Abstract
Although microvascular proliferation can be observed in glioblastoma, obvious vascularity coupled with coexisting cerebral arteriovenous malformation (AVM) is extremely rare. This report is of a rare case of glioblastoma, coexisting with a cerebral AVM. A 20-year-old male presented with progressive right hemiparesis within 1 month. Cranial magnetic resonance imaging revealed a large bleeding tumor with surrounding dilated vessels. Cerebral angiography demonstrated a left frontal AVM with a 1.2 cm nidus. The patient underwent preoperative embolization and radical resection. The coincidence of glioma and AVM was a rare association. However, the concept of hypervascular glioblastoma has been used in different states from different literature reviews; therefore, the role of proangiogenic factors should be addressed
Keywords: Angioglioma, arteriovenous malformation, glioblastoma, hypervascular glioblastoma
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumors in adults. Although microvascular endothelial proliferation is one of the criteria for a histological diagnosis, grossly hypervascular glioblastoma is an unusual manifestation. The authors described a patient, who suffered from a combination between glioblastoma and cerebral arteriovenous malformation (AVM). This case also highlights the rare angiomatous manifestation of glioblastoma.
CASE REPORT
A 20-year-old male developed progressive right hemiparesis on the right side within 1 month. Cranial magnetic resonance imaging (MRI) scans demonstrated a heterogeneously enhancing mass, 5.5 cm × 8 cm in size, with internal hemorrhage at the left frontal lobe. In addition, surrounding dilated arteries and veins at the adjacent cortex were noted on T1-weighted with gadolinium. On cranial computed tomography, the dense calcification located an inferior portion of the mass. Cranial magnetic resonance angiography revealed dilated peripheral branches of the anterior cerebral artery and middle cerebral artery (MCA) with dilated cortical veins [Figure 1a-d].
Figure 1.
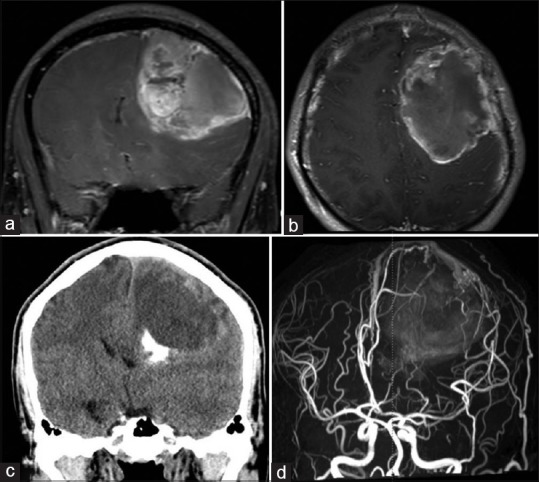
Preoperative neuroimaging. (a) T1-weighted coronal (b) and T1-weighted axial images with gadolinium administration demonstrated a large left frontal mass with flow void signs. (c) Cranial computed tomography revealed calcification at the inferior portion of the mass. (d) Cranial magnetic resonance angiography revealed multiple dilated vessels surrounding the tumor
Moreover, a cerebral angiographic study revealed a nidus, approximately 1.2 cm wide in the subcortical region of the left precentral sulcus. This was fed from the distal branches of the left precentral artery and anterior parietal artery of the left MCA. The flow drained into a single cortical vein to the superior sagittal sinus [Figure 2a and b].
Figure 2.
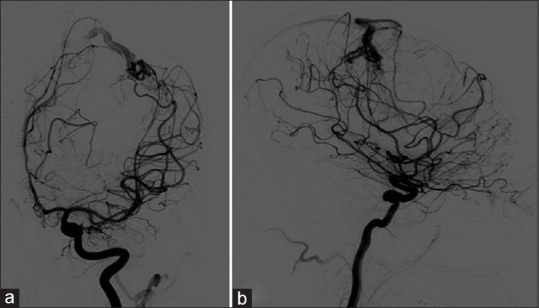
Preoperative cerebral angiography. (a) Anterior-posterior view and (b) lateral view showed a nidus in the subcortical region of the left frontal lobe. It was supplied by distal branches of the left precentral artery and anterior parietal artery of the left middle cerebral artery. The venous drainage was to the cortical vein to the superior sagittal sinus
A preoperative embolization was performed for reducing blood loss during surgery before a craniotomy. Intraoperative findings revealed a well-defined, reddish tumor with obvious abnormal vessels occupying both superficial and deep portions of the tumor. Interestingly, the hypervascular tumor was toughly attached with dura. The tumor was completely removed.
Pathologic examination
Gross characteristics of the tumor were extra-axial mass, reddish-gray color, dural attachment, firm-solid consistency, and fed from multiple dilated arteries. In addition, calcification was observed at the inferior portion of the tumor. Therefore, the gross features mimic meningioma [Figure 3a-c].
Figure 3.
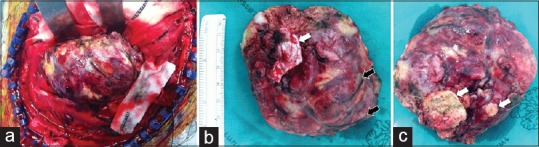
Gross appearance of the tumor. (a) Extra-axial mass was observed in the operative field. (b) The superior aspect of the tumor could be observed, the tumor attached dura matter (white arrow), and multiple feeding arteries (black arrow) were around the tumor. (c) The inferior aspect of the tumor could be observed, dense calcification (white arrow)
A histopathological examination demonstrated nuclear polymorphism astrocytes and necrosis without sarcomatous spindle morphology. Therefore, gliosarcoma was excluded. The tumor was composed of numerous enlarged blood vessels of varying sizes, and dural morphology was normal. Finally, the diagnosis was glioblastoma associated with AVM [Figure 4a-d].
Figure 4.
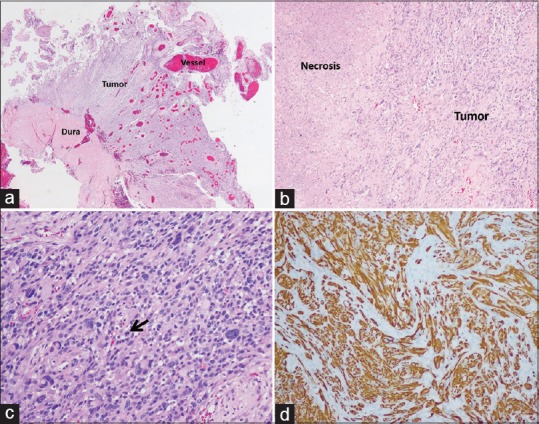
Histological examination of the tumor. (a) Tumor attached normal dura matter and numerous blood vessels in varying sizes were within the tumor. (b) The necrotic area was observed. (c) The neoplastic glial cells had nuclear enlargement with hyperchromatic nuclei, and irregular nuclear membrane and mitotic activity could be observed (black arrow). (d) Immunoreactivity of the tumor was positive for GFAP
After tumor resection, the patient received postoperative adjuvant radiation. Six months after the first operation, the patient presented with alteration of consciousness and tumor recurrence was observed by neuroimaging. Due to these factors, he then underwent the second operation. Postoperatively, his consciousness improved, but tumor regrowth occurred, again, 1 year later, after the second operation. His family refused the third operation, and subsequently, he died in 2 years after the first operation.
DISCUSSION
Glioblastoma is an aggressive primary brain tumor that has composites of necrosis and microscopic endothelial proliferation.[1] The term “angioglioma” had been used in glioma associated with various vascular abnormalities; however, this term was primarily defined by Russell and Rubinstein, who described angioglioma from patients with the cystic cerebellar tumors with coexisting glioma and hemangioblastoma.[2] Hence, the authors reviewed literature using the keywords such as “angioglioma,” “glioblastoma AND arteriovenous malformation,” “hypervascular glioma,” “glioma AND arteriovenous malformation,” and “vascular malformation AND glioma.” We excluded “angiocentric glioma,” because this entity is a distinct group of epileptogenic tumors, according to the 2016 WHO classification of tumors of the central nervous system. As a result, the cases are summarized in Supplementary Table 1. Histopathology of angiocentric glioma demonstrated spindled cells forming occasionally perivascular pseudorosettes.[3]
Supplementary Table 1.
Summary of 68 cases of hypervascular glioma and angioglioma in English literature
| Author and year | Patients profile | Histology of tumor | Vascular malformation/co-existing findings |
|---|---|---|---|
| Russell and Rubinstein, 1989[2] | 70, male | Glioma | Hemangioblastoma |
| Zuccarello et al.,1979 [4] | 50, male | Malignant astrocytoma | AVM (angiogram) |
| Foy et al., 1981[5] | 70, female | Oligodendroglioma | AVM (angiogram) |
| Fischer et al., 1982[6] | 11, male | Oligodendroglioma | Cavernous hemangioma |
| 19, female | Glial tumor | Cavernous hemangioma | |
| Bonnin et al., 1983[7] | 52, female | Cerebellar gemistocytic astrocytoma | Hemangioblastoma |
| 65, female | Spinal intramedullary mixed astrocytoma, ependymoma | Hemangioblastoma | |
| 51, female | Mixed glioma | Hemangioblastoma | |
| 56, female | GBM | Hemangioblastoma | |
| Chee et al., 1985[8] | 25, male | Oligodendroglioma | Cerebral cavernous angioma |
| Licata et al., 1986[9] | 44, male | Gliosarcoma | AVM (angiogram) |
| 25, male | GBM | AVM (angiogram) | |
| Goodkin et al., 1990[10] | 9, female | Anaplastic astrocytoma | AVM (angiogram) |
| Lombardi et al., 1991[11] | 17, male | Thalamic oligodendroglioma | AVM-like lesion (angiogram) |
| 28, male | Occipital oligodendroglioma | AVM-like lesion (angiogram) | |
| 58, male | Frontal oligodendroglioma | AVM-like lesion (no angiogram) | |
| 40, female | Frontal oligodendroglioma | AVM-like lesion (no angiogram) | |
| 26, male | Cerebellar pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 24, male | Cerebellar pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 23, male | Cerebellar pilocytic astrocytoma | AVM-like lesion (angiogram) | |
| 21, male | Cerebellar pilocytic astrocytoma | AVM-like lesion (angiogram) | |
| 23, female | Cerebellar pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 30, female | Supratentorial pilocytic astrocytoma | AVM-like lesion (angiogram) | |
| 2, female | Temporal pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 12, female | Supratentorial pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 8, female | Thalamic pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 23, female | Temporal pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 19, male | Parietal pilocytic astrocytoma | AVM-like lesion (no angiogram) | |
| 37, male | Oligodendroglioma | AVM (angiogram) | |
| 14, female | Oligodendroglioma | AVM (angiogram) | |
| 42, female | Oligodendroglioma | AVM (angiogram) | |
| 23, female | Oligodendroglioma | AVM (angiogram) | |
| 16, female | Oligodendroglioma | AVM (angiogram) | |
| 27, male | Oligodendroglioma | AVM (angiogram) | |
| 17, male | Oligodendroglioma | AVM (angiogram) | |
| 24, male | Oligodendroglioma | AVM (angiogram) | |
| 65, female | Glioma | AVM (angiogram)* | |
| Hasegawa et al., 1995[12] | 54, female | Low-grade glioma | Cavernous angioma |
| Lee et al., 1996[13] | 45, male | Pleomorphic xanthoastrocytoma | AVM (angiogram) |
| Kasantikul et al., 1996[14] | 5, male | Astrocytoma | AV, capillary angioma |
| 15, female | Astrocytoma | Capillary, cavernous angioma | |
| 31, male | Oligodendroastrocytoma | AV, capillary angioma | |
| 35, male | Astrocytoma | Capillary, cavernous angioma | |
| 41, female | Oligodendroglioma | Capillary, cavernous angioma | |
| 58, male | Astrocytoma | Angioma | |
| 60, female | Astrocytoma | AV, angioma | |
| 15, male | Astrocytoma | Capillary, cavernous angioma | |
| 22, male | Oligodendroastrocytoma | AV, angioma | |
| 57, female | Oligodendroastrocytoma | AV, capillary | |
| Tews et al., 1998[15] | 20, male | Oligodendroglioma | Cavernous angioma |
| Harris et al., 2000[16] | 57, male | Anaplastic astrocytoma | AVM (Angiogram) |
| Ziyal et al., 2004[17] | 58, male | High-grade glioma | AVM (Angiogram) |
| Cemil et al., 2009[18] | 58, male | Glioblastoma | AVM (Angiogram) |
| Pallud et al., 2009[19] | 14 , male | Pleomorphic xanthoastrocytoma | AVM like lesion (MRI) |
| Gazzeri et al., 2011[20] | 16, female | Ganglioglioma with glial component | Cavernous angioma |
| 38, male | Oligodendroglioma | Cavernous angioma | |
| Aucourt et al., 2012[21] | 65, male | GBM | AVM (angiogram) |
| Soltanolkotabi et al., 2012[22] | 8, female | Pilocytic astrocytoma | AVM (angiogram) |
| Gmeiner et al., 2013[23] | 72, female | GBM | AVM-like lesion (MRI) |
| Khanna et al., 2013[24] | 53, male | GBM | AVM (angiogram) |
| Nagańska et al., 2013[25] | 36, female | Pleomorphic xanthoastrocytoma | AVM-like lesion (pathology) |
| Boikov et al., 2014[26] | 29, female | GBM | AV fistula (angiogram) |
| Imai et al., 2015[27] | 66, male | GBM | AVM (angiogram) |
| Li et al., 2015[28] | 47, male | Spinal pilocytic astrocytoma | Spinal hemangioblastoma |
| Linsenmann et al., 2015[29] | 35, male | Primary spinal GBM | Secondary cranial GBM with extensive vascular components in contiguous regions |
| Lohkamp et al., 2016[30] | 71, female | GBM | AVM (angiogram) |
| Joshi et al., 2016[31] | 15, male | Spinal pilocytic astrocytoma | Extensive vascular components in contiguous regions |
| Present case 2017 | 20, male | GBM | AVM (angiogram) |
*AVM separate from glioma. AV=Arteriovenous, AVM=Arteriovenous malformation, GBM=Glioblastoma multiforme, MRI=Magnetic resonance imaging
Form the literature review, the coexistence of glioma and vascular malformation is a rare entity. To the best of the authors’ knowledge, at least 67 cases have been described before this present case. From Table 1, hypervascular gliomas are common in males (55.9%) and the distribution of age is between 2 and 72 years (mean age was 35 years).[2,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] In vascular malformation, AVM is the common coexisting vascular malformation. Using cerebral angiography, high blood flow passes through the arteriovenous shunt.[4,5,9,10,11,16,17,18,19,21,22,23,24,25,26,27,30] In addition, various tumors have been reported as having the coexistence of glial cell tumors as well as other various tumors, either vascular tumor (cavernous, capillary hemangioma)[6,8,12,14,15,20] or other hypervascular tumors (hemangioblastoma).[2,7,32]
These coexistences have been found in both low-grade and high-grade gliomas. The common glial cell tumors are GBM, pilocytic astrocytoma, and oligodendroglioma. In histological features, GBMs usually found endothelial cell proliferation and glomeruloid appearance while pilocytic astrocytoma and oligodendroglioma found other characteristics of abnormal vessel pattern. Hyalinized and glomeruloid vessels could be prominent features in pilocytic astrocytoma whereas oligodendroglioma typically shows a dense network of branching capillaries resembling the pattern of chicken wire.[1] However, the overexpression of proteins of tumor angiogenesis pathway is proposed in the hypothesis of the pathophysiology of this coexistence.
Upregulation of angiogenetic-driven events has been hypothesized that induced abnormal vasculatures in hypervascular glioma.[33] C-X-C chemokine receptor type 4 (CXCR4) is a chemokine receptor for C-X-C motif chemokine ligand 12 (CXCL12) that is associated with angiogenesis and tumor progression. The receptors are upregulated by hypoxia-inducible factor-1 (HIF-1) and vascular endothelial growth factors (VEGFs). Reactivity of the HIF-1α subunit and VEGF was highly expressed in pseudopalisading tumor cells adjacent to necrosis. However, a high VEGF expression and overexpression of CXCL12-CXCR4 axis were observed in angiogenic vessels.[23,34]
In vascular malformation, it has been shown that VEGF expression is a fundamental part of intracerebral AVM pathogenesis. VEGF levels are increased in intracerebral AVM.[35] Upcoming molecular research should be studied to demonstrate the common angiogenetic-driven mechanisms between coexisting lesions. As the results, hypervascular glioma is the presence of dark vessel-like signals (flow void signs) on T2-weighted imaging located within the tumor. These represented the prominent serpiginous vessels.[21] Moreover, the histopathologic characteristics reveal clusters of numerous blood vessels of various size.[14]
As for the treatment strategy, radical resection is the treatment of choice in hypervascular glioma, but these tumors are challenging for total tumor resection. Unfortunately, significant intratumoral hemorrhage frequently occurs in this tumor. Emergency decompressive craniectomy with/without lesionectomy should be performed for saving of life. However, the risk of enormous bleeding during surgery has to be considered. Hence, in elective conditions, preoperative endovascular embolization is an alternative method for reducing blood loss, when the tumor is resected. Imai et al. proposed preoperative embolization as a strategy that tumors which located in eloquent areas need provocation testing beforehand. Presurgical embolization for tumor feeder vessels is recommended in negative provocation testing.[27]
CONCLUSIONS
We described a rare manifestation of GBM. Proangiogenic factors are most likely involved in the existence of both entities at the same location. Future molecular studies may reveal a common genetic pathway to explain this association. Surgical treatment is challenging due to the risk of massive intraoperative bleeding. Preoperative endovascular embolizations are alternative methods for controlling intraoperative blood loss.
What is already known on this topic?
GBM is the most common primary malignancy of the brain in adults. One of the histological characteristics involves microvascular proliferation such as endothelial proliferation and glomeruloid appearance.
What does this study add?
This case report demonstrated the rare coexistence between GBM and cerebral AVM. The hypothesis of pathophysiology is overexpression of proteins of tumor angiogenesis pathway.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Louis DN, Brat DJ, Ohgaki H, Stupp R, Suva ML, Biernat W, et al. Glioblastoma, IDH-wildtype. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization Classification of tumours of the Central Nervous System. Revised 4th ed. Lyon: The International Agency for Research on Cancer; 2016. pp. 28–45. [Google Scholar]
- 2.Russell DS, Rubinstein LJ, editors. 5th ed. London: Edward Arnold; 1989. Pathology of Tumours of the Nervous System. [Google Scholar]
- 3.Burger PC, Jouvet A, Preusser M, Rosenblum MK, Ellison DW. Angiocentric glioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization Classification of Tumours of the Central Nervous System. Revised 4th ed. Lyon: The International Agency for Research on Cancer; 2016. pp. 119–20. [Google Scholar]
- 4.Zuccarello M, Giordano R, Scanarini M, Mingrino S. Malignant astrocytoma associated with arteriovenous malformation. Case report. Acta Neurochir (Wien) 1979;50:305–9. doi: 10.1007/BF01808529. [DOI] [PubMed] [Google Scholar]
- 5.Foy PM, Lozada L, Shaw MD. Vascular malformation simulating a glioma on computerized tomography. Case report. J Neurosurg. 1981;54:125–7. doi: 10.3171/jns.1981.54.1.0125. [DOI] [PubMed] [Google Scholar]
- 6.Fischer EG, Sotrel A, Welch K. Cerebral hemangioma with glial neoplasia (angioglioma?). Report of two cases. J Neurosurg. 1982;56:430–4. doi: 10.3171/jns.1982.56.3.0430. [DOI] [PubMed] [Google Scholar]
- 7.Bonnin JM, Peña CE, Rubinstein LJ. Mixed capillary hemangioblastoma and glioma. A redefinition of the “angioglioma”. J Neuropathol Exp Neurol. 1983;42:504–16. doi: 10.1097/00005072-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chee CP, Johnston R, Doyle D, Macpherson P. Oligodendroglioma and cerebral cavernous angioma. Case report. J Neurosurg. 1985;62:145–7. doi: 10.3171/jns.1985.62.1.0145. [DOI] [PubMed] [Google Scholar]
- 9.Licata C, Pasqualin A, Freschini A, Barone G, Da Pian R. Management of associated primary cerebral neoplasms and vascular malformations: 2. Intracranial arterio-venous malformations. Acta Neurochir (Wien) 1986;83:38–46. doi: 10.1007/BF01420506. [DOI] [PubMed] [Google Scholar]
- 10.Goodkin R, Zaias B, Michelsen WJ. Arteriovenous malformation and glioma: Coexistent or sequential? Case report. J Neurosurg. 1990;72:798–805. doi: 10.3171/jns.1990.72.5.0798. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi D, Scheithauer BW, Piepgras D, Meyer FB, Forbes GS. “Angioglioma” and the arteriovenous malformation-glioma association. J Neurosurg. 1991;75:589–66. doi: 10.3171/jns.1991.75.4.0589. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa H, Bitoh S, Koshino K, Obashi J, Kobayashi Y, Kobayashi M, et al. Mixed cavernous angioma and glioma (angioglioma) in the hypothalamus – Case report. Neurol Med Chir (Tokyo) 1995;35:238–42. doi: 10.2176/nmc.35.238. [DOI] [PubMed] [Google Scholar]
- 13.Lee TT, Landy HJ, Bruce JH. Arteriovenous malformation associated with pleomorphic xanthoastrocytoma. Acta Neurochir (Wien) 1996;138:590–1. doi: 10.1007/BF01411181. [DOI] [PubMed] [Google Scholar]
- 14.Kasantikul V, Shuangshoti S, Panichabhongse V, Netsky MG. Combined angioma and glioma (angioglioma) J Surg Oncol. 1996;62:15–21. doi: 10.1002/(SICI)1096-9098(199605)62:1<15::AID-JSO4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 15.Tews DS, Bohl JE, Van Lindert E, Ringel K. Association of oligodendroglioma-like cell proliferation and angiomatous vasculature – Coincidence or pathogenetically related lesions? Clin Neuropathol. 1998;17:69–72. [PubMed] [Google Scholar]
- 16.Harris OA, Chang SD, Harris BT, Adler JR. Acquired cerebral arteriovenous malformation induced by an anaplastic astrocytoma: An interesting case. Neurol Res. 2000;22:473–7. doi: 10.1080/01616412.2000.11740703. [DOI] [PubMed] [Google Scholar]
- 17.Ziyal IM, Ece K, Bilginer B, Tezel GG, Ozcan OE. A glioma with an arteriovenous malformation: An association or a different entity? Acta Neurochir (Wien) 2004;146:83–6. doi: 10.1007/s00701-003-0161-8. [DOI] [PubMed] [Google Scholar]
- 18.Cemil B, Tun K, Polat O, Ozen O, Kaptanoglu E. Glioblastoma multiforme mimicking arteriovenous malformation. Turk Neurosurg. 2009;19:433–6. [PubMed] [Google Scholar]
- 19.Pallud J, Belaid H, Guillevin R, Vallee JN, Capelle L. Management of associated glioma and arteriovenous malformation – the priority is the glioma. Br J Neurosurg. 2009;23:197–8. doi: 10.1080/02688690802688146. [DOI] [PubMed] [Google Scholar]
- 20.Gazzeri R, De Bonis C, Carotenuto V, Catapano D, d’Angelo V, Galarza M, et al. Association between cavernous angioma and cerebral glioma. Report of two cases and literature review of so-called angiogliomas. Neurocirugia (Astur) 2011;22:562–6. doi: 10.1016/s1130-1473(11)70112-9. [DOI] [PubMed] [Google Scholar]
- 21.Aucourt J, Jissendi P, Kerdraon O, Baroncini M. Neuroimaging features and pathology of mixed glioblastoma – AVM complex: A case report. J Neuroradiol. 2012;39:258–62. doi: 10.1016/j.neurad.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Soltanolkotabi M, Schoeneman SE, Dipatri AJ, Hurley MC, Ansari SA, Rajaram V, et al. Juvenile pilocytic astrocytoma in association with arteriovenous malformation. Interv Neuroradiol. 2012;18:140–7. doi: 10.1177/159101991201800203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gmeiner M, Sonnberger M, Wurm G, Weis S. Glioblastoma with the appearance of arteriovenous malformation: Pitfalls in diagnosis. Clin Neurol Neurosurg. 2013;115:501–6. doi: 10.1016/j.clineuro.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Khanna A, Venteicher AS, Walcott BP, Kahle KT, Mordes DA, William CM, et al. Glioblastoma mimicking an arteriovenous malformation. Front Neurol. 2013;4:144. doi: 10.3389/fneur.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagańska E, Matyja E, Pucko E, Ząbek M. The coexistence of pleomorphic xanthoastrocytoma and arteriovenous malformation. A case report. Folia Neuropathol. 2013;51:269–74. doi: 10.5114/fn.2013.37712. [DOI] [PubMed] [Google Scholar]
- 26.Boikov AS, Schweitzer AD, Young RJ, Lavi E, Tsiouris AJ, Gupta A, et al. Glioblastoma-arteriovenous fistula complex: Imaging characteristics and treatment considerations. Clin Imaging. 2014;38:187–90. doi: 10.1016/j.clinimag.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Imai T, Ohshima T, Nishizawa T, Shimato S, Kato K. Successful preoperative endovascular embolization of an extreme hypervascular glioblastoma mimicking an arteriovenous malformation. World Neurosurg. 2016;86:512.e1–4. doi: 10.1016/j.wneu.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Li WQ, Wang X, Zhong NZ, Li YM. Spinal hemangioblastoma combined with pilocytic astrocytoma. Neurosciences (Riyadh) 2015;20:280–4. doi: 10.17712/nsj.2015.3.20140225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linsenmann T, Westermaier T, Vince GH, Monoranu CM, Löhr M, Ernestus RI, et al. Primary spinal glioblastoma multiforme with secondary manifestation as a cerebral “Angioglioma.” literature review and case report. J Neurol Surg Rep. 2015;76:e128–34. doi: 10.1055/s-0035-1549227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohkamp LN, Strong C, Rojas R, Anderson M, Laviv Y, Kasper EM, et al. Hypervascular glioblastoma multiforme or arteriovenous malformation associated glioma? A diagnostic and therapeutic challenge: A case report. Surg Neurol Int. 2016;7:S883–8. doi: 10.4103/2152-7806.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi KC, Khanapure K, Hegde N, Ravindra N, Jagannatha AT, Hegde AS, et al. Angioglioma of the spinal cord. World Neurosurg. 2016;96:610.e5–000. doi: 10.1016/j.wneu.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Matyja E, Grajkowska W, Taraszewska A, Marchel A, Bojarski P, Nauman P. Advanced reactive astrogliosis associated with hemangioblastoma versus astroglial-vascular neoplasm (“angioglioma”) Folia Neuropathol. 2007;45:120–5. [PubMed] [Google Scholar]
- 33.Huang WJ, Chen WW, Zhang X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol Lett. 2016;12:2283–8. doi: 10.3892/ol.2016.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: Implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–32. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 35.Walker EJ, Su H, Shen F, Degos V, Amend G, Jun K, et al. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke. 2012;43:1925–30. doi: 10.1161/STROKEAHA.111.647982. [DOI] [PMC free article] [PubMed] [Google Scholar]


