Abstract
Despite advances in diabetes technology and treatment, a majority of children and adolescents with type 1 diabetes (T1D) fail to meet hemoglobin A1c (HbA1c) targets. Among high-income nations, the United States has one of the highest mean HbA1c values. We tracked the HbA1c values of 261 patients diagnosed with T1D in our practice over a 2.5-year period to identify inflection points in the HbA1c trajectory. The HbA1c declined until 5 months postdiagnosis. There was a rise in the HbA1c between the fifth and sixth month postdiagnosis. The HbA1c continued to steadily rise and by 18 months postdiagnosis, the mean HbA1c was 8.2%, which is also our clinic mean. Understanding the HbA1c trajectory early in the course of diabetes has helped to identify opportunities for intensification of diabetes management to flatten the trajectory of HbA1c and improve clinical outcomes.
Keywords: Type 1 diabetes, New diagnosis, Hemoglobin A1c, Pediatrics
Introduction
Type 1 diabetes (T1D) care is a difficult life-long undertaking by patients, their caregivers, and the health care team. The Diabetes Complications and Control Trial (DCCT) established that prevention of long-term microvascular and macrovascular complications of T1D requires intensive glycemic control.1 For children and adolescents with T1D, the American Diabetes Association (ADA) recommends a hemoglobin A1c (HbA1c) target of <7.5%,2 whereas the International Society for Pediatric and Adolescent Diabetes (ISPAD) and the National Institute of Health and Care Excellence (NICE) in the United Kingdom recommend a HbA1c target of <7%3 and <6.5%,4 respectively. Unfortunately, a majority of children and adolescents with T1D do not meet these HbA1c targets.5–7 Among high income countries, the United States has one of the highest mean HbA1c values.5 Current care for children, adolescents, and young adults with T1D has failed to make meaningful progress in lowering HbA1c, despite advances in diabetes technology,8–10 analog insulins, and refinements in care delivery, among others.7,11–13
Understanding the trajectory of HbA1c after diagnosis can target interventions in the course of T1D. Following diagnosis of T1D, there is typically a decline in HbA1c after insulin initiation. With longer T1D duration, endogenous insulin production wanes, glucose control becomes more challenging, and frequently, HbA1c increases. In this article, we describe the trajectory of HbA1c in patients with newly diagnosed T1D within our clinic to identify inflection points in the HbA1c trajectory that may serve as opportunities to intensify intervention and to identify clinical and demographic factors that affect HbA1c trajectory to deliver more precise care.
Materials and Methods
Subjects
Lucile Packard Children's Hospital at Stanford has ∼900 patients with T1D and ∼100 patients with new onset diabetes every year. The electronic medical record (EMR) was reviewed and all individuals diagnosed with T1D between May 24, 2014 (current EMR start date) and December 31, 2016 were included in this study. The data were extracted in January 2018. All participants were censored at 18 months postdiagnosis since we were concerned with the early trajectory of changes in HbA1c values postdiagnosis. Furthermore, 18 months was the duration achieved by a majority of the cohort. Manual chart review was performed to verify the date of diagnosis, initial HbA1c, mode of insulin delivery, and use of continuous glucose monitor (CGM) technology. Individuals were categorized as being of minority status if the EMR identified them of being of non-Caucasian race. Individuals with Medi-Cal/Medicaid, Medicare, or California Children's Services (CCS) were categorized as having public insurance. If patients left our practice, their data were included in the analysis until their last clinic visit, at which point they were censored. On average, 75% of all patients had follow-up measurements in the EMR until 18 months postdiagnosis.
Measurement of HbA1c
HbA1c measurements were performed using a DCA Vantage Analyzer (Siemans Medical Solution Diagnostics) and measurements were obtained at every clinic visit.
Statistical methods
We summarized baseline characteristics of patients with newly diagnosed T1D by providing the mean (standard deviation) for continuous variables and a frequency distribution for categorical variables. To visually describe the trajectory of HbA1c over time, we used locally weighted scatter plot smoothing techniques (LOESS) with estimates obtained for each time point through those patients able to provide values at that given point. The level of smoothing in LOESS is determined by the span parameter, and we relied on 10-fold cross-validation that corresponded to the lowest mean squared error. We calculated the mean HbA1c at each month and created a LOESS mean trajectory curve along with the standard deviation at each point. In addition, LOESS was displayed by levels of demographic variables.
To understand individual longitudinal trajectories for each patient, we fit longitudinal models of HbA1c over time to assess their relationship with key patient-level characteristics and additionally modeled specific features from the individual trajectories as a function of those key patient-level characteristics. More specifically, to understand the association between demographic factors and HbA1c over time, we used generalized linear mixed effects regression models of HbA1c as a function of time and the variable of interest, where time was modeled flexibly with both linear and quadratic terms. Interaction terms between the variable of interest and the terms representing time were also included as key parameters of interest, as they represented the difference in HbA1c trajectory over time by the patient-level characteristic. We used regression techniques to evaluate whether patient-level characteristics were associated with two key features of the trajectory curve: (1) the minimum level of HbA1c achieved for a given patient and (2) the time at which the minimum value was observed for a given patient. For each of these two models, we also provided the beta coefficienct corresponding to the variable of interest, which represents the degree of change in the outcome variable for every 1 U of change in the variable of interest. A likelihood ratio test was used to assess significance of both interaction terms and beta coefficients from the models.
Finally, we evaluated trajectories of HbA1c among patients who initiated CGM or pump within 18 months of diagnosis. For CGM and pump use trajectory analysis, we evaluated changes in HbA1c from the time of treatment initiation through 18 months. Similar regression techniques were applied for this purpose. Tests were two-sided and conducted at the 0.05 level of significance. Statistical analyses were performed using the R 3.5.2 software package.
Results
Baseline characteristics
We identified 261 individuals who were diagnosed with T1D during the study period. In this cohort, 50.2% were male, 43.7% were non-Hispanic white, and 74.3% were on private insurance. By 18 months postdiagnosis, 40.2% used an insulin pump and 42.1% were on CGM (Supplementary Table S1). The mean age of diagnosis was 9.6 ± 4.0 years.
Descriptive HbA1c trajectory over 18 months
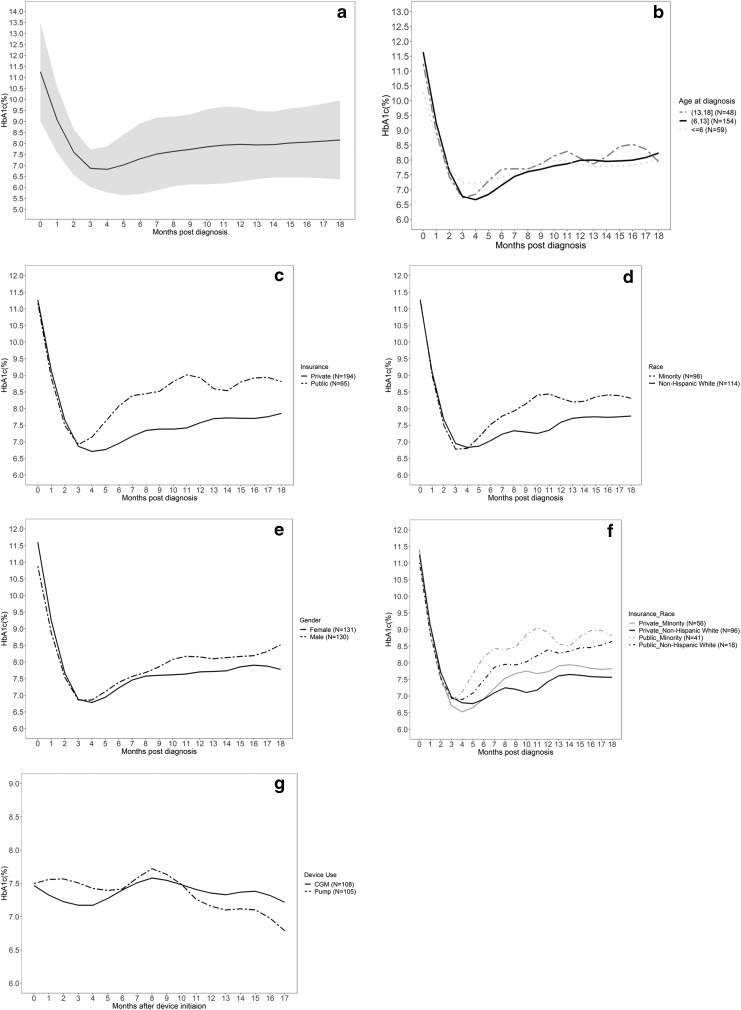
HbA1c data were nonnormally distributed with a right skew. HbA1c mean (minimum/maximum) at 0, 6, 12, and 18 months were as follows: 0 months—11.1 (5.3–14.8); 6 months—7.3 (4.4–14.0); 12 months—8.0 (5.6–13.5); 18 months—8.2 (5.6–13.5) (Supplementary Table S2). The median time to nadir for our population was 5 months, and the average nadir HbA1c value was 6.7% (95% confidence interval: 6.6–6.9). Among those measured at 6 months postdiagnosis, the mean HbA1c value was higher than the value observed at 5 months and among those measured at 1 year postdiagnosis, the observed mean HbA1c was 8.0% ± 1.7%. At 18 months postdiagnosis, the observed mean HbA1c was 8.2% ± 1.9%. Each patient had an average of six HbA1c measurements during the study period.
Demographic associations with trajectory of HbA1c over 18 months, nadir, and time to nadir
Table 1 shows findings from analyses for trajectory, nadir, and time to nadir. The trajectory of HbA1c differed by age group over the study period (P = 0.001; Table 1). Children younger than 6 years of age at diagnosis had the highest HbA1c at nadir (Fig. 1b). The trajectory of HbA1c for children with public insurance was significantly different from children with private insurance (P < 0.001). The mean HbA1c increased faster in individuals with public insurance compared to individuals with private insurance (Fig. 1c). The trajectory of HbA1c also differed by race (P = 0.04). Figure 1d shows that mean HbA1c tended to increase at a higher rate in minority groups compared to non-Hispanic white individuals postnadir. We found the trajectory (P = 0.11) did not significantly differ by gender (Fig. 1e). To gain further insight, when insurance type was stratified by race, publicly insured children who were of minority race tended to have higher HbA1c throughout the study period (Fig. 1F). However, we did not observe any significant associations with time to nadir.
Table 1.
Model Results for Variables of Interest
| Demographic factors | Trajectory | Time to nadir | Nadir | ||
|---|---|---|---|---|---|
| Interaction P value | Beta coefficient (95% CI) | P value | Beta coefficient (95% CI) | P value | |
| Age at diagnosis (reference: 6 to <13) | 0.009 | 0.17 | 0.14 | ||
| ≤6 | 0.28 (−0.99 to 1.55) | 0.32 (−0.04 to 0.68) | |||
| 13 to <18 | −1.16 (−2.52 to 0.21) | −0.10 (−0.49 to 0.29) | |||
| Insurance type (public vs. private) | <0.001 | −0.14 (−1.33 to 1.04) | 0.82 | 0 (−0.33 to 0.34) | 0.99 |
| Race (minority vs. non-Hispanic white) | 0.04 | −0.40 (−1.54 to 0.74) | 0.49 | −0.14 (−0.48 to 0.21) | 0.43 |
| Insurance_Race (referece = “Private non-Hispanic white”) | 0.11 | 0.82 | 0.32 | ||
| Private minority | −0.65 (−2.04 to 0.75) | 0 (−0.41 to 0.41) | |||
| Public non-Hispanic white | −0.27 (−2.40 to 1.86) | 0.35 (−0.27 to 0.98) | |||
| Public minority | −0.03 (−1.57 to 1.52) | −0.29 (−0.74 to 0.17) | |||
| Gender (male vs. female) | 0.11 | −0.10 (−1.13 to 0.93) | 0.85 | −0.15 (−0.45 to 0.14) | 0.31 |
| CGM (yes vs. no) | 0.55 | −0.04 (−1.23 to 1.14) | 0.94 | −0.17 (−0.46 to 0.12) | 0.24 |
| Pump (yes vs. no) | 0.02 | 0.32 (−0.82 to 1.45) | 0.58 | −0.06 (−0.33 to 0.22) | 0.69 |
CGM, continuous glucose monitor; CI, confidence interval.
FIG. 1.
LOESS curve of mean HbA1c (a), LOESS curve of mean HbA1c by age at diagnosis (b), insurance type (c), race (d), sex (e), insurance type and race (f), and device use after initiation within 18 months postdiagnosis (g). HbA1c, hemoglobin A1c; LOESS, locally weighted scatter plot smoothing techniques.
Use of diabetes technology on trajectory of HbA1c
In our study population, 108 (41.4%) individuals were started on a CGM within 18 months postdiabetes diagnosis and the average time from diagnosis to initiation was 6 months (range 0–16.4 months). We found the trajectory of HbA1c did not vary by CGM use (Table 1). There were 105 (40.2%) children who started on an insulin pump within 18 months postdiagnosis and the average time from diagnosis to device initiation is 7.8 months (range 0–17.9 months) (Fig. 1g). We found insulin pump use to be significantly associated with trajectory of HbA1c (P = 0.02) (Table 1).
Discussion
Despite the outcomes of the DCCT establishing the effectiveness of intensive glucose control to reduce diabetic complications in 1993, a minority of individuals with T1D worldwide meet current HbA1c goals. Even among developed countries, there exists a difference between the HbA1c levels in the United States compared with European countries.5 For example, the mean HbA1c in 17-year olds in the T1D Exchange in 2010–12 was similar to the conventional arm of the DCCT (9%).1,7,14 In contrast, significant decreases in pediatric mean HbA1c are documented internationally. For example, in the Diabetes Prospective Follow-Up Registry (DPV) HbA1c decreased from 9% to 8.2% from 1995 to 2010 based on benchmarking and quality improvement methods.15,16 Numerous other countries have reported reductions in HbA1c leaving the mean HbA1c in pediatric diabetes in the United States among the highest worldwide.5 While these countries have dramatically different health care systems, all are in developed countries such as the United States. In contrast, the SWEET registry reports that the mean HbA1c in individual clinics in many developing countries such as India, Nepal, and Mexico is in the 8%–9.5% range, suggesting that even in resource poor situations, better glucose control can be achieved. Therefore, other countries have effectively implemented the DCCT message of intensive glucose control leaving the United States as an outlier in achieved pediatric HbA1c.5 Part of this may be attributed to efforts in other parts of the developed world to benchmark data and institute quality improvement measures. Such efforts are still lagging in the United States.
To identify opportunities for improvement in our clinic, we performed this retrospective analysis of our 261 patients with newly diagnosed T1D between May 2014 and December 2016. We observed that patients experienced a decline in their HbA1c over the first 5 months of diabetes diagnosis. Children who were diagnosed with T1D before 6 years of age had the highest HbA1c at this time point. This is consistent with previous data that younger children tend to have a low rate of partial remission of T1D.17 By 6 months after diagnosis, the mean HbA1c increased in all age groups. At 18 months postdiagnosis, the mean HbA1c was 8.2%, which is in line with our clinic mean. Individuals with public insurance, a marker of lower socioeconomic ststus, had higher mean HbA1c. We were not able to perform finer analyses based on factors such as household income or highest parental education. Analyses of a larger number of patients across the age spectrum would be needed to generalize our findings.
Previous studies have indicated that CGM use can improve HbA1c and time in range while minimizing hypoglycemia.18,19 Use of insulin pumps has also been associated with improved glycemic control.20,21 Data indicate that those using diabetes technology have lower A1c, but this has failed to translate into overall A1c data in clinic populations.12 More research is required on how to best implement diabetes technology to achieve better outcomes for the pediatric T1D population in the United States.
This study provides a real-world description of HbA1c trajectory in patients with newly diagnosed T1D. This trajectory is similar to trajectories previously described by the Pediatric Diabetes Consortium.22 Our data are limited to a single site. Although we analyzed 18 months of data, not all patients had data available at all time points.
In conclusion, the first 6–12 months of T1D diagnosis provides an opportunity to intensify management and flatten the trajectory of HbA1c. In our patient population, the HbA1c at 18 months postdiagnosis appears to be the steady state HbA1c of patients in our clinic. This suggests that interventions targeting the new onset period have the opportunity to shift the baseline of the HbA1c curve down and improve long-term outcomes. Further studies need to be performed to determine if this pattern of HbA1c trajectory is observed at other sites within the United States and around the world. Similar to previous studies,23 interventions involving early use of technology have the potential to improve HbA1c, but require education to fully reach their potential to improve diabetes care. These data also suggest that targeted approaches should be developed for those of lower socioeconomic status.
Supplementary Material
Author Disclosure Statement
Dr. Hood has research support from Dexcom, Inc. for investigator-initiated research; Consultant fees from Lilly Innovation Center, Lifescan Diabetes Institute, Insulet, Inc., and Roche Diagnostics. Dr. Maahs has research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust and his institution has research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. Dr. Maahs has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, and Insulet.
Supplementary Material
References
- 1. The Diabetes Complications and Control Trial Research Group: The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Chiang JL, Maahs DM, Garvey KC, et al. : Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2026–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiMeglio LA, Acerini CL, Codner E, et al. : ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19(Suppl 27):105–114 [DOI] [PubMed] [Google Scholar]
- 4. National Institute for Health and Care Excellence (UK): Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management. NICE Guideline, No. 18, London; August 2015
- 5. Charalampopoulos D, Hermann JM, Svensson J, et al. : Exploring variation in glycemic control across and within eight high-income countries: A cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care 2018;41:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clements MA, Foster NC, Maahs DM, et al. : Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D Exchange clinic registry. Pediatr Diabetes 2016;17:327–336 [DOI] [PubMed] [Google Scholar]
- 7. Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 8. Prahalad P, Tanenbaum M, Hood K, Maahs DM: Diabetes technology: improving care, improving patient-reported outcomes and preventing complications in young people with type 1 diabetes. Diabet Med 2018;35:419–429 [DOI] [PubMed] [Google Scholar]
- 9. Sherr JL, Hermann JM, Campbell F, et al. : Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87–91 [DOI] [PubMed] [Google Scholar]
- 10. Szypowska A, Schwandt A, Svensson J, et al. : Insulin pump therapy in children with type 1 diabetes: analysis of data from the SWEET registry. Pediatr Diabetes 2016;17 Suppl 23:38–45 [DOI] [PubMed] [Google Scholar]
- 11. Cameron FJ, de Beaufort C, Aanstoot HJ, et al. : Lessons from the Hvidoere International Study Group on childhood diabetes: be dogmatic about outcome and flexible in approach. Pediatr Diabetes 2013;14:473–480 [DOI] [PubMed] [Google Scholar]
- 12. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwandt A, Hermann JM, Rosenbauer J, et al. : Longitudinal trajectories of metabolic control from childhood to young adulthood in type 1 diabetes from a large German/Austrian registry: a group-based modeling approach. Diabetes Care 2017;40:309–316 [DOI] [PubMed] [Google Scholar]
- 14. Diabetes Control and Complications Trial Research Group: Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 15. Karges B, Rosenbauer J, Kapellen T, et al. : Hemoglobin A1c Levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med 2014;11:e1001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenbauer J, Dost A, Karges B, et al. : Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 2012;35:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes 2008;9:197–201 [DOI] [PubMed] [Google Scholar]
- 18. Danne T, Nimri R, Battelino T, et al. : International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cooper MN, O'Connell SM, Davis EA, Jones TW: A population-based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia 2013;56:2164–2170 [DOI] [PubMed] [Google Scholar]
- 21. Karges B, Schwandt A, Heidtmann B, et al. : Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA 2017;318:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cengiz E, Connor CG, Ruedy KJ, et al. : Pediatric diabetes consortium T1D New Onset (NeOn) study: clinical outcomes during the first year following diagnosis. Pediatr Diabetes 2014;15:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kordonouri O, Hartmann R, Pankowska E, et al. : Sensor augmented pump therapy from onset of type 1 diabetes: late follow-up results of the Pediatric Onset Study. Pediatr Diabetes 2012;13:515–518 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.