Abstract
While the organization of inanimate systems such as gases or liquids is predominantly thermodynamically driven—a mixture of two gases will tend to mix until they reach equilibrium—biological systems frequently exhibit organization that is far from a well-mixed equilibrium. The anisotropies displayed by cells are evident in some of the dynamic processes that constitute life including cell development, movement, and division. These anisotropies operate at different length-scales, from the meso- to the nanoscale, and are proposed to reflect self-organization, a characteristic of living systems that is becoming accessible to reconstitution from purified components, and thus a more thorough understanding. Here, some examples of self-organization underlying cellular anisotropies at the cellular level are reviewed, with an emphasis on Rho-family GTPases operating at the plasma membrane. Given the technical challenges of studying these dynamic proteins, some of the successful approaches that are being employed to study their self-organization will also be considered.
INTRODUCTION
In a Perspective article on what important problems lay ahead in biological research in the 21st century, self-organization was proposed as a relatively underdeveloped, yet important area of biological research (Kirschner et al., 2000). A more recent review by Karsenti also highlighted the importance of self-organization in cell biology (Karsenti, 2008). These reviews provide an excellent historical and conceptual background on key experiments in the physical and biological sciences that illustrate the importance of self-organization, distinguishing it from other types of physical organization such as self-assembly (see Table 1 for similarities, differences, and examples of each). However, preceding these thoughtful commentaries by some 50 years, Schrödinger had grappled with the problem of whether the laws of physics and chemistry could adequately explain the organization observed in living matter, especially the “organism’s astonishing gift of concentrating a ‘stream of order’ on itself, thus escaping the decay into atomic chaos—of ‘drinking orderliness’ from a suitable environment” (Schrödinger, 1944, p. 77). Schrödinger’s elixir that bestows orderliness is energy, since it is the dissipation of energy that fuels the cell’s ability to organize its constituents, thus propagating life and, at least fleetingly, holding entropy and the second law of thermodynamics at bay (Prigogine and Stengers, 1984).
TABLE 1:
Characteristic properties and examples of structures generated by self-assembly versus self-organization.
| Involves multiple, interacting components? | Displays increase in order in space/time? | Requires energy input for maintenance? | Distance from thermodynamic equilibrium | Example of structure or system | |
|---|---|---|---|---|---|
| Self-assembly | Yes | Yes | No | Near | Liposome, ribosome, proteasome, bacteriophage |
| Self-organization | Yes | Yes | Yes | Far | Mitotic spindle, actin cytoskeleton, cell cycle |
Note how the structures ensuing from self-organized systems tend to display higher rates of flux, or turnover of components, than structures derived via self-assembly.
How does energy consumption generate order and contribute to self-organization? Energy consumption enables cells to actively organize their constituents away from thermodynamic equilibrium, underpinning remarkable phenomena associated with life: cell growth, movement, and multicellular development (Figure 1). Consider the properties that actin confers on these processes, for example. The polymerization of monomeric actin into filamentous actin (F-actin), and the assembly of these filaments into higher-order structures via actin-binding proteins, contribute to a cytoskeletal network capable of concentrating or dispersing cellular constituents on demand (Pollard and Cooper, 2009). At a molecular scale, some of these dynamic properties emerge from the differential rate of ATP–actin incorporation into opposite ends of the polymerizing filament (Drenckhahn and Pollard, 1986; Pollard, 1986) and from the ATP-dependent motility of filament-associated motors along the filament (Kuznetsov et al., 1992). This type of system is capable of transporting vesicles in a directed manner, resulting in the concentration of lipids and protein at specific sites on the plasma membrane at higher concentrations than would be expected from random, diffusion-based movement (Schott et al., 2002; Schuh, 2011). By concentrating reactants locally on the plasma membrane, active transport increases the rates of reaction kinetics and concomitant signaling. This example illustrates how energy dissipation contributes to cellular organization, but in what sense does this reflect self-organization? To appreciate this, we might first consider how actin dynamics are integrated within signal transduction pathways that control the actin cytoskeleton temporally and spatially. We will then see how the actin cytoskeleton, in turn, influences signaling proteins via feedback mechanisms. The ordered structures that emerge constitute a self-organized system endowed with self-regulatory properties.
FIGURE 1:
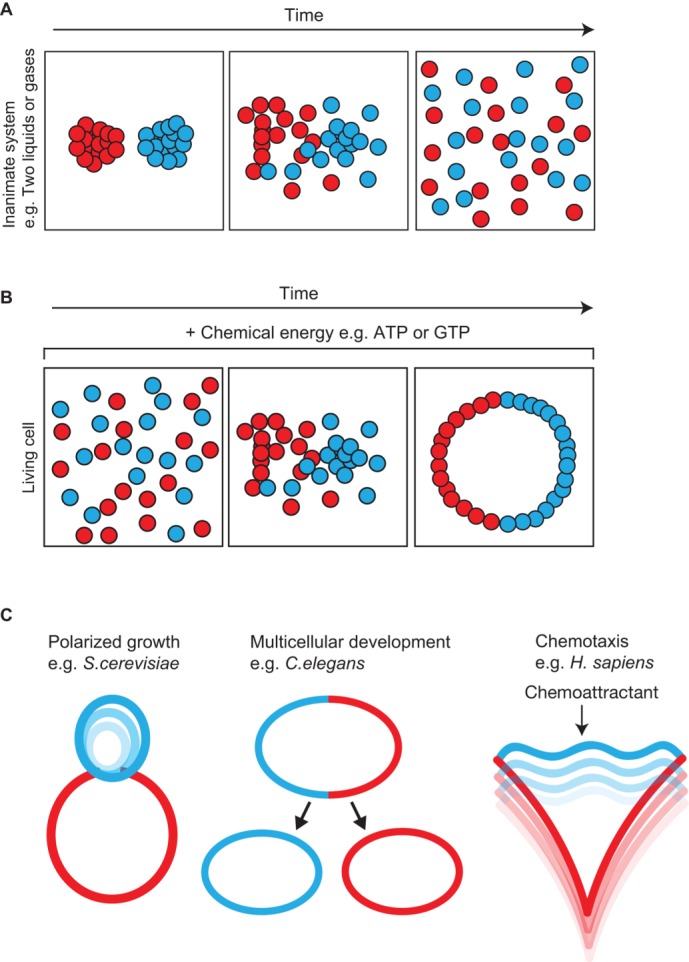
The dissipation of energy is used to drive dynamic cellular processes via self-organization. (A) When added together, two different colored liquids mix until they reach thermodynamics equilibrium. (B) Living cells are energy-dissipating systems that harness chemical energy to drive their constituents far from thermodynamic equilibrium. (C) Examples of nonequilibrium processes in biology include polarized growth, asymmetric cell divisions associated with stem cells during development, and chemotaxis, a stimulus-driven form of cellular migration.
THE MESOSCALE: RHO GTPase REGULATION, DYNAMICS AND RELATIONSHIP WITH ACTIN
Actin organization is intimately linked to Rho guanine triphosphate (GTP)ase function in eukaryotic cells. Rho-family GTPases use GTP as an energy currency to drive conformational changes in GTPase structure that toggle them between active and inactive states. This is a feature shared among the wider Ras superfamily of GTPases to which Rho GTPases belong (Bourne et al., 1990, 1991). When bound to GTP and active, Rho GTPases interact with effector proteins, including actin nucleation-promoting factors such as formins and neuronal Wiskott–Aldrich syndrome protein (n-WASP) (Kohno et al., 1996; Evangelista et al., 1997; Rohatgi et al., 1999) and membrane trafficking regulators such as the exocyst tethering complex (Guo et al., 2001; Inoue et al., 2003). At steady state, the localization of active Rho GTPases on membranes is frequently anisotropic, reflecting their function as critical regulators of cellular polarity. This anisotropic localization and activation is essential, enabling Rho GTPases to exert control over the actin cytoskeleton and cellular polarity more generally. Thus, Rho GTPases have been likened to the cell’s compass, enabling the organization and definition of the cell’s front and back (Bourne and Weiner, 2002; Yang et al., 2016).
While many Rho GTPases appear organized anisotropically at steady state—they localize to the front of migrating cells, the tips of growing yeast, and the equatorial plane of dividing cells—they nevertheless display striking dynamics. For example, during wound healing in Xenopus laevis (frog) oocytes, concentric rings of active Rho and Cdc42 constrict independently of myosin contractility (Burkel et al., 2012). In plant pollen tubes, the Rho-like GTPase (ROP)1 displays pulsatile oscillations at the cell tip during polarized growth (Hwang et al., 2005). Similarly, in the rod-shaped fission yeast Schizosaccharomyces pombe, which grows at the cell tips, the Rho GTPase Cdc42 and its activators oscillate in an anticorrelated manner at the growing cell ends (Das et al., 2012). Oscillations involve rapid signal amplification and attenuation and can display ultrasensitive, nonlinear kinetics that are driven by positive and negative feedback (Ferrell and Ha, 2014a,b,c). Thus, the enrichment of Cdc42 at one cell tip in fission yeast is thought to require autocatalytic Cdc42-GTP production by an activating guanine nucleotide exchange factor (GEF) and an associated scaffold protein, while subsequent depletion at this tip, which precedes accumulation at the opposite tip, requires delayed negative feedback generated by a p21-activated kinase, which phosphorylates and inhibits the GEF (Das et al., 2015a). Oscillations in the Cdc42 GTPase module have also been observed in the budding yeast Saccharomyces cerevisae (Howell et al., 2012). These oscillations may also be driven by scaffold-mediated GTPase module regulation (Kozubowski et al., 2008; Smith et al., 2013; Rapali et al., 2017). Thus, a simple GTPase module that comprises a GTPase, an activator, and an inhibitor can generate a complex oscillatory switch that contributes to the pattern of cell growth and cell shape in an apparently self-organized manner.
The use of positive feedback coupled to delayed negative feedback appears to be a systems-level property employed by other Rho GTPases. This has resulted in distinct, but equally striking, Rho dynamics in diverse species. For example, during furrow ingression in Xenopus and Patiria miniata (starfish) eggs and embryos, Rho-GTP and F-actin have been observed to move along the cortex as traveling waves where F-actin follows active Rho (Bement et al., 2015). Similar to the oscillations in fission yeast, Rho-GTP is thought to be produced in an autocatalytic manner at the leading front of the wave by positive feedback. High spatial and temporal resolution imaging revealed that the product of Rho-GTP, F-actin, follows in the wake of the GTPase, inhibiting Rho-GTP production. While the mechanism by which F-actin inhibits Rho-GTP is not currently known in frogs, F-actin could inhibit Rho by recruiting Rho GTPase activating proteins, as has been demonstrated during pulsatile contractions of the Caenorhabditis elegans embryo (Michaux et al., 2018). Collectively, these dynamics of the GTPase module fit well with the definition of an excitable system; that is, a system in which a threshold signal is exceeded, inducing an accumulation of the active signaling protein that subsequently produces its own inhibitor (Allard and Mogilner, 2013). The Rho-actin waves in frogs and starfish are coupled to the cell cycle machinery via cyclin-dependent kinase 1 (Cdk1) activity, facilitating the coordination of GTPase activation with cell cycle progression so that furrow ingression is initiated at the correct time during the cell cycle (Bement et al., 2015). Interestingly, the activity of Cdk1 has itself been demonstrated to spread via a wave in Xenopus eggs (Chang and Ferrell, 2013). Traveling waves of Cdk1 are biologically important because frog eggs are very large cells (approximately 600 µm in radius) in which the propagation of biochemical signals via waves is much faster than propagation via passive diffusion through the cytoplasm. Theoretically, an average-sized protein would take hours to diffuse across a frog egg, whereas a wave takes tens of seconds to traverse the same distance. As a consequence, the organization of a biochemical signal within a traveling wave enables large cells and embryos to rapidly and decisively respond to changes in Cdk1 activity. Consistently, Cdk1 activity is also organized as waves in large Drosophila embryos during syncytial nuclear divisions (Deneke et al., 2016; Deneke and Di Talia, 2018). Examples of self-organization are widespread in multicellular development and morphogenesis (Schweisguth and Corson, 2019; Shahbazi et al., 2019).
While self-organizing oscillations and traveling waves have only been discussed here in the context of polarized growth and furrow ingression during cytokinesis, similar dynamic phenomena have also been documented in diverse biological contexts (Inagaki and Katsuno, 2017), including neuronal outgrowth (Ruthel and Banker, 1998; Winans et al., 2016), neutrophil chemotaxis (Weiner et al., 2007), Dictyostelium locomotion, and keratinocyte migration (Vicker, 2000; Barnhart et al., 2011). Many of these cells migrate. A common theme in cell migration, and cellular polarity more generally, is the generation of a cell “front” and “back.” In the example of migration, actin polymerization is harnessed at the front to power protrusion and myosin contractility is concentrated at the cell back. These two activities are under the control of Rac and Rho GTPases, respectively, and importantly, while each GTPase is thought to be enriched to the front or back via positive feedback, the two GTPases also display mutual antagonism (Xu et al., 2003). Positive feedback within GTPase modules and antagonism between modules at the front and back of cells appears to be a mechanism that establishes distinct functional domains. For example, the Par6-aPKC module and the Par1 module display mutual antagonism in worm embryos, fly oocytes, and mammalian epithelia that underpins cell development and tissue organization (Figure 2) (St Johnston and Ahringer, 2010). Mechanistically, positive feedback combined with mutual antagonism are proposed to make polarity pathways more robust, and when incorporated into synthetic regulatory signaling networks, these systems-level properties have been observed to support the self-organization of stable lipid domains in the plasma membrane of cells (Chau et al., 2012).
FIGURE 2:
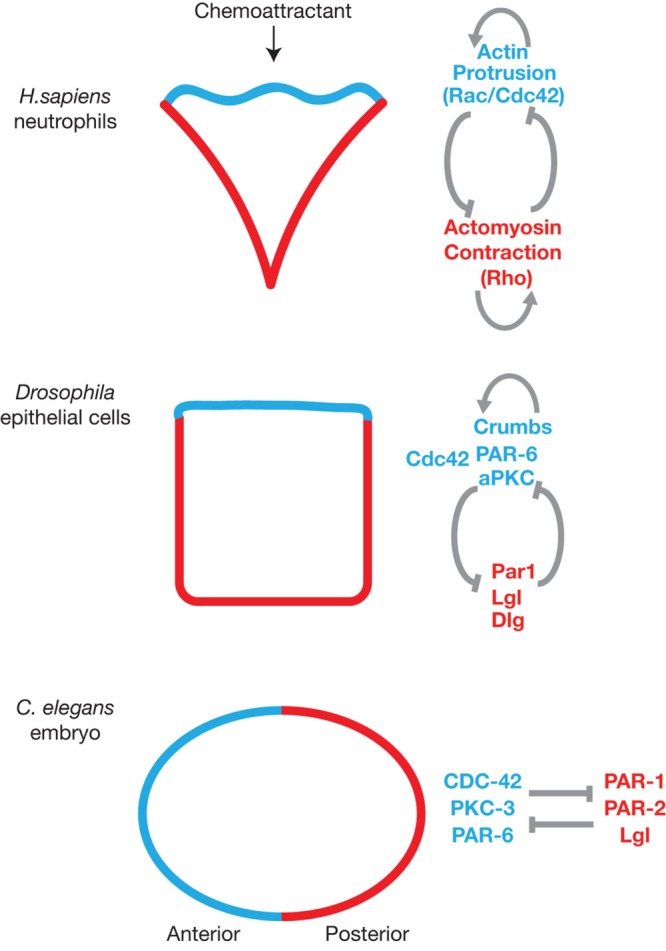
Examples of signaling network configurations associated with self-organizing systems. The signaling networks shown on the right are required for the formation of the discrete domains illustrated in red and blue on the left. Feedback is thought to play a role in these examples of self-organization, although additional mechanisms are also likely to be important. Positive and negative feedback are illustrated as gray arrows and lines, respectively. References for the examples shown: neutrophil chemotaxis (Wong et al., 2006); Drosophila epithelial cells (Fletcher et al., 2012; Tian and Deng, 2008); C. elegans embryo (Motegi et al., 2011; Ramanujam et al., 2018).
THE NANOSCALE: MEMBRANES, LIPIDS, AND GTPases
The function of Rho GTPases and the wider Ras superfamily requires their association with membrane (Buss et al., 1989; Hancock et al., 1990). In the case of Rho GTPases, they frequently interact with the plasma membrane via isoprenylation and carboxyl-methylation of a C-terminal cysteine residue (Ghomashchi et al., 1995). This, together with juxtaposed polybasic residues, facilitates the high affinity interaction of these GTPase with membrane lipids (Finegold et al., 1991). Lipids provide an essential physicochemical environment in which GTPases of the Rho and the wider Ras superfamily reside. Lipids regulate GTPase activity, mobility in the plasma membrane, and the recruitment of downstream effectors. The diffusion of peripheral membrane proteins such as GTPases is reduced by around two orders of magnitude in membrane compared with their diffusion in the cytosol, potentially increasing the probability of encounters with protein partners via local concentration effects, although such effects must be investigated case by case (Groves and Kuriyan, 2010). Therefore, the mechanisms that confine GTPase diffusion in membranes, for example by promoting their clustering, contribute to signaling. Before focusing on the contribution of specific lipids to GTPase self-organization via these mechanisms, it is worth considering some aspects of membrane organization in cells that may be indicative of self-organization more generally.
Along the secretory pathway, the anionic nature of lipid polar head groups tends to increase, as does acyl chain saturation, sterol content, and membrane thickness, being most pronounced at the plasma membrane (Bigay and Antonny, 2012). Transmembrane (TM) proteins tend to self-organize along the secretory pathway to minimize energy cost, such that TM length matches membrane thickness. Consequently, the TM domain of plasma membrane proteins tends to be longer (around 20 amino acids) than Golgi-resident TM proteins (around 15 amino acids) (Bretscher and Munro, 1993; Munro, 1995; Sharpe et al., 2010). This physical means of self-organization makes the plasma membrane thicker and thus a more protective barrier to the outside world.
The chemistry of the plasma membrane also contributes to self-organization (McLaughlin and Murray, 2005). The negative charge of anionic lipids serves as a beacon for the recruitment of peripheral membrane proteins containing clusters of basic residues (Honigmann et al., 2013). Electrostatic interactions between anionic lipids and basic residues in low-complexity regions of proteins can locally concentrate anionic lipids such as phosphatidylinositol 4,5-bisphosphate (PIP2) (Denisov et al., 1998), forming clusters in the plasma membrane to which additional proteins containing basic clusters are recruited (Picas et al., 2014, 2016). Electrostatic interactions between specific protein domains such as bim, amphiphysin, and rvs (BAR) domains and PIP2 can also initiate stable domain formation (Zhao et al., 2013). The result of this lipid-protein feedback may be the demixing of anionic lipids from the membrane milieu and the emergence of organized membrane domains containing clustered proteins (van den Bogaart et al., 2011; Recouvreux and Lenne, 2016).
The dynamic organization of signaling proteins is now amenable to investigation at single molecule resolution in living cells, facilitating insight into self-organization at the nanoscale (Garcia-Parajo et al., 2014; Goyette and Gaus, 2017). For example, canonical Ras superfamily members H-, K-, and N-Ras are not organized randomly, but are concentrated in discrete nanoscale ensembles termed nanoclusters in the plasma membrane (Murakoshi et al., 2004; Plowman et al., 2005). Nanoclusters are signaling hotspots from which Ras signals emanate. Different Ras isoforms contain distinct polybasic sequences in their C-termini. These sequences, in combination with different Ras prenylation signatures, are thought to target the GTPases to specific nanoclusters (Zhou et al., 2017). Recent work indicates that the propensity of Ras GTPases to undergo nanoclustering extends to the Rho family, since Rac has been observed in nanoclusters in the plasma membrane of human cells and Rho-related proteins are observed in nanoclusters in plants (Das et al., 2015b; Remorino et al., 2017; Platre et al., 2019). Moreover, in budding yeast, the Rho GTPase Cdc42 has also been reported to be organized in nanoclusters, whose size correlates with GTPase activation (Sartorel et al., 2018; Meca et al., 2019). Both K-Ras and Cdc42 nanoclustering are controlled by specific anionic lipids including phosphatidylserine, and by regulatory components of their respective GTPase modules (Cho et al., 2012; Blazevits et al., 2016; Zhou et al., 2017). These factors influence nanoclustering by controlling the lateral diffusion of GTPases in the plasma membrane and via mechanisms that await identification. Thus, nanoclustering is emerging as a critical property shared among Ras superfamily members in evolutionary distant species. The conservation of these features implies their importance. However, it is presently unclear how mechanisms operating on these nanoscale ensembles are propagated at the mesoscale to generate the complex, ensemble behavior exhibited by signaling systems in vivo, including the oscillations, traveling waves, and decisive, switch-like properties discussed previously (Bement et al., 2015; Deneke and Di Talia, 2018; Fukushima et al., 2019). Nevertheless, theoretical work has highlighted that positive feedback within signaling networks has the potential to promote protein clustering on membranes and the rapid dissemination of active species across membranes (Das et al., 2009).
The reconstitution of dynamic protein assemblies in model membranes is proving to be a promising avenue to explore how self-organization may arise in the plasma membrane. For example, in addition to the effect of BAR domains on model membranes mentioned previously, actin polymerization has also been found to trigger the demixing of homogeneous lipid mixtures into domains enriched in PIP2 (Liu and Fletcher, 2006). The addition of myosin to actin networks tethered to supported lipid bilayers has also enabled the reconstitution of a self-organizing actomyosin-membrane network (Koster et al., 2016). Importantly, the system depended on a source of chemical energy, displayed actin organized in bundles or asters depending on the ratio of components added, and was capable of clustering an actin-associated protein. Remarkably, when an energy regenerating system was incorporated into this reconstituted network, the system displayed constant remodeling and confinement due to interactions between the system’s components (Koster et al., 2016). The formation of actin asters observed in the reconstituted system is also associated with the self-organization of the actin cortex observed in living cells (Gowrishankar et al., 2012; Fritzsche et al., 2017). Moreover, in the plasma membrane of human cells, a sizeable fraction of phosphatidylserine appears to be corralled in an actin-dependent manner, suggesting that the reconstituted systems recapitulate salient properties of plasma membrane self-organization (Kay et al., 2012).
A final example of an anionic lipid involved in cellular self-organization is the product of phosphoinositide 3-kinase (PI3K), phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 has been identified as an important constituent of Ras and Rho-family GTPase self-organization in Dictyostelium migration (Sasaki et al., 2004, 2007), mammalian neuronal polarity (Fivaz et al., 2008), and neutrophil chemotaxis (Xu et al., 2003; Wong et al., 2006). In these systems, the GTPases activate PI3K at the leading edge of the cell, generating PIP3 that is proposed to activate additional GTPase via positive feedback. Mechanistically, it seems likely that PIP3 recruits cognate GEFs of the GTPases; however, this is not presently well understood. Additionally, the enrichment of PIP3 levels at the cell front in Dictyostelium antagonizes the membrane recruitment of its own inhibitor, the PIP3 phosphatase PTEN, providing another example of inhibition being used as a means of self-organization (Matsuoka and Ueda, 2018). In these cells, PIP3 also forms waves on the plasma membrane that colocalize with active Ras, again underscoring the close relationship between GTPase activation and lipid dynamics during self-organization (Gerisch et al., 2011; Shibata et al., 2012; Fukushima et al., 2019).
CONCLUSION
Self-organization appears to be an intrinsic property of at least some, and perhaps all, GTPase-driven pathways. Energy dissipation in these systems enables the emergence of a highly dynamic steady state in which multiple system components become organized in space and time. These properties appear to endow cells with timely responsiveness when perturbations are experienced. For example, migrating cells will rapidly move around encountered barriers before continuing on their path. Similarly, plasma membrane wounds are rapidly sensed and healed. Whether general principles of self-organization emerge from the study of GTPase-dependent processes is impossible to predict, but the use of diverse experimental models and approaches is providing insight into Schrödinger’s wonder at the “organism’s astonishing gift of concentrating a ‘stream of order’ on itself” (Schrödinger, 1944, p. 77).
Acknowledgments
Anne Royou, Aurélie Massoni-Laporte, and Andrew Weatherall are thanked for their input during the drafting of the article. Work in my lab is supported by the Centre National de la Recherche Scientifique, ANR-13-BSV2-0015-01, the Regional Council of Aquitaine, and the University of Bordeaux through the Synthetic Biology in Bordeaux program (SB2).
Abbreviations used
- Cdk1
cyclin-dependent kinase
- F-actin
filamentous actin
- GEF
guanine nucleotide exchange factor
- GTP
guanine triphosphate
- n-WASP
neuronal Wiskott–Aldrich syndrome protein
- PI3K
phosphoinositide 3-kinases
- PIP 2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- ROP
Rho-like GTPase
- TM
transmembrane.
Footnotes
REFERENCES
- Allard J, Mogilner A. (2013). Traveling waves in actin dynamics and cell motility. Curr Opin Cell Biol , 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart EL, Lee KC, Keren K, Mogilner A, Theriot JA. (2011). An adhesion-dependent switch between mechanisms that determine motile cell shape. PLoS Biol , e1001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement WM, Leda M, Moe AM, Kita AM, Larson ME, Golding AE, Pfeuti C, Su KC, Miller AL, Goryachev AB, et al. (2015). Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat Cell Biol , 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Antonny B. (2012). Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev Cell , 886–895. [DOI] [PubMed] [Google Scholar]
- Blazevits O, Mideksa YG, Solman M, Ligabue A, Ariotti N, Nakhaeizadeh H, Fansa EK, Papageorgiou AC, Wittinghofer A, Ahmadian MR, et al. (2016). Galectin-1 dimers can scaffold Raf-effectors to increase H-ras nanoclustering. Sci Rep , 24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. (1990). The GTPase superfamily: a conserved switch for diverse cell functions. Nature , 125–132. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. (1991). The GTPase superfamily: conserved structure and molecular mechanism. Nature , 117–127. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Weiner O. (2002). A chemical compass. Nature , 21. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Munro S. (1993). Cholesterol and the Golgi apparatus. Science , 1280–1281. [DOI] [PubMed] [Google Scholar]
- Burkel BM, Benink HA, Vaughan EM, von Dassow G, Bement WM. (2012). A Rho GTPase signal treadmill backs a contractile array. Dev Cell , 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss JE, Solski PA, Schaeffer JP, MacDonald MJ, Der CJ. (1989). Activation of the cellular proto-oncogene product p21Ras by addition of a myristylation signal. Science , 1600–1603. [DOI] [PubMed] [Google Scholar]
- Chang JB, Ferrell JE., Jr (2013). Mitotic trigger waves and the spatial coordination of the Xenopus cell cycle. Nature , 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau AH, Walter JM, Gerardin J, Tang C, Lim WA. (2012). Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell , 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KJ, Kasai RS, Park JH, Chigurupati S, Heidorn SJ, van der Hoeven D, Plowman SJ, Kusumi A, Marais R, Hancock JF. (2012). Raf inhibitors target ras spatiotemporal dynamics. Curr Biol , 945–955. [DOI] [PubMed] [Google Scholar]
- Das M, Drake T, Wiley DJ, Buchwald P, Vavylonis D, Verde F. (2012). Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science , 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Kardar M, Chakraborty AK. (2009). Positive feedback regulation results in spatial clustering and fast spreading of active signaling molecules on a cell membrane. J Chem Phys , 245102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Nunez I, Rodriguez M, Wiley DJ, Rodriguez J, Sarkeshik A, Yates JR, 3rd, Buchwald P, Verde F. (2015a). Phosphorylation-dependent inhibition of Cdc42 GEF Gef1 by 14-3-3 protein Rad24 spatially regulates Cdc42 GTPase activity and oscillatory dynamics during cell morphogenesis. Mol Biol Cell , 3520–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Yin T, Yang Q, Zhang J, Wu YI, Yu J. (2015b). Single-molecule tracking of small GTPase Rac1 uncovers spatial regulation of membrane translocation and mechanism for polarized signaling. Proc Natl Acad Sci USA , E267–E276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke VE, Di Talia S. (2018). Chemical waves in cell and developmental biology. J Cell Biol , 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke VE, Melbinger A, Vergassola M, Di Talia S. (2016). Waves of Cdk1 activity in S phase synchronize the cell cycle in Drosophila embryos. Dev Cell , 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. (1998). Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys J , 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D, Pollard TD. (1986). Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J Biol Chem , 12754–12758. [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. (1997). Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science , 118–122. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Ha SH. (2014a). Ultrasensitivity part I: Michaelian responses and zero-order ultrasensitivity. Trends Biochem Sci , 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Ha SH. (2014b). Ultrasensitivity part II: multisite phosphorylation, stoichiometric inhibitors, and positive feedback. Trends Biochem Sci , 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Ha SH. (2014c). Ultrasensitivity part III: cascades, bistable switches, and oscillators. Trends Biochem Sci , 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold AA, Johnson DI, Farnsworth CC, Gelb MH, Judd SR, Glomset JA, Tamanoi F. (1991). Protein geranylgeranyl transferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci USA , 4448–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivaz M, Bandara S, Inoue T, Meyer T. (2008). Robust neuronal symmetry breaking by Ras-triggered local positive feedback. Curr Biol , 44–50. [DOI] [PubMed] [Google Scholar]
- Fletcher GC, Lucas EP, Brain R, Tournier A, Thompson BJ. (2012). Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr Biol , 1116–1122. [DOI] [PubMed] [Google Scholar]
- Fritzsche M, Li D, Colin-York H, Chang VT, Moeendarbary E, Felce JH, Sezgin E, Charras G, Betzig E, Eggeling C. (2017). Self-organizing actin patterns shape membrane architecture but not cell mechanics. Nat Commun , 14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima S, Matsuoka S, Ueda M. (2019). Excitable dynamics of Ras triggers spontaneous symmetry breaking of PIP3 signaling in motile cells. J Cell Sci , jcs224121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Parajo MF, Cambi A, Torreno-Pina JA, Thompson N, Jacobson K. (2014). Nanoclustering as a dominant feature of plasma membrane organization. J Cell Sci , 4995–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G, Ecke M, Wischnewski D, Schroth-Diez B. (2011). Different modes of state transitions determine pattern in the phosphatidylinositide-actin system. BMC Cell Biol , 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghomashchi F, Zhang X, Liu L, Gelb MH. (1995). Binding of prenylated and polybasic peptides to membranes: affinities and intervesicle exchange. Biochemistry , 11910–11918. [DOI] [PubMed] [Google Scholar]
- Gowrishankar K, Ghosh S, Saha S, Rumamol C, Mayor S, Rao M. (2012). Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell , 1353–1367. [DOI] [PubMed] [Google Scholar]
- Goyette J, Gaus K. (2017). Mechanisms of protein nanoscale clustering. Curr Opin Cell Biol , 86–92. [DOI] [PubMed] [Google Scholar]
- Groves JT, Kuriyan J. (2010). Molecular mechanisms in signal transduction at the membrane. Nat Struct Mol Biol , 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Tamanoi F, Novick P. (2001). Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol , 353–360. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Magee AI, Childs JE, Marshal CJ. (1990). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell , 1167–1177. [DOI] [PubMed] [Google Scholar]
- Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, Mullar S, Diederichsen U, Fasshauer D, Grubmuller H, et al. (2013). Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat Struct Mol Biol , 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AS, Jin M, Wu CF, Zyla TR, Elston TC, Lew DJ. (2012). Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell , 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JU, Gu Y, Lee YJ, Yang Z. (2005). Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell , 5385–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Katsuno H. (2017). Actin waves: origin of cell polarization and migration? Trends Cell Biol , 515–526. [DOI] [PubMed] [Google Scholar]
- Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. (2003). The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature , 629–633. [DOI] [PubMed] [Google Scholar]
- Karsenti E. (2008). Self-organization in cell biology: a brief history. Nat Rev Mol Cell Biol , 255–262. [DOI] [PubMed] [Google Scholar]
- Kay JG, Koivusalo M, Ma X, Wohland T, Grinstein S. (2012). Phosphatidylserine dynamics in cellular membranes. Mol Biol Cell , 2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Gerhart J, Mitchison T. (2000). Molecular “vitalism.” Cell , 79–88. [DOI] [PubMed] [Google Scholar]
- Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, et al. (1996). Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J , 6060–6068. [PMC free article] [PubMed] [Google Scholar]
- Koster DV, Husain K, Iljazi E, Bhat A, Bieling P, Mullins RD, Rao M, Mayor S. (2016). Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proc Natl Acad Sci USA , E1645–E1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Saito K, Johnson JM, Howell AS, Zyla TR, Lew DJ. (2008). Symmetry-breaking polarization driven by a Cdc42p GEF-PAK complex. Curr Biol , 1719–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DG. (1992). Actin-dependent organelle movement in squid axoplasm. Nature , 722–725. [DOI] [PubMed] [Google Scholar]
- Liu AP, Fletcher DA. (2006). Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys J , 4064–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Ueda M. (2018). Mutual inhibition between PTEN and PIP3 generates bistability for polarity in motile cells. Nat Commun , 4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. (2005). Plasma membrane phosphoinositide organization by protein electrostatics. Nature , 605–611. [DOI] [PubMed] [Google Scholar]
- Meca J, Massoni-Laporte A, Martinez D, Sartorel E, Loquet A, Habenstein B, McCusker D. (2019). Avidity-driven polarity establishment via multivalent lipid-GTPase module interactions. EMBO J . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux JB, Robin FB, McFadden WM, Munro EM. (2018). Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. J Cell Biol , 4230–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F, Zonies S, Hao Y, Cuenca AA, Griffin E, Seydoux G. (2011). Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat Cell Biol , 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. (1995). An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J , 4695–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Iino R, Kobayashi T, Fujiwara T, Ohshima C, Yoshimura A, Kusumi A. (2004). Single-molecule imaging analysis of Ras activation in living cells. Proc Natl Acad Sci USA , 7317–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picas L, Gaits-Iacovoni F, Goud B. (2016). The emerging role of phosphoinositide clustering in intracellular trafficking and signal transduction. F1000Res , F1000 Faculty Rev-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picas L, Viaud J, Schauer K, Vanni S, Hnia K, Fraisier V, Roux A, Bassereau P, Gaits-Iacovoni F, Payrastre B, et al. (2014). BIN1/M-Amphiphysin2 induces clustering of phosphoinositides to recruit its downstream partner dynamin. Nat Commun , 5647. [DOI] [PubMed] [Google Scholar]
- Platre MP, Bayle V, Armengot L, Bareille J, Marques-Bueno MDM, Creff A, Maneta-Peyret L, Fiche JB, Nollmann M, Miege C, et al. (2019). Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science , 57–62. [DOI] [PubMed] [Google Scholar]
- Plowman SJ, Muncke C, Parton RG, Hancock JF. (2005). H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci USA , 15500–15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. (1986). Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol , 2747–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. (2009). Actin, a central player in cell shape and movement. Science , 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigogine I, Stengers I. (1984). Order Out of Chaos: Man’s New Dialogue with Nature, New York: Bantam Books. [Google Scholar]
- Ramanujam R, Han Z, Zhang Z, Kanchanawong P, Motegi F. (2018). Establishment of the PAR-1 cortical gradient by the aPKC-PRBH circuit. Nat Chem Biol , 917–927. [DOI] [PubMed] [Google Scholar]
- Rapali P, Mitteau R, Braun C, Massoni-Laporte A, Unlu C, Bataille L, Arramon FS, Gygi SP, McCusker D. (2017). Scaffold-mediated gating of Cdc42 signalling flux. eLife , e25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recouvreux P, Lenne PF. (2016). Molecular clustering in the cell: from weak interactions to optimized functional architectures. Curr Opin Cell Biol , 18–23. [DOI] [PubMed] [Google Scholar]
- Remorino A, De Beco S, Cayrac F, Di Federico F, Cornilleau G, Gautreau A, Parrini MC, Masson JB, Dahan M, Coppey M. (2017). Gradients of Rac1 nanoclusters support spatial patterns of Rac1 signaling. Cell Rep , 1922–1935. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell , 221–231. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Banker G. (1998). Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: a novel form of axonal transport? Cell Motil Cytoskeleton , 160–173. [DOI] [PubMed] [Google Scholar]
- Sartorel E, Unlu C, Jose M, Massoni-Laporte A, Meca J, Sibarita JB, McCusker D. (2018). Phosphatidylserine and GTPase activation control Cdc42 nanoclustering to counter dissipative diffusion. Mol Biol Cell , 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki AT, Chun C, Takeda K, Firtel RA. (2004). Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol , 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, Meili R, Devreotes PN, Firtel RA. (2007). G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol , 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DH, Collins RN, Bretscher A. (2002). Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol , 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger E. (1944). What Is Life? The Physical Aspect of the Living Cell, Cambridge, UK: Cambridge University Press, 77. [Google Scholar]
- Schuh M. (2011). An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol , 1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth F, Corson F. (2019). Self-organization in pattern formation. Dev Cell , 659–677. [DOI] [PubMed] [Google Scholar]
- Shahbazi MN, Siggia ED, Zernicka-Goetz M. (2019). Self-organization of stem cells into embryos: a window on early mammalian development. Science , 948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe HJ, Stevens TJ, Munro S. (2010). A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell , 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Nishikawa M, Matsuoka S, Ueda M. (2012). Modeling the self-organized phosphatidylinositol lipid signaling system in chemotactic cells using quantitative image analysis. J Cell Sci , 5138–5150. [DOI] [PubMed] [Google Scholar]
- Smith SE, Rubinstein B, Mendes Pinto I, Slaughter BD, Unruh JR, Li R. (2013). Independence of symmetry breaking on Bem1-mediated autocatalytic activation of Cdc42. J Cell Biol , 1091–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J. (2010). Cell polarity in eggs and epithelia: parallels and diversity. Cell , 757–774. [DOI] [PubMed] [Google Scholar]
- Tian AG, Deng WM. (2008). Lgl and its phosphorylation by aPKC regulate oocyte polarity formation in Drosophila. Development , 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, et al. (2011). Membrane protein sequestering by ionic protein-lipid interactions. Nature , 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicker MG. (2000). Reaction-diffusion waves of actin filament polymerization/depolymerization in Dictyostelium pseudopodium extension and cell locomotion. Biophys Chem , 87–98. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Marganski WA, Wu LF, Altschuler SJ, Kirschner MW. (2007). An actin-based wave generator organizes cell motility. PLoS Biol , e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans AM, Collins SR, Meyer T. (2016). Waves of actin and microtubule polymerization drive microtubule-based transport and neurite growth before single axon formation. eLife , e12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Pertz O, Hahn K, Bourne H. (2006). Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci USA , 3639–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. (2003). Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell , 201–214. [DOI] [PubMed] [Google Scholar]
- Yang HW, Collins SR, Meyer T. (2016). Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol , 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Michelot A, Koskela EV, Tkach V, Stamou D, Drubin DG, Lappalainen P. (2013). Membrane-sculpting BAR domains generate stable lipid microdomains. Cell Rep , 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Prakash P, Liang H, Cho KJ, Gorfe AA, Hancock JF. (2017). Lipid-sorting specificity encoded in K-Ras membrane anchor regulates signal output. Cell , 239–251.e216. [DOI] [PMC free article] [PubMed] [Google Scholar]


