Abstract
Discovery of evolutionarily conserved, nonprotein-coding, endogenous microRNAs has induced a paradigm shift in the overall understanding of gene regulation. Now, microRNAs are considered and classified as master regulators of gene expression as they regulate a wide range of processes – gene regulation, splicing, translation and posttranscriptional modifications. Besides, dysregulated microRNAs have been related to many diseases, including Parkinson's and related disorders. Several studies proposed that differentially expressed microRNAs as a potential biomarker. So far, there is no accepted clinical diagnostic test for Parkinson's disease based on biochemical analysis of biological fluids. However, circulating microRNAs possess many vital features typical of reliable biomarkers and discriminates Parkinson's patients from healthy control with much higher sensitivity and specificity. Though they show tremendous promise as a putative biomarker, translating these research findings to clinical application is often met with many obstacles. Most of the candidate microRNAs reported as a diagnostic biomarker is not organ-specific, and their overlap is low between studies. Therefore this review aimed to highlight the challenges in the application of microRNA in guiding disease discrimination decisions and its future prospects as a diagnostic biomarker in Parkinson's Disease.
Keywords: Biomarker, biofluid, miRNA, Parkinsonism, Parkinson's disease
PARKINSON'S DISEASE
Parkinson's Disease (PD) is the most common progressive movement disorder characterized by motor and non-motor symptoms. Additionally, we can see the selective loss of dopaminergic neurons and abnormal protein inclusions in the brain.[1] At present, even though several medications are available to manage symptoms of PD. Nothing can be done to halt the death of dopaminergic neurons or the accumulation of abnormal protein inclusions in the brain.[2] Presently, sporadic PD is identified based on the patients’ history, clinical assessment, disease presentation, response to the treatment, and functional neuroimaging.[3,4] Based on these criteria, identification of PD specifically from other clinical mimics is challenging and are prone to diagnostic inaccuracies in the early stages of disease.[5,6] Several studies tried to address this limitation by finding circulating biomolecules relevant to neurodegeneration as a diagnostic biomarker.[3,7,8,9,10,11] However, to date, none of them correctly predict the disease or monitor their progression. An ideal diagnostic marker should be safe and easy to measure, cost-efficient, modifiable with treatment, consistent across gender and ethnic groups. Consequently, this highlights the need to identify and validate new early-stage and disease-specific biomarkers, which will complement current practices and increase diagnostic accuracy.[12]
MICRORNAS
MicroRNAs (miRNAs or miRs) are endogenous, non-coding, single-stranded, stable small RNAs which negatively regulate gene expression by promoting target messenger RNA degradation or translational inhibition.[13] In the past years, much effort had focused on understanding their biological significance. Several studies have demonstrated the importance of miRNAs in a range of biological processes and viewed them as critical regulators in differentiation, maturation, apoptosis, and immune signalling pathways.[14,15] There is growing evidence that in many human diseases, including PD, miRNA expression is dysregulated. These dysregulated miRNAs could majorly contribute to pathogenesis by modulating genes central to the pathology.[16,17,18,19,20,21,22,23]
Additionally, the identification and detection of dysregulated miRNAs in body fluids with much higher sensitivity than proteins caught the attention of researchers, to develop miRNA-based biomarkers for diverse diagnostic applications. The profiling of circulating biofluids identified differentially expressed miRNAs, which discriminate patients of various neurological disorders including PD[21,22,23] from healthy individuals with high sensitivity and specificity.[24,25,26,27,28,29,30,31] As a result, differentially expressed miRNAs in various biological fluids have been proposed as putative biomarkers to assist in the PD diagnosis.[32,33,34,35,36] Even though our understanding of the mechanisms regulating the selective secretion of circulating miRNAs and their function remains elusive, they possess many vital features typical of reliable biomarker.[37] Yet, miRNAs could not be able to reach clinical settings for diagnosis of PD irrespective of understanding their biological significance over a decade and effort of several groups to propose many putative candidate miRNAs. Underneath, we discuss the limitations and challenges of using differentially expressed miRNAs as diagnostic biomarker for PD reported over a decade.
Potential candidate miRNAs from various biofluids
In order to list out all studies on circulating miRNAs as a potential diagnostic biomarker for PD, a systematic search of PubMed was performed as depicted in Figure 1 (and in supplementary) and the results were presented in Table 1. Table 1 revealed several putative miRNAs as biomarker from each study with a surprising inconsistency in the proposed candidate miRNAs between studies as discussed below.
Figure 1.
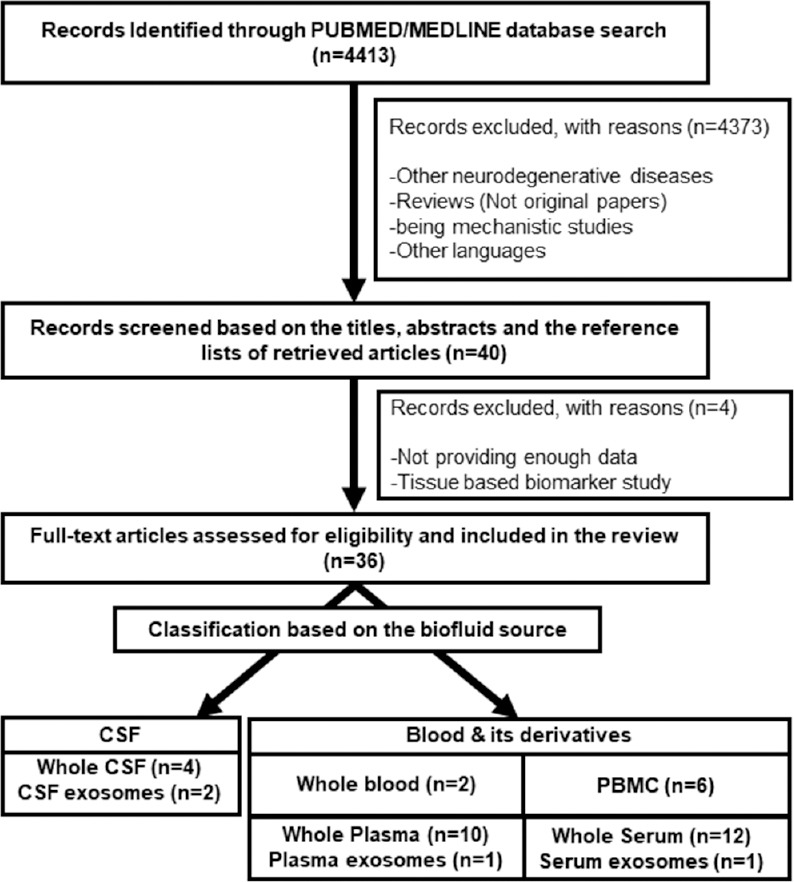
The process of literature search and eligible record selection; n: The number of studies
Table 1.
Proposed candidate miRNAs in various body fluids of PD compared to healthy controls
| Author and the year of the study | Source | Volume used (mL) | Isolation method | miRNA integrity Analysis | Study cohort derived from | Cohort size | Detection Method/Kits used/ Instrument | Up-regulated miRNA | Down-regulated miRNA | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Burgos et al., 2014 | CSF | 1 | miRVana PARIS kit (Invitrogen) | Sun Health Research Institute, Sun City, Arizona, United States of America | PD-65 HC-70 | NGS [Illumina HiSeq2000] | miR-19a-3p, miR-19b-3p, let-7g-3p | miR-132-5p, miR-485-5p, miR-127-3p, miR-128, miR-409-3p, miR-433, miR-370, miR-431-3p, miR-873-3p, miR-136-3p, miR-212-3p, miR-10a-5p, miR-1224-5p, miR-4448 | (38) | |
| Gui, Liu, Zhang, Lv, & Hu, 2015 | CSF Exosome | Qiagen miRNeasy Serum/Plasma Kit (Qiagen, Valencia, CA) | Bioanalyzer (Agilent) | Zhengzhou University School of Medicine in Henan Province, Hangzhou, Zhejiang, China | HC-27 PD-47 for array (PD-78 HC-35 for validation) | RT-qPCR TaqMan Low-Density Array (Applied BioSystems) [7900HT thermocycler (Applied Biosystems)] | miR-1, miR-103a, miR-22, miR-29, miR-30b, miR-16-2, miR-26a, miR-331-5p, miR-153, miR-374, miR-132-5p, miR-119a, miR-485-5p, miR-127-3p, miR-126, and miR-409-3p | miR-433, miR-370, let-7g-3p, miR-151, miR-28, miR-301a, miR-873-3p, miR-136-3p, miR-19b-3p, miR-10a-5p, and miR-29c | (41) | |
| Mo et al., 2017 | CSF | 1 | mirVana PARIS Kit (Ambion, PN AM1556) | Han ethnic population in Guangdong province, China (South China) | HC-42 PD -44 | RT-qPCR [ABI Prism 7500 system (Applied Biosystems, Warrington, UK)] | miR-144-5p, miR-542-3p, miR-200a-3p | (39) | ||
| Marques et al., 2017 | CSF | 0.5 | miRCURY RNA Isolation kit for biofluids (Exiqon, Vedbaek, Denmark) | Radboud University Medical Center (Nijmegen, the Netherlands) | PD-28, MSA-17 HC-28 | qPCR Thermal Cycler (Applied Biosystems, Nieuwerkerk aan den IJssel, The Netherlands)] | miR-205 | miR-24 | (40) | |
| Starhof et al., 2018 | CSF | 0.25 | Exiqon’s miRCURY RNA Isolation Kit (Exiqon A/S, Vedbaek, Denmark) | Department of Neurology, Bispebjerg Hospital, Copenhagen, Denmark | PD-10 HC-10 for profiling (PD-37 HC-23 for validation) | RT-qPCR Exiqon miRCURY PCR Panel I [Roche Lightcycler 480 (Roche Diagnostics, Indianapolis, IN)] | miR-7-5p, miR-331-5p, | miR-145-5p | (34) | |
| Dos Santos et al., 2018 | CSF Exosomes | 0.25 | miRCURY™ Exosome and RNA Isolation Kit (Exiqon, Denmark) | Nanodrop UV-VIS Spectrophotometer (Thermo Fisher Scientific, USA) and Bioanalyzer (Agilent, USA) | outpatient clinic at the Neurodegenerative Department of the University of Tübingen, Germany | Early PD-40 HC-40 | NGS | Let-7f-5p, miR-151a-3p, miR-10b-5p | miR-27a-3p and miR-423-5p, miR-125a-5p, miR-22-3p | (42) |
| Margis, Margis, & Rieder, 2011 | Blood | Movement Disorders Clinic of a university hospital in Southern Brazil | Untreated PD-08 Early-onset PD-07 HC-08 | RT-qPCR | miR-1, miR-22* and miR-29a | (43) | ||||
| Serafin et al., 2015 | Blood | TRIzol reagent (cat. no. 15596-018; Life Technologies, Monza, Italy) | Experion Automated Electrophoresis System (Bio-Rad Laboratories s.r.l., Milano, Italy) | Clinic of the General Regional Hospital of Bolzano (Italy) | 36 L-dopa–treated PD patients. 10 drug-naive PD. Unaffected controls matched 1:1 by sex and age | RT-qPCR [96CFX instrument (Bio-Rad Laboratories s.r.l.)] | miR-103a-3p, miR-30b-5p, and miR-29a-3p | (44) | ||
| Martins et al., 2011 | PBMC | miRNeasy Mini kit (Qiagen) | Lisbon University Hospital Santa Maria, Lisboa, Portugal | PD-19 HC-13 | miRCURY™ LNA Microarrays (version 10.0) [Tecan HS4800 hybridization station] | miR-15b and miR-550 | miR-126*, miR-32, and miR-101 | (45) | ||
| Pasinetti, 2012 | PBMC | Department of Neurology, The Mount Sinai School of Medicine, New York, USA | PD-13 Non-PD-10 | NGS/RT-qPCR | miR-29c, miR-424 and miR-30e5p | (47) | ||||
| Soreq et al., 2013 | PBMC (Leukocyte) | 9 | TRI-Reagent™, (Ambion) | Bioanalyzer 2100 (Agilent, USA) | Hadassah University Hospital, Jerusalem, Israel | PD (male)-76 HC-6 | NGS | miR-199b, miR-1274b, miR-21, miR-150, miR-671, miR-1249, miR-20a, miR-18b, miR-378c and miR-4293 | miR-320a, miR-320b, miR-320c, miR-769, miR-92b, miR-16 | (46) |
| Alieva et al., 2015 | PBMC | TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA). | Qubit fluorimeter (Invitrogen, Carlsbad, CA, USA). | Russians residing in the European part of Russia) were diagnosed with PD at the Research Center of Neurology. | HC-24 untreated PD -20 treated PD -18 | RT-qPCR [StepOnePlus™System (Applied Biosystems, Foster City, CA, USA)] | miR-7, miR-9-3p, miR-9-5p, miR-129, and miR-132 | (27) | ||
| Caggiu et al., 2018 | PBMC | 10 | miRNeasy Mini kit (Qiagen, USA) | Nano Drop spectrometer (Thermo Scientific, USA) | Sardinian PD patients, enrolled at the Neurology Clinic of the University Hospital of Sassari, Italy | L-dopa treated PD-37 HC-43 | Custom miScript miRNA PCR Array | miRNA-155-5p | miRNA-146a-5p | (49) |
| Yang, Li, Li, et al., 2019 | PBMC | mirVana™ miRNA Isolation Kit (Ambion, Carlsbad, CA, USA) | Hospital of Dalian Medical University, China | PD-269 HC-222 | RT-qPCR [ABI 7500 fast real-time PCR system (Applied 262 Biosystems, Foster City, CA)] | miR-132-3p | (48) | |||
| Khoo et al., 2012 | Plasma | TRI reagent RT-blood protocol (Molecular Research Center, Cincinnati, OH) | Saint Mary’s Health Care Hauenstein Parkinson’s Center (SMHCPC), Grand Rapids, MI, USA | PD-32 HC-32 | Agilent whole human genome miRNA microarray v. 3 [Agilent, Santa Clara, CA)] Validated with StepOnePlus RT-PCR system ]Applied Biosystems, Foster City, CA] | miR-222, miR-626, and miR-505 | (50) | |||
| Cardo et al., 2013 | Plasma | 0.35 | TRIzol_ LS Reagent (Ambion) | Genética Molecular-Laboratorio de Medicina Hospital Universitario Central de Asturias, Oviedo, Spain | PD-31 HC-25 | RT-qPCR TaqMan low density miRNA array [ABI 7990 HT Fast RT-PCR equipment (Applied Biosystems)] | miR-181c, miR-331-5p, miR-193a-3p, miR-196b, miR454, miR-125a-3p, and miR-137 | (52) | ||
| Sheinerman et al., 2017 | Plasma | 1 | TRIzol treatment (Life Technologies, Carlsbad, CA, USA) | University of Pennsylvania Health System, Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia, PA, USA | PD-50 HC-50 | RT-qPCR | miR-9*/ miR-129-3p, miR-99b/miR-874 and miR-9*/ miR-411 | (36) | ||
| Zhang et al., 2017 | Plasma | 0.5 | miRcute miRNA isolation Kit (Tiangen, Beijing, China) | Han Chinese individuals | PD-46 HC-49 | RT-qPCR [CFX ConnectTM Real- Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, United States)] | miR-433 and miR-133b (miR-34b and miR-153) | (51) | ||
| Y. Chen et al., 2017 | Plasma | TRI reagent BD (MRCgene, TB-126) | Department of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China | PD-169 HC-170 | Microarray miRCURY™ LNA Array (v. 18.0; Exiqon) | miR-34c-3p, miR-148b-5p, let-7i-3p, miR-4639-5p | miR-181a-5p miR-30a-5p | (16) | ||
| Li et al., 2017 | Plasma | Trizol reagent (TaKaRa, Japan) | Department of Neurology at the Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China | HC-60 PDD-24 PD-36 | RT-qPCR [FTC-3000Smart-Q fluorescence quantitative PCR SOP system (Funglyn Biotech, Canada)] | miR-137 | miR-124 | (53) | ||
| Schwienbacher & Foco, 2017 | Plasma | 0.2 | mirVanaPARIS™Kit (Ambion) | Clinic of the Bolzano Hospital (Italy). | L-dopa-treated PD- 50 HC-49 drug naïve PD-10 | RT-qPCR [96CFX instrument (Bio-Rad)] | miR-30a-5p | (54) | ||
| L. Chen et al., 2018 | Plasma | 0.2 | TRIzol reagent (Life Technologies) | Clinic of Tianjin Union Medical Center (Tianjin, China) | PD-25 HC-25 | RT-qPCR | miR-27a | let-7a, let-7f, miR-142-3p, and miR-222 | (32) | |
| Yang, Li, Li, et al., 2019 | Plasma | 0.2 | miRNA isolation system (Tiangen Biotech 242 (Beijing) Co., Ltd., Beijing, China) | Hospital of Dalian Medical University, China | PD-269 HC-222 | RT-qPCR [ABI 7500 fast real-time PCR system (Applied 262 Biosystems, Foster City, CA)] | miR-132-3p | (48) | ||
| Yao, Qu, Li, Zhang, & Rui, 2018 | Plasma Exosomes | 0.5 | Exsomal RNA and Protein Extraction kit (101 Bio, Palo Alto, CA, USA) | Cangzhou Central Hospital, Cangzhou, China | PD-52 HC-48 Other neurological disease control-176 | RT-qPCR [7900 RT- PCR machine (Applied Biosystems, Danvers, MA, USA)] | miR-331-5p | miR-505 | (35) | |
| Yang, Li, Cui, et al., 2019 | Plasma | miRNA isolation system [Tiangen Biotech (Beijing) Co., Ltd., Beijing, China] | First Affiliated Hospital of Dalian Medical University | IPD-319 HC-273 Other neurological disease control-305 | RT-qPCR [ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA, United States)] | miR-105-5p | (55) | |||
| Burgos et al., 2014 | Serum | 1 | miRVana PARIS kit (Invitrogen) | Sun Health Research Institute, Sun City, Arizona, United States of America | PD-60 HC-72 | NGS [Illumina HiSeq2000] | miR-338-3p, miR-30e-3p and miR-30a-3p | miR-16-2-3p and miR-1294 | (38) | |
| Vallelunga et al., 2014 | Serum | 0.4 | Qiagen miRNeasy mini kit (Qiagen, GmbH, Hilden, Germany), | Fluorometer and spectrophotometer. | San Camillo Hospital (Venice, Italy) and University Hospital of Padua (Padua, Italy). | PD-25 HC-25 | RT-qPCR TaqMan Low Density Array (7900HT Fast Real Time PCR System (Applied Biosystem|Life Technologies™ Monza, Italy).) | miR-24, miR-223*, miR-324-3p | miR-30c and miR-148b | (56) |
| Botta-Orfila et al., 2014 | Serum | 0.2 | miRNA-Easy Mini kit (Qiagen, Valencia, CA) | NanoDrop ND-3300 fluorospectrometer | Parkinson’s Disease and Movement Disorders Unit (Neurology Service, Hospital Clınic-IDIBAPS, Barcelona, Spain) | IPD-10 LRRK2 PD-10, HC-10 (IPD 65 and HC-65 for validation) | RT-qPCR TaqMan Low Density MicroRNA array [Viia7 1.0 Real-Time PCR system (ABI)] | miR-29a, miR-29c, miR-19a, and miR-19b | (29) | |
| Zhao, Jin, Fei, Zheng, & Zhong, 2014 | Serum | 0.4 | mirVana™ miRNA Isolation Kit (Ambion, CA) | Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China | PD-46 HC-46 | RT-qPCR [7500HT Fast RT-PCR System] | miR-133b | (61) | ||
| Fernandez - Santiago et al., 2015 | Serum | 0.2 | miRNA-easy mini kit (Qiagen, Valencia, CA) | NanoDrop ND-3300 Fluorospectrometer (Thermo Scientific, Waltham, MA) | Hospital Clınic of Barcelona, Barcelona, Spain | HC-28 PD-08 | RT-qPCR [StepOnePlus RT-PCR System (ABI)] | miR-19b, miR-29a, and miR-29c | (59) | |
| Ma et al., 2016 | Serum | Trizol Reagent (Invitrogen, Carlsbad, Calif) | Department of Neurology, Qilu Hospital of Shandong University, People’s Republic of China | PD-138 HC-112 | RT-qPCR | miR-29c, miR-146a, miR-214, and miR-221 | (62) | |||
| Dong et al., 2016 | Serum | TRIzol reagent (Invitrogen, Carlsbad, CA) | Nanjing Brain Hospital (Nanjing, China) | PD-77 HC-106 for sequencing (PD-122 HC-104 for validation) | Solexa sequencing followed by a RT-qPCR [Illumina’s Solexa Sequencer (Illumina Inc., San Diego, CA)] | miR-141, miR-214, miR-146b-5p, and miR-193a-3p | (57) | |||
| Ding et al., 2016 | Serum | 90 | Jiangsu province hospital and the Nanjing brain hospital, Nanjing, China | PD-106 HC-91 | Solexa sequencing followed by a RT-qPCR | miR-195 | miR-185, miR-15b, miR-221 and miR-181a | (58) | ||
| Bai et al., 2017 | Serum | 0.1 | miRNeasy Serum/Plasma Kit (Qiagen, Germany). | Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China. Tongde Hospital, Zhejiang Province., Shanghai, China | PD-80 HC-80 | RT-qPCR | miR-29a and miR-29c | (28) | ||
| Cao et al., 2017 | Serum exosomes | miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) | NanoDrop 1000 UV-spectrophotometer (Thermo Fisher Scientific) | Nanjing First Hospital and Huai’An First People’s Hospital, Nanjing Medical University (Nanjing, China) | PD-109 HC-40 | RT-qPCR [ABI-7500 instrument (Applied Biosystems Life Technologies, Foster City, CA, USA)] | miR-195 and miR-24 | miR-19b | (33) | |
| Jin et al., 2018 | Serum | mirVana™ miRNA Isolation Kit (Ambion, CA, USA) | Departments of neurology, Zhongshan Hospital (Shanghai) and Huashan Hospital of Fudan University (Shanghai) | HC-46 PD-46 | RT-qPCR | miR-520d-5p | (60) | |||
| Rosas-Hernandez et al., 2018 | Serum | 0.02 | miRNeasy Serum Kit | NGS | miR-19b, miR-124, miR-126a and miR-133b | (63) | ||||
| Patil et al., 2019 | Serum | Nanodrop 2000 (Thermo Scientific) | Patients and controls from the Norwegian ParkWest study, the Swedish NYPUM study and the Norwegian DemWest study | Drug naïve PD-16 HC-8 | Affymetrix GeneChip® miRNA 4.0 arrays/RT-qPCR | miR-335-5p, miR-3613-3p, and miR-6865-3p | (12) |
PD=Parkinson patients, HC=Healthy control, PDD=Parkinson disease with depression, LRRK2 PD=PD patient’s carriers of the LRRK2 G2019S mutation, PBMC=Peripheral Blood Mononuclear Cells, and mL=millilitre
The CSF directly exchanges materials with the brain parenchyma and circulates in a closed system. Hence the analysis of biomolecules in CSF might provide more specific insights into the neurodegenerative disease, such as PD. Consequently, CSF can be a promising source of biomarker to predict PD. Interestingly, in the systematic search, we found 6 studies that carried out CSF miRNA profile using either quantitative real-time PCR (RT-qPCR), microarray, or next-generation sequencing (NGS) to discriminate healthy controls from PD patients.[34,38,39,40,41,42] Among them, 4 studies used whole CSF,[34,38,39,40] and two studies used CSF derived exosomes.[41,42] The direct comparison of proposed miRNAs from each of these 6 studies revealed an overlap of 5 differentially expressed miRNAs (miR-10a-5p, miR-19b-3p, miR-136-3p, miR-331-5p, and miR-433). The miR-10a-5p and miR-433 were down regulated in at least 2 studies, whereas miR-19b-3p, miR-136-3p, and miR-331-5p were up regulated in at least 2 studies. On the other hand, the results of let-7g-3p, miR-19b-3p, miR-132-5p, miR-127-3p, miR-485-5p, and miR-409-3p expression were contrasting in at least 2 studies.
Beside CSF, the usage of easily accessible venepuncture blood and their derivatives are also suggested as a non-invasive source of biomarker due to the risk (patients may suffer from headache and nausea) and limitations (requires expertise) associated with CSF collection. In the systematic search, we found that only 2 studies assessed the differential expression of miRNAs using whole blood. The first study contained 7 early-onset PD patients treated with levodopa/carbidopa, 8 untreated PD patients, and eight healthy individuals. The data revealed a set of differentially expressed miRNAs; miR-29a-3p, miR-22-5p, and miR-1-3p that permitted discrimination of PD patients from healthy controls, as well as miR-16-2-3p, miR-26a-2-3p, and miR30a-5p which differentiated untreated and treated PD groups.[43] Unfortunately, not even a single comparable study is available to date. Another study reported a different set of up regulated miRNAs (miR-29a-3p, miR-30b-5p, and miR-103a-3p) to differentiate L-dopa-treated patients from healthy controls where they recruited Ten drug-naive PD patients, 36 L–dopa-treated PD patients, and unaffected controls matched by 1:1 age and sex. Unfortunately, these miRNAs failed to differentiate drug-naive PD from healthy controls despite using a similar detection method.[44] This variability in candidates could be due to smaller cohort size or different reference genes used for data normalization between these two studies.
Six studies analysed dysregulated miRNA expression in peripheral blood mononuclear cells (PBMCs) of PD patients and compared to healthy controls using either arrays or NGS.[27,45,46,47,48,49] The direct comparison of suggested miRNAs from each of these six studies revealed overlap of only miR-132 in at least two studies.[27,48]
Differential expression of miRNAs in PD patients’ plasma sample was investigated in eleven studies using either microarray or RT-qPCR[16,32,35,36,48,50,51,52,53,54,55] and proposed several candidate miRNAs in each study [Table 1]. To our surprise, the comparison of proposed candidate miRNAs from all 11 studies revealed only 2 miRNAs, miR-137[52,53] and miR-331-5p[35,52] overlapped in at least 2 studies. Rest of the miRNAs were unique to each study. Whereas, 13 studies analysed the expression of miRNAs in serum using either NGS or qPCR array or individual qPCR assays.[12,28,29,33,38,56,57,58,59,60,61,62,63] Fascinatingly, 6 miRNAs (miR-19b, miR-24, miR-29c, miR-133b, miR-195, and miR-214) were overlapped. MicroRNA-19b[29,33,63] and miR-29c[28,29,62] were down-regulated in at least 3 studies irrespective of study design or detection method used. MicroRNA-133b[61,63] and miR-214[57,62] were down-regulated, and miR-24[33,56] and miR-195[33,58] were up-regulated in at least 2 studies.
So far, as we can see in Table 1, several studies proposed differentially expressed circulating miRNAs as a potential diagnostic biomarker to identify PD. However, merely a fraction of human miRNome has been detected in biofluids, and their expression pattern is obscure. Of the potential candidate biofluid miRNAs, serum candidates had better overlap. The better overlap of serum candidates could attribute to a number of studies carried out with a higher sample size. However, the practical application of these candidate miRNAs for clinical diagnosis requires accurate optimization of the protocols used and their validation in large sample sets across a different population.[64,65]
Limitations and strength of selected studies
Several inconsistencies can be observed among the studies [Table 1] in several aspects, such as the source materials, the quantity of the specimen, the isolation methods, the detection methods, and technologies used. Moreover, the study population, age, and cohort size varied between studies and not all included an independent cohort for validation. Multiple studies have demonstrated that these differences influence and affect miRNA quantification in circulating biofluids.[66,67,68,69,70] Similarly, this could have substantially contributed to the poor overlap of the candidate miRNAs while comparing studies [Table 1]. Besides, many candidate miRNAs listed in Table 1 are not brain-specific, and several of them could have been derived from peripheral tissues and organs.[71] The essential foundation for recognition and interpretation of the changes in the expression pattern of miRNAs associated with the disease could be possible only if we can understand and characterize the temporal and individual variations of miRNAs in healthy individuals, which was not carried out in any studies. Further, none of the studies considered the extent of inter-individual variation in miRNA expression or their unique expression pattern in PD pathology. This is an essential and relatively unexplored area.
In an attempt to identify miRNAs most commonly overlapped between studies, the direct comparison of CSF, blood, and their derivatives unveiled a limited number of overlapping miRNAs (miR-19b, miR-29c, miR-132, miR-133b, and miR-331-5p) in at least three or more studies. MiR-19b was down-regulated in four studies[29,33,41,63] and their predicted target genes are as follows: FMR1, LRRK2, COQ2, HIP1R, ATP13A2, SYT11, RAB39B, CHCHD2, PLA6G2, EDN1, and SNCA, all of which have been previously reported to be associated with PD.[72] Additional prediction of the GO processes regulated by miR-19b and miR-24-3p were those relevant to synaptic transmission and dopamine secretion.[72] Recently, Chen et al. demonstrated that miR-19b could inhibit activation of iNOS and promote up-regulated levels of DAT, PCNA, and Bcl-2, and decreased levels of cleaved-caspase 3 and Bax in PD patients via negative regulation of p38 signalling pathways.[73] Brain specific expression of miR-29 is required for neuronal survival.[74] Several studies reported down regulation of circulating levels of miR-29c and its other family members miR-29a/b[28,29,41,62] in PD patients, and they tend to reduce with disease severity. Candidate targets of the miR-29 family of miRNAs include oxidative stress sensor PARK7 (DJ-1), Parkin substrate GPR37, targets related to apoptotic processes Puma, Bim, Bak, Bcl2, IGF1 and AKT1, microglial phagocytosis-related CDC42, and the epigenetic molecules DNMT3A, DNMT3B and HDAC4.[28] This suggests that decreased miR-29 family members lead to alterations in the homeostasis of oxidative stress, neuronal survival and protection leading neuronal apoptosis, more specifically dopaminergic neurons. Also, miR-29 expression is also downregulated in idiopathic rapid eye movement behaviour disorder after they were diagnosed with PD and dementia with Lewy bodies.[59] Four studies have shown overexpression of miR-132 and their increased transcription shown to activate Rac1-Pak actin-remodeling pathway, which could play a possible role in protein accumulation.[75] In addition, microRNA expression profiling of early symptomatic α-synuclein (A30P)-transgenic mice revealed significantly alteration in several miRNAs (miR-10a, -10b, -212, -132, -495).[76] One of them miR-132 is highly inducible by growth factors and to be a key regulator of neuronal dendritic spine formation.[75,76] Further, miR-133b is found enriched explicitly in midbrain dopaminergic neurons of healthy individuals and decreased in PD patients.[77] Correspondingly, here three reported studies have shown underexpression of miR-133b in the circulation. The physiological target of miR-133b is paired-like homeodomain transcription factor (Pitx 3), a transcription factor that plays a key role in dopaminergic neuron differentiation.[78] Interestingly, Pitx 3 promotes transcription of miR-133b,[77] which in turn decreases Pitx 3 expression in a negative-feedback loop mechanism. Therefore, miR-133b could regulate the maturation and function of midbrain dopaminergic neurons by a negative feedback mechanism. MiR-34b and miR-34c have been previously shown to be down-regulated in the brains (frontal cortex, cerebellum, and amygdala as well as substantia nigra) of patients with PD and they repress the expression of α-synuclein, a key protein in PD pathogenesis.[79] When expression of miR-34b and miR-34c decreased, dopaminergic SH-SY5Y cells increased α-synuclein levels and stimulated aggregate formation.[79] Also, brain specific miR-7 and miR-433 regulate the SNCA gene expression in healthy and PD brains of both human and animal models.[20,76]
MiR-34b and c were also suggested to indirectly reduce the expressions of both Parkin and Parkinson protein 7 (DJ1), as well as increase the rate of cell death whereas miR-133b expression was not found to be altered in any of the areas in which miR-34b and c downregulation was observed.[80] MiR-331-5p was shown to overexpress in at least 4 studies,[34,35,41,52] and they were implicated in neuroprotection in the ischemic cortex.[81] MiR-331-3p gene target neuropilin 2 (NRP2) is shown to promote the cell growth and proliferation of glioblastoma.[82] However, its exact role in the PD is unknown. This suggests that changes in the expression of these candidate miRNAs might reflect the pathogenesis of or the pathological changes in PD, even though currently their exact mechanism is elusive. Therefore, miRNA may thus represent novel biomarkers for neuronal malfunction and potential therapeutic targets for human neurodegenerative diseases.
In contrast, many miRNAs’ (let-7g-3p, miR-19b-3p, miR-132-5p, miR-127-3p, miR-485-5p, and miR-409-3p) differential expression is contradicted within the same biofluid source. The differences in the candidate miRNA expression could be explained by differences in study design, the source of miRNA, sample size, clinical features of patients, and possible differences in the pharmacological treatment or the drug dose, including reference genes used for data analysis. Otherwise, it can be explained to an extent based on their origin. For example, the level of miRNAs expressed, and their pattern in serum and plasma might not be the same. Because while blood clotting, activated platelets may contribute a substantial proportion of miRNAs to serum compared to plasma. Therefore, the duration of clotting might also affect the levels of serum miRNAs. When it comes to the organ specificity of differentially expressed miRNA, especially brain-specific one could have decreased due to dilution (while crossing the blood-brain barrier) or taken up by peripheral tissues/organs. Additionally, the chronic inflammation and other non-specific causes could have also influenced the differentially expressed circulating miRNA.[83] Therefore, the precise selection and comparison of a specific fraction of the circulating body fluid, cohort size, the isolation method, detection technology, and adequate inter-study or multicentre data is of paramount importance and prerequisite for the reproducibility and inter-study comparison. Similarly, it is of vital importance to accept the best practices and strictly adhere to the uniform protocol and statistical apparatus to have comparable measurements. The development of suitable statistics to compare results of different study design and techniques could be an alternative to overcome the current limitations to an extent. Further, the addition of other atypical Parkinsonian disorders would yield more detailed insight into the association of specific miRNAs with PD.
Despite dissimilarities in several aspects, direct comparison of all the studies in Table 1 displayed overlap of miR-19b, miR-29c, miR-132, miR-133b, and miR-331-5p in multiple studies. Even in different biofluid sources and Table 2 highlights the outstanding potential of some of the candidate miRNAs as a putative biomarker to assist in PD diagnosis with high discrimination power. However, currently, it is challenging to use them in the clinical diagnosis due to limited knowledge in the miRNA expression pattern with respect to age, race, disease severity, or medical therapies. Therefore, different population-based studies are needed to confirm the variations based on age, ethnic origin, disease stage, and the effects of treatment. Additionally, miRNA expression is also regulated at various levels—transcription, Drosha, and Dicer processing, transfer of pre-miRNAs from the nucleus to the cytoplasm, RNA editing and loading into the RISC complex.[84,85,86] Contrastingly, relatively little is known about the regulation of miRNA genes themselves and their pattern of dysregulation in PD pathology. Therefore, understanding the elements modulating the miRNA expression, stability, and decay in the physiological and pathological conditions required along with overcoming the limitations of the current profiling approaches could pave new avenues for the practical application of miRNAs as a biomarker.
Table 2.
Potential candidate miRNAs to assist PD diagnosis with high sensitivity and specificity
| Author and the year of the study | Differentially expressed miRNA | Discriminatory accuracy ( AUC ) | Ref |
|---|---|---|---|
| Pasinetti, 2012 | miR-29c, miR-424 and miR-30e5p | 0.892, 0.927 and 0.762 | (47) |
| Ma et al., 2016 | miR-221 | 0.787 | (62) |
| Ding et al., 2016 | miR-195, miR-15b, miR-221, miR-181a, and miR-185 | 0.733, 0.897, 0.854, 0.822 and 0.820 respectively | (58) |
| Cao et al., 2017 | miR-19b, miR-24 and miR-195 | 0.753, 0.908, and 0.697 respectively | (33) |
| Li et al., 2017 | miR-137/miR-124 | 0.707 and 0.709 respectively | (53) |
| Mo et al., 2017 | miR-144-5p, miR-200a-3p, and miR-542-3p | 0.73, 0.75, and 0.87 respectively | (39) |
| Sheinerman et al., 2017 | miR-9*/miR-129-3p; miR-99b/miR-874 and miR-9*/miR-411 | 0.91, 0.81 and 0.81 respectively | (36) |
| Chen et al., 2018 | miR-27a, let-7a, let-7f, miR-142-3p, and miR-222 | 0.8 | (32) |
| Starhof et al., 2018 | miR-7-5p, miR-331-5p, and miR-145-5p | 0.88 | (34) |
| Yao, Qu, Li, Zhang, & Rui, 2018 | miR-331-5p and miR-505 | 0.849 and 0.898 respectively | (35) |
| Patil et al., 2019 | (miR-335-5p/miR-3613-3p), (miR-335-5p/miR-6865-3p), and (miR-335-5p/miR-3613-3p/miR-6865-3p) combination | 0.9-1.0 | (12) |
| Yang et al., 2019 | miR-105-5p | 0.7 | (55) |
AUC=Area under the receiver operating characteristic curve
FACTORS AFFECTING DIFFERENTIAL MIRNA EXPRESSION
Patient cohort
Clinically defined Parkinsonian syndrome, its phenotypic presentation, dopaminergic therapy, and duration of illness are essential factors that are imperative for the identification of unique expression pattern. Most of the studies done so far have not used uniform criteria for recruitment, and also there is variability in the factors as mentioned earlier.
MicroRNA expression profile
The development of miRNA-based biomarker essentially requires the precise determination of its expression in a given specimen. Many properties unique to miRNAs – such as its short length, lack of common sequence and its variability in the source fluids – could influence the miRNA profiling and data analysis.[87] Numerous high-throughput miRNA profiling techniques (RT-qPCR, microarray, next-generation sequencing (NGS), and nanopores) have been developed to detect and quantify small RNAs. All of them come with different limitations.[87] At this time, qRT-PCR is the most common, reliable, inexpensive method with high sensitivity and specificity available to quantify small amount of miRNAs in biofluids and the subsequent validation in clinical samples.[88,89]
Though Table 1 demonstrates a considerable number of miRNA profiling studies to differentiate early Parkinson's disease from healthy individuals, the screening of PD using them is still nascent.[37] Exploiting the informative potential of miRNA profile requires thorough understanding and optimization of procedures and methods used for miRNA extraction, detection and their limitations.[66,67,68,69,70]
General concepts and challenges in miRNA isolation and quality control
MicroRNAs are stable in a variety of specimen types (circulating biofluids, cells in culture, and fresh or fixed tissue) and can be extracted easily in all of them. MicroRNAs can be extracted either directly from the biofluid or separate microvesicles first and then extract miRNA to achieve an enrichment. The amount, type of input material and method of choice influence the recovery of miRNA during the extraction procedure. The issue is that multiple extraction techniques exist for extracellular miRNA isolation. Most of the RNA extraction approaches include a lysis step followed by precipitation, phase separation, and RNA elution. The RNA yield, quantity and size profile vary depending on the isolation method due to differences in lysis and recovery capacities of that particular method.[68,69,70,90] A comparison of the vesicular RNA profiles resulting from different isolation methods showed marked differences in miRNA expression,[91] which is likely due to distinctive RNA profiles in the respective vesicle subpopulations.[90] These studies imply practicing standard procedures and methods across laboratories for miRNA extraction.
Several efforts have been made to understand the effects of commonly used isolation methods on small RNA profiling and their optimization for miRNA.[92,93,94,95,96] Most commonly used commercially available miRNA extraction kits such as miRNeasy® kit and miRCURY™ biofluid kit, typically use a chemical extraction combined with a purification step involving binding and eluting from an adsorption column. miRCURY™ biofluid kit can efficiently extract miRNAs from circulating biofluids. However, the column of this kit poorly recovers miRNAs from a small quantity of biofluids or cells. Besides, miRCURY™ column is rapidly saturated by large RNA species and biofluid components, thus impeding the recovery of miRNAs from high specimen input, whereas miRNeasy® kit permits better miRNA detection despite less pure extracted RNA. The Trizol (phenol) based RNA isolation yield a very low RNA purity from biofluids, which affects miRNA quantification efficiency. Therefore a careful selection of the isolation method and consideration of the amount and type of input material is highly recommended to avoid biased results.
The reliable profiling of miRNA expression is not possible in the degraded RNA sample or the sample contaminated with protein, genomic DNA, nucleases, and enzymatic inhibitor. Therefore, care and precaution need to be taken to get excellent miRNA quality and quantity. The loss of RNA integrity or contamination increases the unpredictability of array and qPCR based miRNA expression profiles. Currently available ways to test the quality and quantity of RNA is by assessing them in spectrophotometry and automated capillary electrophoresis with the Bioanalyzer or Experion. The specimens of CSF, serum, plasma and urine usually have too low total RNA yields. Therefore, the recovery of synthetic spiked-in oligos and quantification of suitable housekeeping miRNAs could be a useful surrogate to quantify miRNA expression accurately. Even after getting good quality and quantity of miRNAs from the extraction procedure, the chances of variability could arise due to the detection method and technology used.
Challenges in miRNA profiling
Ideally, the selected method should be rapid, highly sensitive, and allow an unbiased analysis of the target; even with minimal input material. Thus far, RT-qPCR generally has the most extensive dynamic range (detects as low as 10 ng of RNA) with the highest accuracy. This is the only method that has the potential for absolute quantification by generating standard curves using a known concentration of synthetic oligos.[32,97] However, some limitations of qRT-PCR, such as primers and housekeeping genes used for data normalization, profoundly influence the results. Several miRNAs (miR-16, miR-30b, miR-93, miR-145, miR-142-3p, U6, U6B, RNU43, RNU48, RNU62, SNORD68, and 5sRNA) are put forward as housekeeping RNA to normalize qPCR data. However, there is no single housekeeping RNA that can be used as the standard endogenous reference gene in normalizing the expression of circulating miRNAs, and is a critical issue.[89]
Furthermore, all miRNAs present in the circulation cannot be consistently quantified with a cycle threshold value of <35, even after pre-amplification. Also, it can detect only known miRNAs. Profiling of hundreds of miRNAs in parallel is a major challenge due to the presence of significant miRNA-specific biases such as wide variance in their GC content and melting temperature. This is of major concern because miRNAs represent a small fraction of the total RNA mass, and therefore must be selectively detected in a background of diverse RNA species, including miRNA precursors that also contain the nucleotide sequence of the mature miRNA species. Clearly, like any other technologies, there are limitations for RT-qPCR based detection of miRNAs as a potential diagnostic biomarker.
If we take microarrays, they are inexpensive but have the lowest dynamic range and sensitivity. Therefore, it is best used as a discovery tool. When microarrays are used for initial screening, many tissue-enriched miRNAs were not detectable. As a consequence of this, the ubiquitous miRNA, including those associated with common pathologic processes such as carcinogenesis and inflammation were often selected as potential biomarkers for PD. These ubiquitous miRNA can effectively differentiate PD patients from healthy controls, but not necessarily from patients with similar pathology such as atypical Parkinsonism since there is a high possibility that the circulating levels of these miRNA will also be affected in such patients.
Small RNA sequencing is a relatively new technique continuously improved for the miRNA sequencing, which allows the unbiased analysis of all the detectable small RNA's at once in a given specimen without target pre-selection. Even so, the small RNA sequencing and miRNA microarrays are not sufficiently sensitive to detect a miRNA whose concentration in body fluids is relatively low,[98] and promising observations are required to validate candidate miRNAs by alternate methods like RT-qPCR. Newer technologies such as single-molecule real-time sequencing that does not require PCR-based amplification is becoming more established,[99] and in future absolute quantification by sequencing may become possible.
PROSPECTIVE OF MIRNAS AS A PD BIOMARKER
The asymptomatic early phase in PD is associated with specific changes in miRNA expression.[100] Therefore, many studies [Table 1] tried to establish miRNA signatures specific to PD over a decade. The major weakness of comparing these studies is that unaccountability of the consistently increasing known and predicted human miRNAs over a period (www.mirbase.org). Most of the earlier studies contain only a part of them. Thus, these expression profiles may not reflect the correct and complete miRNA signature in this context. Therefore, to compare the data between studies, re-performing of expression profile in the same samples is warranted. Though the miRNA detection technology is quickly evolving, there is a lack of consensus among scientists in using an optimal approach to analyse large-scale miRNA profile. Also, lack of databases providing information regarding temporal and inter-individual miRNA expression variations are limiting the identification of miRNA pattern. There are significant hurdles in understanding the sample-to-sample biological variability that is not related to the disease condition of interest. If we overcome these barriers, the richness of information associated with miRNA profiles could partake eventual clinical translation.
To design and evaluate more effective diagnostic and therapeutic interventions based on miRNA ultimately requires appropriate interpretation of differentially expressed miRNAs and their related family members that underpin the PD development and progression. A signature pattern of a family of miRNA can considerably strengthen their diagnostic value over single candidate miRNA. The future investigations should also focus on normal variations of miRNAs associated with PD and related disorders within and between individuals, over time with age, gender, and other aspects of the disease condition. This might give fascinating results to interpret the levels of individual or family of miRNAs significantly varied between individuals without any pathological significance or discern donor-specific variations. Besides, this could help us to define and build a database to understand the human individuality and their association with the disease.
Though there is much to link the differential expression of miRNAs to various diseases, the clinical utility and their validity have not been demonstrated due to its heterogeneity, mutagenic regulation, and miRNA specific limitations [Table 3]. The identification of miRNA signatures involved in the regulation and progression of a particular disease is a methodological and technological challenge. Currently, miRNAs are manually extracted and processed for profiling, presenting the biggest obstacle to increase throughput. If a new technological platform provides an opportunity for faster miRNA extraction or direct analysis without an extraction step is established, that could significantly improve usability of miRNAs in clinical settings. Further, the technological and methodological advances in multi-omics data generation, integration, and interpretation with respect to PD will benefit the diagnostic value of miRNAs. We speculate using network inference approaches by integrating all the levels of data from transcriptomics to miRNomics to proteomics as well as radiological imaging approaches can provide an opportunity for early detection of PD and development of alternative therapeutic approaches. Recently, the Nanopore-based methods for miRNA measurement are also being developed, if perfected, that could enable simple, rapid, and highly sensitive miRNA profiling assays[101] and pave the way for broadly available clinical tests based on miRNA.
Table 3.
Advantages and challenges in using miRNAs as diagnostic marker
| Advantages | Challenges |
|---|---|
| MicroRNAs present in various source materials (i.e., whole blood, plasma, serum, blood cells and tissues) and that can be easily isolated and quantified | Liquid biopsies present special difficulties compared with tissue sampling, as miRNA levels are very sensitive to pre-processing and post-processing factors |
| miRNAs are highly stable in the circulation, minimal or no differences have been found between fresh and frozen specimens, even after repeated freeze-thaw cycles | Specific standard operating procedures (SOPs) for blood collection and plasma/serum preparation are not followed |
| No standard endogenous control for the normalization of miRNA levels in blood has been established Tissue-specific housekeeping miRNAs are not suitable and global normalization approach could not be appropriate for normalization of miRNA profiling data because it assumes that the same total amount of miRNAs is expected in all samples and that only a small percentage of miRNAs is differentially expressed, as both up- and downregulated | |
| Several methods have been developed to quantify circulating miRNAs: qRT-PCR, droplet digital PCR, quantitative stem-loop RT-PCR) and chip-based digital PCR, as well as RNAseq and microarrays | |
| Digital PCR (dPCR) provides a quasi-absolute readout or copy number for miRNAs and eliminates the need for standard curves as well as the influence of normalization strategies (at least for assays that have been thoroughly validated) | |
| The availability of powerful approaches for global miRNA characterization and simple, universally applicable assays for quantitation (e.g., qRT-PCR) suggests that the discovery–validation pipeline for miRNA biomarkers will be more efficient than traditional proteomic biomarker discovery– validation pipelines, which typically encounter bottlenecks at the point of antibody generation and quantitative assay development for validation of biomarker candidates | Expression pattern of miRNAs between different liquid biopsies (e.g., platelet-rich plasma, platelet-poor plasma, serum and whole blood) vary due to difference in their method of separation or composition of blood cells in them |
| Potential differences in sample/patient number, sampling time, methods for miRNA isolation, quantification, miRNA normalization parameters and co-morbidities could attribute miRNA expression and profiling | |
| Significant number of miRNAs were identified with the potential for becoming targets in order to understand more about disease pathology | Most miRNAs are expressed widely in a non-cell/tissue-specific manner, and they do not differ drastically in level between cases and controls |
| Many miRNAs proposed as biomarkers for one disease have been found in association with a bewildering variety of other conditions | |
| Compared to DNA or RNA-based tests that indicate the presence of a mutation (s), miRNA tests produce results that are difficult to interpret | |
| At present, the effect of controllable (gender, age, drug assumption etc.,) and uncontrollable (individual genetics, diet, life style etc.,) pre-analytical factor on the miRNA profiling is not established completely | |
| Very low percentage of hemolysis can elicit a considerable increase in erythrocyte-specific miRNA levels. Therefore, miRNA-based methods could turn out to be not sufficiently accurate to discriminate hemolyzed samples from samples presenting altered erythrocyte-contained miRNA expression due to other conditions | |
| No consistent results have been obtained regarding differences in column-based miRNA isolation methods, suggesting that a great effort is still needed in comparing different extraction methods and working toward standardization |
Despite the encouraging progress in the functional areas, there remain enigmas on several theoretical and technical difficulties on miRNA [Table 3]. Firstly, more investigations are required to discern the functional role of miRNAs in PD pathology. Although miRNAs can inhibit messenger RNA translation, other possible unknown mechanisms could also be influencing translational or post-translational repression. Secondly, the biological functions of extracellular miRNAs need to be better elucidated. Finally, before translating miRNAs’ diagnostic capability, it requires additional steps of validation of all the findings on circulating miRNAs and accurate standardization of every procedures and method used, to avoid all potential technical biases.
SUMMARY
The analysis of circulating miRNA as a biomarker for the diagnosis of PD and related disorder is a nascent field with many advantages and limitations in its use tabulated in Table 3, yet rapidly evolving. In this review, Table 1 highlighted several putative candidate miRNAs as a diagnostic biomarker and judged by their advantageous properties and the continuously increasing amount of studies circulating miRNAs hold great promise as a diagnostic biomarker for PD. However, as we have discussed in this review, before translation into clinical practice, all circulating miRNA findings require further steps of validation and a proper standardization of all pre-analytical and analytical procedures and methods, in order to control all potential technical biases. These reports helps to understand the extent and nature of the variability in miRNA expression in circulating body fluids and provides insight into the factors that contribute to this variation and direction for future studies to focus on the factors affecting the miRNA biogenesis, its stability and the mechanism of their decay in physiological and pathological conditions as well as intra and inter-individual variations in the miRNA expression that could help to determine PD-related miRNA signature and their prognostic value.
In conclusion, in order to develop clinical diagnostic biomarker based on circulating miRNA, it requires stringent protocol optimization of all the methodological aspects, broad acceptance of suitable source material and re-confirmation of carefully chosen PD cases in multiple centers to ensure diagnostic accuracy. Moreover, along with PD inclusion of atypical Parkinsonian disorders could get us detailed insight into the association of PD-specific miRNA signature.
Financial support and sponsorship
This work supported by the Indian Council of Medical Research [Grant numbers BMS/TF/Trans-Neuro/2014-3368/July-15/20/KA/Govt].
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 2.Dexter DT, Jenner P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic Biol Med. 2013;62:132–44. doi: 10.1016/j.freeradbiomed.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Malek N, Swallow D, Grosset KA, Anichtchik O, Spillantini M, Grosset DG. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson's disease-A systematic review. Acta Neurol Scand. 2014;130:59–72. doi: 10.1111/ane.12247. [DOI] [PubMed] [Google Scholar]
- 4.Chahine LM, Stern MB. Diagnostic markers for Parkinson's disease. Curr Opin Neurol. 2011;24:309–17. doi: 10.1097/WCO.0b013e3283461723. [DOI] [PubMed] [Google Scholar]
- 5.Poewe W, Wenning G. The differential diagnosis of Parkinson's disease. Eur J Neurol. 2002;9(Suppl 3):23–30. doi: 10.1046/j.1468-1331.9.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 6.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30:1591–601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 7.Espay AJ, Vizcarra JA. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology. 2019;92:329–37. doi: 10.1212/WNL.0000000000006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim R, Kim HJ, Kim A, Jang M, Kim A, Kim Y, et al. Peripheral blood inflammatory markers in early Parkinson's disease. J Clin Neurosc. 2018;58:30–3. doi: 10.1016/j.jocn.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 9.Chang KH, Cheng ML, Tang HY, Huang CY, Wu YR, Chen CM. Alternations of metabolic profile and kynurenine metabolism in the plasma of Parkinson's disease. Mol Neurobiol. 2018;55:6319–28. doi: 10.1007/s12035-017-0845-3. [DOI] [PubMed] [Google Scholar]
- 10.Atik A, Stewart T, Zhang J. Alpha-Synuclein as a biomarker for Parkinson's disease. Brain Pathol. 2016;26:410–8. doi: 10.1111/bpa.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88:930–7. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patil KS, Basak I, Dalen I, Hoedt E, Lange J, Lunde KA, et al. Combinatory microRNA serum signatures as classifiers of Parkinson's disease. Parkinsonism Relat Disord. 2019;64:202–10. doi: 10.1016/j.parkreldis.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Zeng Y. Regulation of mammalian microRNA expression. J Cardiovasc Transl Res. 2010;3:197–203. doi: 10.1007/s12265-010-9166-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhu S, Pan W, Qian Y. MicroRNA in immunity and autoimmunity. J Mol Med. 2013;91:1039–50. doi: 10.1007/s00109-013-1043-z. [DOI] [PubMed] [Google Scholar]
- 15.Zibert JR, Løvendorf MB, Litman T, Olsen J, Kaczkowski B, Skov L. MicroRNAs and potential target interactions in psoriasis. J Dermatol Sci. 2010;58:177–85. doi: 10.1016/j.jdermsci.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Gao C, Sun Q, Pan H, Huang P, Ding J, et al. MicroRNA-4639 Is a regulator of DJ-1 expression and a potential early diagnostic marker for Parkinson's disease. Front Aging Neurosci. 2017;9:232. doi: 10.3389/fnagi.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Sun Y, Chen J. Identification of critical genes and miRNAs associated with the development of Parkinson's disease. J Mol Neurosci. 2018;65:527–35. doi: 10.1007/s12031-018-1129-8. [DOI] [PubMed] [Google Scholar]
- 18.Gao JX, Li Y, Wang SN, Chen XC, Lin LL, Zhang H. Overexpression of microRNA-183 promotes apoptosis of substantia nigra neurons via the inhibition of OSMR in a mouse model of Parkinson's disease. Int J Mol Med. 2019;43:209–20. doi: 10.3892/ijmm.2018.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, et al. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82:283–9. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106:13052–7. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Xu Z, Wang K, Wang N, Zhu M. Network analysis of microRNAs, genes and their regulation in human bladder cancer. Biomed Rep. 2013;1:918–24. doi: 10.3892/br.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Guire V, Robitaille R, Tetreault N, Guerin R, Menard C, Bambace N, et al. Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: Promises and challenges. Clin Biochem. 2013;46:846–60. doi: 10.1016/j.clinbiochem.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Van Roosbroeck K, Pollet J, Calin GA. miRNAs and long noncoding RNAs as biomarkers in human diseases. Expert Rev Mol Diagn. 2013;13:183–204. doi: 10.1586/erm.12.134. [DOI] [PubMed] [Google Scholar]
- 24.Lai CY, Lee SY, Scarr E, Yu YH, Lin YT, Liu CM, et al. Aberrant expression of microRNAs as biomarker for schizophrenia: From acute state to partial remission, and from peripheral blood to cortical tissue. Transl Psychiatry. 2016;6:e717. doi: 10.1038/tp.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren RJ, Zhang YF, Dammer EB, Zhou Y, Wang LL, Liu XH, et al. Peripheral blood MicroRNA expression profiles in Alzheimer's disease: Screening, validation, association with clinical phenotype and implications for molecular mechanism. Mol Neurobiol. 2016;53:5772–81. doi: 10.1007/s12035-015-9484-8. [DOI] [PubMed] [Google Scholar]
- 26.Vistbakka J, Elovaara I, Lehtimaki T, Hagman S. Circulating microRNAs as biomarkers in progressive multiple sclerosis. Mult Scler. 2017;23:403–12. doi: 10.1177/1352458516651141. [DOI] [PubMed] [Google Scholar]
- 27.Alieva A, Filatova EV, Karabanov AV, Illarioshkin SN, Limborska SA, Shadrina MI, et al. miRNA expression is highly sensitive to a drug therapy in Parkinson's disease. Parkinsonism Relat Disord. 2015;21:72–4. doi: 10.1016/j.parkreldis.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Bai X, Tang Y, Yu M, Wu L, Liu F, Ni J, et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson's disease. Sci Rep. 2017;7:5411. doi: 10.1038/s41598-017-03887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botta-Orfila T, Morato X, Compta Y, Lozano JJ, Falgas N, Valldeoriola F, et al. Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson's disease. J Neurosci Res. 2014;92:1071–7. doi: 10.1002/jnr.23377. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson's disease. Mol Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasso M, Piscopo P, Confaloni A, Denti MA. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules (Basel, Switzerland) 2014;19:6891–910. doi: 10.3390/molecules19056891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Yang J, Lu J, Cao S, Zhao Q, Yu Z. Identification of aberrant circulating miRNAs in Parkinson's disease plasma samples. Brain Behav. 2018;8:e00941. doi: 10.1002/brb3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS, et al. MicroRNA biomarkers of Parkinson's disease in serum exosome-like microvesicles. Neurosci Lett. 2017;644:94–9. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 34.Starhof C, Hejl AM, Heegaard NHH, Carlsen AL, Burton M, Lilje B, et al. The biomarker potential of cell-free microRNA from cerebrospinal fluid in Parkinsonian syndromes. Mov Disord. 2018 doi: 10.1002/mds.27542. Epub 2018/12/18. doi: 10.1002/mds. 27542. PubMed PMID: 30557454. [DOI] [PubMed] [Google Scholar]
- 35.Yao YF, Qu MW, Li GC, Zhang FB, Rui HC. Circulating exosomal miRNAs as diagnostic biomarkers in Parkinson's disease. Eur Rev Med Pharmacol Sci. 2018;22:5278–83. doi: 10.26355/eurrev_201808_15727. [DOI] [PubMed] [Google Scholar]
- 36.Sheinerman KS, Toledo JB, Tsivinsky VG, Irwin D, Grossman M, Weintraub D, et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimers Res Ther. 2017;9:89. doi: 10.1186/s13195-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramaswamy P, Christopher R, Pal PK, Yadav R. MicroRNAs to differentiate Parkinsonian disorders: Advances in biomarkers and therapeutics. J Neurol Sci. 2018;394:26–37. doi: 10.1016/j.jns.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS One. 2014;9:e94839. doi: 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mo M, Xiao Y, Huang S, Cen L, Chen X, Zhang L, et al. MicroRNA expressing profiles in A53T mutant alpha-synuclein transgenic mice and Parkinsonian. Oncotarget. 2017;8:15–28. doi: 10.18632/oncotarget.13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques TM, Kuiperij HB, Bruinsma IB, van Rumund A, Aerts MB, Esselink RAJ, et al. MicroRNAs in cerebrospinal fluid as potential biomarkers for Parkinson's disease and multiple system atrophy. Mol Neurobiol. 2017;54:7736–45. doi: 10.1007/s12035-016-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6:37043–53. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dos Santos MCT, Barreto-Sanz MA, Correia BRS, Bell R, Widnall C, Perez LT, et al. miRNA-based signatures in cerebrospinal fluid as potential diagnostic tools for early stage Parkinson's disease. Oncotarget. 2018;9:17455–65. doi: 10.18632/oncotarget.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margis R, Margis R, Rieder CR. Identification of blood microRNAs associated to Parkinsonis disease. J Biotechnol. 2011;152:96–101. doi: 10.1016/j.jbiotec.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Serafin A, Foco L, Zanigni S, Blankenburg H, Picard A, Zanon A, et al. Overexpression of blood microRNAs 103a, 30b, and 29a in L-dopa-treated patients with PD. Neurology. 2015;84:645–53. doi: 10.1212/WNL.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 45.Martins M, Rosa A, Guedes LC, Fonseca BV, Gotovac K, Violante S, et al. Convergence of miRNA expression profiling, alpha-synuclein interacton and GWAS in Parkinson's disease. PLoS One. 2011;6:e25443. doi: 10.1371/journal.pone.0025443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soreq L, Salomonis N, Bronstein M, Greenberg DS, Israel Z, Bergman H, et al. Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Front Mol Neurosci. 2013;6:10. doi: 10.3389/fnmol.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasinetti GM. Role of personalized medicine in the identification and characterization of Parkinson's disease in asymptomatic subjects. J Alzheimers Dis Parkinsonism. 2012;2 doi: 10.4172/2161-0460.1000e118. Epub 2012/10/02. doi: 10.4172/2161-0460.1000e118. PubMed PMID: 23024925; PubMed Central PMCID: PMCPmc3459238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z, Li T, Li S, Wei M, Qi H, Shen B. Altered expression levels of MicroRNA-132 and Nurr1 in peripheral blood of Parkinson's disease: Potential Disease Biomarkers. ACS Chem Neurosci. 2019;10:2243–9. doi: 10.1021/acschemneuro.8b00460. [DOI] [PubMed] [Google Scholar]
- 49.Caggiu E, Paulus K, Mameli G, Arru G, Sechi GP, Sechi LA. Differential expression of miRNA 155 and miRNA 146a in Parkinson's disease patients. eNeurologicalSci. 2018;13:1–4. doi: 10.1016/j.ensci.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khoo SK, Petillo D, Kang UJ, Resau JH, Berryhill B, Linder J, et al. Plasma-based circulating MicroRNA biomarkers for Parkinson's disease. J Parkinsons Dis. 2012;2:321–31. doi: 10.3233/JPD-012144. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Yang R, Hu BL, Lu P, Zhou LL, He ZY, et al. Reduced circulating levels of miR-433 and miR-133b are potential biomarkers for Parkinson's disease. Front Cell Neurosci. 2017;11:170. doi: 10.3389/fncel.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardo LF, Coto E, de Mena L, Ribacoba R, Moris G, Menendez M, et al. Profile of microRNAs in the plasma of Parkinson's disease patients and healthy controls. J Neurol. 2013;260:1420–2. doi: 10.1007/s00415-013-6900-8. [DOI] [PubMed] [Google Scholar]
- 53.Li N, Pan X, Zhang J, Ma A, Yang S, Ma J, et al. Plasma levels of miR-137 and miR-124 are associated with Parkinson's disease but not with Parkinson's disease with depression. Neurol Sci. 2017;38:761–7. doi: 10.1007/s10072-017-2841-9. [DOI] [PubMed] [Google Scholar]
- 54.Schwienbacher C, Foco L. Plasma and white blood cells show different miRNA expression profiles in Parkinson's disease. J Mol Neurosci. 2017;62:244–54. doi: 10.1007/s12031-017-0926-9. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Li T, Cui Y, Li S, Cheng C, Shen B, et al. Elevated plasma microRNA-105-5p level in patients with idiopathic Parkinson's disease: A potential disease biomarker. Front Neurosci. 2019;13:218. doi: 10.3389/fnins.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallelunga A, Ragusa M, Di Mauro S, Iannitti T, Pilleri M, Biundo R, et al. Identification of circulating microRNAs for the differential diagnosis of Parkinson's disease and Multiple System Atrophy. Front Cell Neurosci. 2014;8:156. doi: 10.3389/fncel.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong H, Wang C, Lu S, Yu C, Huang L, Feng W, et al. A panel of four decreased serum microRNAs as a novel biomarker for early Parkinson's disease. Biomarkers. 2016;21:129–37. doi: 10.3109/1354750X.2015.1118544. [DOI] [PubMed] [Google Scholar]
- 58.Ding H, Huang Z, Chen M, Wang C, Chen X, Chen J, et al. Identification of a panel of five serum miRNAs as a biomarker for Parkinson's disease. Parkinsonism Relat Disord. 2016;22:68–73. doi: 10.1016/j.parkreldis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Santiago R, Iranzo A, Gaig C, Serradell M, Fernandez M, Tolosa E, et al. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann Neurol. 2015;77:895–901. doi: 10.1002/ana.24384. [DOI] [PubMed] [Google Scholar]
- 60.Jin L, Wan W, Wang L, Wang C, Xiao J, Zhang F, et al. Elevated microRNA-520d-5p in the serum of patients with Parkinson's disease, possibly through regulation of cereloplasmin expression. J Mol Neurosci. 2018;687:88–93. doi: 10.1016/j.neulet.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 61.Zhao N, Jin L, Fei G, Zheng Z, Zhong C. Serum microRNA-133b is associated with low ceruloplasmin levels in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:1177–80. doi: 10.1016/j.parkreldis.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 62.Ma W, Li Y, Wang C, Xu F, Wang M, Liu Y. Serum miR-221 serves as a biomarker for Parkinson's disease. Cell Biochem Funct. 2016;34:511–5. doi: 10.1002/cbf.3224. [DOI] [PubMed] [Google Scholar]
- 63.Rosas-Hernandez H, Chigurupati S, Raymick J, Robinson B, Cuevas E, Hanig J, et al. Identification of altered microRNAs in serum of a mouse model of Parkinson's disease. Neurosci Lett. 2018;687:1–9. doi: 10.1016/j.neulet.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 64.Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med. 2014;18:371–90. doi: 10.1111/jcmm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witwer KW. Circulating microRNA biomarker studies: Pitfalls and potential solutions. Clin Chem. 2015;61:56–63. doi: 10.1373/clinchem.2014.221341. [DOI] [PubMed] [Google Scholar]
- 66.El-Khoury V, Pierson S, Kaoma T, Bernardin F, Berchem G. Assessing cellular and circulating miRNA recovery: The impact of the RNA isolation method and the quantity of input material. Sci Rep. 2016;6:19529. doi: 10.1038/srep19529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang WX, Wilfred BR, Baldwin DA, Isett RB, Ren N, Stromberg A, et al. Focus on RNA isolation: Obtaining RNA for microRNA (miRNA) expression profiling analyses of neural tissue. Biochim Biophys Acta. 2008;1779:749–57. doi: 10.1016/j.bbagrm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eldh M, Lotvall J, Malmhall C, Ekstrom K. Importance of RNA isolation methods for analysis of exosomal RNA: Evaluation of different methods. Mol Immunol. 2012;50:278–86. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Burgos KL, Javaherian A, Bomprezzi R, Ghaffari L, Rhodes S, Courtright A, et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA. 2013;19:712–22. doi: 10.1261/rna.036863.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McAlexander MA, Phillips MJ, Witwer KW. Comparison of methods for miRNA extraction from plasma and quantitative recovery of RNA from cerebrospinal fluid. Front Genet. 2013;4:83. doi: 10.3389/fgene.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C, et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–77. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uwatoko H, Hama Y, Iwata IT, Shirai S, Matsushima M, Yabe I, et al. Identification of plasma microRNA expression changes in multiple system atrophy and Parkinson's disease. Molecular Brain. 2019;12:49. doi: 10.1186/s13041-019-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen L, Gan L, Zhou HY, Liang JH. Protective effects of miR-19b in Parkinson's disease by inhibiting the activation of iNOS through negative regulation of p38 signaling pathways. Int J Clin Exp Med. 2019;12:4735–44. [Google Scholar]
- 74.Roshan R, Shridhar S, Sarangdhar MA, Banik A, Chawla M, Garg M, et al. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA. 2014;20:1287–97. doi: 10.1261/rna.044008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, et al. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci. 2010;43:146–56. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, et al. MicroRNA and proteome expression profiling in early-symptomatic alpha-synuclein (A30P)-transgenic mice. Proteomics Clin Appl. 2008;2:697–705. doi: 10.1002/prca.200780025. [DOI] [PubMed] [Google Scholar]
- 77.Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–4. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 2003;100:4245–50. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kabaria S, Choi DC, Chaudhuri AD, Mouradian MM, Junn E. Inhibition of miR-34b and miR-34c enhances alpha-synuclein expression in Parkinson's disease. FEBS Lett. 2015;589:319–25. doi: 10.1016/j.febslet.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minones-Moyano E, Porta S, Escaramis G, Rabionet R, Iraola S, Kagerbauer B, et al. MicroRNA profiling of Parkinson's disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet. 2011;20:3067–78. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- 81.Hunsberger JG, Fessler EB, Wang Z, Elkahloun AG, Chuang DM. Post-insult valproic acid-regulated microRNAs: Potential targets for cerebral ischemia. Am J Transl Res. 2012;4:316. [PMC free article] [PubMed] [Google Scholar]
- 82.Epis MR, Giles KM, Candy PA, Webster RJ, Leedman PJ. miR-331-3p regulates expression of neuropilin-2 in glioblastoma. J Neurooncology. 2014;116:67–75. doi: 10.1007/s11060-013-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Ann Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 84.Davis BN, Hata A. Regulation of MicroRNA biogenesis: A miRiad of mechanisms. Cell Commun Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slezak-Prochazka I, Durmus S, Kroesen B-J, van den Berg A. MicroRNAs, macrocontrol: Regulation of miRNA processing. RNA. 2010;16:1087–95. doi: 10.1261/rna.1804410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 87.de Planell-Saguer M, Rodicio MC. Analytical aspects of microRNA in diagnostics: A review. Anal Chim Acta. 2011;699:134–52. doi: 10.1016/j.aca.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 88.de Planell-Saguer M, Rodicio MC. Detection methods for microRNAs in clinic practice. Clin Biochem. 2013;46:869–78. doi: 10.1016/j.clinbiochem.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 89.Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, et al. Real-time PCR quantification of precursor and mature microRNA. Methods. 2008;44:31–8. doi: 10.1016/j.ymeth.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20677. Epub 2013/11/14. doi: 10.3402/jev.v2i0.20677. PubMed PMID: 24223256; PubMed Central PMCID: PMCPmc3823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. Epub 2014/10/16. doi: 10.3402/jev.v3.24858. PubMed PMID: 25317274; PubMed Central PMCID: PMCPmc4169610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sourvinou IS, Markou A, Lianidou ES. Quantification of circulating miRNAs in plasma: Effect of preanalytical and analytical parameters on their isolation and stability. J Mol Diagn. 2013;15:827–34. doi: 10.1016/j.jmoldx.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Monleau M, Bonnel S, Gostan T, Blanchard D, Courgnaud V, Lecellier C-H. Comparison of different extraction techniques to profile microRNAs from human sera and peripheral blood mononuclear cells. BMC Genomics. 2014;15:395. doi: 10.1186/1471-2164-15-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaarz A, Debey-Pascher S, Classen S, Eggle D, Gathof B, Chen J, et al. Bead array–based microRNA expression profiling of peripheral blood and the impact of different RNA isolation approaches. J Mol Diagn. 2010;12:335–44. doi: 10.2353/jmoldx.2010.090116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moret I, Sánchez-Izquierdo D, Iborra M, Tortosa L, Navarro-Puche A, Nos P, et al. Assessing an improved protocol for plasma microRNA extraction. PLoS One. 2013;8:e82753. doi: 10.1371/journal.pone.0082753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan GW, Khoo ASB, Tan LP. Evaluation of extraction kits and RT-qPCR systems adapted to high-throughput platform for circulating miRNAs. Sci Rep. 2015;5:9430. doi: 10.1038/srep09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanders R, Mason DJ, Foy CA, Huggett JF. Considerations for accurate gene expression measurement by reverse transcription quantitative PCR when analysing clinical samples. Anal Bioanal Chem. 2014;406:6471–83. doi: 10.1007/s00216-014-7857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leidner RS, Li L, Thompson CL. Dampening enthusiasm for circulating microRNA in breast cancer. PLoS One. 2013;8:e57841–e. doi: 10.1371/journal.pone.0057841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ardui S, Ameur A, Vermeesch JR, Hestand MS. Single molecule real-time (SMRT) sequencing comes of age: Applications and utilities for medical diagnostics. Nucleic Acids Res. 2018;46:2159–68. doi: 10.1093/nar/gky066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hesse M, Arenz C. miRNAs as novel therapeutic targets and diagnostic biomarkers for Parkinson's disease: A patent evaluation of WO2014018650. Expert Opin Ther Pat. 2014;24:1271–6. doi: 10.1517/13543776.2014.965679. [DOI] [PubMed] [Google Scholar]
- 101.Gu LQ, Wanunu M, Wang MX, McReynolds L, Wang Y. Detection of miRNAs with a nanopore single-molecule counter. Expert Rev Mol Diagn. 2012;12:573–84. doi: 10.1586/erm.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]


