed-facility screening at CHC with mobile screening at PHC is likely to increase coverage. Two other factors that could contribute to the success of screening for DR are the involvement of ASHA workers and providing transport to facilities providing services for diagnosis and treatment of DR.
Financial support and sponsorship
The Queen Elizabeth Diamond Jubilee Trust, London, UK.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and pre diabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase 1 results of the Indian council of medical research – India diabetes (ICMR – INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Scanlon PH. The english national screening programme for diabetic retinopathy 2003-2016. Acta Diabetol. 2017;54:515–25. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohan D, Raj D, Shanthirani CS, Datta M, Unwin NC, Kapur A, et al. Awareness and knowledge of diabetes in Chennai – The Chennai urban rural epidemiology study [CURES-9] JAPI. 2005;53:283–7. [PubMed] [Google Scholar]
- 4.Singla A, Sharma T, Kaeley N. Awareness of diabetic patients towards diabetes mellitus: A survey based study. Ntl J Commun Med. 2017;8:606–10. [Google Scholar]
- 5.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 6.Murthy G, Das T. Diabetic care initiatives to prevent blindness from diabetic retinopathy in India. Indian J Ophthalmol. 2016;64:50–4. doi: 10.4103/0301-4738.178152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raman R, Gella G, Srinivasan S, Sharma T. Diabetic retinopathy: An epidemic at home and around the world. Indian J Ophthalmol. 2016;64:69–75. doi: 10.4103/0301-4738.178150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt BR. ASHAs in rural India, the ray of hope for diabetes care. J Soc Health Diabetes. 2014;2:18–24. [Google Scholar]
- 9.Rani PK, Raman R, Agarwal S, Paul PG, Uthra S, Margabandhu G, et al. Diabetic retinopathy screening model for rural population: Awareness and screening methodology. Rural Remote Health Res. 2005;5:350. [PubMed] [Google Scholar]
- 10.Vashist P, Singh S, Gupta N, Saxena R. Role of early screening for diabetic retinopathy in patients with diabetes mellitus: An overview. Indian J Community Med. 2011;36:247–52. doi: 10.4103/0970-0218.91324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain R, Rajesh B, Giridhar A, Gopalakrishnan M, Sadasivan S, James J, et al. Knowledge and awareness about diabetes mellitus and diabetic retinopathy in suburban population of a south Indian state and its practice among the patients with diabetes mellitus: A population-based study. Indian J Ophthalmol. 2016:64:272–6. doi: 10.4103/0301-4738.182937. [DOI] [PMC free article] [PubMed] [Google Scholar]