Abstract
PURPOSE
In patients with diffuse large B-cell lymphoma (DLBCL), most relapses occur within the first 2 years of diagnosis. We sought to define the rate and outcome of late relapses that occurred after achieving event-free survival at 24 months (EFS24).
METHODS
We prospectively followed 1,324 patients with newly diagnosed DLBCL from 2002 to 2015 and treated with immunochemotherapy. Cumulative incidences of late DLBCL and indolent lymphoma relapses were analyzed as competing events. Postrelapse survival was defined as time from first relapse to death from any cause.
RESULTS
In 847 patients who achieved EFS24, the cumulative incidence of late relapse was 6.9% at 3 years, 9.3% at 5 years, and 10.3% at 8 years after EFS24. The incidence of DLBCL relapse was similar in patients with DLBCL alone at diagnosis (6.3% at 5 years), compared with patients with concurrent indolent lymphoma at diagnosis (5.2%; P = .46). However, the rate of indolent lymphoma relapse was higher in patients with concurrent indolent lymphoma (7.4% v 2.1% at 5 years; P < .01). In patients with DLBCL alone, the rate of DLBCL relapse was similar in the germinal center B-cell–like (GCB) (4.1% at 5 years) and non-GCB (4.0%; P = .71) subtypes, whereas the rate of indolent lymphoma relapse was higher in patients with the GCB subtype (3.9% v 0.0% at 5 years; P = .02). Postrelapse survival was inferior for patients who relapsed with DLBCL than for those who relapsed with indolent lymphoma (median 29.9 months v unreached; P < .01).
CONCLUSION
Patients with DLBCL with a concurrent indolent lymphoma and those with the GCB subtype had a higher rate of late relapse, owing to increased relapses with indolent lymphoma. Patients who relapsed with DLBCL had a worse prognosis than those who relapsed with indolent lymphoma.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the Western world. It is a potentially curable disease with the current standard of care of immunochemotherapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or similar regimens. Approximately 50% to 70% of patients can achieve long-term disease-free survival with the standard frontline immunochemotherapy.1-5 The majority of the disease relapses after frontline immunochemotherapy occur within the first 2 years after diagnosis.6 The outcome of patients with an early relapse is generally poor, although some fit patients can receive salvage treatment with high-dose chemotherapy and autologous stem-cell transplantation (ASCT).7-9 On the other hand, as a group, patients who remain event free for 2 years have an excellent prognosis, with minimal to no loss of subsequent survival time compared with the age- and sex-matched general population at 5 years.10-12
However, it is important to note that achieving event-free survival status at 2 years after diagnosis does not necessarily mean a cure for all patients. Although infrequent, late relapses after being event free for 2 years do occur, and in a subset of patients, the relapse is an indolent lymphoma rather than DLBCL. The incidence, clinical characteristics, and outcome of such late relapses have not been well characterized in the immunochemotherapy era. In this study, we investigated the cumulative incidence of late relapse in a prospectively followed cohort of patients with newly diagnosed DLBCL who were treated with R-CHOP or R-CHOP–like immunochemotherapy. We also examined the impact of a concurrent indolent lymphoma identified at diagnosis and DLBCL cell of origin (COO) on the incidence and histologic characterization of late relapses. Finally, we summarized the treatment pattern and clinical outcome of the patients who experienced a late relapse.
METHODS
Patients
This study was reviewed and approved by the institutional review boards at Mayo Clinic and University of Iowa. All patients were from the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence. The MER is a prospective cohort study of lymphoma outcomes that started in 200213 and includes consecutive patients with newly diagnosed lymphoma (within 9 months of diagnosis) who consented to participate. For the current study, patients with newly diagnosed DLBCL from March 2002 to June 2015 who received R-CHOP or R-CHOP–like immunochemotherapy were included. DLBCL was classified per the World Health Organization 2008 classification.14 Patients with MYC and BCL2 and/or BCL6 translocations were not excluded. Patients with concurrent DLBCL and indolent lymphoma at diagnosis (also referred to as composite or discordant lymphoma) were included. Patients with primary mediastinal large B-cell lymphoma, primary cutaneous large B-cell lymphoma who did not receive immunochemotherapy, or primary CNS lymphoma were excluded.
Statistical Analysis
Event-free survival was defined as the time from diagnosis to disease progression or relapse, unplanned retreatment for lack of efficacy, or death from any cause. Event-free survival at 24 months (EFS24) was defined on the basis of the EFS status at 24 months.10 Patients who achieved EFS24 were included for late-relapse analysis.
Cumulative incidences of late-relapse and nonrelapse mortality after achieving EFS24 were analyzed as competing events, using Gray’s test.15 Univariate and multivariable associations between clinical characteristics and late relapse were assessed using the Fine-Gray Method.16 Postrelapse survival was defined as time from first relapse to death from any cause and analyzed using the Kaplan-Meier method. Statistical analyses were performed using R, version 3.4.2 (https://cran.r-project.org/bin/windows/base/old/3.4.2/).
RESULTS
Patients
A total of 1,324 patients with newly diagnosed DLBCL and treated with immunochemotherapy were included. At diagnosis, 1,153 patients (87%) had DLBCL alone, and 171 patients (13%) had concurrent DLBCL and indolent lymphoma: 109 with follicular lymphoma (FL), 15 with marginal zone lymphoma (MZL), 14 with chronic lymphocytic leukemia/small lymphocytic lymphoma, two with lymphoplasmacytic lymphoma, and 31 with unspecified low-grade B-cell lymphoma, mainly in the bone marrow. After a median follow-up of 83.2 months from diagnosis, 457 patients had an event within 24 months after diagnosis, 20 patients remained event free with a follow-up of less than 24 months, and 847 patients remained event free at 24 months after diagnosis (ie, achieved EFS24). Among these, 361 (42.7%) had stage I/II and 485 (57.3%) had stage III/IV disease (stage missing for one patient). For patients with stage I/II disease, 243 (67.3%) were treated with immunochemotherapy alone, with 35 of 213 (16.4%) completing fewer than six cycles of treatment; and 118 (32.7%) were treated with both immunochemotherapy and radiation therapy, with 111 of 116 patients (95.7%) completing either three or four (n = 79) or at last six (n = 32) cycles of immunochemotherapy. For patients with stage III/IV disease, 460 (94.8%) were treated with immunochemotherapy alone, and 25 (5.2%) were treated with both immunochemotherapy and radiation therapy; 59 of 431 patients (13.7%) completed fewer than six cycles of immunochemotherapy. A total of 74 patients (8.7%) received methotrexate-based CNS prophylaxis.
Cumulative Incidence of Late Relapses
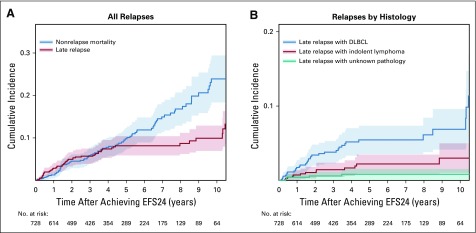
Among the 847 patients who had achieved EFS24, at a median follow-up of 62.9 months from EFS24, 78 experienced a late relapse during follow-up. The cumulative incidence of late relapse was 6.9% (95% CI, 5.3% to 8.9%) at 3 years, 9.3% (95% CI, 6.7% to 11.7%) at 5 years, and 10.3% (95% CI, 7.2% to 13%) at 8 years after achieving EFS24 (Fig 1A). As a reference, the cumulative incidence of nonrelapse mortality (as a competing event) was 5.6% (95% CI, 4.1% to 7.5%) at 3 years, 9.8% (95% CI, 6.4% to 12.5%) at 5 years, and 16.0% (95% CI, 8.7% to 19.8%) at 8 years after achieving EFS24 (Fig 1A).
FIG 1.
Cumulative incidence of (A) late relapse and nonrelapse mortality, and (B) late relapse by histology in patients with diffuse large B-cell lymphoma (DLBCL). EFS24, event-free survival at 24 months.
Clinical Characteristics of Late-Relapse Cases
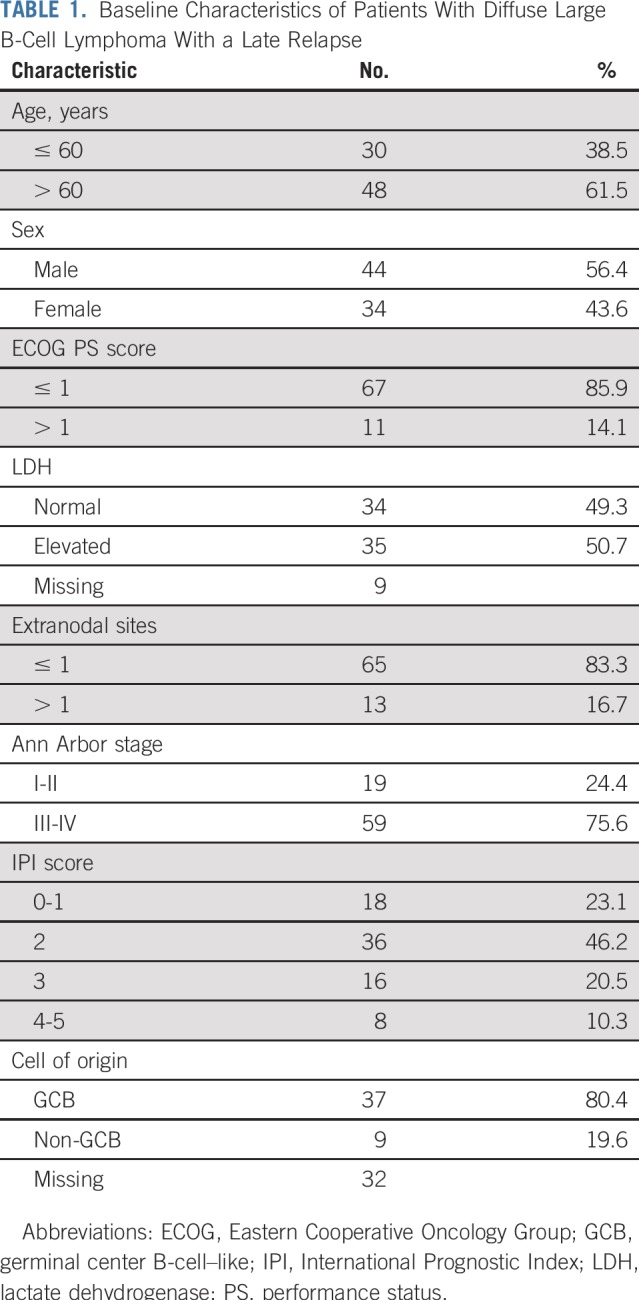
The baseline clinical characteristics at initial diagnosis of the 78 patients who relapsed after achieving EFS24 are listed in Table 1. The median age at diagnosis was 63 (range, 35 to 92) years, and 44 of patients (56.4%) were men. At diagnosis, 11 (14.1%) had an Eastern Cooperative Oncology Group Performance Status score of greater than 1, 34 (49.3%) had lactate dehydrogenase elevation, 59 (75.6%) had stage III-IV disease, 13 (16.7%) had more than one extranodal site, and 24 (30.8%) had intermediate-high or high-risk disease by International Prognostic Index (IPI) score. A total of 55 patients (70.5%) had DLBCL alone at diagnosis, and 23 patients (29.5%) had concurrent DLBCL and indolent lymphoma at initial diagnosis. COO by Hans algorithm was predominantly germinal center B-cell-like (GCB) (n = 37 of 46; 80.4%).
TABLE 1.
Baseline Characteristics of Patients With Diffuse Large B-Cell Lymphoma With a Late Relapse
In the 55 patients with DLBCL alone at diagnosis who experienced a relapse after 2 years, 36 (73.5%) relapsed with DLBCL, 13 (26.5%) relapsed with indolent lymphoma alone (12 FL and one MZL), and the pathology was unknown in six patients (biopsy not done or results not available). In contrast, among the 23 patients with concurrent indolent lymphoma at diagnosis who experienced a relapse after 2 years, nine (45.0%) relapsed with DLBCL, 11 (55.0%) relapsed with indolent lymphoma alone (seven with FL, one with MZL, one with chronic lymphocytic leukemia, one with unspecified indolent lymphoma—all consistent with the pathology of the indolent component at diagnosis; and one mantle cell lymphoma in a patient who initially had concurrent DLBCL and FL), and the pathology was unknown in three patients (biopsy not done or results not available). The cumulative incidence of DLBCL relapse over time was approximately twice as high as that of indolent lymphoma relapse: 4.1% versus 2.0% at 3 years, and 5.4% versus 2.9% at 5 years after achieving EFS24 (Fig 1B).
Impact of Concurrent Indolent Lymphoma and COO on the Late-Relapse Rate
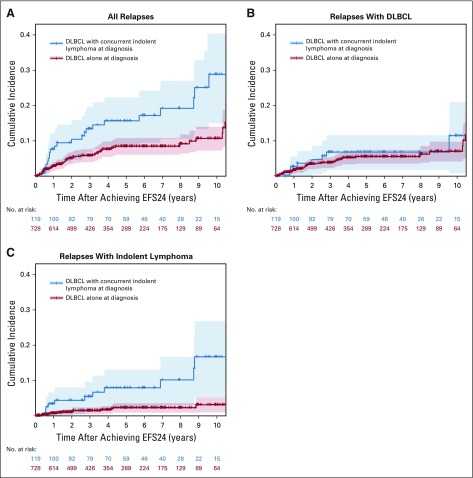
Compared with patients with DLBCL alone at diagnosis, patients who had a concurrent indolent lymphoma at initial diagnosis had a higher cumulative incidence of late relapse (13.3% v 5.7% at 3 years, 15.4% v 8.1% at 5 years; P < .01; Fig 2A). This difference was accounted for by relapses of indolent lymphoma. The cumulative incidences of late relapse with DLBCL were similar in patients with concurrent indolent lymphoma at initial diagnosis and those with DLBCL alone at diagnosis (6.3% v 3.7% at 3 years, 6.3% v 5.2% at 5 years; P = .46; Fig 2B). However, patients with concurrent indolent lymphoma at initial diagnosis had a significantly higher cumulative incidence of late relapse with indolent lymphoma (5.3% v 1.4% at 3 years, 7.4% v 2.1% at 5 years; P < .01; Fig 2C).
FIG 2.
Cumulative incidence of late relapse for (A) all histologies combined, (B) diffuse large B-cell lymphoma (DLBCL), and (C) indolent lymphoma in patients with DLBCL alone and in patients with concurrent DLBCL and indolent lymphoma. EFS24, event-free survival at 24 months.
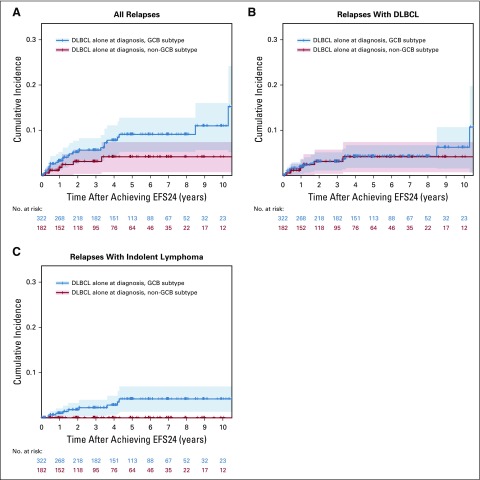
In patients with DLBCL alone at diagnosis, there was a trend in those with the GCB subtype for higher cumulative incidence of late relapse compared with the non-GCB subtype (5.6% v 3.0% at 3 years, 8.8% v 4.0% at 5 years; P = .05; Fig 3A). Interestingly, this difference was also driven by differences in relapses of indolent lymphoma. The cumulative incidences of DLBCL relapse were similar between the GCB and non-GCB subtypes (3.1% v 3.0% at 3 years, 4.1% v 4.0% at 5 years; P = .71; Fig 3B). However, patients with the GCB, but not the non-GCB, subtype had relapses with indolent lymphoma (cumulative incidence 2.2% v 0.0% at 3 years, 3.9% v 0.0% at 5 years; P = .02; Fig 3C).
FIG 3.
Cumulative incidence of late relapse for (A) all histologies combined, (B) diffuse large B-cell lymphoma (DLBCL), and (C) indolent lymphoma in patients with germinal center B-cell–like (GBC) DLBCL and in patients with non-GCB DLBCL. EFS24, event-free survival at 24 months.
In additional univariate analyses, advanced stage (III/IV v I/II) and a higher IPI score (≥ 2 v 0 to 1) were associated with a higher risk of late relapse (Data Supplement). In multivariable analyses (Data Supplement), the associations of concurrent indolent lymphoma and the GCB subtype with the risk of late relapse were independent of stage and IPI. Interestingly, in patients with DLBCL alone, although the GCB subtype was associated with a higher risk of indolent lymphoma but not DLBCL relapse, advanced stage and a higher IPI score were associated with a higher risk of DLBCL but not indolent lymphoma relapse (Data Supplement).
Treatment and Outcome of Late-Relapse Cases
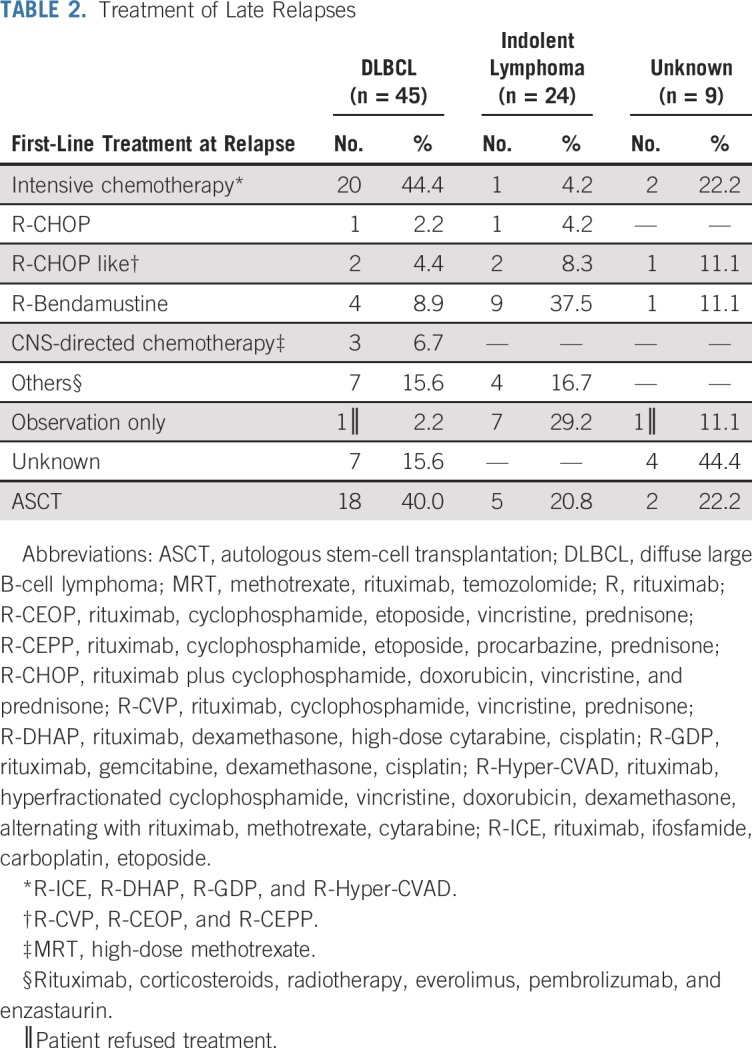
At the time of late relapse, the pathology was DLBCL in 45 patients, indolent lymphoma in 24 patients, and unknown in nine patients (biopsy not done or results unavailable). The pattern of first-line treatment is listed in Table 2. In patients who had a relapse of DLBCL, 20 (44.4%) received platinum or high-dose cytarabine containing intensive salvage chemotherapy, three (6.7%) received R-CHOP or R-CHOP–like regimen, four (8.9%) received rituximab plus bendamustine, three (6.7%) received methotrexate-based chemotherapy for CNS relapse, and 18 (40.0%) went on to undergo ASCT. In patients who had a relapse with indolent lymphoma, seven (29.2%) initially were observed, three (12.5%) received R-CHOP or R-CHOP–like regimen, nine (37.5%) received rituximab plus bendamustine, one (4.2%) received intensive salvage chemotherapy, and five (20.8%) underwent ASCT after subsequent progression.
TABLE 2.
Treatment of Late Relapses
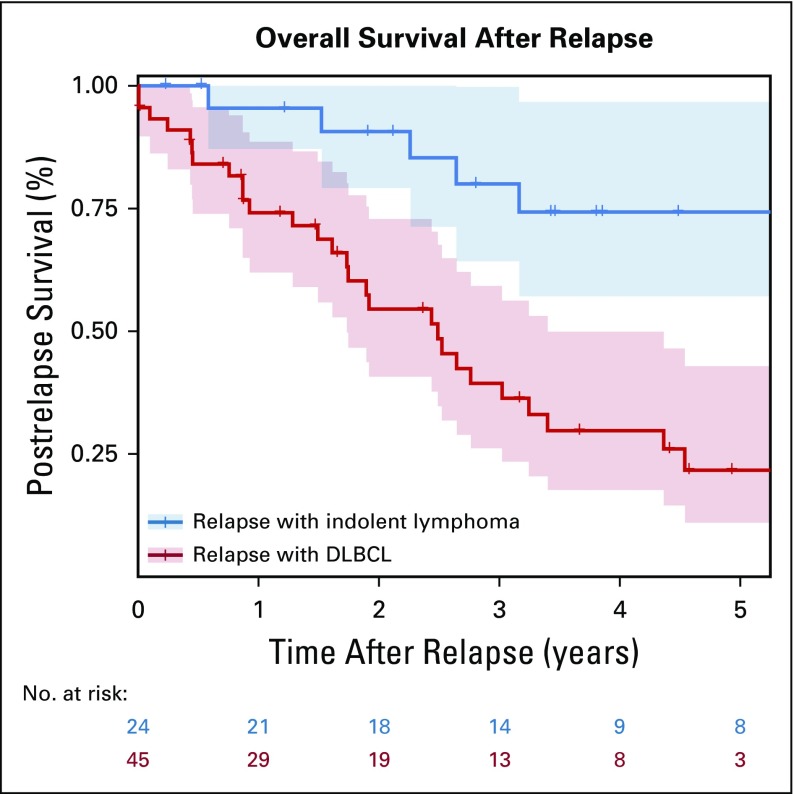
The median postrelapse survival for all patients with a relapse after achieving EFS24 was 38.9 (95% CI, 30.3 to not reached) months. The median postrelapse survival was 29.9 (95% CI, 20.8 to 40.8) months for patients who had a relapse with DLBCL, and was not reached (95% CI, 67.5 to not reached) for patients who had a relapse with indolent lymphoma (P < .01; Fig 4). For the 18 patients who had a relapse with DLBCL and underwent ASCT, the median post-transplantation survival was 2.2 (95% CI, 1.2 to 4.2) years (Data Supplement).
FIG 4.
Postrelapse survival in patients with late relapses with diffuse large B-cell lymphoma (DLBC) or indolent lymphoma.
DISCUSSION
This is a report of a comprehensive study of the cumulative incidence, clinical characteristics, and outcome of late relapses in patients with DLBCL treated with R-CHOP or R-CHOP–like immunochemotherapy. Strengths of this study include the prospective cohort study design, central pathology review, large sample size, validation of key outcomes, virtually complete follow-up, and modeling of cumulative incidence with competing risk of death. Limitations include missing data on COO in some patients and the use of the Hans algorithm; lack of standardized follow-up assessments or surveillance strategies, as found in a clinical trial (but consistent with real-world practice); and lack of geographic or ethnic or racial diversity.
The novelties of our study include EFS24-based definition of late relapse, decomposition of late relapses by histology, and identification of clinical factors that affect the cumulative incidence of late relapse. Most prior studies on DLBCL late relapse were based on data derived in the pre-rituximab era,6,17-22 primarily reporting absolute numbers of late relapses and total number of patients (or patients achieving complete remission) instead of cumulative incidence of late relapse, as in the current study. The definitions of late relapse in these studies were inconsistent and arbitrarily defined ranging from 2 to 5 years after diagnosis or achieving complete remission. Two recent studies from our group10 and Jakobsen et al11 demonstrated that in the rituximab era, as a group, patients with DLBCL who achieve EFS24 have an excellent clinical outcome, with a life expectancy similar to that of the age- and sex-matched general population at 5 years. In this context, the patients who do experience relapse after achieving EFS24 may represent a distinct population that requires clinical attention. Therefore, in the current study, we defined late relapses as those occurring 2 years or later after diagnosis. In our MER study, the cumulative incidence of all relapses was not trivial: 9.3% at 5 years after EFS24 (DLBCL relapse, 5.4%; indolent relapse, 2.9%). In the Jakobsen et al11 study, the 5-year cumulative incidence of relapse was 8% (95% CI, 7% to 10%) in patients achieving posttreatment EFS24, although it is unclear whether all relapses were biopsy-proven DLBCL relapses.
Patients with DLBCL with a concurrent indolent lymphoma at diagnosis had a higher risk of late relapse compared with patients with DLBCL alone at diagnosis. This increased risk was the result of a higher incidence of relapse with the indolent component. Although DLBCL is potentially curable, indolent lymphoma tends to have a continuous risk of relapse. Interestingly, in patients with DLBCL alone at diagnosis, the GCB subtype had a higher risk of late relapse compared with the non-GCB subtype, also as a result of a higher incidence of relapse with an indolent lymphoma, predominantly FL. This raises the question of whether these patients had an undiagnosed FL component at the time of initial DBLCL diagnosis. Unsurprisingly, a concurrent FL component at the time of diagnosis was predominantly seen in the GCB subtype of DLBCL (> 90%; unpublished data). It is possible that a small fraction of patients with GCB-subtype DLBCL have an undetected FL component at diagnosis, which may lead to a late relapse. The association of COO with late relapse should be interpreted with caution though, because COO results were missing in approximately 30% of patients with DLBCL who achieved EFS24.
The long-term follow-up of the SWOG S8736 and S0014 studies showed a continued risk of relapse independent of treatment modality (eight cycles of CHOP v three cycles of CHOP plus radiotherapy) in limited-stage DLBCL, with a cumulative incidence of progressive disease of approximately 20% at 5 years and 30% at 10 years.23 The cumulative incidence of late relapse seemed to be lower in our study; however, it is difficult to compare results of the two studies, because of their different designs (all relapses in the SWOG studies v late relapses in patients who achieved EFS24 in our study), follow-up time (much longer in SWOG studies), treatment (S8736 did not include rituximab), and patient population (S0014 included older and higher-risk patients only). Our attempt to analyze the association of treatment modality (immunochemotherapy only v immunochemotherapy plus radiotherapy) with the estimated cumulative incidence of late relapse was limited by the low event rate in the radiotherapy group. Although the findings of the SWOG studies are intriguing, a longer follow-up of our study is required to further evaluate the impact of limited stage and treatment modality on the late-relapse rates in the immunochemotherapy era.
Several studies reported that patients with DLBCL with late relapses had better outcome compared with those with early relapses.9,19,22,24 It is not entirely clear whether the late relapses were exclusively relapses with DLBCL in these studies. In the current study, we found that patients who had a relapse with an indolent lymphoma had relatively favorable outcome, but those who had a relapse with DLBCL had a significantly worse survival, similar to a prior study.6 Patients who had a relapse with DLBCL in our study had a median overall survival (OS) of approximately 2.5 years, a 3-year OS of approximately 40%, and a 5-year OS of approximately 20%, despite that approximately 40% of the patients underwent aggressive salvage chemotherapy and ASCT. The outcome is similar to that reported in the Collaborative Trial in Relapsed Aggressive Lymphoma study (salvage chemotherapy and ASCT for relapsed and refractory DLBCL).7 Though prognosis may not be as poor as with early relapse, late relapse with DLBCL still represents a life-threatening event.
Our results have implications for clinical practice and clinical trial design. It is critical to counsel patients that achieving EFS24 does not mean a cure and the late-relapse risk is not trivial, with particular attention not only to those with advanced stage or a high IPI score (higher risk of DLBCL relapse) but also those with concurrent indolent lymphoma or the GCB subtype (higher risk of indolent lymphoma relapse). Although routine surveillance imaging has no demonstrated survival benefit,25 it is important for the patients and the primary care providers to recognize the signs and symptoms of lymphoma relapse. The distinct outcome of DLBCL relapse and indolent lymphoma relapse highlights the need of repeated biopsy. In terms of clinical trials, longer follow-up may be necessary for some frontline DLBCL trials, because patients with advanced-stage disease, high IPI score, or the GCB subtype have continued risk of relapse even after passing the EFS24 mark. Like COO, the genetic subtypes of DLBCL26,27 may also be associated with risks of relapse and may also affect clinical trial follow-up in the near future. Patients who experience a late relapse of DLBCL still have a poor outcome, and novel therapies such as chimeric antigen receptor T-cell therapy should be evaluated in clinical trials for these patients. There is a need to develop biomarkers such as minimal residual disease, circulating tumor DNA,28 or gene profiling that could capture early and late relapses, which, in turn, could be incorporated in frontline DLBCL clinical trials, hopefully to guide future studies to improve the long-term outcome of DLBCL. Furthermore, given the substantial competing risk of nonrelapse mortality, progression-free survival (excluding unrelated death as progression) and lymphoma-specific survival would be important end points when reporting long-term outcomes in DLBCL clinical trials.
In conclusion, patients with newly diagnosed DLBCL who achieve EFS24 after immunochemotherapy have a risk of late relapse of almost 10% at 5 years after EFS24. Patients with a concurrent indolent lymphoma at initial diagnosis have a higher incidence of late relapse compared with those with DLBCL alone at diagnosis, because of the added risk of indolent component relapse. GCB DLBCL has a higher incidence of late relapse compared with non-GCB DLBCL, owing to more relapses with indolent lymphoma. Patients who had a relapse with DLBCL had a worse prognosis than those who had a relapse with indolent lymphoma. A repeated biopsy at the time of late relapse is essential.
Footnotes
Supported by the National Cancer Institute (Grants No. P50 CA97274 and U01 CA195568) and the Predolin Foundation.
Listen to the podcast by Dr LaCasce at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Yucai Wang, Matthew J. Maurer, Thomas M. Habermann, Stephen M. Ansell, James R. Cerhan, Grzegorz S. Nowakowski
Financial support: Brian K. Link, James R. Cerhan
Administrative support: Thomas E. Witzig, James R. Cerhan
Provision of study material or patients: Brian K. Link, Carrie A. Thompson, Ivana N. Micallef, Thomas M. Habermann, Thomas E. Witzig, Stephen M. Ansell
Collection and assembly of data: Yucai Wang, Umar Farooq, Brian K. Link, Melissa C. Larson, Rebecca L. King, Matthew J. Maurer, Cristine Allmer, Thomas M. Habermann, Thomas E. Witzig, James R. Cerhan, Grzegorz S. Nowakowski
Data analysis and interpretation: Yucai Wang, Umar Farooq, Brian K. Link, Melissa C. Larson, Matthew J. Maurer, Cristine Allmer, Mehrdad Hefazi, Carrie A. Thompson, Ivana N. Micallef, Patrick B. Johnston, Thomas M. Habermann, Thomas E. Witzig, Stephen M. Ansell, James R. Cerhan, Grzegorz S. Nowakowski
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Late Relapses in Patients With Diffuse Large B-Cell Lymphoma Treated With Immunochemotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Umar Farooq
Honoraria: Celgene
Research Funding: Kite Pharma (Inst)
Travel, Accommodations, Expenses: Kite Pharma
Brian K. Link
Consulting or Advisory Role: Roche, AbbVie, Gilead Sciences, Celgene
Research Funding: Roche (Inst), Pharmacyclics/Janssen (Inst)
Travel, Accommodations, Expenses: Roche, Celgene
Matthew J. Maurer
Consulting or Advisory Role: MorphoSys
Research Funding: Celgene (Inst), NanoString Technologies (Inst)
Patrick B. Johnston
Consulting or Advisory Role: BTG International
Thomas E. Witzig
Consulting or Advisory Role: Karyopharm Therapeutics, Immune Design, AbbVie/Genentech (Inst), Spectrum Pharmaceuticals (Inst), Sandoz, Seattle Genetics (Inst), Celgene (Inst), Incyte (Inst)
Research Funding: Celgene (Inst), Novartis (Inst), Spectrum Pharmaceuticals (Inst), Acerta Pharma (Inst)
Stephen M. Ansell
Honoraria: WebMD, Research to Practice
Research Funding: Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Affimed Therapeutics (Inst), Regeneron (Inst), Pfizer (Inst), LAM Therapeutics (Inst), Trillium Therapeutics (Inst)
James R. Cerhan
Consulting or Advisory Role: Janssen
Research Funding: NanoString Technologies, Celgene
Grzegorz S. Nowakowski
Consulting or Advisory Role: Celgene (Inst), MorphoSys (Inst), Genentech (Inst)
Research Funding: Celgene (Inst), NanoString Technologies (Inst), MorphoSys (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-Year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 5.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 6.Larouche JF, Berger F, Chassagne-Clément C, et al. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: Clinical characteristics and outcome. J Clin Oncol. 2010;28:2094–2100. doi: 10.1200/JCO.2009.24.5860. [DOI] [PubMed] [Google Scholar]
- 7.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology (Am Soc Hematol Educ Program) 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 9.Farooq U, Maurer MJ, Thompson CA, et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol. 2017;179:50–60. doi: 10.1111/bjh.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32:1066–1073. doi: 10.1200/JCO.2013.51.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobsen LH, Bøgsted M, Brown PN, et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: A Danish population-based study. J Clin Oncol. 2017;35:778–784. doi: 10.1200/JCO.2016.70.0765. [DOI] [PubMed] [Google Scholar]
- 12.Maurer MJ, Habermann TM, Shi Q, et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. 2018;29:1822–1827. doi: 10.1093/annonc/mdy203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerhan JR, Link BK, Habermann TM, et al. Cohort profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) Cohort Study. Int J Epidemiol. 2017;46:1753–1754i. doi: 10.1093/ije/dyx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Cabanillas F, Velasquez WS, Hagemeister FB, et al. Clinical, biologic, and histologic features of late relapses in diffuse large cell lymphoma. Blood. 1992;79:1024–1028. [PubMed] [Google Scholar]
- 18.Lee AY, Connors JM, Klimo P, et al. Late relapse in patients with diffuse large-cell lymphoma treated with MACOP-B. J Clin Oncol. 1997;15:1745–1753. doi: 10.1200/JCO.1997.15.5.1745. [DOI] [PubMed] [Google Scholar]
- 19.Sanz L, López-Guillermo A, Martínez C, et al. Risk of relapse and clinico-pathological features in 103 patients with diffuse large-cell lymphoma in complete response after first-line treatment. Eur J Haematol. 1998;61:59–64. doi: 10.1111/j.1600-0609.1998.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 20.Ko AH, Yuen AR. Clinical outcomes associated with very late relapses in diffuse large cell lymphoma. Leuk Lymphoma. 2002;43:1789–1793. doi: 10.1080/1042819021000006466. [DOI] [PubMed] [Google Scholar]
- 21.Vose JM, Weisenburger DD, Loberiza FR, et al. Late relapse in patients with diffuse large B-cell lymphoma. Br J Haematol. 2010;151:354–358. doi: 10.1111/j.1365-2141.2010.08330.x. [DOI] [PubMed] [Google Scholar]
- 22.Modvig L, Vase M, d’Amore F. Clinical and treatment-related features determining the risk of late relapse in patients with diffuse large B-cell lymphoma. Br J Haematol. 2017;179:75–82. doi: 10.1111/bjh.14822. [DOI] [PubMed] [Google Scholar]
- 23.Stephens DM, Li H, LeBlanc ML, et al. Continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: Final and long-term analysis of Southwest Oncology Group Study S8736. J Clin Oncol. 2016;34:2997–3004. doi: 10.1200/JCO.2015.65.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1729–1736. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32:3506–3512. doi: 10.1200/JCO.2014.55.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. doi: 10.1038/s41591-018-0016-8. Chapuy B, Stewart C, Dunford AJ, et al: Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:679-690, 2018 [Errata: Nat Med 24(8):1292, 2018; and Nat Med 24(8):1290-1291,2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol. 2018;36:2845–2853. doi: 10.1200/JCO.2018.78.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]