Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is currently the major cause of chronic liver disease globally. Bile acids (BAs) have emerged as relevant signaling molecules that are associated with NAFLD development. This study was aimed to examine the association of serum total bile acids (TBAs) with NAFLD in a large population of Chinese subjects.
Methods
This cross sectional study recruited 152,336 participants from the Second Xiangya Hospital, China. NAFLD was diagnosed based on the presence of hepatic steatosis on ultrasonography, without significant alcohol consumption and other known causes for chronic liver disease. A multivariate logistic regression model was used to test for the association of serum TBAs with NAFLD, adjusting for conventional risk factors of NAFLD.
Results
A total of 27.4% of the participants had NAFLD. Patients with NAFLD had slightly higher TBA levels than those without, 3.4 vs. 3.0 μmol/L (p < 0.001). However, TBA levels were not associated with NAFLD in the multivariate logistic regression model, which adjusted for age, gender and other acknowledged risk factors for NAFLD (OR = 1.00. 95% CI: 1.00–1.00, p = 0.797).
Conclusions
We found that the serum TBA levels were not associated with NAFLD. Future studies in a large population, focusing on serum BA composition may improve the understating of the role of BAs in NAFLD.
Keywords: Non-alcoholic fatty liver disease, Bile acid, Farnesoid X receptor, Takeda G-protein-coupled receptor 5, Type 2 diabetes, Ziyu Zhang and Wen Dai contributed equally to this work and were listed co-first authors.
Background
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and is currently the major cause of chronic liver disease globally [1–3]. The estimated prevalence of NAFLD worldwide is approximately 25% in the general population [4]. NAFLD includes a broad clinical and histological spectrum ranging from simple hepatic steatosis (HS) to non-alcoholic steatohepatitis (NASH), with varying degrees of inflammation and fibrosis, which can progress to cirrhosis. It has been reported that approximately 20–30% of subjects with HS would develop NASH [5]. Moreover, NASH has become the leading cause of liver failure and the major indication for liver transplantation [6–9].
Bile acids (BAs) are amphipathic steroid acids that are derived from hepatic cholesterol catabolism in a series of enzyme-catalyzed reactions [10]. Upon synthesis, BAs are subsequently secreted and drained into the gallbladder for temporary storage via the biliary ducts. Postprandial contraction of the gallbladder releases BAs to the duodenum. In the intestine, BAs facilitate the emulsification and absorption of dietary lipids, as well as lipophilic vitamins [10]. After reaching the terminal ileum, BAs are almost completely (95%) reabsorbed and recirculated to the liver via the portal vein. The reabsorbed BAs are secreted back into the bile ducts together with newly synthesized BAs. This process is known as bile acid enterohepatic circulation, which allows for efficient recovery and reuse of the vast majority of BAs [10]. However, a small fraction of reabsorbed BAs bypass enterohepatic circulation and reach the systemic circulation [10].
Interestingly, circulating BAs have emerged as relevant signaling molecules, which act on hepatic and extrahepatic tissues to regulate lipid and carbohydrate metabolic pathways, as well as energy homeostasis, via activation or modulation of bile acid receptors, such as the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5) [11–14]. It has been reported that the BA-FXR/TGR5 pathways regulate hepatic lipid and glucose metabolism, as well as inflammatory activity [15–19].
Moreover, recent studies have shown that blood BAs are closely associated with NAFLD in humans. Serum total bile acid (TBA) levels in NAFLD patients (n = 16) were approximately 3 times higher than those of healthy controls (n = 11) [20]. Similarly, Rebecca et al. found that patients with type 2 diabetes (T2D), a major risk factor for NAFLD, had a nearly two-fold elevation in their plasma TBA [21].
A major limitation of these studies was their small sample sizes and to our knowledge, the association between serum TBAs and NAFLD has not been examined in a large-scale epidemiologic study before. Thus, this study was aimed to evaluate whether serum TBA levels were independently associated with NAFLD in a large population of Chinese subjects.
Methods
Study design and population
In this cross-sectional study, participants were recruited from the Health Management Center, The Second Xiangya Hospital, Changsha, China, from 2010 to 2017. We included adult participants who were scheduled to receive systemic medical evaluation, including subjective medical history, physical exam, blood chemistries, electrocardiogram and abdominal ultrasound. Subjects were excluded if they 1) were less than 18 years old; 2) reported excess alcohol consumption or a history of viral hepatitis, autoimmune hepatitis, or other known causes of chronic liver disease. 152,336 subjects were ultimately included in this study. This study was approved by the ethics committee institutional review board of The Second Xiangya Hospital of Central South University. The study was carried out in accordance with the 1975 Declaration of Helsinki and pertinent regulations. Informed written consent was obtained from all participants.
Data collection
Demographic characteristics, medical histories, blood chemistries and abdominal ultrasound data were gathered for all participants at the time of enrollment. Specific blood chemistries included fasting serum BAs, glucose and lipids, as well as liver and renal function tests. As previously described [22, 23], blood samples were drawn by venipuncture after overnight fasting. The blood specimens were processed and measured at the central laboratory in The Second Xiangya hospital, which is standardized and certified. An automatic biochemistry analyzer (Hitachi 7360; Hitachi Ltd., Tokyo, Japan) and commercially available kits were used to measure serum biochemistries according to accompanying manuals. Specifically, serum BAs were measured using enzymatic assay [24].
NAFLD was diagnosed based on the evidence of fatty liver upon abdominal ultrasonography and the exclusion of other known etiology of chronic liver diseases [25]. Hypertension was referred to as blood pressure ≥ 140/90 mmHg in more than two measurements and/or the requirement of or treatment with anti-hypertension drugs [26]. Diabetes was diagnosed as the existence of fasting serum glucose levels ≥7.0 mmol/L, and/or random serum glucose ≥11.1 mmol/L, and/or 2-h post-prandial serum glucose ≥11.1 mmol/L on oral glucose tolerance test (OGTT) in multiple measurements and/or the requirement of treatment with hypoglycemic agents [27]. Patients were diagnosed with coronary artery disease (CAD) based on the clinical manifestation, such as chest pain, and evidences indicating myocardial ischemia [28, 29].
Statistical analysis
Numerical variables were expressed as the mean (standard deviation) or as medians (Q1-Q3 quartiles), depending on the pattern of data distribution. Categorical variables were expressed as percentages (numbers). Differences in numerical variables between groups were analyzed by the independent t test, analysis of the variance (ANOVA), Mann-Whitney U test or Kruskal-Wallis H test, as appropriate. Differences in categorical variables were analyzed by the chi-square test. Logistic regression analyses were performed to examine the association of serum TBA with NAFLD and T2D. SPSS software (version 20.0; SPSS Inc., Cary, Chicago, USA) was used to perform the statistical analyses. For all analyses, two-tailed p values < 0.05 were considered statistically significant.
Results
Clinical characteristics
The demographic and clinical characteristics of the study population were shown in Table 1. The mean age and body mass index (BMI) of participants was 45 years old and 24.0 kg/m2, respectively, and 54.2% (82,533/152,336) were males. 27.4% (41,771/152,336) of individuals were diagnosed with NAFLD. We also found 19.5% (29,767/152,336), 3.7% (5623/152,336) and 1.3% (1920/152,336) of participants had hypertension, T2D and CAD, respectively. Individuals with NAFLD were noted to be older, have a higher BMI and increased prevalence of hypertension, T2D and CAD than those without (all p < 0.001).
Table 1.
Demographic and clinic characteristics of the study population
| Total (n = 152,336) | With NAFLD (n = 41,771) | Without NAFLD (n = 110,565) | p | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 45 (13) | 48 (12) | 44 (14) | <.001 |
| Male percentage, % (n) | 54.2% (82,533) | 75.4% (31,510) | 46.1% (51,023) | <.001 |
| BMI, kg/m2 | 24.0 (3.3) | 27.1 (2.8) | 22.9 (2.8) | <.001 |
| Systolic pressure, mm Hg | 120 (17) | 128 (16) | 117 (17) | <.001 |
| Diastolic pressure, mm Hg | 76 (11) | 81 (11) | 74 (10) | <.001 |
| Hypertension, % (n) | 19.5% (29,767) | 33.8% (14,129) | 14.1% (15,638) | <.001 |
| T2D, % (n) | 3.7% (5623) | 6.9% (2870) | 2.5% (2753) | <.001 |
| CAD, % (n) | 1.3% (1920) | 1.8% (733) | 1.1% (1187) | <.001 |
| Biochemical parameters | ||||
| TBA, μmol/L | 2.0 (3.1–5.0) | 3.4 (2.3–5.4) | 3.0 (1.9–4.8) | <.001 |
| AST, U/L | 23 (17) | 26 (14) | 21 (17) | <.001 |
| ALT, U/L | 26 (26) | 37 (28) | 22 (24) | <.001 |
| BUN, mmol/L | 5.0 (1.4) | 5.2 (1.4) | 5.0 (1.5) | <.001 |
| Cr, μmol/L | 67.3 (22.6) | 71.9 (19.0) | 65.6 (23.6) | <.001 |
| UA, μmol/L | 312.3 (83.1) | 359.3 (81.2) | 294.6 (75.8) | <.001 |
| FBG, mmol/L | 5.04 (1.29) | 5.48 (1.76) | 4.90 (1.01) | <.001 |
| OGTT-2 h BG †, mmol/L | 7.10 (3.07) | 7.74 (3.45) | 6.74 (2.77) | <.001 |
| TC, mmol/L | 4.66 (0.93) | 4.92 (1.00) | 4.56 (0.88) | <.001 |
| HDLC, mmol/L | 1.31 (0.30) | 1.16 (0.24) | 1.37 (0.30) | <.001 |
| LDLC, mmol/L | 2.67 (0.77) | 2.81 (0.83) | 2.62 (0.74) | <.001 |
| RLP-C, mmol/L | 0.68 (0.61) | 0.95 (0.88) | 0.57 (0.43) | <.001 |
| TG, mmol/L | 1.24 (0.86–1.86) | 1.87 (1.33–2.74) | 1.07 (0.78–1.53) | <.001 |
Data are shown as mean (standard deviation), median (Q1–Q3 quartiles), or percentages (n). P values from analysis of the independent t test, Mann-Whitney U test, or chi-square test. Two-tailed p < 0.05 was considered statistically significant. BMI body mass index, NALFD non-alcoholic fatty liver disease, T2D type 2 diabetes, CAD coronary artery disease, TBA total bile acid, FBG fasting blood glucose, AST aspartate aminotransferase, ALT alanine aminotransferase, BUN blood urea nitrogen, Cr creatinine, UA uric acid, TC total cholesterol, LDL-C LDL cholesterol, HDL-C HDL cholesterol, RLP-C remnant lipoprotein cholesterol, TG triglyceride. OGTT-2 h BG oral glucose tolerance test 2 h-post blood glucose. † 7870 individuals had the OGTT-2 h data
Serum TBA levels
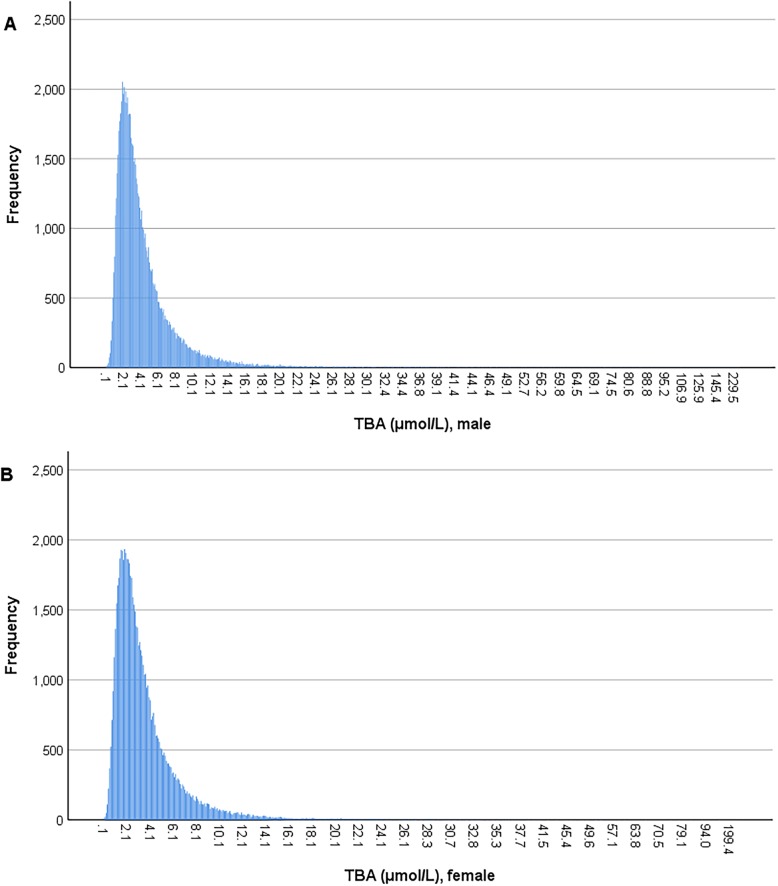
Consistent with previous findings [30], the distribution of serum TBA levels was positively skewed in both male and female populations in our study (Figure 1). The median level of TBA was 3.4 μmol/L in males, which was significantly higher than the level of 2.8 μmol/L in females (p < 0.001). Moreover, this was applied to all age strata in the study population (Table 2). Currently, the reference range of TBA is less than 10 μmol/L for both males and females in the clinical setting. According to this criterion, 6.2% of males and 3.5% of females had elevated TBA in this study.
Fig. 1.
Distribution of serum total bile acid levels in males and females. a. distribution of serum total bile acid levels in males, b. distribution of serum total bile acid levels in females
Table 2.
Gender and age stratum specific median levels of serum TBA
| Age | Male | Female | p | ||
|---|---|---|---|---|---|
| n | TBA, μmol/L | n | TBA, μmol/L | ||
| < 20 | 189 | 2.9 (1.9–4.6) | 179 | 3.0 (1.8–5.1) | < 0.01 |
| 20~29 | 9099 | 3.4 (2.2–5.4) | 10,283 | 2.9 (1.9–4.5) | < 0.01 |
| 30~39 | 18,094 | 3.2 (2.1–5.1) | 16,062 | 2.6 (1.7–4.1) | < 0.01 |
| 40~49 | 22,738 | 3.3 (2.1–5.2) | 18,851 | 2.7 (1.7–4.2) | < 0.01 |
| 50~59 | 18,852 | 3.4 (2.2–5.4) | 15,243 | 2.9 (1.9–4.7) | < 0.01 |
| 60~69 | 8377 | 3.6 (2.4–5.8) | 6445 | 3.2 (2.1–5.2) | < 0.01 |
| ≥ 70 | 5184 | 4.2 (2.8–6.9) | 2740 | 3.9 (2.5–6.2) | < 0.01 |
| Total | 82,533 | 3.4 (2.2–5.4) | 69,803 | 2.8 (1.8–4.5) | < 0.01 |
Data are shown as median (Q1–Q3 quartiles). P values from analysis of the Kruskal-Wallis H test. Two-tailed p < 0.05 was considered statistically significant. TBA total bile acid
Serum TBA and NAFLD
Patients with NAFLD were noted to have slightly higher TBA levels than those without (3.4 vs. 3.0 μmol/L, p < 0.001) (Table 1). We then calculated the prevalence of NAFLD categorized by TBA quintiles and we found that the prevalence of NAFLD increased with the elevation in TBA levels. The 5th quintile (Q5) group had the highest (31.3%) while the 1st quintile (Q1) group had the lowest prevalence (20.8%) of NAFLD (Table 3). Furthermore, we used a multivariate logistic regression model to test whether serum TBA was associated with NAFLD after adjusting for age, gender, BMI and other acknowledged risk factors for NAFLD. After this adjustment, we found that the association between serum TBA level and NAFLD was lost (Table 4).
Table 3.
Basic characteristics of the study population categorized by TBA quintiles
| Serum TBA quintiles | P | |||||
|---|---|---|---|---|---|---|
| Q1 (n = 31,267) | Q2 (n = 30,136) | Q3 (n = 31,298) | Q4 (n = 29,245) | Q5 (n = 30,390) | ||
| Clinical characteristics | ||||||
| Age, years | 44 (12) | 45 (13) | 45 (13) | 46 (14) | 48 (15) | <.001 |
| Male percentage, % (n) | 42.5% (13,284) | 52.0% (15,679) | 55.7% (17,428) | 58.6% (17,129) | 62.6% (19,013) | <.001 |
| BMI, kg/m2 | 23.7 (3.3) | 24.0 (3.3) | 24.1 (3.4) | 24.2 (3.4) | 24.3 (3.4) | <.001 |
| Systolic pressure, mm Hg | 117 (17) | 119 (17) | 120 (17) | 121 (17) | 123 (18) | <.001 |
| Diastolic pressure, mm Hg | 74 (11) | 75 (11) | 76 (11) | 76 (11) | 77 (11) | <.001 |
| NALFD, % (n) | 20.8% (6512) | 26.0% (7840) | 28.8% (9012) | 30.4% (8895) | 31.3% (9512) | <.001 |
| Hypertension, % (n) | 14.7% (4581) | 17.6% (5309) | 19.3% (6028) | 21.9% (6403) | 24.5% (7446) | <.001 |
| T2D, % (n) | 2.0% (630) | 3.1% (931) | 3.7% (1161) | 4.6% (1358) | 5.1% (1543) | <.001 |
| CAD, % (n) | 0.9% (269) | 0.9% (282) | 1.2% (387) | 1.4% (407) | 1.9% (575) | <.001 |
| Biochemical parameters | <.001 | |||||
| AST, U/L | 20 (7) | 21 (9) | 22 (9) | 23 (10) | 26 (32) | <.001 |
| ALT, U/L | 22 (14) | 25 (18) | 26 (20) | 27 (22) | 32 (44) | <.001 |
| BUN, mmol/L | 4.8 (1.4) | 4.9 (1.4) | 5.1 (1.5) | 5.1 (1.5) | 5.1 (1.5) | <.001 |
| Cr, μmol/L | 64.6 (21.7) | 66.7 (21.5) | 67.9 (26.6) | 68.3 (21.7) | 69.2 (20.5) | <.001 |
| UA, μmol/L | 299.1 (80.3) | 310.4 (82.6) | 314.7 (83.2) | 317.3 (83.8) | 320.6 (84.2) | <.001 |
| FBG, mmol/L | 4.90 (1.01) | 4.98 (1.18) | 5.04 (1.31) | 5.11 (1.41) | 5.16 (1.49) | <.001 |
| TC, mmol/L | 4.65 (0.91) | 4.66 (0.92) | 4.66 (0.92) | 4.67 (0.95) | 4.66 (0.97) | 0.103 |
| HDLC, mmol/L | 1.35 (0.29) | 1.32 (0.29) | 1.30 (0.29) | 1.29 (0.30) | 1.28 (0.31) | <.001 |
| LDLC, mmol/L | 2.70 (0.76) | 2.69 (0.77) | 2.68 (0.77) | 2.67 (0.78) | 2.62 (0.78) | <.001 |
| RLP-C, mmol/L | 0.60 (0.47) | 0.65 (0.56) | 0.68 (0.60) | 0.71 (0.66) | 0.76 (0.74) | <.001 |
| TG, mmol/L | 1.11 (0.80–1.61) | 1.21 (0.84–1.81) | 1.25 (0.87–1.90) | 1.30 (0.89–1.97) | 1.34 (0.92–2.06) | <.001 |
Data are shown as mean (standard deviation), median (Q1–Q3 quartiles), or percentages (n). P values from analysis of the ANOVA, Kruskal-Wallis H test, or chi-square test. Two-tailed p < 0.05 was considered statistically significant. BMI body mass index, NALFD non-alcoholic fatty liver disease, T2D type 2 diabetes, CAD coronary artery disease, TBA total bile acid, FBG fasting blood glucose, AST aspartate aminotransferase, ALT alanine aminotransferase, BUN blood urea nitrogen, Cr creatinine, UA uric acid, TC total cholesterol, LDL-C LDL cholesterol, HDL-C HDL cholesterol, RLP-C remnant lipoprotein cholesterol, TG triglyceride
Table 4.
The association of serum TBA with NAFLD by multivariate logistic regression analysis
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.02 | 1.01–1.02 | < 0.001 |
| Gender (male vs. female) | 1.47 | 1.42–1..52 | < 0.001 |
| BMI | 1.59 | 1.58–1.60 | < 0.001 |
| History of hypertension (with vs. without) | 1.28 | 1.23–1.33 | < 0.001 |
| History of T2D (with vs. without) | 1.58 | 1.48–1.70 | < 0.001 |
| TC | 0.98 | 0.92–1.04 | 0.501 |
| LDL-C | 1.39 | 1.31–1.48 | < 0.001 |
| HDL-C | 0.38 | 0.34–0.42 | < 0.001 |
| TG | 1.35 | 1.31–1.39 | < 0.001 |
| TBA | 1.00 | 1.00–1.00 | 0.797 |
P values were from multivariate logistic regression. Two-tailed p < 0.05 was considered statistically significant. TBA total bile acid, NAFLD non-alcoholic fatty liver disease, BMI body mass index, T2D type 2 diabetes, TC total cholesterol, LDL-C LDL cholesterol, HDL-C HDL cholesterol, TG triglyceride
Serum TBA and T2D
Since NAFLD is closely associated with T2D and insulin resistance is considered one of the major cause for NAFLD development, we also checked the association of serum TBA with T2D. Similarly, we found that the prevalence of T2D rose with increasing TBA levels. Notably, the Q5 group had the highest and Q1 group had the lowest prevalence of T2D, 5.1% vs. 2.0%, respectively (Table 3). However, in our multivariate logistic regression analysis, serum TBA was not associated with T2D in the model controlling for age, gender, BMI and known T2D risk factors (Table 5).
Table 5.
The association of serum TBA with T2D by multivariate logistic regression analysis
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.07 | 1.07–1.07 | < 0.001 |
| Gender (male vs. female) | 1.33 | 1.25–1.42 | < 0.001 |
| BMI | 1.01 | 1.00–1.02 | 0.136 |
| History of hypertension (with vs. without) | 1.61 | 1.52–1.72 | < 0.001 |
| History of NAFLD (with vs. without) | 1.73 | 1.62–1.85 | < 0.001 |
| TC | 1.04 | 0.97–1.11 | 0.253 |
| LDL-C | 0.88 | 0.82–0.95 | < 0.001 |
| HDL-C | 0.34 | 0.29–0.39 | < 0.001 |
| TG | 1.06 | 1.04–1.09 | < 0.001 |
| TBA | 1.00 | 1.00–1.01 | 0.178 |
P values were from multivariate logistic regression. Two-tailed p < 0.05 was considered statistically significant. TBA total bile acid, NAFLD non-alcoholic fatty liver disease, BMI body mass index, T2D type 2 diabetes, TC total cholesterol, LDL-C LDL cholesterol, HDL-C HDL cholesterol, TG triglyceride
Discussion
The understandings of the function and effect of BA modulated receptors in the liver, such as FXR, suggest the potential role of BAs in NAFLD development. However, in this study, we found that serum TBA levels were not independently associated with NAFLD or T2D when controlling for age, gender, BMI and additional risk factors.
BAs are a group of diverse amphipathic steroids, rather than a single type of molecule, derived from the catabolism of cholesterol in hepatocytes in a process that involves two principal pathways [10]. The classical pathway, which generates the majority of BAs, is regulated by the rate-limiting enzyme, cholesterol 7α-hydroxylase (CYP7A1). The alternative pathway is initially catalyzed by sterol-27-hydroxlase (CYP27A1), followed by BA hydroxylation through oxysterol 7α-hydroxylase (CYP7B1). These two pathways account for the synthesis of the primary BAs, namely chenodeoxycholic acid (CDCA) and cholic acid (CA). They, in turn, are modified by gut microbiota to yield lithocholic acid (LCA) and deoxycholic acid (DCA) respectively, which represent two major type of secondary BAs.
Different types of BAs differ in in their physicochemical properties. For example, whereas CDCA is a potent agonist of FXR, DCA and LCA have been suggested as partial antagonists of FXR [10]. This suggests they may have a distinct regulatory effect on metabolism. Recent findings indicated that alterations in circulating BA composition were closely associated with NAFLD and T2D. Na et al. measured the serum BA composition of NAFLD patients and healthy controls and found that the percentage of FXR antagonistic DCA was increased, while the agonistic CDCA was decreased in patients with NAFLD compared to healthy subjects [20]. Rebecca et al. showed that increased plasma levels of 12a-hydroxylated BA were associated with insulin resistance in both T2D patients and healthy subjects [21]. These interesting findings suggest that the alterations in BA composition rather than quantitative changes in TBA levels are more relevant to metabolic homeostasis.
Currently, no convenient/applicable serum BA composition assessment techniques are available in non-academic clinics. So far, penetration of "classical" laboratory methods, including high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS), into the clinics is minimal due to the need of expensive equipment and time-consuming protocols [24]. Because of that, we had to resort to the conventional enzymatic assessment of blood TBA in this study, which is widely used in clinical settings
In addition to this, we acknowledge several other limitations to our study. Due to the nature of cross-sectional studies, we were not able to follow participants to determine the predictive values of serum TBA levels in NAFLD development. Besides, we only measured the fasting serum BA levels. The role of postprandial BA levels in metabolism should also be studied in the future.
Conclusion
Serum TBA was not associated with NAFLD. Future studies in a large population focusing on the serum BA composition may strengthen our understating of the role of BAs in NAFLD development and progression.
Acknowledgments
None.
Abbreviations
- ANOVA
Analysis of the variance
- BAs
Bile acids
- BMI
Body mass index
- CA
Cholic acid
- CAD
Coronary artery disease
- CDCA
Chenodeoxycholic acid
- CYP27A1
Sterol-27-hydroxlase
- CYP7A1
Cholesterol 7α-hydroxylase
- CYP7B1
Oxysterol 7α-hydroxylase
- DCA
Deoxycholic acid
- FXR
Farnesoid X receptor
- GC-MS
Gas chromatography-mass spectrometry
- HPLC
High-performance liquid chromatography
- HS
Hepatic steatosis
- LCA
Lithocholic acid
- LC-MS
Liquid chromatography-mass spectrometry
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- OGTT
Oral glucose tolerance test
- T2D
Type 2 diabetes
- TBA
Total bile acid
- TGR5
Takeda G-protein-coupled receptor 5
Authors’ contributions
Conceived and designed the study: DP. Performed the study: ZZ, WD, SW and ML. Analyzed the data: ZZ, WD, JF and JAZ. Wrote the paper: ZZ, WD, JAZ, SS and DP. All authors reviewed drafts and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [No. 81870336 and 81670426 to Daoquan Peng]; the Fundamental Research Funds for the Central University of Central South University [No. 2019zzts805 to Ziyu Zhang].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethics committee review board of The Second Xiangya Hospital of Central South University. Informed written consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ziyu Zhang and Wen Dai contributed equally to this work.
Contributor Information
Ziyu Zhang, Email: ziyuzhang@csu.edu.cn.
Wen Dai, Email: owen115122@csu.edu.cn.
Shuwei Weng, Email: wengshuwei@csu.edu.cn.
Mengdie Luo, Email: luomengdie@csu.edu.cn.
Jiahao Fu, Email: jhfu.acc@gmail.com.
John A. Zadroga, Email: zadroga@rowan.edu
Stefano Spolitu, Email: ss4602@cumc.columbia.edu.
Daoquan Peng, Email: daoquanpeng2019@sina.com.
References
- 1.Araujo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: what we need in the future. Liver Int. 2018;38(Suppl 1):47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 3.Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):11–17. doi: 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 4.Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221–235. doi: 10.1055/s-0035-1562943. [DOI] [PubMed] [Google Scholar]
- 5.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 6.Cholankeril G, Wong RJ, Hu M, Perumpail RB, Yoo ER, Puri P, et al. Liver transplantation for nonalcoholic Steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci. 2017;62(10):2915–2922. doi: 10.1007/s10620-017-4684-x. [DOI] [PubMed] [Google Scholar]
- 7.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most rapidly increasing indication for liver transplantation in young adults in the United States. J Clin Gastroenterol. 2018;52(4):339–346. doi: 10.1097/MCG.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 8.Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatol. 2017;65(6):2100–2109. doi: 10.1002/hep.29068. [DOI] [PubMed] [Google Scholar]
- 9.Chedid MF. Nonalcoholic Steatohepatitis: the second leading indication for liver transplantation in the USA. Dig Dis Sci. 2017;62(10):2621–2622. doi: 10.1007/s10620-017-4724-6. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215(2):383–396. doi: 10.1084/jem.20171965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 12.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatol. 2017;65(1):350–362. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copple BL, Li T. Pharmacology of bile acid receptors: evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66(4):948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatol. 2011;54(4):1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 Inflammasome. Immun. 2016;45(4):802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17(2):259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67(10):1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 21.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62(12):4184–4191. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai W, Long J, Cheng Y, Chen Y, Zhao S. Elevated plasma lipoprotein(a) levels were associated with increased risk of cardiovascular events in Chinese patients with stable coronary artery disease. Sci Rep. 2018;8(1):7726. doi: 10.1038/s41598-018-25835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang QY, Tian F, Lin QZ, Du X, Zhang SL, Gui YJ, et al. Comparison of remnant cholesterol levels estimated by calculated and measured LDL-C levels in Chinese patients with coronary heart disease. Clin Chim Acta. 2020;500:75–80. doi: 10.1016/j.cca.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Danese E, Salvagno GL, Negrini D, Brocco G, Montagnana M, Lippi G. Analytical evaluation of three enzymatic assays for measuring total bile acids in plasma using a fully-automated clinical chemistry platform. PLoS One. 2017;12(6):e0179200. doi: 10.1371/journal.pone.0179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalasani Naga, Younossi Zobair, Lavine Joel E., Charlton Michael, Cusi Kenneth, Rinella Mary, Harrison Stephen A., Brunt Elizabeth M., Sanyal Arun J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2017;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 26.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71(6):1269–324. [DOI] [PubMed]
- 27.Buse John B., Wexler Deborah J., Tsapas Apostolos, Rossing Peter, Mingrone Geltrude, Mathieu Chantal, D’Alessio David A., Davies Melanie J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2019;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 29.Qaseem A, Fihn SD, Williams S, Dallas P, Owens DK, Shekelle P. Diagnosis of stable ischemic heart disease: summary of a clinical practice guideline from the American College of Physicians/American College of Cardiology Foundation/American Heart Association/American Association for Thoracic Surgery/preventive cardiovascular nurses association/Society of Thoracic Surgeons. Ann Intern Med. 2012;157(10):729–734. doi: 10.7326/0003-4819-157-10-201211200-00010. [DOI] [PubMed] [Google Scholar]
- 30.Frommherz L, Bub A, Hummel E, Rist MJ, Roth A, Watzl B, et al. Age-related changes of plasma bile acid concentrations in healthy adults--results from the cross-sectional KarMeN study. PLoS One. 2016;11(4):e0153959. doi: 10.1371/journal.pone.0153959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.