Abstract
Background: The U.S. Food and Drug Administration (FDA) has made efforts to encourage adequate assessment of women, racial/ethnic minorities, and geriatric participants in clinical trials through regulations and guidance documents. This study surveyed the demographics of clinical trial participants and the presence of efficacy and safety analyses by sex for new drugs approved between 2013 and 2015 by the FDA Center for Drug Evaluation and Research.
Methods: New drug marketing applications submitted to FDA were surveyed for demographic data (sex, race, ethnicity, and age) and the presence of sex-based analyses for efficacy and safety. The Ratio of the Proportion of women in clinical trials for the indicated disease population relative to the estimated Proportion of women in the disease population (PPR) was calculated for new drug indications.
Results: Of the 102 new drugs in this cohort (defined as new molecular entity drugs and original therapeutic biologics), sex was reported for >99.9% of trial participants, and women accounted for 40.4% of these participants. An estimated 77.2% of participants were White, 6.4% were Black/African American, and 29.1% were aged ≥65 years. Sex-based analyses for both efficacy and safety were conducted for 93.1% of applications. PPR was calculated for 82 new drugs for a total of 60 indications, of which 50 indications (83.3%) had a PPR ≥0.80.
Conclusions: Sex data are now collected for almost all study participants, and this study shows appropriate sex participation for most new drugs when estimated disease prevalence by sex (PPR) is considered. Therapeutic area and disease indication are important considerations when assessing the sex of participants because variation occurs depending on the disease under study. Some racial minorities, especially Blacks/African Americans, are still not well represented in most drug development programs and remain an area where improvement is needed.
Keywords: : United States Food and Drug Administration (FDA), sex distribution, demography, clinical trial
Introduction
Adequate assessment of men and women in clinical trials is essential for the identification of potential sex differences in a drug's efficacy and safety. Several known examples of sex differences in the treatment effects associated with drugs have been incorporated into drug labeling.1,2 Some differences in the way men and women respond to drugs may be due to anatomical differences, such as body weight.3 Women are also more likely than men to experience QT prolongation and related ventricular arrhythmias while taking some medications.4,5 One drug, amlodipine, a calcium channel blocker used for treating hypertension, causes more drug- and dose-related adverse events in women than in men.6,7
Several drugs require a lower starting dose in women, as pharmacokinetic (PK) sex differences cause variations in absorption, distribution, metabolism, and excretion (ADME).8–10 For example, administration of zolpidem, indicated for the treatment of insomnia, resulted in higher levels of zolpidem in women the next morning. Because women eliminated zolpidem more slowly, this difference led to a greater risk of next-morning driving impairment.11 This finding is reflected in the labeling, where a lower initial dose is recommended for women.12 Similarly, flurazepam, a sedative and skeletal muscle relaxant for the treatment of insomnia, was found to have lower clearance in women, and, thus, flurazepam also has a lower recommended initial dose for women.13
The United States Food and Drug Administration (FDA) reviews demographics of clinical trial participants and subgroup analyses during its regulatory assessment of new drug and biological product (“drug”) marketing applications. The information from these clinical trials is used to support regulatory decisions (e.g., approvals, nonapprovals) for drug applications. Drugs that are approved may then be prescribed for use in a larger, more diverse population than is typically exposed to the drug during premarket clinical development. Over the years, the FDA has published regulations and guidance for industry about expectations regarding the need for diverse enrollment in clinical trials and the conduct of sex analyses.10,14,15
Other demographic subgroups—specifically, race, ethnicity, and age—may also contribute to differences in disease response and in response to drugs (e.g., the drug effect). For example, in a Veterans Administration Cooperative Group study on anti-hypertensives, several classes of anti-hypertensive agents were found to be not as effective in Black/African American patients.16 More recently, studies looking at the combination of isosorbide dinitrate and hydralazine in patients with advanced heart failure demonstrated increased survival in Black/African American patients.17,18 Collection of race and ethnicity data is encouraged for FDA-regulated products in the 2016 guidance, Collection of Race and Ethnicity Data in Clinical Trials.19
Age has also been found to be an important factor in the response to drugs. The FDA has required age data to be reported in New Drug Applications (NDAs) since 1985.20 Beginning in 2006, the FDA has required the inclusion of specific sections in approved drug labeling for special populations, such as pediatric (0–16 years) and geriatric (≥65 years) populations.21 Information, such as dosing adjustments, regarding recommendations for certain age groups, may be available in the “Pediatric Use” and “Geriatric Use” sections of labeling.
Guidance has also been published regarding the conduct of subgroup analyses used to support NDAs. A 1989 guidance specifically recommended analysis of safety and effectiveness data by age,22 and a 1993 guidance on the study and evaluation of gender differences, similarly recommended such analyses by sex.10 Both restated prior guidelines from 1988 for the format and content of efficacy and safety analyses in clinical trials.23 This 1988 guideline recommended addressing PK and pharmacodynamics (PD) differences in different demographic subgroups and for various patient characteristics (e.g., renal function).23 Differences between patients in response to a drug can be the result of variation in the PK (the drug's ADME in the body), variation in the PD (the drug's effect on the body), or other factors for which there are known demographic differences.2,3,8,9
In 1998, the FDA issued a final rule on investigational new drug (IND) applications and NDAs (the “Demographic Rule”), which required the analyses of efficacy and safety data for important demographic subgroups in NDAs.14 The Demographic Rule also required that the enrollment of participants in clinical trials for drugs be calculated for key demographic subgroups in IND annual reports.14 The FDA continues to work on improving both data collection and analysis of demographic subgroups in clinical trials.
In August 2013, the FDA released a report to Congress describing demographics and subset analyses included in 72 applications for drugs, biologics, and medical devices approved in 2011.24 This report was congressionally mandated as part of the Food and Drug Administration Safety and Innovation Act, Section 907.25 Subsequently, the FDA published an action plan with recommendations for improving the completeness and the quality of data analyses on demographic subgroups, and for including these analyses in product labeling and in information distributed to patients and healthcare providers.26,27
The participation of demographic subgroups in clinical trials submitted to support drug applications has been periodically studied by the FDA and other organizations, such as the Government Accountability Office (GAO).28–33 These previous studies have noted improvements compared with the 1992 GAO report28 in the percentage of women enrolled in clinical trials and the conduct of sex analyses, but they have also identified areas in which the percentage of women is lower than expected based on prevalence estimates of the disease.
Our descriptive, retrospective study is an update on the status of the participation of demographic subgroups in all clinical trials (phases 1, 2, and 3) submitted to support the approval of new drugs. We use the term “new drugs” to refer to new molecular entity (NME) drugs and original therapeutic biologics that have been approved by the FDA's Center for Drug Evaluation and Research (CDER) between 2013 and 2015. This study also assessed compliance with the 1998 Demographic Rule requiring sponsors to submit sex-based efficacy and safety analyses in applications for new drugs.14 To assess trends in the percentages of women, minorities, and geriatrics participating in clinical trials, this study compared findings for the years 2013–2015 with those of previous studies.28–33
Methods
Demographic data collection
A list of new drug approvals was obtained from CDER's webpage of approved NME NDAs and original therapeutic biologics license applications (BLAs).34 New drugs approved between January 1, 2013 and December 31, 2015 were included in this study. Drugs approved for sex-specific (n = 6) or predominantly pediatric (n = 5) indications were excluded. Clinical trials for the remaining new drugs that included only pediatric patients were also excluded because pediatric populations are covered under different legislation. Products approved by the Center for Biologics Evaluation and Research, such as blood products and vaccines, were not reviewed.
Drug indications were obtained from drug labeling found on the Drugs@FDA website.35 Abbreviated indications were abstracted from the drug labeling and may not have been representative of the full indication. For example, “chronic hepatitis C virus” (HCV) was inclusive of five new drugs indicated for one or more HCV genotypes, and these indications are abbreviated as chronic HCV. The CDER division that reviewed the new drug was used as a surrogate for the drug's therapeutic grouping, although we recognize that in some cases, the groupings (e.g., Pulmonary/Allergy/Rheumatology) are not limited to a single therapeutic area.
Data extracted from sponsor-submitted final clinical study reports included: trial name, trial phase, total enrollment, demographic subgroup enrollment by sex, race, ethnicity, and age group (<65 years, ≥65 years, ≥75 years, ≥85 years), and presence of sex-based efficacy and safety analyses. Only data in the source documents were captured, and no additional calculations or assumptions were made (e.g., participants ≥85 years were captured only when presented in that specific age group; otherwise, no data were extracted for that field). A sponsor's marketing application of their entire drug development program may have consisted of trials conducted in only the United States, only foreign countries, or both the United States and foreign countries. Further analysis of demographics by trial location was not performed in this study.
Race was captured in eight categories with definitions adapted from the 2016 guidance on race and ethnicity data collection19: “White,” “Black/African American,” “Asian,” “American Indian/Alaska Native,” “Native Hawaiian/Other Pacific Islander,” “Hispanic/Latino,” “Other,” and “Unknown (reported).” Hispanic/Latino was not consistently reported in study demographics—it was reported as a race category, an ethnicity category, or not at all. If reported as an ethnic group, then the three possible categories were, “Hispanic/Latino,” “Not Hispanic/Latino,” or “Unknown (reported).” The following are some examples of additional terms used to describe race if they were captured in the clinical trials data differently from the racial and ethnic categories given earlier:
• White also included “Caucasian”
• Black/African American also included “Afro-Caribbean”
• Asian also included “Oriental” or “Japanese”
• American Indian/Alaska Native also included “Native American”
• Native Hawaiian/Other Pacific Islander also included participants from New Guinea or New Zealand
• Hispanic/Latino also included participants labeled “Puerto Rican”
• Other also included “Multiracial” or mixed race
• Unknown (reported) also included participants “Not reported,” “Missing,” “Not collected,” or “Not specified”
A quality assurance/quality control (QA/QC) was performed for each clinical trial after data collection to ensure the quality and accuracy of the extracted data. During the QA/QC process, data entry was independently confirmed by two or more investigators (A.C., H.W., H.I., M.E., A.I.).
Demographic data analysis
Participation by sex, race, ethnicity, and age group was calculated as a percentage of the total participants for whom the corresponding demographic information was available. Percent participation by sex, race, ethnicity, and age group was further assessed by approval year and by clinical trial phase (phases 1, 2, and 3). “Phase 1/2” trials were coded as “phase 2” trials, and “phase 2/3” trials were coded as “phase 3.” Participation by sex, race, and age group was assessed by therapeutic grouping as well. Of note, percent participation of pooled racial minorities included participants reported as being of the following races: Black/African American, Asian, American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, Hispanic/Latino (Race), and Other. The participation of these minority racial groups was pooled to contrast with participation by the White racial group.
Estimated prevalence data collection for sex
The estimated prevalence of a disease (e.g., human immunodeficiency virus) or a procedure (e.g., percutaneous coronary intervention) by sex was obtained by means of a comprehensive literature search of peer-reviewed journal articles and public source databases, such as PubMed, those from the Centers for Disease Control and Prevention, and those from the National Institutes of Health. The estimated prevalence is the number of cases of disease in a population at risk for the disease. Because prevalence was not always available, incidence, the number of newly diagnosed cases, was estimated when available.
The prevalence or incidence data by sex were reported in these sources in various forms, sometimes as prevalence rate or incidence rate, which is the percent of prevalence or incidence cases divided by the population at risk. Sources were identified by using keyword searches that included “woman,” “women,” “female,” “sex,” “gender,” “prevalence,” “incidence,” “demographics,” or “epidemiology,” and disease names or procedures. The most recently published source was utilized when possible.
If prevalence information was unavailable for relapsed or refractory populations with the disease or for genetic or molecularly defined subsets, then estimated prevalence for the more general indication was used. Data from studies conducted in North America and Europe were preferred to best estimate prevalence.32,33 For new drugs indicated for disease symptoms, estimated prevalence or incidence data were found for the underlying disease. For example, one of the new drugs was approved for “long-term, once-daily, maintenance treatment of airflow obstruction and for reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD).” Prevalence was, therefore, found for COPD and then utilized in further analyses.
Comparison of the proportion of women
A ratio of the proportion of women in the clinical trials for the indicated disease population (%CT) relative to the estimated proportion of women in the disease population (%P) (i.e., the prevalence rate) was used to compare the clinical trial with the source population by the metric, PPR:
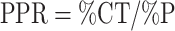 |
The proportion of women in the clinical trials for the indicated disease population (%CT) was calculated by dividing the total number of women in trials that included patients with the indicated disease by the total participants in those trials whose sex was reported. The estimated proportion of women in the disease population (%P) was calculated by dividing women's estimated prevalence or incidence by the total prevalence or incidence in the source population.
PPR was calculated for all indications for which appropriate prevalence or incidence information was available. The proportion of women in the clinical trials was assessed as being comparable to the estimated proportion of women in the disease population if the PPR fell between 0.8 and 1.2, as previously proposed by Poon et al.32 and Eshera et al.33
Presence of sex-based analyses of efficacy and safety
To evaluate whether a sponsor conducted and reported an analysis by sex, the sponsors' integrated summaries of efficacy and safety submitted to FDA in NDAs and BLAs were surveyed for the presence of sex-based analyses of the drug's efficacy and safety. A coding criterion similar to that used by the GAO in its 2001 report29 was employed, where analysis consisting of at least one sentence, table, or graphic summarizing efficacy or safety analysis by sex was sufficient to classify a new drug as presenting sex-based analysis. No statement that the analysis was statistically powered to draw conclusions was necessary. Both efficacy and safety analyses were surveyed and coded separately.
This descriptive, retrospective study was not designed with a prespecified statistical hypothesis. Calculations were performed, and graphics were prepared by using Microsoft Excel 2010.
Results
One hundred and thirteen (113) new drugs (88 NDAs and 25 BLAs) were approved by the FDA CDER from January 2013 through December 2015. New drugs with the following sex-specific indications were excluded (n = 6): prostate cancer, menopausal vasomotor symptoms and postmenopausal osteoporosis, menopausal dyspareunia, ovarian cancer, breast cancer in postmenopausal women, and female hypoactive sexual desire disorder. One breast cancer drug was included because the drug labeling did not exclusively indicate its use in women. New drugs with the following predominantly pediatric indications were excluded (n = 5): neuroblastoma, perinatal-, infantile-, and juvenile-onset hypophosphatasia, bile acid synthesis disorders, hereditary orotic aciduria, and lysosomal acid lipase deficiency. Of the 113 new drugs, 102 were analyzed.
Overall participation in clinical trials
In this study, a total of 2,455 clinical trials (enrolling 484,896 participants) were used to evaluate 102 new drug applications distributed among the 13 therapeutic groupings. Overall participation by sex, race, ethnicity, and age group was as follows:
• Participation by sex: The participant's sex was reported for 484,876 of the 484,896 total participants (>99.9%), of whom 40.4% were women and 59.6% were men (Table 1). When the new drug indicated for breast cancer (n = 1) was excluded, women accounted for 40.2% of trial participants. Women's participation accounted for 46.6% of trial participants in 2013, 37.7% in 2014, and 39.2% in 2015 (Table 1).
• Participation by race: Race was reported for an estimated 472,843 (97.5%) of trial participants (in certain cases, the sponsor may have recorded multiple races for one trial participant, thus 97.5% is an approximation). By year, race was reported for 96.4%, 99.8%, and 96.5% of participants in 2013, 2014, and 2015, respectively. Overall, of the participants for whom race was reported, 77.2% were White, 12.2% were Asian, 6.4% were Black/African American, 2.6% were Other, 0.6% were American Indian/Alaska Native, 0.6% were Hispanic/Latino, 0.2% were Unknown (reported), and 0.1% were Native Hawaiian/Other Pacific Islander (Table 1).
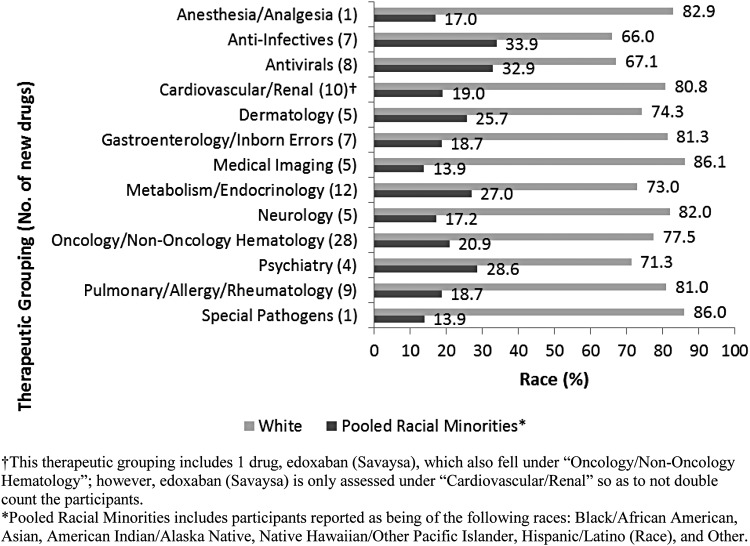
A breakdown of race participation by therapeutic grouping shows that Whites accounted for 66.0% to 86.1% of clinical trial participants. After pooling minority racial groups, participation ranged from 13.9% to 33.9% (Fig. 1). Minority racial groups were pooled so that smaller racial groups are collectively represented to better contrast with the majority racial group.
• Participation by ethnicity: Ethnicity was reported for 313,928 (64.7%) of trial participants, of whom 83.6% were Not Hispanic/Latino, 13.3% were Hispanic/Latino, and 3.1% were reported as Unknown (Table 1). Because >35% of participants were missing ethnicity data, the percent participation can only be used to describe those who reported their ethnicity; therefore, these percentages may not reflect actual participation of the entire study population.
• Participation by age group: Age group (defined as <65 years, ≥65 years) was reported for 350,064 (72.2%) of trial participants. By year, reporting rates were 43.8% in 2013, 82.0% in 2014, and 79.2% in 2015. Among participants whose age group could be coded according to this study's parameters, 70.9% were <65 years of age and 29.1% were ≥65 years of age (Table 1). For a limited number of new drugs, participant age was further characterized as those ≥75 years and ≥85 years.
Table 1.
Demographics of Clinical Trial Participants of New Drugs Approved by FDA CDER (2013–2015)
| 2013 | 2014 | 2015 | Entire study period | |
|---|---|---|---|---|
| New drugs approved, na | 24 | 40 | 38 | 102 |
| Total sex reported | 108,132 | 152,609 | 224,135 | 484,876 (>99.9%)b |
| Men | 57,743 (53.4%) | 95,012 (62.3%) | 136,379 (60.8%) | 289,134 (59.6%) |
| Women | 50,389 (46.6%) | 57,597 (37.7%) | 87,756 (39.2%) | 195,742 (40.4%) |
| Total race reported | 104,269 | 152,266 | 216,308 | 472,843 (97.5%)b |
| White | 80,120 (76.8%) | 117,761 (77.3%) | 166,986 (77.2%) | 364,867 (77.2%) |
| Black/AA | 6,824 (6.5%) | 9,896 (6.5%) | 13,590 (6.3%) | 30,310 (6.4%) |
| Asian | 11,837 (11.4%) | 17,493 (11.5%) | 28,424 (13.1%) | 57,754 (12.2%) |
| AI/AN | 1,127 (1.1%) | 1,053 (0.7%) | 841 (0.4%) | 3,021 (0.6%) |
| NH/OPI | 120 (0.1%) | 217 (0.1%) | 338 (0.2%) | 675 (0.1%) |
| Hispanic/Latino | 155 (0.1%) | 1,597 (1.0%) | 892 (0.4%) | 2,644 (0.6%) |
| Other | 3,656 (3.5%) | 3,962 (2.6%) | 4,887 (2.3%) | 12,505 (2.6%) |
| Unknown (reported) | 430 (0.4%) | 287 (0.2%) | 350 (0.2%) | 1,067 (0.2%) |
| Total ethnicity reported | 77,211 | 106,807 | 129,910 | 313,928 (64.7%)b |
| Hispanic/Latino | 11,698 (15.2%) | 16,632 (15.6%) | 13,406 (10.3%) | 41,736 (13.3%) |
| Not Hispanic/Latino | 64,106 (83.0%) | 86,464 (81.0%) | 111,746 (86.0%) | 262,316 (83.6%) |
| Unknown (reported) | 1,407 (1.8%) | 3,711 (3.5%) | 4,758 (3.7%) | 9,876 (3.1%) |
| Total age group reportedc (year) | 47,312 | 125,171 | 177,581 | 350,064 (72.2%)b |
| <65 | 40,189 (84.9%) | 91,571 (73.2%) | 116,432 (65.6%) | 248,192 (70.9%) |
| ≥65 | 7,123 (15.1%) | 33,600 (26.8%) | 61,149 (34.4%) | 101,872 (29.1%) |
| ≥75d | 570 (1.2%) | 7,658 (6.1%) | 20,011 (11.3%) | 28,239 (8.1%) |
| ≥85e | 7 (<0.1%) | 28 (<0.1%) | 7 (<0.1%) | 42 (<0.1%) |
Excludes sex-specific and pediatric indications (n = 11).
This is the percentage of participants out of the 484,896 total enrollment who reported their information within each demographic subgroup. In subgroups where a higher percentage of participants were not reported, then results of participation should only be based on those reporting their information and should not be extrapolated to the entire population.
The information provided in the applications for age was reported in variable formats. Applications that included a breakdown of participants by <65 years and ≥65 years usually did not further delineate the additional geriatric subgroupings by age ≥75 or ≥85. The numbers included in the table are only for those applications that reported/presented the data in those age groups, and they will underestimate the actual number of patients for the ≥75 and ≥85 year age groups participating in the clinical trials.
≥75 years is a subset of ≥65 years and is included in the ≥65 years age group numbers.
≥85 years is a subset of ≥75 years and is included in both the ≥65 and ≥75 years age group numbers.
AA, African American; AI/AN, American Indian/Alaska Native; CDER, Center for Drug Evaluation and Research; FDA, Food and Drug Administration; NH/OPI, Native Hawaiian/Other Pacific Islander.
FIG. 1.
Percent race participation in new drug trials by therapeutic grouping.
Women's participation by therapeutic grouping and drug indication
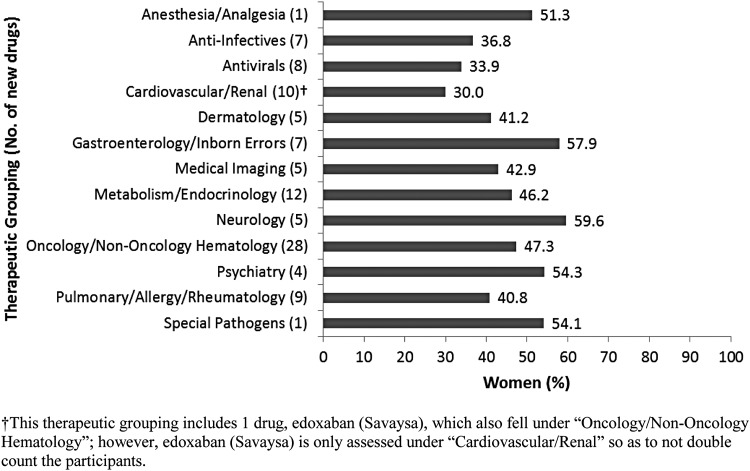
When all new drugs were categorized into 1 of 13 therapeutic groupings,32,33 the number of drugs in each grouping ranged from 1 to 28. Women's participation by therapeutic grouping ranged from 30.0% (Cardiovascular/Renal) to 59.6% (Neurology) (Fig. 2). Only two therapeutic groupings, Psychiatry and Pulmonary/Allergy/Rheumatology, had a mean PPR <0.80 (at 0.78 and 0.72, respectively) (Table 2).
FIG. 2.
Percent women participation in new drug trials by therapeutic grouping.
Table 2.
The Ratio of Women's Participation in Clinical Trials for the Indicated Disease Relative to the Estimated Proportion of Women in the Disease Population (PPR) by Therapeutic Grouping
| Therapeutic grouping (no. of new drugsa) | Mean PPR | Standard error | 95% confidence interval |
|---|---|---|---|
| Anti-infectives (3) | 1.13 | 0.13 | 0.56 to 1.71 |
| Antivirals (8) | 0.99 | 0.06 | 0.84 to 1.14 |
| Cardiovascular/renal (8) | 1.05 | 0.17 | 0.65 to 1.45 |
| Dermatology (7) | 0.80 | 0.11 | 0.53 to 1.07 |
| Gastroenterology/inborn errors (8) | 1.11 | 0.09 | 0.90 to 1.32 |
| Metabolism/endocrinology (8) | 1.03 | 0.03 | 0.97 to 1.08 |
| Neurology (3) | 0.97 | 0.07 | 0.68 to 1.25 |
| Oncology/nononcology hematology (34) | 1.10 | 0.06 | 0.98 to 1.22 |
| Psychiatry (6) | 0.78 | 0.08 | 0.57 to 1.00 |
| Pulmonary/allergy/rheumatology (9) | 0.72 | 0.10 | 0.49 to 0.95 |
| Special pathogens (1) | 1.00 | N/A | N/A |
This only includes new drugs with indications for which estimated prevalence or incidence data were found and, therefore, a PPR could be calculated. Thus, there was no PPR available for the therapeutic groupings, “Anesthesia/Analgesia” and “Medical Imaging.”
The sum of the number of new drugs (n = 95) is greater than the number of drugs for which estimated prevalence or incidence was found (n = 82) because several drugs have multiple indications and, thus, were captured more than once.
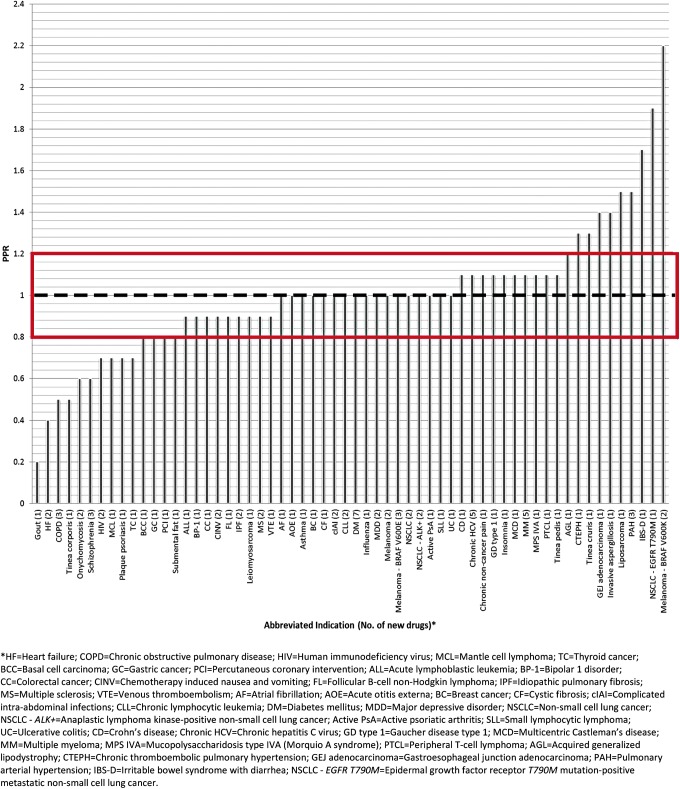
Prevalence and incidence data by sex obtained from published literature were used to estimate the PPR, as described in the Methods section. Twenty new drugs were excluded from the ratio analysis because appropriate epidemiology data for their indication could not be found. For the remaining 82 new drugs, epidemiology data were found (Supplementary Table S1; Supplementary Data are available online at www.liebert pub.com/jwh) for 60 indications (Supplementary Table S2). Fifty (of 60) indications had a PPR ≥0.80 and 9 (of these 50) indications had a ratio >1.2 (Fig. 3). This suggests that the proportion of women in the clinical trials was comparable to or greater than the estimated proportion of women in the disease population for 83.3% of the approved indications.
FIG. 3.
The ratio of women's participation in clinical trials for the indicated disease relative to the estimated proportion of women in the disease population (PPR).
Presentation of sex-based analyses of efficacy and safety
Of the 102 new drugs evaluated, 99 new drugs (97.1%) had sex-based analyses of efficacy and 97 new drugs (95.1%) had sex-based analyses of safety, whereas 95 new drugs (93.1%) had both. There were seven applications that lacked either type of sex-based analysis or both analyses; six of these seven applications lacking only an efficacy (n = 2) or safety (n = 4) analysis were for rare disease indications (“orphan drugs”), and their clinical trials did not have enough participants to warrant subgroup analysis by sex. These six applications were indicated for: previously untreated chronic lymphocytic leukemia, non-24-hour sleep-wake disorder, congenital or acquired generalized lipodystrophy, unresectable or metastatic melanoma with BRAF V600E or V600K mutation, relapsed or refractory metastatic melanoma, and reversal of anticoagulant effects in patients treated with dabigatran.
The one application that lacked both efficacy and safety analyses was indicated for a disease found predominantly in women (HER2-positive metastatic breast cancer) and did not have enough male participants in the pivotal trials to warrant subgroup analysis by sex.
Comparison with previously conducted studies
A comparison of this study with similar studies conducted in previous years on the percent participation of women and the percent of applications with sex-based analyses of both efficacy and safety can be found in Table 3. For five previous studies conducted in various time periods between 1988 and 2012, participation of women ranged from 44% to 56% compared with 40.4% for this study. However, previous studies included clinical trials at different phases (e.g., one study included phases 1–3, two studies included phases 2 and 3 only), which may affect comparisons between individual cohorts (see Fig. 4a and participation by trial phase section later). It should be noted that not all of these previous studies assessed PPR, which is a more relevant measure of participation by women.
Table 3.
A Comparison Between Similar Studies in Participation of Women and Presence of Sex-Based Analyses
| Study group | Study period | Focus of study | % women | % applications with sex-based analyses of both efficacy and safety |
|---|---|---|---|---|
| U.S. GAO28 | January 1988 to June 1991 | Phases 2 and 3 | 44 | 47.2a |
| U.S. GAO29 | August 1998 to December 2000 | Phases 2 and 3 | 56 | 72 |
| Yang et al.31 | January 2000 to December 2002 | Phases 1, 2, and 3 | 48.5 | 70.7a |
| Poon et al.32 | January 2007 to December 2009 | Late-phase trialsb | 44.7 | 72.1 |
| Eshera et al.33 | January 2010 to December 2012 | Pivotal trialsc | 47.0 | 91.8 |
| Chen et al. (current study) | January 2013 to December 2015 | Phases 1, 2, and 3 | 40.4 | 93.1 |
Source is unclear as to whether this is the percent with sex-based analyses of both efficacy and safety versus only one or the other.
Defined late-phase clinical trials as phase 3 and some late phase 2 studies that generally confirm the efficacy outcome and safety profiles of a drug or biologic product in the targeted patient population.
Defined pivotal clinical trials as those phase 2 and/or 3 trials described in the medical and statistical reviews or the product labeling as supporting the drug or biological approval.
U.S. GAO, United States General Accounting Office.
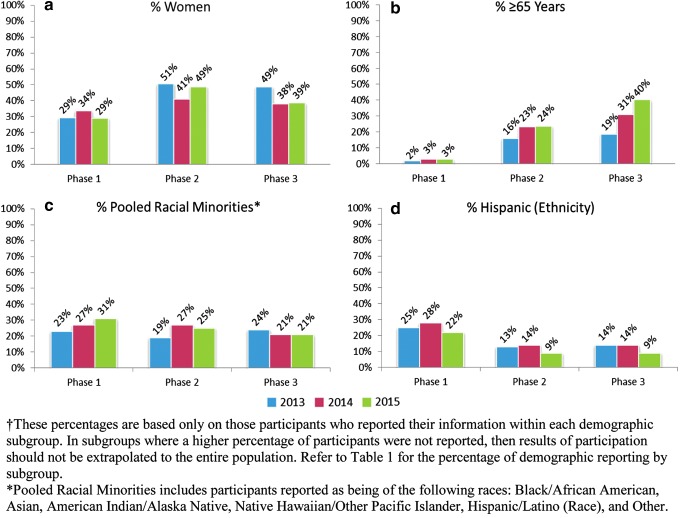
FIG. 4.
Demographics of clinical trial participants by phase and year according to reported: sex (a), age (b), race (c), and ethnicity (d) subgroups.†
The percentage of applications with sex-based analyses for both efficacy and safety ranged from 47.2% to 91.8% in previous studies compared with 93.1% for this study.
Participation by trial phase
Percent participation by demographic subgroup by trial phase and by year was explored (Fig. 4). Women's participation was 29%–34% in phase 1, 41%–51% in phase 2, and 38%–49% in phase 3, suggesting an increased rate of participation of women in later phase (phase 2, 3) trials than in phase 1. There was a similar trend for greater participation of older patients (age ≥65 years) in later phases, but not for racial or ethnic minorities.
Discussion
For new drugs approved at the FDA CDER between January 1, 2013 and December 31, 2015, women accounted for 40.4% of clinical trial participants enrolled in all phases (phases 1, 2, and 3) of clinical trials supporting the applications (Table 1). The percentage of women varied considerably by indication and therapeutic grouping (Fig. 2). When the participation of women was examined as an estimated proportion of women in the disease population (PPR), 83.3% of the newly approved indications examined in this study had participation of women in the clinical trials supporting the drug's approved indication that was similar to or greater than the estimated proportion of women in the disease population for that indication (i.e., PPR >0.8) (Fig. 3).
A trend toward higher participation of women in later phase trials than in phase 1 was seen (Fig. 4a) and is not unexpected. Phase 2 and 3 trials are typically conducted in the indicated disease population, and phase 3 trials are usually the largest and longest trials, designed and conducted for the purpose of demonstrating the benefits and risks of the drug in the population for which the drug is labeled for use in the postmarketing period. Therefore, later phase trials are expected to more closely approximate the population with the disease than early phase trials, which are typically conducted mainly for safety and PK/PD exploration.
Compliance with Federal regulations for demographic reporting of sex in patient-level data submitted to the FDA by drug sponsors was high, with sex demographic subgroup data having been reported for virtually all of the clinical trial participants (>99.9%). By comparison, a 2001 GAO report found that sex was unknown for 9% of trial participants.29 Further, the percentage of submissions by sponsors that included sex-based analyses for both safety and efficacy (93.1%) was higher than what was observed in earlier studies (Table 3). Since 2015, the FDA has also been enhancing the reporting of demographics of participants in the clinical trials that support regulatory decisions via publication of these data on the FDA's “Drug Trials Snapshots” website in an effort to increase agency transparency so that this information can be directly communicated to the public.27
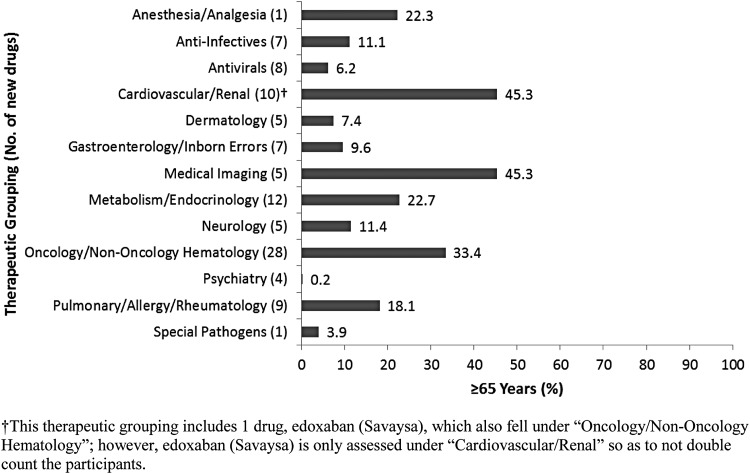
The increased reporting of participant age (<65 years, ≥65 years) in 2015 and 2014 compared with 2013 may be due to the 2012 amendment to the International Council for Harmonisation E-7 guidance, which emphasized the importance of including patients ≥65 years of age.36 Analysis of phase 3 data showed that the percentage of trial participants with ages ≥65 years increased from 19% to 40% from 2013 to 2015 (Fig. 4b); however, this increase is based on a limited sample of drugs surveyed in the 3-year study period, and no definite conclusions regarding percent participation for older patients can be drawn. The therapeutic groupings with the greatest percentage of trial participants ≥65 years of age were Oncology/Nononcology Hematology, Medical Imaging, and Cardiovascular/Renal (Fig. 5), which is likely due to cancer (oncology), cognitive decline (medical imaging), and heart conditions (cardiovascular) being more prevalent in older patients.
FIG. 5.
Percent geriatric participation in new drug trials by therapeutic grouping.
For race and ethnicity, the results show that although collection and reporting of race subgroup data by sponsors was high (97.5%), collection of ethnicity data was more limited (64.7%) and was inconsistent across applications. Some applications reported “Hispanic/Latino” as race, whereas others reported “Hispanic/Latino” as ethnicity; thus, there is a possibility that some trials reported race without reporting ethnicity separately. Although this study found 13.3% Hispanic/Latino participation within the ethnicity subgroup, missing ethnicity data in >35% of participants makes it difficult to draw conclusions on the overall participation by ethnic group in clinical trials.
The majority of clinical trial participants surveyed during the study period were White and Not Hispanic/Latino (Table 1). Relative to the U.S. population, the mean percent participation of Asians was high (12.2%), whereas the mean percentage of Blacks/African Americans in clinical trials was lower than anticipated (6.4%). Although the percent participation varied somewhat by disease indication, in most instances, Black/African American participation was generally lower than would be expected based on the percentage of Blacks/African Americans in the U.S. population (13.3%)37 and estimated percentages in the disease population. This finding is consistent with numerous recent studies in the medical literature and remains an area of drug development in need of improvement.24,38–40
In contrast to the higher participation of women and older patients in later phase clinical trials, participation of pooled racial minorities was relatively consistent by phase (Fig. 4c). Participation by Hispanic ethnicity for those who reported this information also did not show a trend toward higher participation in the later phases (Fig. 4d). The reasons for these trends are not clear; however, previous reports indicate that racial minority participation varies substantially by the geographic location in which these trials are conducted,30 which may have affected percent participation by race when assessed by overall clinical trial demographics and by trial phase. Additional assessment of participation by racial and ethnic subgroups is needed and remains an area of additional study.
Limitations
This study has several limitations. First, the drug approvals included in this cohort were limited to a recent 3-year period. Drug development and approvals can vary substantially from year to year, and they often reflect scientific and pharmaceutical advances within a given period. Because the percent female (and other demographic subgroup) participation varies depending on the disease indication for which the drug is being administered, differences in drugs approved in a limited period may affect the demographic make-up of the clinical trial population.
Second, the scope of this study did not expand on clinical trial location, which limited the analytic detail required for conclusions based on the participants' geographic location. Because the majority of applications were multi-national, clinical trials conducted in foreign countries may not reflect the demographics of the United States and limited conclusions for U.S. participation.
Third, due to the higher number of participants for whom data on ethnicity and age were missing, it was difficult to draw conclusions on the overall participation in clinical trials by ethnicity and age. Also, some sponsors collected Hispanic/Latino as a race category, and this might have added to the lower number of patients reporting it under ethnicity.
Fourth, although PPR is more informative than %CT alone (as it corrects for differences in disease prevalence by sex), there are limitations to the use of PPR.32,33 PPR sources only provide estimates of the sex prevalence by disease. When an indication is very narrow or specific, it may be difficult to identify epidemiologic data on a similar patient population. For instance, although prevalence information may be available for a general indication, it may not be available for relapsed or refractory populations with the disease or for genetic or molecularly defined subsets.
“Precision medicine,” where drugs are increasingly being developed for underlying disease-related targets (e.g., mutationally defined tumors for cancer drugs), is an increasing area of drug development, and estimates of the disease prevalence in these narrow patient populations can be difficult to define. One example is that the demographics of the population affected by nonsmall cell lung cancer (NSCLC) resulting from an anaplastic lymphoma kinase-positive (ALK+) mutation41 may be different from the demographics of the population affected by NSCLC resulting from an epidermal growth factor receptor (EGFR) T790M mutation,42 or even from the overall NSCLC population.43
Another example is refractory gout. The lowest PPR calculated in this study was for gout (0.2), with a %CT of 4.7% and a %P of 26.5% for women. Gout overall is a male-predominant disease for which prevalence estimates are available44; however, the drug included in this study was indicated for refractory gout, which is expected to be an overwhelmingly male disease, but for which reliable sex-prevalence data are not available. Hence, percent women in this specific disease population was likely overestimated, which may, in part, have contributed to the low PPR.
Similar difficulties in estimating disease and demographic subpopulations are also seen for some orphan drugs, which are approved for rare diseases and disorders affecting <200,000 people in the United States,45 and in some cases, have little epidemiologic data available in the published literature.
Disease prevalence estimates by demographic subgroup may also vary in different parts of the world, which may have affected both the demographic composition of the clinical trials30 and the calculation of PPR because disease prevalence estimates were based predominantly on prevalence in Western nations32,33; however, even among Western nations or among different regions within the United States, disease prevalence may be variable.
In this study, >95% of the approved drugs had clinical development programs that were supported by results from multi-national trials; two drugs (miltefosine for leishmaniasis and ivabradine for congestive heart failure) relied entirely on foreign trials to support approval, and one drug (metreleptin for leptin deficiency) relied entirely on trials conducted in the United States.
One example of demographic differences by geographic region is COPD. COPD in the United States is a female-predominant disease,46 but it is male predominant in some other regions of the world.47 For the three COPD drugs included in this study, the percent female participation in the trials conducted in the United States was similar to the percent of women with COPD in the U.S. population; however, the PPR was found to be low (0.5) when the multi-national clinical trial populations were pooled (Fig. 3), which may have been due to an over-estimation of the female prevalence in the multi-national populations in which the trials were conducted.
Finally, the statistical variability in mean PPR by therapeutic grouping is influenced by the number of drugs approved by the division during the study period (Table 2). Therapeutic groupings with more new drugs approved may give a better assessment of mean PPR and vice versa.
Conclusions
This descriptive, retrospective study demonstrates important current trends in clinical trial demographic data submitted to the U.S. FDA: Sex data are now collected for almost all patients, and there appears to be appropriate sex participation for most new drugs included in this study when estimated disease prevalence by sex (PPR) is considered. We recognize, however, that better data on the prevalence of disease in demographic subsets are needed for some indications, particularly for subsets of diseases (e.g., mutationally defined cancers) and orphan diseases.
Participation of some racial minorities—most notably, Blacks/African Americans—is still not well represented in many programs and remains an area in need of improvement. One contributing factor could be that many clinical development programs are now multi-national, and, hence, a large proportion of patients enrolled were from outside the United States where there were fewer Blacks/African Americans.30 In contrast, Asian participation is now 11%–13%, which is above the percent of Asians in the U.S. population (5.6%).37 Analyzing demographic data by geographic region could be an area of future research.
Lastly, these data represent a limited 3-year period and are dependent on the indications that received approval during this time. Therefore, in the future, it will be important to look at specific applications when assessing the sex of participants since participation of women is expected to vary depending on the disease under study. Future studies should also provide more granular information about specific therapeutic areas that appear to show disparities in enrolling clinical trial participants.
Supplementary Material
Acknowledgments
This project was supported in part by the appointments of A.C., H.W., H.I., M.E., and A.I. to the Research Participation Program at the Office of Women's Health, U.S. FDA, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the FDA. The authors would like to thank Drs. Pamela Scott and Marjorie Jenkins of the Office of Women's Health, Dr. Jonca Bull of the Office of Minority Health, and Drs. John Whyte, Robert Temple, Robert O'Neill, and ShaAvhrée Buckman-Garner of CDER for their comments and insight on the draft manuscript. They are also grateful to Yao-Jen Lu, Kenneth Geh, and Natasha Duggal for their assistance with the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Parekh A, Fadiran EO, Uhl J, Throckmorton DC. Adverse effects in women: Implications for drug development and regulatory policies. Expert Rev Clin Pharmacol 2011;4:453–466 [DOI] [PubMed] [Google Scholar]
- 2.Fadiran EO, Zhang L. Effects of sex differences in the pharmacokinetics of drugs and their impact on the safety of medicines in women. In: Harrison-Woolrych M, ed. Medicines for women. Switzerland: Springer International Publishing, 2015:41–68 [Google Scholar]
- 3.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 2004;44:499–523 [DOI] [PubMed] [Google Scholar]
- 4.Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther 2000;67:413–418 [DOI] [PubMed] [Google Scholar]
- 5.Drici MD, Clément N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf 2001;24:575–585 [DOI] [PubMed] [Google Scholar]
- 6.Pfizer Inc. Drug labeling: Norvasc® (amlodipine besylate), 2013. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2013/019787s054lbl.pdf Accessed August25, 2016
- 7.Abad-Santos F, Novalbos J, Galvez-Mugica MA, et al. Assessment of sex differences in pharmacokinetics and pharmacodynamics of amlodipine in a bioequivalence study. Pharmacol Res 2005;51:445–452 [DOI] [PubMed] [Google Scholar]
- 8.Anderson GD. Sex and racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health 2005;14:19–29 [DOI] [PubMed] [Google Scholar]
- 9.Franconi F, Campesi I. Sex and gender influences on pharmacological response: An overview. Expert Rev Clin Pharmacol 2014;7:469–485 [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Guidance for industry: Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs, 1993. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072044.pdf Accessed August25, 2016
- 11.U.S. Food and Drug Administration. Drug safety communications: Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist), January 10, 2013. Available at: www.fda.gov/downloads/Drugs/DrugSafety/UCM335007.pdf Accessed August25, 2016
- 12.Sanofi-Aventis U.S. LLC. Drug labeling: Ambien (zolpidem tartrate), 2013. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2013/019908s032s034,021774s013s015lbl.pdf Accessed August25, 2016
- 13.Mylan Pharmaceuticals Inc. Drug labeling: Flurazepam hydrochloride, 2015. Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2f2db2f5-49d3-4d47-a08a–628df49d2120 Accessed August25, 2016
- 14.U.S. Food and Drug Administration. Final Rule. 63 FR 6854. 21 CFR 312 and 21 CFR 314: Investigational New Drug Applications and New Drug Applications. Federal Register 1998;63:6854–686210177736 [Google Scholar]
- 15.U.S. Food and Drug Administration. Guidance for industry and Food and Drug Administration staff: Evaluation of sex-specific data in medical device clinical studies, 2014. Available at: www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf Accessed August25, 2016
- 16.Veterans Administration Cooperative Study Group on Antihypertensive Agents. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension: II. Results of long-term therapy. JAMA 1982;248:2004–2011 [PubMed] [Google Scholar]
- 17.Taylor AL, Ziesche S. Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–2057 [DOI] [PubMed] [Google Scholar]
- 18.Temple R, Stockbridge NL. BiDil for heart failure in black patients: The U.S. Food and Drug Administration perspective. Ann Intern Med 2007;146:57–62 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Guidance for industry and Food and Drug Administration staff: Collection of race and ethnicity data in clinical trials, 2016. Available at: www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf Accessed November12, 2016
- 20.U.S. Food and Drug Administration. Code of Federal Regulations, Title 21, Sec. 314.50: Content and format of an Application. 2015. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=314.50 Accessed August25, 2016
- 21.U.S. Food and Drug Administration. Code of Federal Regulations, Title 21, Sec. 201.56: Requirements on content and format of labeling for human prescription drug and biological products, 2015. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=201.56 Accessed August25, 2016
- 22.U.S. Food and Drug Administration. Guidance for industry: Guideline for the study of drugs likely to be used in the elderly, 1989. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072048.pdf Accessed August25, 2016
- 23.U.S. Food and Drug Administration. Guideline for the format and content of the clinical and statistical sections of an application, 1988. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071665.pdf Accessed August25, 2016
- 24.U.S. Food and Drug Administration. FDA Report: Collection, analysis, and availability of demographic subgroup data for FDA-approved medical products, 2013. Available at: www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM365544.pdf Accessed August25, 2016
- 25.Food and Drug Administration Safety and Innovation Act. Public Law 112–144, July 9, 2012. Available at: www.gpo.gov/fdsys/pkg/PLAW-112publ144/pdf/PLAW-112publ144.pdf Accessed August25, 2016
- 26.U.S. Food and Drug Administration. FDA report: FDA action plan to enhance the collection and availability of demographic subgroup data, 2014. Available at: www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM410474.pdf Accessed August25, 2016
- 27.U.S. Food and Drug Administration. Drug trials snapshots, 2016. Available at: www.fda.gov/Drugs/InformationOnDrugs/ucm412998.htm Accessed August25, 2016
- 28.U.S. General Accounting Office. Women's health: FDA needs to ensure more study of gender differences in prescription drug testing, 1992. Available at: www.gao.gov/assets/220/216966.pdf Accessed August25, 2016
- 29.U.S. General Accounting Office. Women's health: Women sufficiently represented in new drug testing, but FDA oversight needs improvement, 2001. Available at: www.gao.gov/new.items/d01754.pdf Accessed August25, 2016
- 30.Evelyn B, Toigo T, Banks D, et al. Participation of racial/ethnic groups in clinical trials and race-related labeling: A review of new molecular entities approved 1995–1999. J Natl Med Assoc 2001;93(12 Suppl):18S–24S [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Carlin AS, Faustino PJ, et al. Participation of women in clinical trials for new drugs approved by the Food and Drug Administration in 2000–2002. J Womens Health 2009;18:303–310 [DOI] [PubMed] [Google Scholar]
- 32.Poon R, Khanijow K, Umarjee S, et al. Participation of women and sex analyses in late-phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007–2009. J Womens Health 2013;22:604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eshera N, Itana H, Zhang L, Soon G, Fadiran EO. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am J Ther 2015;22:435–455 [DOI] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration. New drugs at FDA: CDER's new molecular entities and new therapeutic biological products, 2016. Available at: www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/default.htm Accessed August25, 2016
- 35.U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products, 2016. Available at: www.accessdata.fda.gov/scripts/cder/daf Accessed August25, 2015
- 36.U.S. Food and Drug Administration. Guidance for industry: E7 Studies in support of special populations: Geriatrics–questions and answers, 2012. Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm189544.pdf Accessed August25, 2016 [PubMed]
- 37.U.S. Census Bureau. QuickFacts: United States, 2016. Available at: www.census.gov/quickfacts/table/PST045215/00 Accessed August25, 2016
- 38.Wissing MD, Kluetz PG, Ning YM, et al. Under-representation of racial minorities in prostate cancer studies submitted to the US Food and Drug Administration to support potential marketing approval, 1993–2013. Cancer 2014;120:3025–3032 [DOI] [PubMed] [Google Scholar]
- 39.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004;291:2720–2726 [DOI] [PubMed] [Google Scholar]
- 40.Chen MS, Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: Enhancing minority participation in clinical trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual: Renewing the case for enhancing minority participation in cancer clinical trials. Cancer 2014;120(7 Suppl):1091–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Wang S, Xu S, Qu J, Liu B. Clinicopathologic features of patients with non-small cell lung cancer harboring the EML4-ALK fusion gene: A meta-analysis. PLoS One 2014;9:e110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res 2006;66:7854–7858 [DOI] [PubMed] [Google Scholar]
- 43.NIH National Cancer Institute: Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: Lung and bronchus cancer, 2016. Available at: http://seer.cancer.gov/statfacts/html/lungb.html Accessed August25, 2016
- 44.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63:3136–3141 [DOI] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration. Developing products for rare diseases & conditions, 2016. Available at: www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/ucm2005525.htm Accessed August25, 2016
- 46.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance—United States, 1999–2011. Chest 2013;144:284–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health 2015;5:020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.