Abstract
Background
The aim of this study was to evaluate the clinical characteristics of 2 rat models of sepsis for improved cecal ligation/puncture (CLP) and feces intraperitoneal-injection (FIP), including systemic inflammation, organ dysfunction, and blood coagulation.
Material/Methods
Sixty-two male SD rats were randomly divided into 3 groups: a normal control group (NC, n=6), a CLP group (n=28), and a FIP group (n=28). Ten rats each in the CLP and FIP groups were observed for 72-h mortality rate. The remaining 18 rats in each group were divided into 3 subgroups (n=6) according to their post-operation period (6, 12, and 24 h). Abdominal arterial blood was collected to determine the lactic acid (Lac) concentration, prothrombin time (PT), active partial prothrombin time (APTT), plasmic interleukin-6 (IL-6) level, and cardiac troponin (cTnI) level. The intestines, lung, and heart were collected for pathological examination.
Results
The 72-h mortality rates in the CLP and FIP groups were 60% and 100%, respectively. The Lac level in both groups was significantly elevated at 6, 12, and 24 h after modeling. Compared with the NC group, PT in the CLP and FIP groups was prolonged at 12 and 24 h, and APTT was significantly prolonged at 6 h. IL-6 levels in the CLP and FIP groups peaked at 6 h. The cTnI level in the FIP group was significantly higher at 12 h after modeling compared with the NC group. The intestines, lung, and heart were pathologically damaged at 6 h, and this damage worsened over time.
Conclusions
Both modeling methods induced sepsis in rats and closely mimicked the clinical conditions, but FIP was easier to establish and was more suitable for standardization.
MeSH Keywords: Cytokines, Multiple Organ Failure, Sepsis
Background
Sepsis is a common and severe disease in the Intensive Care Unit (ICU), characterized by multiple organ dysfunction induced by a systemic infection. It remains an important topic for research in this field due to its high incidence and high mortality rates [1]. In 2016, the Sepsis-3 Conference defined sepsis as a “life-threatening organ dysfunction caused by a deregulated host response to infection”, and septic shock was defined as a “subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality” [1].
Many animal models for sepsis have been developed to study the pathogenesis and treatment of sepsis [2]. Currently available sepsis models were established mainly by bacteria/endotoxin-attacking or host-barrier sabotaging. However, the bacteria-attacking models have limited applications owing to the inconvenience of bacterial culture and quantification. The International Expert Consensus for Pre-Clinical Sepsis Studies [3] recommended that challenge with lipopolysaccharide (LPS), an endotoxin, is not an appropriate model for replicating human sepsis [4]. Methods used to establish sepsis models by host-barrier sabotaging include cecal ligation/puncture (CLP), colon ascendens stent plantation (CASP), and feces intraperitoneal-injection (FIP). The CLP model was accepted as a typical model of sepsis, as it closely replicates the pathology and physiology of human sepsis [5,6]. However, extensive studies have proved certain limitations of the CLP model, such as instability and difficulty in establishing the model [7,8]. CASP is a model modified from CLP. The advantage of CASP over CLP is that 24 h after CLP, the ligated cecum is covered by adhesive small-bowel loops, whereas in CASP mice the intestinal leakage is still present [8]. A recent study of the FIP model found that it is easy to establish and provides good simulation of human sepsis [9].
Despite the inevitable limitations of animal models of sepsis [2], it is critical find an appropriate animal model of the condition in humans. Hence, in the present study, we compared the 2 rat models of sepsis – improved CLP and FIP – with respect to their inflammatory cytokine release, coagulation function, myocardial injury markers, organ pathogenesis, and mortality, and we discuss their differences, stability, and adaptability.
Material and Methods
Animals
Sixty-two Sprague-Dawley (SD) rats, weighing 270 to 330 g, aged 8 to 10 weeks, and housed in a specific pathogen-free (SPF) environment, were obtained from the Animal Center of Guizhou Medical University (Certificate Number: SCXK [Qian] 2018-0001). Throughout the experiments, tap water and standard laboratory rat chow were provided ad libitum. The study was conducted in accordance with the ethical procedures and policies approved by the Committee of Animal Care and Use of Guizhou Medical University.
Grouping
As shown in Figure 1, 62 rats were randomly (according to random number table) assigned to a normal control group (NC, n=6), a cecal ligation/puncture group (CLP, n=28), or a feces intraperitoneal-injection group (FIP, n=28). Ten rats in the CLP or FIP group were reserved for 72-h mortality. The other 18 rats in the 2 groups were divided into 3 subgroups for parameter determination at 6, 12, and 24 h.
Figure 1.
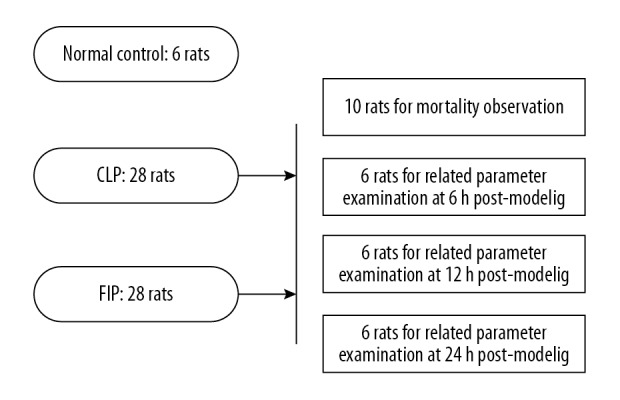
The grouping scheme of the study.
Modeling
The CLP rat model was established according to the method proposed by Wichterman et al. [10], with modifications. In brief, after anesthetization by 2% sodium pentobarbital (40 mg/kg), the colon of the rat was exposed through a longitudinal incision of 1–2 cm. The colon was then ligated at 1/2 to the root and punctured using a No. 12 needle. A thin silicone tube was stabilized to the punctured hole by external purse-string suture. Saline (0.5 mL) was injected through the tube, and diluted feces were made to flow to the abdomen from the colon. The abdomen was sutured after induction of the exposed colon.
The FIP rat model was established according to a method previously described by Shrum et al. and Tyml et al. [11,12]. The feces were collected and suspended in saline, and the feces saline solution was then injected intraperitoneally at 1 g per kg body weight.
Parameters for detection
Blood was collected from abdominal aortas of rats under anesthesia, of which 0.3 mL was used for the detection of lactic acid (Lac) concentration using a blood gas analyzer (i-STAT 300, Abbott, USA), 3 mL was used for assessment of prothrombin time (PT) and active partial prothrombin time (APTT) examination using an Automatic Coagulometer (STA-R Evolution, STAGO, France), and 2 mL was used for interleukin-6 (IL-6) and cardiac troponin I (cTnI) detection using ELISA kits (Elabscience, Wuhan, China). The rats in the 6 h and 24 h subgroups of both CLP and FIP groups were euthanized, and the cardiac apex, inferior lobe of the left lung, and the small intestine (15 cm to the ileocecal area) were collected for HE staining.
Statistical analysis
SPSS 24.0 was used for data analysis. Values are expressed as mean±SD. The Kolmogorov-Smirnov test was used to test the normality of distribution. One-way ANOVA was used for comparison among multiple groups, followed by the LSD test. Kaplan-Meier survival curve analysis was used to compare 72-h mortality rates. P<0.05 was considered statistically significant.
Results
General conditions
Six hours after modeling, the rats exhibited increased oronasal secretion, accelerated respiration, faster abdominal breathing, and curling up of the body. Twelve hours after the operation, the oronasal secretion was increased, the otic skin became pale, and cyanosis in the acral limbs was present. At 24 h after modeling, we observed diarrhea, exacerbated curling up of the body, and systemic shivering. Generally, symptoms in the CLP group were relatively than in the FIP group, as some rats in the CLP group were able to eat and drink independently.
Mortality of both models
Death of the rats in the CLP or FIP groups peaked from 6 h to 12 h after modeling, with the mortality rates at 6 h and 12 h being 30% and 70%, respectively. The 72-h mortality rates of the CLP and FIP groups were 60% and 100%, respectively, and Kaplan-Meier survival curve analysis revealed a significant difference between the 2 groups (P<0.05, Figure 2).
Figure 2.
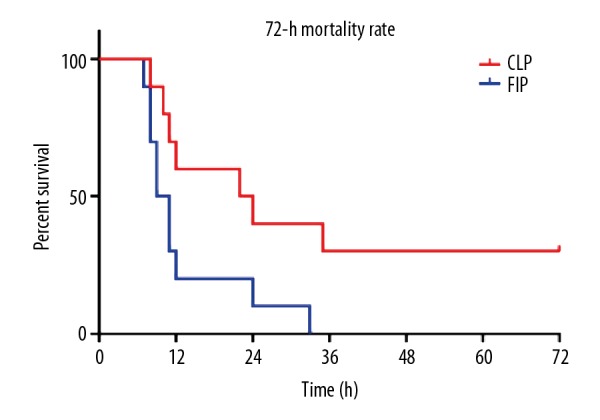
The 72-h mortality rate and K-M survival curve of the CLP and FIP modeling groups. n=10.
Levels of Lac
Compared with the NC group, the level of Lac in the CLP and FIP groups was significantly elevated at 6 h, 12 h, and 24 h after modeling (aa P<0.01 vs. NC). Compared with that in the CLP group, the Lac level was significantly elevated at 6 h after modeling (b P<0.05 vs. CLP). Moreover, the level of Lac in both models increased with time (Figure 3).
Figure 3.
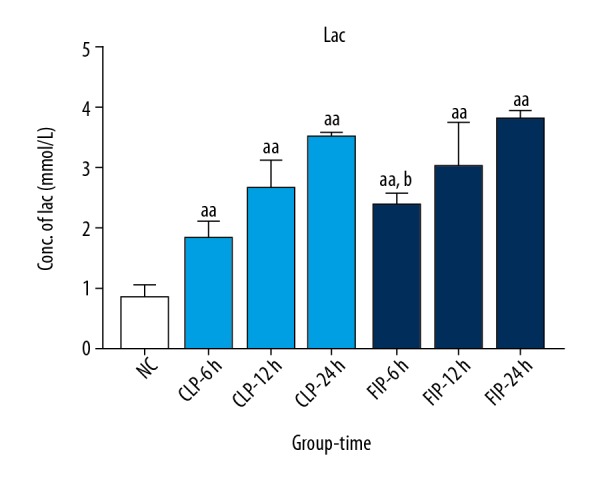
The Lac level of both CLP (light blue) and FIP (deep blue) models at 6 h, 12 h, and 24 h. Values are mean±SD. n=6. aa P<0.01 vs. NC, b P<0.05 vs. CLP-6 h.
Coagulation function
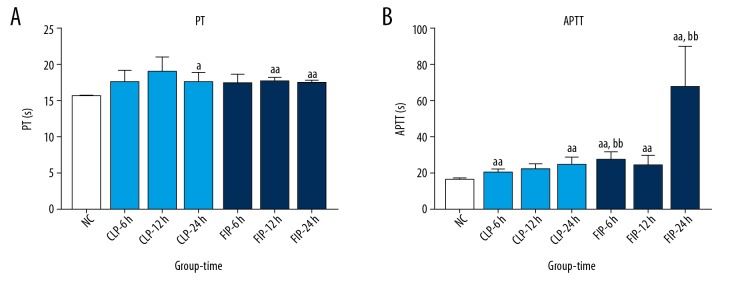
Compared with the NC group, the PT in the CLP group was markedly prolonged at 24 h after modeling (a P<0.05 vs. NC), and that in the FIP group was significantly extended at 12 h and 24 h after modeling (aa P<0.01 vs. NC). No significant differences were observed at any of the time points between the 2 groups. Compared with the NC group, the APTT in the CLP group was significantly prolonged at 6 h and 24 h (aa P<0.01 vs. NC), while APTT in the FIP group was significantly extended at 6 h, 12 h, and 24 h after modeling (aa P<0.01 vs. NC). Compared with the CLP group, the APTT in the FIP group was remarkably longer at 6 h and 24 h after modeling (bb P<0.01 vs. CLP) (Figure 4).
Figure 4.
The PT (A) and APTT (B) of both CLP (light blue) and FIP (deep blue) models at 6 h, 12 h, and 24 h. Values are mean±SD. n=6. a P<0.05 vs. NC, aa P<0.01 vs. NC; bb P<0.01 vs. CLP-6 h or -24 h.
Plasma levels of cTnI and IL-6
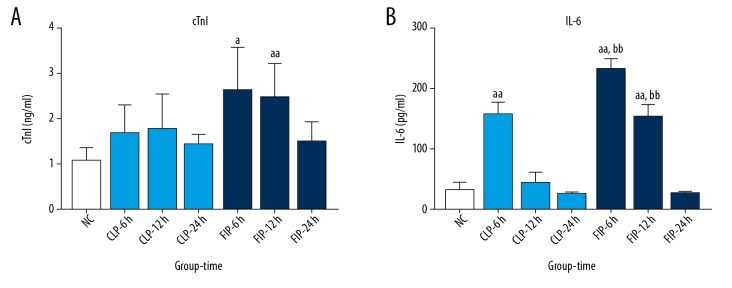
The cTnI level increased in both models compared with that in the NC group. In the CLP group, the increase in the cTnI level was not significant, but at 6 h and 12 h after modeling, the cTnI level in the FIP group was significantly higher compared with that in the NC group (a P<0.05 vs. NC). No significance difference was observed in the cTnI level between the CLP group and FIP group.
The IL-6 level peaked at 6 h after modeling, and the difference was statistically significant compared with that in the NC (aa P<0.01 vs. NC). Compared with that in the CLP group, the IL-6 level in the FIP group was markedly higher at 6 h and 12 h after modeling (bb P<0.01 vs. CLP) (Figure 5).
Figure 5.
The plasmic cTnI (A) and IL-6 (B) of both CLP (light blue) and FIP (deep blue) models at different 6 h, 12 h, and 24 h. Values are mean SD. n=6. a P<0.05, aa P<0.01 vs. NC; bb P<0.01 vs. CLP-6 h or -12 h.
Pathological alteration
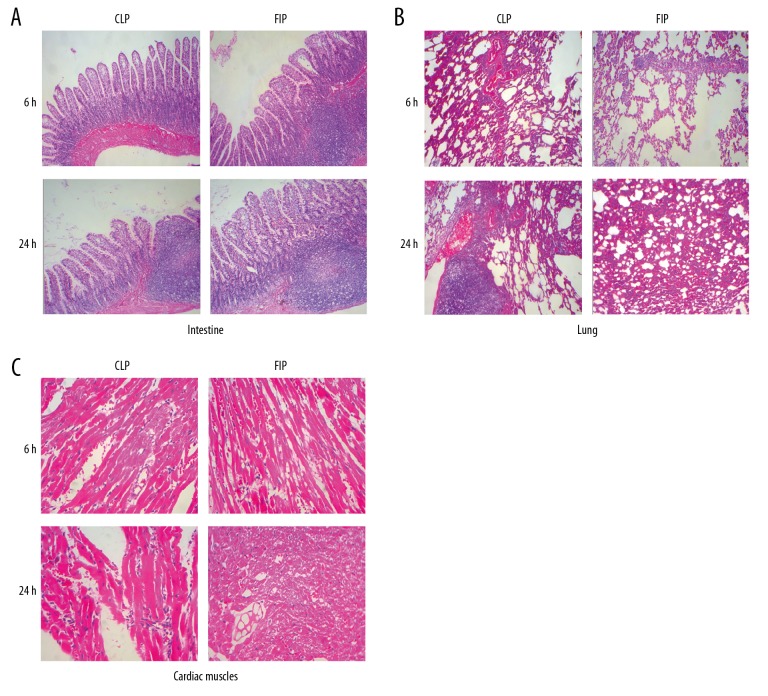
At 6 h after modeling, we observed lesion and inflammatory cell infiltration of different degrees in the small intestine, lung, and heart of both groups, with more observable lesions at 24 h after modeling. In the intestinal mucosa of both modeling groups, we observed a massive inflammatory cell infiltration, some glands disappearing, interstitial vessels dilating and congesting, and tissue edema. In the cardiac tissue of both modeling groups, disarray of myocardial fiber, massive inflammatory cell infiltration, and necrosis were observed, while in the lungs of both modeling groups, we observed destruction of the alveolar septum, dilation, and fusion of pulmonary alveoli, as well as massive inflammatory cell infiltration. Notably, exudation of erythrocytes was found in the alveoli of the FIP group. In general, the degree and range of the intestine, lung, and cardiac muscle from the FIP group were all more severe than those from the CLP group (Figure 6).
Figure 6.
Pathological alteration of CLP and FIP models in the intestines (A), lung (B), and cardiac muscle (C) at 6 h and 24 h. Magnification: 10×.
Discussion
In recent years, considerable progress has been made in investigating sepsis in the ICU. It is important to have a model that mimics the pathological and physiological processes as they occur in clinical practice [10]. For this purpose, the present study compared 2 rat models of sepsis in terms of their methods of establishment, related biomarkers, disease severity, and prognosis.
The Lac level, which is a critical index of diagnosis and prognosis of the disease, can reflect the severity of hypoxia and insufficient perfusion [1,13]. It was found that, compared with that in the NC group, the plasma Lac levels in the 2 models were higher at 6 h after modeling, and increased with time, suggesting that microcirculatory dysfunction of ischemia and hypoxia was present at 6 h. IL-6, a key inflammatory cytokine in sepsis, is mainly produced in monocytes and endothelial cells, and can directly or indirectly induce lesions in vital organs, making it an important index for early diagnosis, severity, and prognosis [14,15]. In this study, early and acute inflammation was present after modeling. At 6 h after modeling, the plasma IL-6 level peaked, indicating possible early inflammatory injury in the 2 models.
Recent studies showed that the interaction of inflammation-coagulation was a cause of multiple organ dysfunctions [16]. Over-release of inflammatory cytokines in sepsis can injure endothelial cells, induce increased expression of tissue factors, activate the coagulation pathway, downregulate the physiological anticoagulant system, and inhibit the fibrinolytic system, inducing massive microthrombus production, enhancing inflammation, and leading to tissue hypoxia, ischemia, and multiple organ dysfunctions [16,17]. In the present research, PT and APTT were longer after modeling, and this is in accordance with previous studies [18]. This condition of insufficient coagulation can be attributed to the depletion of coagulation factors. Clinical studies have shown that the incidence of myocardial injury in sepsis patients is as high as 64%, and the severity of myocardial injury is closely related to early mortality [19,20]. Our experiment showed that the cTnI level, an index of myocardial injury [21], was more evident in the FIP group than in the CLP group, suggesting more severe myocardial damage.
Song et al. [7] demonstrated that multiple organ dysfunction and death occurred at 6–12 h after CLP modeling. The present study verified, through modified CLP and FIP methods, that pathological damage of the intestine, lung, and heart was present as early as 6 h after modeling, and was exacerbated with time. Evidence demonstrated that not all sepsis patients die from the acute stage of the inflammatory cascade; most patients die at the stage of immune inhibition, or due to secondary infection [22]. The results of the present study suggested that alteration in coagulation, microcirculation, and multiple organ dysfunction induced by the FIP modeling method was more severe than in the CLP group, as illustrated by the 100% 72-h mortality rate in the former. This condition could be possibly attributed to the massive release of inflammatory factors in the early stage. Therefore, we recommend that FIP modeling be used to investigate the acute stage of sepsis. In contrast, in the CLP model, the levels of inflammatory factors peaked at 6 h, organ dysfunction was progressively aggravated, and the 72-h mortality rate was maintained at 60%, suggesting that the CLP model could be used in investigations in the acute inflammatory stage, as well as the immune-inhibition stage.
However, although the CLP model has been widely used, its stability, standardization, and repeatability between different labs remain difficult to assure [7,8]. In the CLP method, the hole punctured by a normal needle tends to heal by itself, and the lesion is easily restricted by omentum and peripheral tissue, causing a self-healing process that occurs in the post-operation stage, leading to the failure of this model [8,23]. On the contrary, the typical FIP method is easy to conduct and standardize, and the severity of the disease can be conveniently regulated by the altering the dose of injected feces. Specifically, in our experiment, we modified the typical modeling method of CLP by setting a thin and flexible tube in the cecal hole, followed by dilation of the feces by saline. Therefore, in our modified model, the severity of the disease was closely related to the tube diameter.
Conclusions
Optimization of the animal model is fundamental to sepsis research. Through comparison, the present study shows the extent of applicability of both the improved CLP and FIP models, and the key factors in the process of their modeling. To conclude, the FIP model mimics sepsis induced by peritonitis, while the CLP model mimics sepsis induced by peritoneal abscess. The establishment of FIP is much easier than with CLP, which allows researchers from different labs to much more easily establish the same or more similar sepsis models. Therefore, FIP is a better model than CLP.
Footnotes
Source of support: This study was financially supported by the Guizhou Provincial Science and Technology Cooperation Program (grant number 2016-7249)
Conflict of interest
None.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink MP. Animal models of sepsis. Virulence. 2014;5(1):143–53. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osuchowski MF, Ayala A, Bahrami S, et al. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Intensive Care Med Exp. 2018;6(1):26. doi: 10.1186/s40635-018-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–59. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 5.Starr ME, Steele AM, Saito M, et al. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One. 2014;9(12):e115705. doi: 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Song L, Zou Y, Cao Z. Comparison of two different models of sepsis induced by cecal ligation and puncture in rats. J Surg Res. 2018;229:277–82. doi: 10.1016/j.jss.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 8.Maier S, Traeger T, Entleutner M, et al. Cecal ligation and puncture versus colon ascendens stent peritonitis: Two distinct animal models for polymicrobial sepsis. Shock. 2004;21(6):505–11. doi: 10.1097/01.shk.0000126906.52367.dd. [DOI] [PubMed] [Google Scholar]
- 9.Hayward EN. Electronic Thesis and Dissertation Repository. The university of western Ontario London; Ontario, Canada: 2013. Development of a translational animal model of sepsis. [Google Scholar]
- 10.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock – a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 11.Shrum B, Anantha RV, Xu SX, et al. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 2014;7:233. doi: 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyml K, Swarbreck S, Pape C, et al. Voluntary running exercise protects against sepsis-induced early inflammatory and pro-coagulant responses in aged mice. Crit Care. 2017;21(1):210. doi: 10.1186/s13054-017-1783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englert JA, Rogers AJ. Metabolism, metabolomics, and nutritional support of patients with sepsis. Clin Chest Med. 2016;37(2):321–31. doi: 10.1016/j.ccm.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Liu X, Zhang D, et al. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp Ther Med. 2017;13(6):3479–83. doi: 10.3892/etm.2017.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barre M, Behnes M, Hamed S, et al. Revisiting the prognostic value of monocyte chemotactic protein 1 and interleukin-6 in the sepsis-3 era. J Crit Care. 2018;43:21–28. doi: 10.1016/j.jcrc.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Zarychanski R, Abou-Setta AM, Kanji S, et al. The efficacy and safety of heparin in patients with sepsis: A systematic review and metaanalysis. Crit Care Med. 2015;3(3):511–18. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 18.Li JL, Li G, Jing XZ, et al. Assessment of clinical sepsis-associated biomarkers in a septic mouse model. J Int Med Res. 2018;46(6):2410–22. doi: 10.1177/0300060518764717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulido JN, Afessa B, Masaki M, et al. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87(7):620–28. doi: 10.1016/j.mayocp.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frencken JF, Donker DW, Spitoni C, et al. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes. 2018;11(2):e004040. doi: 10.1161/CIRCOUTCOMES.117.004040. [DOI] [PubMed] [Google Scholar]
- 21.Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: A meta-analysis. Heart Lung. 2015;44(1):75–81. doi: 10.1016/j.hrtlng.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Horiguchi H, Loftus TJ, Hawkins RB, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Wang N, Wei G, et al. Consistency and pathophysiological characterization of a rat polymicrobial sepsis model via the improved cecal ligation and puncture surgery. Int Immunopharmacol. 2016;32:66–75. doi: 10.1016/j.intimp.2015.12.041. [DOI] [PubMed] [Google Scholar]