Immune checkpoint inhibitors target pathways involved in immune regulation, including cytotoxic T lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1). Their therapeutic use is well-established for many solid-organ, haemotological and skin malignancies, such as metastatic melanoma, in which a notable survival benefit has been reported with combination anti-CTLA-4 (Ipilimumab) and anti-PD-1 (Nivolumab) agents.[1], [2] However, immune-related adverse events (IrAEs) are common and when used in combination can affect greater than 95% of patients. The incidence of severe or life-threatening events (Common Terminology Criteria for Adverse Events (CTCAE) grade 3-4) is 55%, and immune-related hepatitis is reported in up to 18% of those on combination therapy, vs. 1–4% and 4–9% with anti-PD1 and anti-CTLA-4 monotherapy, respectively. However, the extent of liver injury is variable and most cases are not severe.3
Oncology guidelines for managing IrAEs are based on algorithms developed for trials of checkpoint inhibitors and extensive experience, but have not themselves been validated.[4], [5] Management is tailored to the CTCAE grade; for those with grade 1 hepatitis (alanine aminotransferase [ALT] ≤ 3 x upper limit of normal [ULN]) immune therapy might continue with monitoring, whereas for those with grade 3 hepatitis (ALT ≥ 5xULN and a raised bilirubin) treatment is stopped and methylprednisolone 1–2 mg/kg/day or equivalent is initiated. In all cases, alternative causes for hepatotoxicity should be excluded. Current guidelines state that liver biopsy is not required to make the diagnosis, but should be reserved for patients refractory to treatment.[4], [5] De Martin and colleagues, in contrast, have recently highlighted the potential benefits of pre-treatment histology to clarify disease pattern and severity, in order to minimise exposure to immunosuppression in self-limiting cases.3 Whilst the majority of cases resolve within 4–6 weeks of treatment, there is a paucity of data about optimal management of steroid-refractory liver IrAEs. The anti-TNF agent, infliximab, is widely used as salvage for IrAE colitis but never for hepatitis. Herein, we report the first case of its successful use as a rescue therapy in life-threatening, refractory immune-mediated hepatitis, in a patient on combination anti-CTLA-4 (Ipilimumab) and anti-PD-1 (Nivolumab) therapy for metastatic melanoma.
Case
A 53-year-old lady had a left abdominal cutaneous melanoma (Breslow > 3.7 mm) resected in 2016, followed by extra-capsular spread on axillary node block dissection in 2017. Widespread hypermetabolic new malignancy was demonstrated on computer tomography (CT)/positron emission tomography (PET) scan in the left breast/axilla/shoulder/subpleural space, right adrenal, small bowel, and bone as well as extensive hepatic metastatic disease. The melanoma carried the V600 BRAF mutation and < 1% of metastatic cells expressed programmed death ligand 1 (PDL1). She was treated from the start with combination ipilimumab 3 mg/kg and nivolumab 1 mg/kg 3-weekly, completing her third cycle in November 2017. Of note, her liver function tests (LFTs) were normal prior to the third cycle of therapy.
She was admitted 18 days after the third cycle with lethargy, nausea, anorexia and dark urine. She was febrile (39oC) with a widespread morbiliform rash. Bilirubin was 93 μmol/L (normal < 22 μmol/L), ALT 1,135 IU/L (normal ≤ 41 U/L), ALP 857 IU/L (normal females ≤ 105 U/L), albumin 35 g/L (normal 34–51 g/L) with normal clotting and C-reactive protein 27.8 mg/L (normal < 10 mg/L). Initial white cell count and renal function were normal. She was a non-smoker, did not drink alcohol and had no other relevant medical/family history. Hepatitis A, B, C, E, cytomegalovirus (CMV) and human immunodeficiency virus were negative. Extended liver autoimmune antibodies were all negative and IgG concentrations were 8.26 g/L (normal range 6–16 g/L). CT showed multiple liver metastases, but no biliary dilatation.
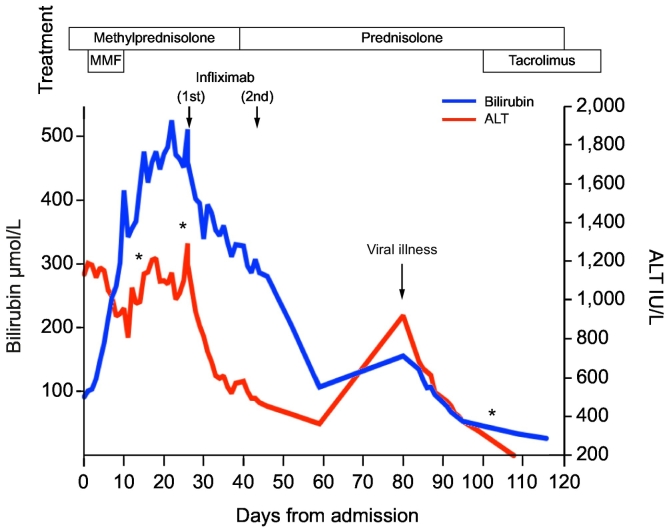
As per guidelines for immune-related hepatitis[4], [5] 200 mg of intravenous methylprednisolone once daily was given. Despite 5 days of such, ALT persisted above 1,000 IU/L and jaundice progressed (Fig. 1). Treatment was escalated with mycophenolate mofetil (MMF) 1 g twice daily to no avail and a percutaneous liver biopsy was performed on day 10 of immunosuppressive treatment (Fig. 2A-B). This showed severe acute lobular hepatitis in the absence of fibrosis and a predominance of CD3+ CD8+ lymphocytes throughout. After 7 days of dual therapy she developed severe neutropenia (< 0.1x109/L) and MMF was discontinued. On day 21 of high-dose corticosteroid therapy the bilirubin was 474 μmol/L and a second liver biopsy was undertaken, which showed severe lobular CD3+ CD8+ T-cells predominant hepatitis with confluent/bridging necrosis and evolving mild peri-portal fibrosis, without malignant cells (Fig. 2C-D). Clinically, there were no features of liver failure (no encephalopathy/ascites, international normalized ratio < 1.5).
Fig. 1.
Serial changes in liver biochemistry and function from commencement of therapy for severe immune-related hepatitis. Pattern of change in ALT and bilirubin values from time of admission to discharge with timeline of immunosuppression, serial biopsies and readmission. Key: * timing of liver biopsies.
ALT, alanine aminotransferase; MMF, mycophenolate mofetil.
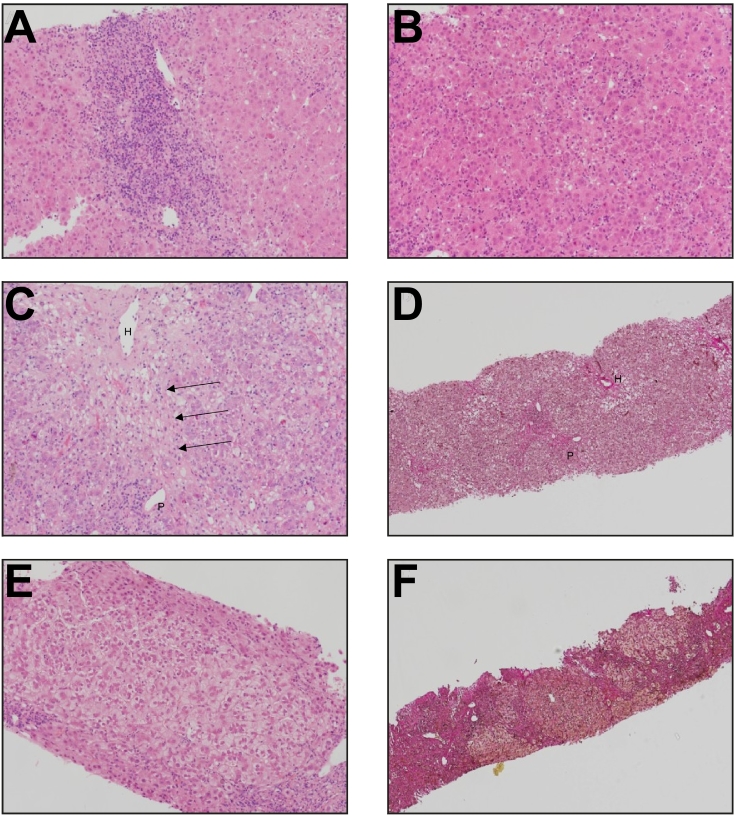
Fig. 2.
Liver histology in a patient with severe immune-related hepatitis
The first biopsy showed (A) portal inflammation and (B) diffuse spotty lobular inflammation and lobular disarray, typical of acute hepatitis. (C) A second biopsy, taken prior to treatment with infliximab, demonstrated continued lobular inflammation with evidence of central-portal bridging necrosis (see arrows). (D) There was mild periportal fibrosis without bridging. The third biopsy, following treatment with infliximab, showed minimal inflammation. (E) Hepatocyte ballooning persisted with (F) progressive periportal and bridging fibrosis associated with early nodule formation.
A, B, C, E = haematoxylin & eosin; D,F = haematoxylin van Gieson.
Key: P = portal tract, H = hepatic venule).
Following informed consent, 2 cycles of infliximab 5 mg/kg were administered 2 weeks apart, with concomitant antibiotic and antifungal prophylaxis. There was a rapid, highly clinically significant and progressive improvement in bilirubin and ALT (Fig. 1); in the absence however of normalisation of LFTs this remains defined as a ‘partial response’. Subsequently, she was discharged to out-patient follow-up with a tapering dose of prednisolone (60 mg/day) and continued to have a biochemical improvement (Fig. 1). However, 8 weeks after infliximab treatment the patient had non-specific symptoms (anorexia, coryzal, myalgia), ALT increased above 1,000 U/L and there was a parallel rise in the bilirubin, in the absence of common opportunistic infections (negative CMV, hepatitis E virus, parvovirus and Epstein-Barr virus) (Fig. 1). As a result, a third liver biopsy was performed (Fig. 2E-F), which revealed significant improvements in lobular inflammation, but new-onset (i.e. presence of collagen fibres without elastic fibres), progressive liver fibrosis with bridging and early nodule formation (Fig. 2E-F). The ALT and bilirubin spontaneously improved without alteration in therapy. Low dose calcineurin inhibitor, tacrolimus, was then initiated (target trough 3–5 ng/ml) in order to taper the prednisolone, avoid neutropenia with MMF, and conceptually to prevent a break-through T-cell mediated immune-related flare. Fourteen weeks after completion of melanoma therapy and 11 weeks after initial presentation with liver injury, CT-PET demonstrated stable liver metastases, with complete metabolic response to treatment at the previous sites of disease. Fourteen months after the last exposure to checkpoint inhibition, the patient was off all immunosuppression and had sustained radiological response in the absence of further cancer immunotherapy.
Discussion
Immune-related hepatitis, is common in people treated with immune checkpoint inhibitors: particularly combination ipilimumab and nivolumab for whom the incidence of transaminitis is up to 20% CTCAE grade 1–2 (ULN to ≤ 5 x ULN), 9% grade 3 (up to 20xULN) and 2% grade 4 (> 20xULN).[1], [2] A prospective pharmacovigilance registry identified 19/536 (3%) with grade 3 hepatitis with checkpoint inhibitors, including 4 that received combination therapy.3 Our case represents a very severe immune-related hepatitis occurring after the third cycle of ipilimumab and nivolumab. Sequential liver biopsies demonstrated an acute lobular, CD8+ T-cell predominant hepatitis, which was refractory to corticosteroids and MMF. Concerningly, the injury progressed to confluent/bridging necrosis and fibrosis, despite improvement in LFTs. Previously reported histological patterns of immune-related hepatitis are diverse; ranging from granulomatous hepatitis with fibrin deposition and central vein endothelitis in recipients of anti-CTLA-4 +/- anti-PD-1 to more heterogeneous lobular and periportal injury in recipients of anti-PD-1 or PDL1 agents.3 Three patients with anti-PD-1 induced grade 4 transaminitis and jaundice had varying degrees of biliary duct damage and ductopenia on biopsy.4 Whether or not the severity of the drug-induced liver injury reported in our case additionally relates to the extent of the underlying liver metastases (i.e. less viable liver tissue) requires further study.
Failure of treatment with corticosteroids and MMF, which was undertaken in line with published guidelines,[4], [5] prompted escalation to targeted anti-T-cell immunosuppression. Anti-thymocyte globulin has also been used to treat steroid-refractory immune-related hepatitis, but is limited to a small number of case reports.[6], [7] Ziemer and colleagues have also reported the successful use of budesonide (with ursodeoxycholic acid ± N-acteylcysteine) in 2 cases, in order to limit exposure to systemic corticosteroids and enable re-introduction of cancer immunotherapy8. This approach is of interest, albeit it remains unclear whether it would be successful in patients with extensive liver metastases and jaundice. We, therefore, made the decision to use anti-TNFα, infliximab, for several reasons: (a) predominance of marked CD8+ T-cell lobular and necrotic hepatitis on histology, (b) prior experience with use in colitis9 and pneumonitis,10 (c) marked progressive jaundice and (d) the urgent need for an anti-inflammatory response to prevent sub-acute liver failure, in a patient in whom liver transplantation was contraindicated. Interestingly, both the American Society of Clinical Oncology and the European Society for Medical Oncology guidelines advocate against the use of anti-TNFα in immune-related hepatitis, highlighting concern over idiosyncratic liver failure without citing evidence. There are no registered cases of infliximab-induced liver failure when used for immune-related colitis and infliximab has been used to reverse liver injury in a small case series of autoimmune hepatitis.11 In our case, infliximab did not result in any adverse liver events, sepsis or identifiable opportunistic infections.
Escalating immunosuppression to prevent liver failure and chronic liver injury must be balanced against the risk of a life-threatening opportunistic infection and/or loss of immune control of the underlying malignancy. As in those with autoimmune hepatitis, resolution of liver injury may lag months behind LFT normalisation. Therefore, decisions around tapering immune suppression require informed agreement between hepatologist, oncologist and patient to guide risk management. Notably in our case, despite the use of systemic corticosteroids, MMF (albeit limited use), infliximab and then maintenance with monotherapy low dose tacrolimus, there was no progression of the underlying melanoma. This suggests that the very severe T-cell immune-related hepatitis reaction coincided with control of extensive hepatic malignancy (i.e. no metabolic activity) and it is plausible that they are associated. In the event of recurrent tumour metabolic activity, there are limited published data concerning re-treatment with checkpoint inhibitors, in particular PD1-inhibitors/combination therapy and the risk of IrAE re-manifestation.
Abnormal LFTs occur in approximately a fifth of patients on combination immune checkpoint inhibitors for advanced malignancy. Early detection of liver injury by close laboratory monitoring of LFTs is thus a key part of caring for patients receiving checkpoint-inhibitor therapies. However, a small number of cases develop steroid-refractory, severe immune-related hepatitis. In the event of severe jaundice and progressive histological injury, we report the first successful, safe use of infliximab as a rescue therapy in a patient with life-threatening, checkpoint-inhibitor induced hepatitis.
Financial support
No funding was obtained to write this case report.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
All authors were involved in the clinical care of the patient and were involved in the conception of this case report. MC, GMH and MJA were involved in the initial drafting of this submission and are guarantors of the data. NS was involved in review and revision of the manuscript from an oncological perspective. SH was involved in review and revision of the manuscript from a histopathology perspective. GH, FT, NR and CP revised the draft. All authors reviewed the final version prior to submission.
Patient consent
Written consent was obtained from the patient for publication of this case for report and use of the accompanying images.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2019.02.001.
Contributor Information
Margaret Corrigan, Email: margaretcorrigan@doctors.org.uk.
Matthew J Armstrong, Email: mattyarm2010@googlemail.com.
Supplementary data
Supplementary material
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;2018 doi: 10.1200/JOP.18.00005. JCO2017z776385. [DOI] [PubMed] [Google Scholar]
- 5.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 6.Chmeil KD, Suan D, Liddle C, Nankivell B, Ibrahim R, Bautista C. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol. 2011;29:e237–e240. doi: 10.1200/JCO.2010.32.2206. [DOI] [PubMed] [Google Scholar]
- 7.Spankuch I, Gassenmaier M, Tampouri I, Noor S, Forschner A, Garbe C. Severe hepatitis under combined immunotherapy: Resolution under corticosteroids plus anti-thymocyte immunoglobulins. Eur J Cancer. 2017;81:203–205. doi: 10.1016/j.ejca.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Ziemer M, Koukoulioti E, Beyer S, Simon JC, Berg T. Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol. 2017;66:657–659. doi: 10.1016/j.jhep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3 doi: 10.1136/esmoopen-2017-000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1 related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529–534. doi: 10.1016/j.jhep.2012.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material