Abstract
Background
According to the World Health Organization, cardiovascular diseases (CVDs) are the leading non-communicable cause of death. Awareness of the individual risk profile is crucial to implement a healthy lifestyle and prevent CVDs. Multiple studies demonstrated that atherosclerosis, the main cause of CVDs, begins early in life. Therefore, it may be necessary to start prevention programs already in childhood.
Methods
The EVA-Tyrol study is a population-based non-randomized controlled trial that will prospectively enroll 2000 participants from high schools and training companies in North- and East-Tyrol (Austria) and South-Tyrol (Italy). Participants will be assigned to either an intervention (n = 1500) or a control (n = 500) group. Intervention group participants will be enrolled at the 10th school grade (mean age 15–16 years), undergo two examinations within a two-year interval, with follow-up at the 12th grade (mean ages 17–18 years). Control group participants will be enrolled at the 12th grade (mean age 17–18 years). Medical examination will include anthropometric measurements, comprehensive lifestyle and dietary questionnaires, a fasting blood sample, high-resolution ultrasound of the carotid arteries, and measurement of carotid-femoral pulse wave velocity. Active intervention will consist of (1) enhancing knowledge about CVDs, (2) individual medical counseling based on the results of the baseline examination, (3) an online health promotion tool and (4) involvement of participants in planning and implementation of health promotion projects. Effectiveness of the intervention will be assessed by comparing the proportion subjects with ideal health metrics as defined by the American Heart Association between study groups.
Discussion
This study aims to improve cardiovascular health in Tyrolean adolescents by demonstrating the efficacy of a multi-layer health promotion program and may yield novel insights into the prevalence of vascular risk conditions and mechanisms of early vascular pathologies in adolescents.
Trial registration
EVA-Tyrol has been retrospectively registered at clinicaltrials.gov under NCT03929692 since April 29, 2019.
Background
According to the world health organization (WHO), 17.7 million people died from CVDs in 2015 [1, 2], with ischemic heart disease and stroke accounting for 26.6% of all deaths worldwide [3]. The Committee on Preventing the Global Epidemic of Cardiovascular Disease recommended that apart from early diagnosis and management of CVDs interventions at ‘all stages of life course’ should be performed in order to ‘promote cardiovascular health by preventing acquisition and augmentation of risk’ [4]. Awareness of the individual risk profile and subsequent modification of risk factors and risk behavior are prerequisites for effective prevention. However, it is unclear at which age best to initiate prevention.
While CVDs occur predominately in the elderly, atherosclerosis starts in early life and might even be influenced by fetal and postnatal development. According to the Barker hypothesis, early life factors such as being born preterm or small for gestational age, or impaired fetal growth or inadequate weight gain in the first years of life may predispose to CVDs later in life [5–8]. Histologic necropsy studies found coronary atherosclerotic plaques in 12% of adolescents and 28% of young adults [9]. Classical cardiovascular risk factors like smoking, physical inactivity and unhealthy diet are present not only in adults and adolescents but also in the pediatric population [10–12]. In addition, unfavorable health behaviors like sedentary lifestyle, smoking and alcohol consumption may be acquired in late childhood [13–15] and frequently persist in adulthood. Cohort studies have demonstrated the effects of risk factors on early atherosclerotic vessel wall thickening using high-resolution ultrasonography in children, adolescents and young adults [16, 17]. Conversely, a more favorable cardiovascular risk profile in childhood (as defined by the AHA) is associated with a lower aortic intima-media thickness (IMT) and a better aortic elasticity [18], and with a reduced risk of hypertension, metabolic-syndrome and elevated low-density lipoprotein cholesterol in adulthood [19]. Early correction of an unfavorable lifestyle can prevent CVDs in later life [20]. Adverse effects of obesity may be reversed by early weight reduction [20]. In Children, lifestyle and dietary counseling exhibits favorable effects on their cardiovascular risk profile without harmful side-effects [18, 21].
In summary, it is well established that cardiovascular risk factors (CVRFs) are related to early vascular ageing and early atherosclerotic wall changes in children and young adults. Promotion of a healthy lifestyle and control of CVRF. In youth presents the opportunity to reverse these changes and prevent persistence of risk conditions into adulthood. The current study will evaluate the efficacy of a defined cardiovascular health promotion program in facilitating risk factor control.
Trial aim and objectives
The aim of EVA-Tyrol is to promote cardiovascular health in high schools/training companies by an interventional health promotion program and to acquire data on its efficacy in a cohort of Tyrolean adolescents with a mean age of 15–16 years.
The primary objectives of EVA-Tyrol are:
To assess health in the Tyrolean youth
To survey the effect of a multi-layer health promotion program in this age group
To study the effects of neonatal and childhood weight gain on CVRF in youth
To assess the effects of being born preterm or small for gestational age on vascular health in youth
To determine the effects of CVRF on vascular health in youth
To explore the effects of lifestyle on vascular health in youth
To establish a serum biobank
Methods and design
Study design
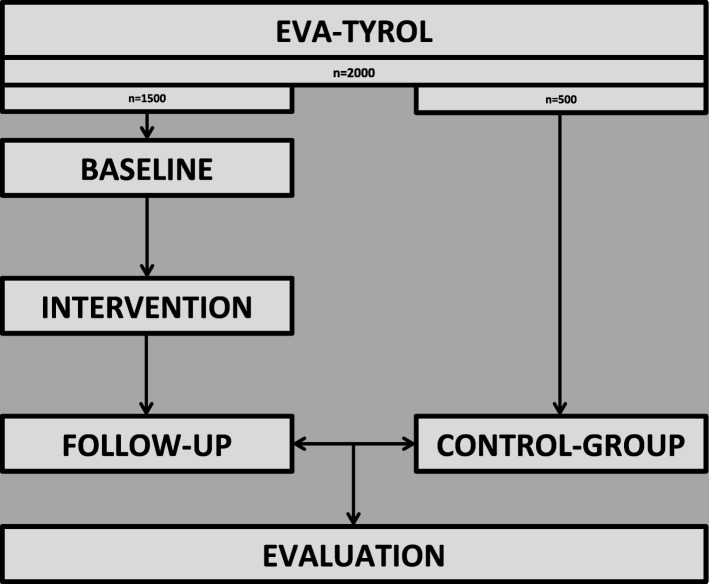
The EVA-study is a non-randomized controlled trial. Two thousand participants will be recruited from high schools and training companies spread over North- and East-Tyrol (Austria) and South-Tyrol (Italy). Study participants will be assigned to i) health intervention group (n = 1500) or ii) control group (n = 500). For participants included in the intervention group two examinations will be scheduled within a two-year interval between the examinations. Participants will be in the 10th grade (mean age, 15–16 years) at the baseline and in the 12th grade (mean age, 17–18 years) at the follow-up examination. Between the examinations the health intervention program will be offered.
Five hundred participants with a mean age of 17–18 years without participation in a health promotion program will serve as a control group. The prevalence of AHA health metrics in the intervention group will be compared to the prevalence in the control group (Fig. 1).
Fig. 1.
Schematic illustration of study design
In addition to health metrics, EVA-Tyrol will evaluate further components and determinants of cardiovascular health of Tyrolean adolescents. In addition, we will collect information on the first 6 years of life including pre-, peri- and postnatal data prospectively documented in the mother-child booklet, the official Austrian pregnancy and early childhood medical record book.
Participants
Recruitment
The local education authority (Landesschulrat for Tyrol) will brief all Tyrolean high schools about the project. Subsequently, the schools will be able to apply for study-inclusion. In order to ensure a homogenous regional and social distribution, principals of selected schools will be actively encouraged to participate. Schools and training companies will not receive financial compensation for participation. The two study centers will be Innsbruck (Austria) for North and East Tyrol and Bruneck (Italy) for South Tyrol. Moreover, Tyrolean training companies will be invited to participate. For participating schools and companies a project presentation on the background of CVD and concept of CVRF will be offered. Pupils and trainees will be briefed about the study procedure. In addition, a video summarizing study aims and procedures will be shown.
Inclusion criteria
Participants in the 10th to 12th grade (mean ages 15–16 at baseline for the intervention group and 17–18 years for the control group) will be enrolled. Informed consent by the participant and, for participants younger than 18 years, their legal representative will be obtained. Consent for inclusion of data from the “mother-child booklet” additional maternal informed consent will be obtained.
Schools and training companies will be randomly assigned to either the intervention or the control group. The examination of the intervention group will start first. Schools will be assigned to either group according to their available schedule. The exact number of participating schools/training companies will depend on the number of participants per school/company.
Exclusion criteria
None; previous or concurrent diseases are not an exclusion criterion.
Data acquisition
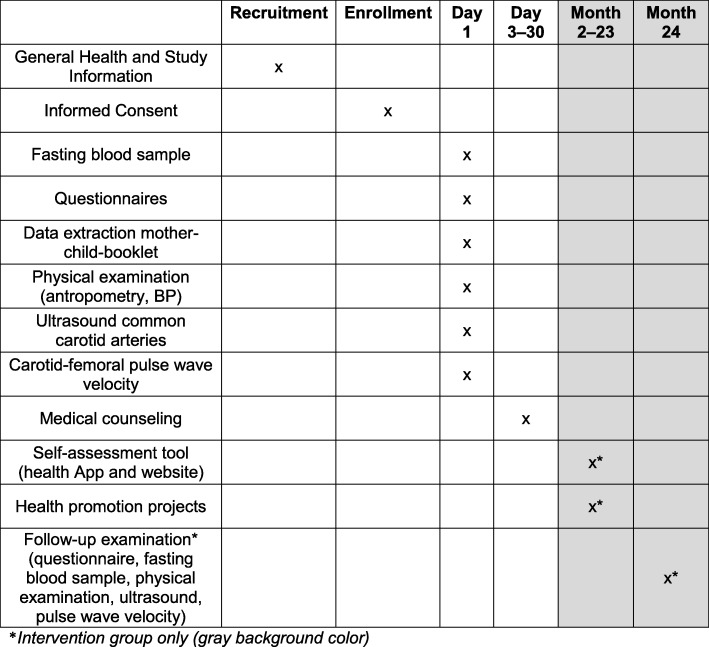
The study will take place at the schools’ or training companies’ sites. An overview of the trial procedures is depicted in Fig. 2. Data will be collected by a paper case report form (CRF) and include a self-administered and assisted questionnaire, a structured interview and a series of examinations (blood sampling, high-resolution ultrasound of the carotid arteries, tonometric measurement of carotid-femoral pulse-wave velocity, blood-pressure measurement and anthropometry). Data acquisition will be done by medical doctors assisted by medical students.
Fig. 2.
SPIRIT diagram: Timeframe of EVA-Tyrol
Questionnaire
Table 1 shows the structure of the employed CRF. The CRF is adapted from the Bruneck- [27], ARMY- [16], ARFY- [17] and HBSC (Health Behavior in School Children Study)- [22] studies and organized in three parts: the first part of the CRF is self-administered, the second part has to be completed under group supervision of an MD. The third part is a structured face-to-face questionnaire by an MD about the study’s main outcome parameters and previous medical history of each participant (Table 1). The family history for diabetes, hypertension or CVDs, is explored separately for each condition and rated as present when at least one first or 2 s degree relatives became diseased at an early age (women < 65 years, men < 55 years).
Table 1.
Overview over the EVA-Tyrol questionnaire
| Self-administered | ||
| Overall health and life satisfaction, lifestyle factors, social background | 21 items | Health Behaviour in School Children -Study [22] |
| Food habits | 23 items | The Adolescent Food Habits Checklist [23] |
| Nutritional knowledge | 16 items | Turconi score Section E, G and H [24] |
| Participation and perception of health promotion | 12 items | |
| Assisted | ||
| Baecke score for physical activity | 20 items | [25] |
| Score for Allergic Rhinitis | 9 items | [26] |
| Traffic-Exposure | 3 items | Taken from the ARMY [16] and ARFY [17] studies |
| Food frequency questionnaire | 90 items | Taken from the ARMY [16], ARFY [17] and Bruneck [27] studies |
| Face-to-face interview | ||
| Dietary interview | 7 items | According to the AHA health metrics for youth [11, 28] |
| Physical activity | 1 item | Moderate- and vigorous-intensity activity (minutes per day), AHA health metrics for youth [11, 28] |
| Smoking and alcohol consumption | 12 items | Adapted from the Bruneck Study [27, 29] |
| Classical cardiovascular risk-factors and previous diseases as well as chronic infections. | 17 items | Structured interview for known hypercholesterolemia, diabetes, hypertonia, diseases of the heart, vasculature, thyroid glands, liver, lung, known neoplasias, chronic infections of lung, sinuses, urinary tract, skin or chronic dental infections. |
| Family history for CVD | 3 items | Premature CVDs (women < 65 years, men < 55 years), hypertension or diabetes in one 1st or two 2nd degree relatives. |
| Allergies and atopic predisposition | 11 items | Structured interview for history of allergies, clinical symptoms and medical therapy. |
| Headache history | 8 items | Classification of the international headache society (ICHD-3) [30] |
| Medication use | 9 items | Structured interview for previous and current medication use |
Table 1 shows an overview of the three parts (self-administered, assisted and face-to-face) of the EVA-Tyrol questionnaire. Left column – topic, middle column – number of questions, right column – source/description of questionnaire
Mother-child booklet data
In 1974 the Austrian government introduced a mother-child booklet (“Mutter-Kind Pass”) aiming to improve the health of pregnant women and children [31]. It consists of predefined examinations of the mother, the fetus, and the newborn and extends until the age of 6 years. Results of these examinations are documented in a booklet. As the child’s continuous financial governmental support is dependent on the completion of all examinations, adherence to the suggested medical examinations is high. Data extracted from the mother-child booklet is shown in Table 2. The Italian mother-child booklet is similar in content except for minor differences in examination intervals.
Table 2.
Data extracted from the mother-child-booklet
| Mother-child-booklet | |
|---|---|
| Maternal characteristics |
Age at pregnancy Singleton/multiple pregnancy Body weight and length (begin and end of pregnancy) Blood pressure (begin and end of pregnancy) Smoking status (begin and end of pregnancy) Pre-existing conditions (diabetes, hypertension) |
| Pregnancy complications (yes/no) |
Pathological oral glucose tolerance test Preeclampsia Hypertension Proteinuria |
| Characteristics of neonate |
Date of birth Gestational age Apgar Score Umbilical blood pH Mode of birth Body weight Body length Head circumference |
| Data of the child of 8 follow-up examinations at week 4–7, month 3–5, 7–9, 10–14, 22–26, 34–38, 46–50 and 58–62 |
Date of examination Body weight Body length Head circumference |
Table 2 shows an overview of the information extracted from the mother-child-booklet
Physical examination
Physical examination will be performed by study personnel. Anthropometric measurements will include size and weight as well as waist- and hip-circumference. Blood pressure will be measured three times in the sitting position after 5 min of rest by a standard digital haemodynamometer (Omron; Omron Healthcare, Lake Forest, Illinois, US) on both arms. High-resolution ultrasound of the carotid arteries will be performed with a portable GE medical Vividi ultrasound with a linear transducer 12 L RS probe (both General Electric, GE-Healthcare, Chicago, US). The intima-media thickness (IMT) will be assessed by experienced sonographers from the anterior and posterolateral view. Representative images will be stored digitally and IMT will be measured offline on the stored images on three representative locations in the distal proportion of the common carotid artery of both sides. Carotid-femoral pulse wave velocity and central blood pressure will be calculated as a surrogate for aortic stiffness from simultaneous recording of ten consecutive pulse waves of artefact-free cardiac cycles by applanation tonometry according to the manufacturer’s instructions (Complior-Analyze®, ALAMmedical, Paris, France).
Sample collection and analysis
Blood samples will be drawn after an overnight fastand will immediately be stored in cooling boxes at approximately 4 °C before direct transfer to the ISO-certified Central Institute for Medical and Chemical Laboratory Diagnostics of Innsbruck University Hospital. Parameters and measurement methods are detailed in Table 3. Long-term storage of serum and plasma samples will be at − 80 °C. The serum biobank will consist of serum and lithium-heparin plasma, as well as full blood for DNA extraction only for subjects that signed an additional informed consent form.
Table 3.
‘Routine lab parameters and methodology'. HPLC High pressure liquid chromatography, ECLIA Electrochemiluminescenceimmunoassay
| Parameter | Unit | Method | Reagent | Analyzer |
|---|---|---|---|---|
| Glucose | mg/dl | Hexokinase method | Roche | Cobas 8000 |
| HbA1c (DCCT/NGSP) | % | HPLC | Menarini | HA 8180 T |
| HbA1c (IFCC) | mmol/mol | HPLC | Menarini | HA 8180 T |
| Insulin | mU/l | ECLIA | Roche | Cobas 8000 |
| Cholesterol | mg/dl | Enzymatic color assay | Roche | Cobas 8000 |
| HDL-Cholesterol | mg/dl | Enzymatic color assay | Roche | Cobas 8000 |
| LDL-Cholesterol | mg/dl | Enzymatic color assay | Roche | Cobas 8000 |
| Triglyceride | mg/dl | Enzymatic color assay | Roche | Cobas 8000 |
| Lipoprotein (a) | nmol/l | Particle-enhanced immunological clouding assay | Roche | Cobas 8000 |
| Total-Homocystein | umol/l | Chemiluminescence microparticle immunoassay | Abbott | Architect |
| Urea | mg/dl | Kinetic test with urease and Glutamate dehydrogenase | Roche | Cobas 8000 |
| Creatinine (enzym.-IDMS) | mg/dl | Enzymatic color assay | Roche | Cobas 8000 |
| Total-protein | g/dl | Biuret test | Roche | Cobas 8000 |
| Uric acid | mg/dl | Enzymatic color assay with uricase | Roche | Cobas 8000 |
| Potassium | mmol/l | Indirect potentiometry | Roche | Cobas 8000 |
| Calcium | mmol/l | Photometric with 5-Nitro-5′-methyl-BAPTA | Roche | Cobas 8000 |
| GOT (ASAT) | U/l | According to IFCC recommendations, though optimized | Roche | Cobas 8000 |
| GPT (ALAT) | U/l | According to IFCC recommendations, though optimized | Roche | Cobas 8000 |
| Gamma-GT | U/l | Enzymatic color assay | Roche | Cobas 8000 |
| Creatine kinase | U/l | Enzymatic UV-Assay | Roche | Cobas 8000 |
| C-reactive protein | mg/dl | Particle-enhanced immunological clouding assay | Roche | Cobas 8000 |
| Ferritin | ug/l | Particle-enhanced immunological clouding assay | Roche | Cobas 8000 |
| TSH | mU/l | ECLIA | Roche | Cobas 8000 |
| Free thyroxine (FT4) | pmol/l | ECLIA | Roche | Cobas 8000 |
| Thyroglobulin | ug/l | ECLIA | Roche | Cobas 8000 |
| Thyroglobulin-Antibodies | kU/l | ECLIA | Roche | Cobas 8000 |
| ESR after 1 h | mm/h | Westergren-Method | Mechatronics | Starrsed Auto Compact |
| Leukocytes | G/l | Biofluorescence/flow cytometry | Sysmex | XE-5000 |
| Absolute number of Neutrophiles | G/l | Biofluorescence/flow cytometry | Sysmex | XE-5000 |
| Erythrocytes | T/l | Impedance method | Sysmex | XE-5000 |
| Haemoglobin | g/l | Photocolorimetric | Sysmex | XE-5000 |
| Hematocrit | l/l | Impedance method | Sysmex | XE-5000 |
| Platelets | G/l | Impedance method | Sysmex | XE-5000 |
| MCH | pg | Calculated | Sysmex | XE-5000 |
| MCHC | g/l | Calculated | Sysmex | XE-5000 |
| MCV | fl | Calculated | Sysmex | XE-5000 |
| Erythrocyte-distributional width | % | Calculated | Sysmex | XE-5000 |
| Band neutrophiles | % | Manual microscopy | manual | Light microscopy |
| Segmented neutrophiles | % | Biofluorescence/flow cytometry and light microscopy | Sysmex | XE-5000 |
| Lymphocytes | % | Biofluorescence/flow cytometry and light microscopy | Sysmex | XE-5000 |
| Lymphocytes | G/l | Biofluorescence/flow cytometry and light microscopy | Sysmex | XE-5000 |
| Monocytes | % | Biofluorescence/flow cytometry and light microscopy | Sysmex | XE-5000 |
| Eosinophils | % | Biofluorescence/flow cytometry and light microscopy | Sysmex | XE-5000 |
| Basophils | % | Biofluorescence/flow cytometry and light microscopy | Sysmex | XE-5000 |
| Plasma cells | % | Manual microscopy | Manual | Light microscopy |
| Folic acid | μg/l | Competitive binding assay | Roche | Cobas 8000 |
| Vitamin B12 | pmol/l | ECLIA | Roche | Cobas 8000 |
Cardiovascular health evaluation
As successfully applied in previous studies [18, 21], healthy lifestyle will be measured by the AHA’s seven health metrics (adapted by Lloyd-Jones [11] and Daniels [28]). Every health metric except smoking is categorized into ideal, moderate and poor health as defined in Table 4. Healthy diet is defined by the AHA based on expert opinion and on the dietary approach to stop hypertension (DASH) score. One point is scored for each of the following components: consumption of at least four portions of vegetables and fruits per day, at least three portions of fiber-rich nutriments per day, of fish at least two-times a week, a salt-free or salt-poor diet (less than 1.5 g/day) and not more than 1 Liter of sugar-rich drinks per week (max. 450 kcal/week) [11].
Table 4.
‘Seven AHA health metrics’
| Health behavior goal: | Poor | Moderate | Ideal health |
|---|---|---|---|
| Smoking-habits | Smoked in the last 30 days | – | Never smoked, never smoked a whole cigarette |
| Body-Mass-Index | >95th percentile | 85-95th percentile | <85th percentile |
| Physical activity | None | < 60 min moderate or intensive physical activity per day | ≥ 60 min moderate or intensive physical activity per day |
| Healthy diet | 0–1 components | 2–3 components | 4–5 components |
| Total-cholesterol | ≥200 mg/dL | 170–199 mg/dL | < 170 mg/dL |
| Blood pressure | >95th percentile | 90-95th percentile or ≥ 120 systolic or ≥ 80 diastolic | <90th percentile |
| Fasting blood sugar | ≥126 mg/dL | 100–125 mg/dL | < 100 mg/dL |
Intervention
Our health promotion program is based on (1) enhancing the knowledge about CVDs and related risk factors, (2) medical counseling and discussion of individual risk conditions and lifestyle, (3) providing a self-assessment tool to control and visualize chances in CVD risk conditions and (4) involvement of participants in the planning of health promotion projects.
Our health-promotion program will combine the running health-promotion programs “Gesunde Schule (healthy school)” and “Do-it-yourself!” of the Tyrolean regional medical insurance company (Tiroler Gebietskrankenkasse, TGKK) with the medical examinations of the EVA-Study and health information focusing on CVDs: Potential participants are introduced into the topic of cardiovascular health in a stepwise way. First, adolescents will receive information on the aims of the EVA-project including healthy lifestyle in their school or company (kick-off event). The concept of vascular ageing as a result of continuous accumulation of cardiovascular risks from early life on will be presented orally and by written information material (flyers). The individual visualization of the vessel wall and blood pressure measurement during medical examination will help the adolescents to link this information to their own body. In a second step, individual risk behaviors and laboratory results will be discussed with each participant. In a third step, the summary result from each school/company class will be presented and compared to other schools/companies.
We will create and provide online tools to promote health among adolescents. A website will be designed with information on healthy lifestyle and will contain an encrypted, password-protected web-app that provides the participants with individual health data. Traffic-lights will be used to represent the seven health metrics defined by the AHA [7, 32]. When adding current personal information on body weight, smoking status or physical activity the colors of the traffic lights will switch from red to green (or vice versa) in order to encourage further health-improvements. Further health games (e.g. health quiz) will be added to the website.
Participants will have the opportunity to record their own physical activity over 7 days using modern movement-trackers (Move 3, Movisense GmbH, Karlsruhe, Germany). The results of these records will be downloadable from the web-app.
Special health-promotion programs of the TGKK will include behavioral- and circumstance-oriented activities specially designed for each school/company. Representatives of each school/company are invited for an afternoon workshop aiming at health promotion by oral presentations and written information material. The scholars will undergo an interview to define their interests and expectations. The results of these workshops and the interview will pick out the most relevant topics for a tailored health promotion program for each school or company class. Local health-promotion projects will be supervised by the TGKK and presented on the EVA website as a health promotion book.
Outcome measures and statistical analysis
The primary outcome measure will be the difference in the proportion of each of AHA’s seven health metrics in the ideal range between the intervention and the control group.
The secondary outcome measure will be the change in the proportion of AHA’s seven health metrics in the ideal range between baseline and follow-up in the intervention group.
Further descriptive and exploratory analysis will focus on the prevalence of unfavorable health behaviors, the prevalence of vascular risk conditions and the change of these factors over age as well as their influence on EVA measured by IMT and the PWV. In addition, the influence of premature birth and early life weight gain (taken from the mother-child-booklet) on obesity and other components of the metabolic syndrome in youth will be explored.
Differences in proportions of the individual health metrics in the ideal range between the intervention and control group (primary outcome parameter) will be analyzed by Chi-squared test. The Prevalence of the individual health metrics in the ideal range in a similar cohort [18] varied from < 5% for ideal diet, over around 50% for ideal physical activity to > 80% for ideal body-mass index. On the basis of a 3:1 intervention to control group split, power analysis indicates that 95% power can be achieved with a sample size of 2000 to detect differences in proportions between intervention and control group of 3% (at a control group proportion of 96%) to 10% (at a control group proportion of 50%) [33] .Multivariate analysis will be done by logistic regression.
Principal investigators and study-centers
Innsbruck: Univ.-Prof. Dr. Ursula Kiechl-Kohlendorfer (Department of Pediatrics II - Neonatology, Medical University of Innsbruck, Austria), Assoc.-Prof. Priv.-Doz. Dr. Michael Knoflach (Department of Neurology, Medical University of Innsbruck, Austria).
Bruneck: Univ. -Prof. Dr. Ralf Geiger (Azienda Sanitaria dell’Alto Adige, Hospital of Bruneck, Deptartment of Paediatrics and Department of Pediatrics III - Cardiology, Medical University of Innsbruck, Austria).
Availability of data and materials
Anonymized data can be shared in academic cooperations. Request for data can be addressed to the principal investigators with an appropriate research question.
Discussion
The EVA-Tyrol study is a large-scale research and prevention program targeting cardiovascular health-promotion in adolescents. The planed 2000 participants will represent about 5% of the Tyrolean population at that age and will allow in-depth insights into vascular risk profile and vascular health of a representative Middle European population. The health promotion program used in our trial could realistically be continued at low costs outside the academic study setting. Our study explores novel grounds, as, even though numerous health promotion programs are targeted to the youth, few did prove efficacy. The STRIP study conducted in Turku, Finland, was able to increase the number of ideal cardiovascular health metrics by accompanying children with dietary and later smoking prevention counselling continuously between the age of 7 month and 20 years. Also, dietary counseling of pre-pubertal children with elevated LDL Cholesterol and their parents effectively reduced LDL Cholesterol [34]. However, other health promotion programs for adolescents were less successful [32, 35]. A recent meta-analysis of 30 studies aiming at improving physical activity in children and adolescents could demonstrate only a modest effect of various interventions [35]. In contrast to our study all these interventions focused on a single health behavior and did not provide comprehensive health counseling on multiple cardiovascular risk factors and behaviors.
The descriptive analysis of the EVA-Tyrol study will substantially enhance our understanding of the distribution of vascular risk behaviors and risk factors in subjects with varying social backgrounds (school types, apprentices) as well as sex and age groups and will help to better focus future health promotion programs.
Furthermore, the EVA-Tyrol cohort will allow to explore mechanisms of early vascular ageing by using well-defined surrogate parameters for vascular health (IMT and PWV) and analyze their association with cardiovascular risk factors and health behaviors. High-quality information from the “mother-child booklet” will elucidate the impact of early life weight gain on adolescent vascular health.
Acknowledgements
Early Vascular Aging (EVA) Study Group consists of the following collaborating authors:
Mandy, Asare, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
Manuela, Bock-Bartl, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Maximilian, Bohl, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
Christina, Burger, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Gregor, Brössner, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
Tatjana, Heisinger, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Christoph, Hochmayr, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Julia, Klingenschmid, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria.
Martina, Kothmayer, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Julia, Marxer, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Raimund, Pechlaner, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
Maximilian, Pircher, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Carmen, Reiter, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Sophia J, Kiechl, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
Bernhard, Winder, Department of Pediatrics II (Neonatology), Medical University of Innsbruck, Innsbruck, Austria
Abbreviations
- AHA
American heart association
- COMET
Competence centers for excellent technologies
- CRF
Case report form
- ctc
Clinical trial center
- CVD
Cardiovascular diseases
- CVRF
Cardiovascular risk-factors
- Dash
Dietary approach to stop hypertension
- ECLIA
Electrochemiluminescenceimmunoassay
- EVA
Early vascular ageing
- FFG
Austrian Research Promotion Agency
- HPLC
High pressure liquid chromatography
- IHS
International headache society
- IMT
Intima-media thickness
- MD
Medical doctor
- NCD
Non-communicable diseases
- PI
Principle investigator
- PWV
Pulse wave velocity
- TGKK
Tiroler Gebietskrankenkasse = Tyrolean regional medical insurance
- VASCage
Research Center of Excellence in vascular ageing - Tyrol
Authors’ contributions
NG, KS, AS (PhD-students) and RP (scientific collaborator) are involved in the EVA-Tyrol-study and contributed to the preparation and detailed elaboration of the study-protocol and the linguistical elaboration of this publication. RG is responsible for the implementation of the EVA-Tyrol-study in Bruneck (South-Tyrol) and is the principal investigator in Bruneck. AG participated in the elaboration and implementation of the laboratory-parameters surveyed in the EVA-Tyrol-study and will be responsible for the correctness of the laboratory-results. SK is the VASCage’s principal investigator and is responsible for the project’s funding and the statistical elaboration of our study-protocol. BB is PhD-student in the EVA-Tyrol-Study and elaborated the present manuscript and was involved in the implementation of the ICHD. UK and MK contributed equally to the elaboration of the study protocol, they are principal investigators in Tyrol (Austria), are EVA-Tyrol’s project directors and further more are supervisors of the medical- and PhD-students involved in the EVA-Tyrol-study. All authors read and approved the final manuscript.
Funding
EVA Tyrol is financially supported by the excellence initiative (Competence Centers for Excellent Technologies—COMET) of the Austrian Research Promotion Agency FFG: “Research Center of Excellence in Vascular Ageing—Tyrol, VASCage” (K Project No. 843536) funded by BMVIT, BMWFW, Wirtschaftsagentur Wien, and Standortagentur Tirol. The funding body had no role in the design of the study, collection, analysis and interpretation of data or in writing the manuscript.
Availability of data and materials
Request for data shall be addressed to the corresponding authors.
Ethics approval and consent to participate
Informed consent has to be approved and signed by the participant and by the legal representative if necessary (under the age of 18 years). For the inclusion of data of the “mother-child booklet” an additional maternal informed consent will be necessary.
The consent-form, as well as the complete EVA-Tyrol-Study, has been approved by the ethics-committee of the medical university Innsbruck (AN2015–0005 345/4.13), in order to GCP and the declaration of Helsinki.
Innrain 43
6020-Innsbruck
Austria
Phone: + 43 (0) 512/504–25444
Contact: Mag. David Bachler
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael Knoflach and Ursula Kiechl-Kohlendorfer contributed equally to this work.
Contributor Information
Benoît Bernar, Email: benoit.bernar@tirol-kliniken.at.
Nina Gande, Email: nina.gande@i-med.ac.at.
Katharina A. Stock, Email: anna.stock@i-med.ac.at
Anna Staudt, Email: anna.staudt@tirol-kliniken.at.
Raimund Pechlaner, Email: raimund.pechlaner@i-med.ac.at.
Ralf Geiger, Email: ralf.geiger@i-med.ac.at.
Andrea Griesmacher, Email: andrea.griesmacher@tirol-kliniken.at.
Stefan Kiechl, Email: stefan.kiechl@i-med.ac.at.
Michael Knoflach, Email: Michael.knoflach@i-med.ac.at.
Ursula Kiechl-Kohlendorfer, Email: ursula.kohlendorfer@i-med.ac.at.
for the Early Vascular Aging (EVA) Study Group:
Mandy Asare, Manuela Bock-Bartl, Maximilian Bohl, Christina Burger, Gregor Brössner, Tatjana Heisinger, Christoph Hochmayr, Julia Klingenschmid, Martina Kothmayer, Julia Marxer, Raimund Pechlaner, Maximilian Pircher, Carmen Reiter, Sophia J. Kiechl, and Bernhard Winder
References
- 1.World Health Organization (WHO) Fact sheet: “Cardiovascular diseases”. 2018. [Google Scholar]
- 2.World Health Organization (WHO) Mainpage: “Cardiovascular diseases (CVDs)”. 2018. [Google Scholar]
- 3.World Health Organization (WHO) Fact sheet: “The top 10 causes of death”. 2018. [Google Scholar]
- 4.Institute of Medicine. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health; chapter 5 reducing the burden of cardiovascular disease: Intervention strategies: The National Academies Press; 2010. 10.17226/12815. [PubMed]
- 5.Barker DJ, Osmond C, Law CM. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43:237–240. doi: 10.1136/jech.43.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal A, Cole TJ, Fewtrell M, Kennedy K, Stephenson T, Elias-Jones A, Lucas A. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115(2):213–220. doi: 10.1161/CIRCULATIONAHA.106.617811. [DOI] [PubMed] [Google Scholar]
- 7.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, De Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105(9):1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 8.Posod A, Odri Komazec I, Kager K, Pupp Peglow U, Griesmaier E, Schermer E, Würtinger P, Baumgartner D, Kiechl-Kohlendorfer U. Former very preterm infants show an unfavorable cardiovascular risk profile at a preschool age. PLoS One. 2016;11(12):e0168162. doi: 10.1371/journal.pone.0168162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velican D, Velican C. Atherosclerotic involvement of the coronary arteries of adolescents and young adults. Atherosclerosis. 1980;36(4):449–460. doi: 10.1016/0021-9150(80)90238-5. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) Published by the World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization. In: Mendis S, Puska P, Norrving B, editors. Global Atlas on cardiovascular disease prevention and control. Section A What are cardiovascular diseases (CVDs)? 2018. [Google Scholar]
- 11.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction. The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, DK MG, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive Summary: Heart Disease and Stroke Statistics—2014 Update; A Report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 13.Health Behavior in School-aged Children (HBSC) HBSC Physical activity focus group. Factsheet: “Physical activity”. 2019. [Google Scholar]
- 14.Health Behavior in School-aged Children (HBSC) HBSC Risk behaviours focus group. Factsheet: “Tobacco”. 2019. [Google Scholar]
- 15.Health Behavior in School-aged Children (HBSC) HBSC Risk behaviours focus group. Factsheet: “Alcohol”. 2019. [Google Scholar]
- 16.Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, van der Zee R, Gaston H, Jarosch E, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (atherosclerosis risk-factors in male youngsters) Circulation. 2003;108(9):1064–1069. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- 17.Knoflach M, Kiechl S, Penz D, Zangerle A, Schmidauer C, Rossmann A, Shingh M, Spallek R, Griesmacher A, Bernhard D, Robatscher P, Buchberger W, Draxl W, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young women: atherosclerosis risk factors in female youngsters (ARFY study) Stroke. 2009;40(4):1063–1069. doi: 10.1161/STROKEAHA.108.525675. [DOI] [PubMed] [Google Scholar]
- 18.Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Rönnemaa T, Niinikoski H, Lagström H, Talvia S, Jula A, Heinonen OJ, Juonala M, Simell O, Raitakari OT. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the special Turku coronary risk factor intervention project for children [STRIP] study) Circulation. 2013;127(21):2088–2096. doi: 10.1161/CIRCULATIONAHA.112.000761. [DOI] [PubMed] [Google Scholar]
- 19.Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkilä V, Jokinen E, Hutri-Kähönen N, Laitinen T, Kähönen M, Lehtimäki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the cardiovascular risk in young Finns study. Circulation. 2012;125(16):1971–1978. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 20.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 21.Simell O, Niinikoski H, Rönnemaa T, Raitakari OT, Lagström H, Laurinen M, Aromaa M, Hakala P, Jula A, Jokinen E, Välimäki I, Viikari J, STRIP Study Group Cohort profile: the STRIP study (special Turku coronary risk factor intervention project), an infancy-onset dietary and life-style intervention trial. Int J Epidemiol. 2009;38(3):650–655. doi: 10.1093/ije/dyn072. [DOI] [PubMed] [Google Scholar]
- 22.Health Behavior in School-aged Children (HBSC). WHO Library Cataloguing in Publication Data. International Report from the 2001/2002 survey, Young people’s health in context. Edited by Candace Currie et al. ISBN 92 890 1372 9. (Last 07.01.2019). URL: http://www.euro.who.int/__data/assets/pdf_file/0008/110231/e82923.pdf
- 23.Johnson F, Wardle J, Griffith J. The adolescent food habits checklist: reliability and validity of a measure of healthy eating behaviour in adolescents. Eur J Clin Nutr. 2002;56(7):644–649. doi: 10.1038/sj.ejcn.1601371. [DOI] [PubMed] [Google Scholar]
- 24.Turconi G, Celsa M, Rezzani C, Biino G, Sartirana MA, Roggi C. Reliability of a dietary questionnaire on food habits, eating behaviour and nutritional knowledge of adolescents. Section E, G and H. Eur J Clin Nutr. 2003;57(6):753–763. doi: 10.1038/sj.ejcn.1601607. [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Frijters ER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 26.Annesi-Maesano I, Didier A, Klossek M, Chanal I, Moreau D, Bousquet J. The score for allergic rhinitis (SFAR) a simple and valid assessment method in population studies. Allergy. 2002;57(2):107–114. doi: 10.1034/j.1398-9995.2002.1o3170.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C, Iglseder B, Weger S, Mairhofer B, Gartner M, Kedenko L, Chmelikova M, Stekovic S, Stuppner H, Oberhollenzer F, Kroemer G, Mayr M, Eisenberg T, Tilg H, Madeo F, Willeit J. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108(2):371–380. doi: 10.1093/ajcn/nqy102. [DOI] [PubMed] [Google Scholar]
- 28.Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Circulation. 2011;124(15):1673–1686. doi: 10.1161/CIRCULATIONAHA.110.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willeit J, Kiechl S, Oberhollenzer F, Rungger G, Egger G, Bonora E, Mitterer M, Muggeo M. Distinct risk profiles of early and advanced atherosclerosis: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol. 2000;20(2):529–537. doi: 10.1161/01.ATV.20.2.529. [DOI] [PubMed] [Google Scholar]
- 30.International Headache Society The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 31.Bundes Ministerium für Gesundheit und Familie, Österreich . Mutter-Kind-Pass. 2019. [Google Scholar]
- 32.Lubans DR, Lubans DR, Morgan PJ, Okely AD, Dewar D, Collins CE, Batterham M, Callister R, Plotnikoff RC. Preventing obesity among adolescent girls: one-year outcomes of the nutrition and enjoyable activity for teen girls (NEAT girls) cluster randomized controlled trial. Arch Pediatr Adolesc Med. 2012;166(9):821–827. doi: 10.1001/archpediatrics.2012.41. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: Lawrence Erlbaum; 1988. pp. 978–0805802832. [Google Scholar]
- 34.Obarzanek E, Kimm SY, Barton BA, Van Horn LL, Kwiterovich PO, Jr, Simons-Morton DG, Hunsberger SA, Lasser NL, Robson AM, Franklin FA, Jr, Lauer RM, Stevens VJ, Friedman LA, Dorgan JF, Greenlick MR, DISC Collaborative research group Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: seven-year results of the Dietary Intervention Study in Children (DISC) Pediatrics. 2001;107(2):256–264. doi: 10.1542/peds.107.2.256. [DOI] [PubMed] [Google Scholar]
- 35.Metcalf B, Henley W, Wilkin T. Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes (EarlyBird 54) BMJ. 2012;345:e5888. doi: 10.1136/bmj.e5888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be shared in academic cooperations. Request for data can be addressed to the principal investigators with an appropriate research question.
Request for data shall be addressed to the corresponding authors.