Abstract
Dyspnea is one of the most common symptoms in advanced cancer patients at the end of their life. It is often multifactorial with diverse malignant, nonmalignant, and cancer treatment related etiologies. Oxygen, opiates, and anxiolytics are commonly administered. Here a complex case of progressive dyspnea and its treatments in a patient with advanced pancreatic cancer is described, and its multiple potential contributing causes are identified and clinical responses evaluated. Literature review is conducted on pulmonary drug toxicity and tumor lymphangitic spread, and the role of corticosteroids in relieving dyspnea in the palliative care setting.
Introduction
Dyspnea or breathlessness is perhaps one of the most common and distressing symptoms experienced by patients with advanced cancer during their course of illness.1 It is subjectively defined as sensations of discomfort in breathing, either physiologically or psychologically.2 Several studies of patients with advanced cancer reveal that dyspnea is highly prevalent and debilitating, and the symptom burden worsens during the dying process.3, 4 Patients, families, and caregivers often feel very disturbed by the sensations of air hunger and suffocation, as well as the severe limitation in their activities of daily living.5 Palliative care physicians are often called upon to relieve dyspnea at the end of life (EOL) and to support patients, families, and caregivers through the dying process.
A retrospective review of 100 patients showed that the etiology of dyspnea was often multifactorial, and on average, patients had five potential causes of dyspnea.6 The most common causes of dyspnea were (1) pulmonary pathologies related to cancer, such as tumor obstruction and malignant pleural effusion; (2) pathologies related to cancer treatments, such as chemotherapy and/or radiation; and (3) nonmalignant causes such as chronic obstructive lung disease (COPD), congestive heart failure (CHF), and anxiety. Up to 30% of patients had no identifiable causes of dyspnea.6
Many treatment modalities have been found to be effective in relieving dyspnea in advanced cancer patients including oxygen, opiates, anxiolytics, as well as focused psychosocial support.7,8 Less well studied strategies such as circulating cool air, nebulized opiates, nebulized furosemide, corticosteroids, non-invasive positive pressure ventilation, and in the case of refractory dyspnea, palliative sedation, have also been reported.7,8
Corticosteroids are commonly used in palliative care settings other than dyspnea.9 Common indications include relief of spinal cord compression; reduction of vasogenic edema from brain metastasis; resolution from malignant bowel obstruction; and symptom control of nausea, vomiting, anorexia, and pain. Potential corticosteroid responsive conditions in the management of dyspnea include bronchospasm, radiation, and chemotherapy-related pneumonitis, superior vena cava (SVC) syndrome, and exacerbations of COPD.10 However, given concerns about significant side effects associated with short- and long-term corticosteroid use, evidence-based guidelines have not advocated their use to palliate EOL dyspnea in cancer patients.7,8
Case Presentation
A 90 year-old female with coronary artery disease, diastolic heart failure, atrial tachycardia status post-ablation and recently diagnosed pancreatic adenocarcinoma presents with progressive exertional dyspnea, fatigue, and febrile episodes. The patient has been receiving monthly chemotherapy with gemcitabine and erlotinib. The last dose of chemotherapy was three weeks ago. A staging computed tomography (CT) the week prior showed increased size of liver metastases, although her liver function tests have been relatively stable.
On admission she is in acute respiratory distress with tachycardia and tachypnea. Rales are auscultated bilaterally throughout both lung fields, and her jugular venous pressure is elevated to the jaw. There is bilateral lower extremity 2+ pitting edema more pronounced on the left. Electrocardiogram shows no ischemic changes. Chest X-ray (CXR) shows bilateral interstitial and alveolar infiltrates (Figure 1a), and arterial blood gas reveals acute hypoxemic respiratory failure. BNP is over 1200, WBC is over 18, hemoglobin is 8, and lower extremity Doppler shows acute left lower extremity deep vein thrombosis (DVT).
FIG. 1a.
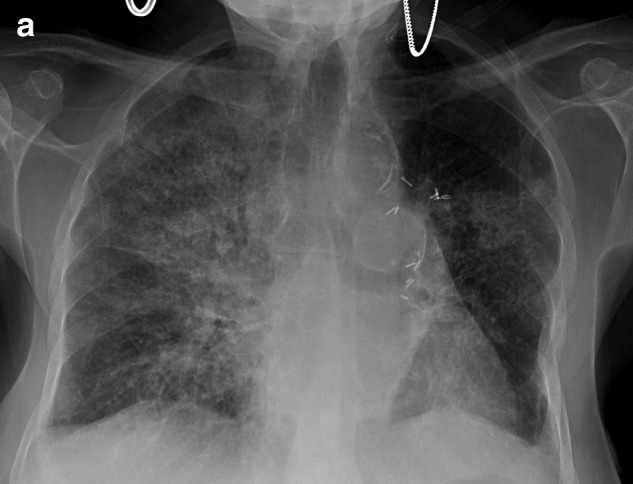
Admission CXR showing interstitial infiltrates.
Given fever, leukocytosis, underlying immunosuppression, and bilateral pulmonary infiltrates, empirical broad-spectrum antibiotics, Vancomycin and Piperacillin/Tazobactam, are started for health care associated pneumonia. She is diuresed aggressively given clinical findings of pulmonary edema and presumed acute exacerbation of congestive heart failure. Anticoagulation is started with low molecular weight heparin for acute DVT with possible concomitant pulmonary embolism. Over the next 48 hours her symptoms are minimally improved. Nasal swabs and polymerase chain reactions for viral etiologies of pneumonia are negative. Transthoracic echocardiogram reveals depression of left ventricular ejection to 45% without associated right ventricular strain or valvular abnormalities. Further treatment with bilevel positive pressure ventilation (BiPAP), opiates, anxiolytics, and a cool air-circulating device are utilized. A CT of the chest reveals nondependent, diffuse bilateral ground glass opacity, concerning for pulmonary edema, interstitial pneumonitis, or lymphagitic spread of her pancreatic cancer (Figure 1b). Amiodarone is discontinued. Corticosteroids in the form of prednisone 30 mg twice a day orally are started empirically for presumed diagnosis of chemotherapy- or amiodarone-induced pulmonary toxicity with possible superimposed lymphagitic spread, leading to rapid improvement of her symptoms as well as CXR findings in one week (Figure 1c).
FIG. 1b.
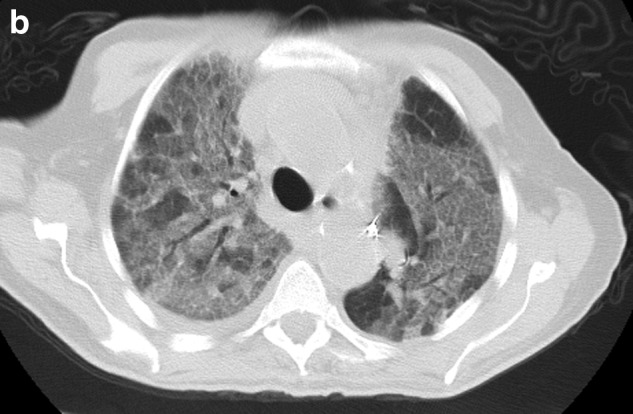
Chest CT on admission showing bilateral diffuse infiltrates.
FIG. 1c.
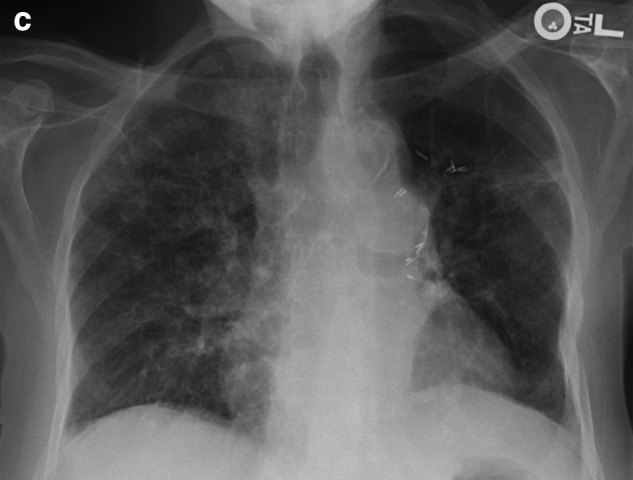
CXR after one week of corticosteroid treatment showing improvement.
She is eventually discharged home with a steroid taper, oxygen, and pulmonary rehabilitation. Chemotherapy is discontinued given pulmonary toxicity and progression of the disease. Home palliative care service is instituted and she is eventually transitioned to hospice.
Discussion
We describe here a case of progressive dyspnea in a patient with advanced pancreatic cancer and dramatic response to corticosteroids. Several potential etiologies are entertained, which include bacterial pneumonia, exacerbation of CHF, pulmonary embolism, pulmonary drug toxicity, and tumor lymphangitic spread. However, given the clinical time course and the rapid response to corticosteroids, the most likely causes are chemotherapy-induced pulmonary toxicity and lymphangitic spread of the tumor. This case highlights the characteristic scenario of dyspnea in the palliative care setting where the cause is often multifactorial and the response is often unpredictable. We advocate for the prompt recognition and treatment of corticosteroid-responsive conditions in EOL dyspnea in cancer patients.
The differential diagnosis of dyspnea in a patient with advanced cancer and diffuse interstitial pulmonary infiltrates on chest radiograph is extensive, including bacterial and viral pneumonia, hydrostatic pulmonary interstitial edema, lung toxicity induced by chemotherapeutic drugs, radiation pneumonitis, and lymphangitic carcinomatosis.1,5 The rational diagnostic approach here is to consider the tempo of the disease, clinical context, and the characteristic pattern on high resolution CT scan to make the most likely diagnosis, since lung biopsies carry a high risk for complications in these patients.11
In our patient, the classic causes of respiratory infection, fluid overload, and pulmonary embolism may have all contributed to her dyspnea, but the acute presentation in the setting of progressive malignancy with recent chemotherapy, as well as the typical radiographic findings of bilateral interstitial and alveolar infiltrates, make drug-induced pneumonitis the most likely culprit and lymphangitic spread a close second. Fortunately, both conditions are corticosteroid-sensitive, and she responded well with prompt resolution of her respiratory symptoms. In contrast, treatments with broad-spectrum antibiotics, diuresis, and anticoagulation were not effective in this case.
With the ever-increasing armamentarium of chemotherapeutic agents at our disposal, one can expect an increasing spectrum of significant side effects, especially pulmonary drug toxicity. Traditionally, pulmonary drug toxicity is characterized by fever, dyspnea, hypoxemia, a radiographic pattern of interstitial fibrosis, and a temporal relationship with the suspected offending drugs.12 It is often overlooked because of other common diagnoses including interstitial lung disease, viral and bacterial pneumonia, CHF, or obstructive pulmonary diseases. It is usually not suspected until after many other etiologies and treatments have been exhausted. Some of the characteristic pulmonary toxicity is related to antibiotics and cardiac drugs such as amiodarone.13 Review of the literature shows that the prevalence of pulmonary drug toxicity is increasingly common in advanced cancer patients, in particular for those receiving certain chemotherapeutics such as bleomycin, gemcitabine, and oral tyrosine kinase inhibitors.12 Pneumonitis associated with gemcitabine and oral tyrosine kinase inhibitors has an incidence rate ranging from 0.02% to 1%, and typically presents as interstitial pneumonitis, diffuse alveolar damage, and noncardiogenic pulmonary edema.12
Cessation of the presumed culprit agent and treatment with corticosteroids often lead to dramatic and rapid improvements of the symptoms and radiographic findings, although this has not been evaluated in randomized controlled trials.14 Interestingly, the temporal relationship of the offending drug and the development of pulmonary symptoms often varies, ranging from days to weeks after the drug was started. This is important for patients who are referred to palliative care and/or hospice care with concurring and/or recent chemotherapy, since their symptoms of dyspnea often have multiple etiologies and chemotherapy-related pulmonary toxicity might be neglected and corticosteroids thus not offered. Similarly, radiation peumonitis, another corticosteroid-responsive condition, may occur months after the radiation treatment.15 It should be noted that the use of corticosteroids at the EOL is a much more common practice in Europe.16,17
Lymphangitic carcinomatosis (LC) is the diffuse infiltration and obstruction of the parenchymal lymphatic channels by cancer. Incidence of LC accounts for 6%-8% of all metastatic diseases to the thorax.18 The common carcinomas associated with LC are breast, larynx, prostate, thyroid, gallbladder, stomach, and pancreas.18 Clinical manifestations of LC such as dyspnea, nonproductive cough, and bilateral pulmonary infiltrates often lead to an incorrect diagnosis; however, the characteristic findings of LC on high resolution CT significantly assists in the differential diagnosis. LC has a poor prognosis, as it indicates advanced metastatic disease, and the use of radiation and/or chemotherapy is generally not helpful. However, systemic corticosteroids are known to have palliative effects.19
Several other pulmonary pathologies in dyspneic cancer patients are also responsive to corticosteroids. Besides pulmonary conditions such as COPD and bronchospasm, corticosteroids have been shown to be effective in cancer associated SVC syndrome, upper airway obstruction, and radiation induced pneumonitis. A systemic review by Rowell and Gleeson finds that corticosteroids, while not a substitute for radiation, chemotherapy, or palliative stenting, may be useful in relieving dyspnea caused by tumor-related SVC syndrome in ill patients.20 Anecdotal reports have been published on the role of corticosteroids in relieving upper airway obstruction, presumably due to reduction of tumor-related airway edema or secretions.21 Finally, radiation-induced pneumonitis is particularly sensitive to low dose of corticosteroids.15
Perhaps the apparent underuse of corticosteroids in relieving cancer-related dyspnea is related to concerns about their significant side effects, particularly at high doses. Data from the spinal cord compression literature reveal significant hyperglycemia, risks of infection, psychomotor agitation, and fluid retention.9 Clinicians, as well as patients and family caregivers, often worry about the side effects and thus are reluctant to use corticosteroids. Two regimens of corticosteroids are widely reported in the literature: a high-dose regimen (e.g., dexamethasone 100 mg followed by 96 mg per day in divided doses), which has been used to relieve spinal cord compression, increased intracranial pressure, and upper airway obstruction; and a low intermediate dose regimen (e.g., dexamethasone 1-6 mg once or twice daily), which has been used in the aforementioned pulmonary conditions. In patients with a limited life expectancy of days to several weeks, long-term side effects are less likely to occur and therefore, we believe that they should not preclude the continuous use of corticosteroids until the patient's death. The benefit of potential rapid improvement in patient's disabling dyspnea clearly outweighs manifestations of corticosteroid side effects. Palliative care physicians should educate patients and their caregivers about the risks and benefits of corticosteroid use in relieving EOL dyspnea, taking into account patients' illness trajectory, prognosis, and goals of care.
Our case illustrates severe debilitating dyspnea in a patient with advanced malignancy and dramatic response to corticosteroids. We highlight several potential corticosteroid-responsive pulmonary conditions that may be prevalent in this patient population, and we recommend a trial of low dose of oral corticosteroids in dyspneic cancer patients even without definitive tissue diagnosis, given the high prevalence of corticosteroid-responsive conditions in these patients, and the favorable risk-benefit ratio of low-dose corticosteroids. Our approach calls for liberal use of high-risk medications and treatment modalities at the EOL, taking into consideration the patient's prognosis and goals of care. With the pleotrophic effects of corticosteroids on many other EOL symptoms in addition to dyspnea, palliative care physicians should consider adding corticosteroids in treating dyspneic cancer patients with other potential corticosteroid-responsive EOL symptoms.
Acknowledgement
No competing financial conflict of interest exists for the authors.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Thomas JR. von Gunten CF. Management of dyspnea. J Support Oncol. 2003;1:23–34. doi: 10.1007/978-1-59745-291-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Manning HL. Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333:1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 3.Reuben DB. Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89:234–236. doi: 10.1378/chest.89.2.234. [DOI] [PubMed] [Google Scholar]
- 4.Dudgeon DJ. Kristjanson L. Sloan JA. Lertzman M. Clement K. Dyspnea in cancer patients: Prevalence and associated factors. J Pain Symptom Manage. 2001;21:92–95. doi: 10.1016/s0885-3924(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 5.Cachia E. Ahmedzai SH. Breathlessness in cancer patients. Eur J Cancer. 2008;44:1116–1123. doi: 10.1016/j.ejca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Dudgeon DJ. Lertzman M. Dyspnea in the advanced cancer patient. J Pain Symptom Manage. 1998;16:212–219. doi: 10.1016/s0885-3924(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Aharon I. Gafter-Gvili A. Paul M. Leibovici L. Stemmer SM. Interventions for alleviating cancer-related dyspnea: A systemic review. J Clin Oncol. 2008;26:2396–2404. doi: 10.1200/JCO.2007.15.5796. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Palliative Care Guideline, 2011 version 1. www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Apr 9;2011 ]. www.nccn.org/professionals/physician_gls/f_guidelines.asp
- 9.Shih A. Jackson KC., 2nd Role of corticosteroids in palliative care. J Pain Palliat Care Pharmacother. 2007;21:69–76. [PubMed] [Google Scholar]
- 10.Viola R. Kiteley C. Lloyd NS. Mackay JA. Wilson J. Wong RK. Supportive care guidelines group of the cancer care Ontario program in evidence-based care. The management of dyspnea in cancer patients: A systemic review. Support Care Cancer. 2008;16:329–337. doi: 10.1007/s00520-007-0389-6. [DOI] [PubMed] [Google Scholar]
- 11.Ryu JH. Olson EJ. Midthun DE. Swensen SJ. Diagnostic approach to the patient with diffuse lung disease. Mayo Clin Proc. 2002;77:1221–1227. doi: 10.4065/77.11.1221. [DOI] [PubMed] [Google Scholar]
- 12.Vahid B. Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 13.Schwaiblmair M. Berghaus T. Haseckel T. Wagner T. von Scheidt W. Amiodarone-induced pulmonary toxicity: An under-recognized and severe adverse effect? Clin Res Cardiol. 2010;99:693–700. doi: 10.1007/s00392-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 14.Camus P. Kudoh S. Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004;91:S18–S23. doi: 10.1038/sj.bjc.6602063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves PR. Siddiqui F. Anscher MS. Movsas B. Radiation pulmonary toxicity: From mechanisms to management. Semin Radiat Oncol. 2010;20:201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Hardy JR. Rees E. Ling J. Burman R. Feuer D. Broadley K. Stone P. A prospective survey of the use of dexamethasone on a palliative care unit. Palliative Med. 2001;15:3–8. doi: 10.1191/026921601673324846. [DOI] [PubMed] [Google Scholar]
- 17.Gannon C. McNamara P. A retrospective observation of corticosteroid use at the end of life in a hospice. J Pain Symptom Manage. 2002;24:328–334. doi: 10.1016/s0885-3924(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 18.Bruce DM. Heys SD. Eremin O. Lymphangitis carcinomatosa: A literature review. J R Coll Surg Edin. 1996;41:7–13. [PubMed] [Google Scholar]
- 19.Storck K. Crispens M. Brader K. Squamous cell carcinoma of the cervix presenting as lymphangitic carcinomatosis: A case report and review of the literature. Gynecologic Oncol. 2004;94:825–828. doi: 10.1016/j.ygyno.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Rowell NP. Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus. Cochrane Database Syst Rev. 2001;4:CD001316. doi: 10.1002/14651858.CD001316. [DOI] [PubMed] [Google Scholar]
- 21.Elasyem A. Bruera E. High-dose corticosteroids for the management of dyspnea in patients with tumor obstruction of the upper airway. Support Care Cancer. 2007;15:1437–1439. doi: 10.1007/s00520-007-0305-0. [DOI] [PubMed] [Google Scholar]


