Summary
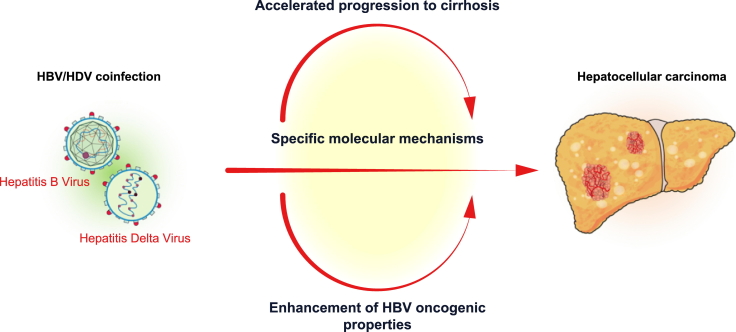
Hepatitis delta virus (HDV) is a small defective virus that needs hepatitis B virus (HBV) to replicate and propagate. HDV infection affects 20-40 million people worldwide and pegylated interferon (PegIFN) is the only recommended therapy. There is limited data on the contribution of HDV infection to HBV-related liver disease or liver cancer. Evidence from retrospective and cohort studies suggests that HBV/HDV coinfection accelerates progression to cirrhosis and is associated with an increased risk of hepatocellular carcinoma (HCC) development compared to HBV monoinfection. Although the life cycle of HDV is relatively well known, there is only ancillary information on the molecular mechanisms that can drive specific HDV-related oncogenesis. No thorough reports on the specific landscape of mutations or molecular classes of HDV-related HCC have been published. This information could be critical to better understand the uniqueness, if any, of HDV-related HCC and help identify novel targetable mutations. Herein, we review the evidence supporting an oncogenic role of HDV, the main reported mechanisms of HDV involvement and their impact on HCC development.
Keywords: Hepatitis B virus; liver cancer; HCC, co-infection; molecular pathogenesis; defective; superinfection
Graphical abstract
Key points
-
•
Hepatitis delta virus (HDV) is a small defective virus that needs hepatitis B virus (HBV) to replicate and propagate
-
•
Initial data suggest that HDV accelerates the progression to cirrhosis and increases the risk of hepatocellular carcinoma (HCC) in patients with HBV
-
•
Proposed mechanisms for HDV enhance HBV-related oncogenesis include activation of pathways related to inflammation and fibrosis
-
•
Unlike HBV, there is limited data to support a direct oncogenic role of HDV in human hepatocarcinogenesis
Alt-text: Unlabelled Box
Introduction
More than 40 years after its discovery,1 key features of hepatitis delta virus (HDV) infection remain unknown. This contrasts with other hepatotropic viruses, such as hepatitis B (HBV) and hepatitis C virus (HCV), which have been thoroughly investigated and for which there are effective treatments. The lower prevalence of HDV compared to HBV or HCV likely justified the limited research efforts dedicated to HDV, even though HDV infection is highly prevalent in certain countries.2 HDV is a defective virus that co-exists with HBV, and is related to the most severe form of liver failure attributable to chronic viral hepatitis. HDV is understood to accelerate the progression to cirrhosis, and it is considered a main driver of the malignant hepatocyte transformation.3 HBV/HDV-coinfected patients seem to be at an increased risk of HCC development compared to HBV-monoinfected individuals, although the evidence is still limited. Thus, HDV is not yet included on the list of oncogenic agents, whilst HBV and HCV are well defined carcinogens.4 Herein, we will review the evidence available regarding the oncogenic role and mechanisms of HDV-related carcinogenesis, as well as discussing the key unmet needs in HDV research.
HDV epidemiology
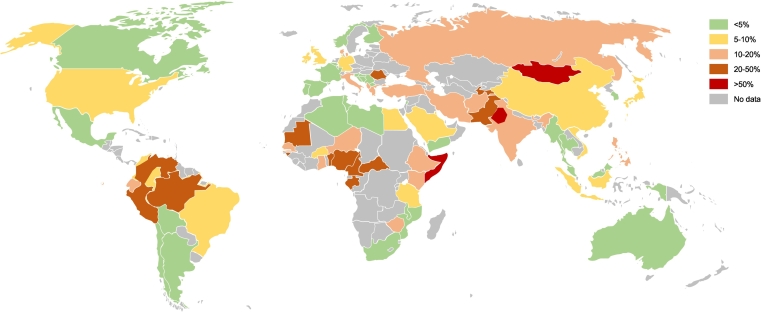
The global prevalence of HDV is remarkably variable (Fig. 1).5 As routine testing for HDV in HBV surface antigen (HBsAg)-positive individuals is not a standard procedure,6 HDV prevalence is unknown in many countries, resulting in a global underestimation of HDV disease burden. To date, few studies have evaluated the global burden of HDV, and available data are mostly biased towards local reports from different regions in specific cohorts. The World Health Organization (WHO) estimates that there are at least 20 million people infected with HDV worldwide, which represents 5% of HBV carriers.7 A recent meta-analysis including 182 articles from 61 countries estimated a pooled HDV prevalence of 10%, even after excluding intravenous drug users and individuals with high-risk sexual behaviour.8 However, methodological errors regarding the definition of HDV infection, extrapolation of data from the different cohorts, and selection bias have been pointed out in this study. Thus, it is likely that such analysis represents an overestimation of the real prevalence.9 In certain areas such as Nigeria, Gabon, Benin, Mauritania, Cameroon, Senegal, Iran, Peru, the Western Brazilian Amazon, the mountain regions of Colombia and Venezuela, Romania, Pakistan, and Tajikistan, the reported HDV prevalence exceeds 20%.[2], [5], [10], [11] HDV is endemic in Punjab, Somalia, and Mongolia, with an estimated prevalence of up to 60–80% in HBV-infected patients.[12], [13] Remarkably, these estimates of prevalence may be biased, as they are usually extrapolated from high-risk cohorts. HDV prevalence also varies across risk factors, with prevalences of 37% and 17% in intravenous drug users and people with high-risk sexual behaviours, respectively.8
Fig. 1.
Global distribution of HDV infection among HBsAg carriers. HDV prevalence is highly different among different countries. The most prevalent areas are Punjab, the Amazon basin, Somalia, and Mongolia. In European countries, the highest prevalences are seen in Romania and Albania. HBsAg, hepatitis B virus surface antigen; HDV, hepatitis delta virus.
Eight different HDV genotypes have been identified,[14], [15] with data suggesting different disease courses depending on genotype.16 Genotype 1, the most common, has worldwide distribution but is predominant in Europe, the Mediterranean countries, Iran, Turkey, and North America. Genotypes 2 and 4 are found in Asia, genotype 3 has only been described in South America, and genotypes 5-8 are found almost exclusively in Africa.17 Genotype 1 has been associated with worse outcomes than genotype 2, including progression to cirrhosis and higher rates of HCC.[18], [19]
HDV pathogenesis
Life cycle
HDV is a circular single-stranded negative-sense RNA virus which encodes for a single protein, the delta protein or delta antigen (HDAg).5 The delta antigen was discovered in Italy in 1977 by Rizzetto and collaborators while examining liver biopsies of chronic HBV patients with direct immunofluorescence. They noticed that some HBV-infected patients reacted and revealed a nuclear fluorescent pattern, which was initially thought to be a new HBV antigen.1 It was not until 1980 when they realised that it was a new RNA virus.20 The HDV genome spams between 1,672 and 1,697 base pairs depending on the genotype, making it the smallest virus infecting humans. HDV shares more characteristics with plant virusoids than with other human pathogens, such as the circular configuration of its RNA genome, its RNA self-cleavage (ribozyme activity) property and its RNA to RNA rolling circle replication. [21], [22] HDV is a defective virus which needs the presence of HBV for infectivity and assembly purposes, as it lacks its own envelope and uses HBsAg instead.1 The preS1 domain of the large HBsAg (L-HBsAg) is necessary to infect hepatocytes by binding to the sodium taurocholate cotransporting polypeptide (NTCP) receptor, and the small HBsAg (S-HBsAg) is essential for HDV assembly. Notably, HDV does not need active HBV DNA synthesis, which is inhibited in patients under effective anti-HBV treatment with nucleos(t)ide analogues (NUCs). HDV is able to replicate as long as the translation of these structural proteins continues. Furthermore, integrated HBV DNA can also provide the necessary envelope proteins for HDV virions, independently of HBV replication.[23], [24] In addition to its dependence on HBV, HDV replication also needs a host (i.e. the hepatocyte), as the virus does not code for an RNA polymerase but uses the host’s machinery.2 The delta protein is expressed in 2 isoforms with complementary functions: the small form, called S-HDAg or p24 for its molecular weight of 24 kDa, regulates the nuclear import of HDV ribonucleoproteins and the replication process. The large form, called L-HDAg or p27, inhibits replication and participates in virion assembly.25 During HDV replication, HDAg proteins form a ribonucleoprotein, which will later get a coat in the endoplasmic reticulum consisting of the 3 HBV HBsAg proteins.16 Thus, the outer envelope is the same for HBV and HDV, which has crucial implications in the interaction between the 2 viruses.26
Potential HDV oncogenic mechanisms
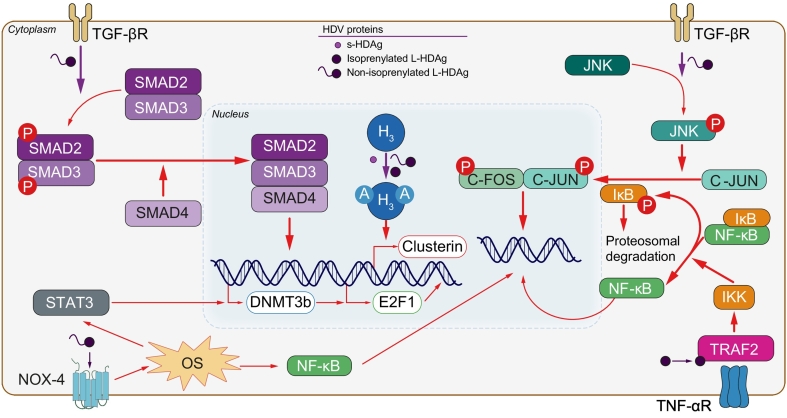
There is no data on the molecular alterations present in patients with HDV-related HCC. This is particularly unfortunate for studies on genome-wide mutation profiling, which could identify druggable mutations particularly enriched in these patients. HDV does not integrate into the genome and lacks the machinery required to propagate in the absence of HBV. Thus, a direct oncogenic mechanism of HDV is unlikely. However, the interactions between HDV and HBV could also help promote HCC development.27 Preliminary data have indicated potential mechanisms by which HDV can modify key signalling pathways related to fibrosis, including epigenetic changes, immune response modifications, specific dysregulation of long non-coding RNAs (lncRNAs), and proteomic changes (Fig. 2). Enhanced transforming growth factor-β (TGF-β) signalling has been proposed as a mechanism behind the accelerated liver disease in HBV/HDV-coinfected patients. TGF-β is involved in multiple cellular processes, including growth, differentiation, wound repair and apoptosis, with a major regulatory role in fibrosis and hepatocarcinogenesis.28 L-HDAg can activate the TGF-β pathway, probably via the Smad3 protein, which could promote HCC development.29 Since HBV can also upregulate TGF-β via the HBx protein,30 this could be a mechanism by which HDV enhances HBV-related oncogenesis. L-HDAg has been shown to activate c-Jun and to antagonize the inhibitory effect of c-Jun over TGF-β.29 This effect could be synergistic with that of the HBx protein, which also activates these 2 signalling cascades.31 Another proposed mechanism involves nuclear factor kappa B (NF-κB), a transcription factor with crucial roles in inflammation, immunity, cell proliferation and apoptosis, and HCC development.32 L-HDAg can induce NF-κB activation via tumor necrosis factor-α (TNF-α) stimulation33 or oxidative stress.34 L-HDAg has also been related to the activation of other known oncogenic pathways including the JAK-STAT pathway35 (via activation of the signal transducer and activator of transcription 3 [STAT-3] downstream protein34) or c-Fos activation.36 Another reported mechanism for HDV-related oncogenesis is downregulation of glutathione S-transferase P1 (GSTP1), a tumour suppressor gene. Transfection with S-HDAg in fetal hepatic cell lines inhibited GSTP1 expression specifically by binding to its mRNA, which resulted in accumulation of reactive oxygen species and increased apoptosis.37
Fig. 2.
Potential mechanism of increased oncogenicity due to HDV. L-HDAg potentiates TGF-β and c-Jun signalling cascades. It can also activate NOX-4 and promote oxidative stress, which enhances NF-kB and STAT3 signalling. STAT3 promotes transcription of DNMT3b, which induces expression of the E2F1 transcription factor. L-HDAg potentiates NF-αB activation via TNF-α receptor by interacting with TRAF2. Finally, both L-HDAg and S-HDAg increase clusterin expression by promoting acetylation of histone 3. HDV, hepatitis delta virus; L-HDAg, large delta antigen; OS, oxidative stress; S-HDAg, small delta antigen.
Inactivation of tumour suppressor genes through aberrant DNA methylation is frequent in HCC.38 Some studies have evaluated the capacity of HDV to interfere with DNA methylation. For example, the chaperon protein clusterin, which is involved in cell death regulation and frequently overexpressed in HCC,39 was found upregulated through histone acetylation in HDV-infected cell lines.40 Also, a small study in an HCC cell line overexpressing HDV found a mild increase in the levels of methyltransferase 3b.41 Other potential epigenetic mechanisms involved long non-coding RNAs (lncRNAs), including the deregulation of Y3 in HDV-related HCC.42 Notably, dysregulation of lncRNAs is crucial in HDV replication.43 In terms of immune dysregulation, there is data on humanised mice showing a higher number of interferon stimulated genes (ISGs) and cytokines such as TGF-β and interleukin-28 in HBV/HDV-coinfected human hepatocytes compared to HBV-monoinfected hepatocytes, suggesting enhanced inflammation in HDV.44 Finally, changes in the cellular proteome have also been linked to HDV infection, including differential expression of 89 proteins, predominantly affecting DNA damage checkpoints and the cell cycle.45 Most of this information comes from small studies, so a thorough evaluation of the key molecular features of HDV-related HCC is eagerly awaited.
HDV infection and risk of HCC development
HDV infection in patients with HBV
HBV is invariably present in all HDV-infected patients. The interaction between these 2 viruses is not completely understood but it seems to be reciprocal, and it may be crucial to the events that lead to disease progression. Intriguingly, HDV may persist for several months after liver transplantation without evidence of HBV replication.46 Besides, it has recently been shown that HDV replication may persist even in the absence of HBV.47 Two types of infection can be distinguished. The acquisition of both viruses at the same time (i.e. coinfection) is an important cause of severe hepatitis, but only 5% of patients will develop a chronic infection, with the vast majority evolving to spontaneous clearance of HDV.23 However, when HDV infection is transmitted in the setting of an already established chronic HBV infection (i.e. superinfection), more than 90% of patients will evolve to chronic hepatitis, with a more severe course to cirrhosis.23 Thus, to decipher the role of HDV in liver oncogenesis it is crucial to understand the interaction between both viruses. Longitudinal studies have shown that the long-term interplay between HDV and HBV is complex, with fluctuating levels of HDV RNA and HBV DNA viremia over time and oscillating predominance at different time points.[48], [49] Importantly, in more than 50% of cases, HBV activity is detected at different time points, which has been associated with worse prognosis.48
Several mechanisms have been proposed behind HDV-related suppression of HBV replication, including a direct effect of HDAg and the induction of an antiviral immune response. Furthermore, it has been shown that both L-HDAg and S-HDAg can repress the HBV enhancers Enh1 and Enh2, and that L-HDAg can activate the transcription of the myxovirus resistance-A (MxA) protein, an interferon (IFN)-κ inducible peptide that inhibits viral replication and contributes to HBV suppression.50 HBV can also downregulate IFN antiviral responses, and inhibit the transcription of the MxA gene, but in the presence of HDV this mechanism seems inefficient.51 HDV replication may induce a strong type-I IFN signal, leading to an anti-HBV response.52 This is further supported by a model that compared HBV-monoinfected and HBV/HDV-coinfected human liver chimeric mice, in which a stronger and more sustained antiviral response, including the activation of ISGs, was observed in coinfected mice.44 Similarly, in a model of HepaRG cells, superinfection with HDV induced a strong type-I IFN response, with activation of ISGs (mainly RSAD2 and MxA), which was associated with suppression of HBV replication.53
HBV can also influence HDV, which would explain why patients with chronic HBV/HDV coinfection may have intermittent periods of HBV replication. The HBsAg, which is different between the HBV genotypes, is essential for HDV infectivity and assembly, and also regulates the nuclear export of HDV ribonucleoproteins. Hence, the number of hepatocytes that can be infected and the number of virions that successfully start replication after entry has been shown to be different between the variants, with genotypes B and D being the most supportive.54 Likewise, the packaging signal located in the C-terminal region of L-HDAg, which differs across HDV genotypes, may mediate its affinity to the HBsAg, determining the difference in assembly efficiency between genotypes.55 Hence, HDV genotype 1 can assemble and secrete more HDV virions than genotype 2,56 which could explain why this genotype is associated with a more aggressive clinical course.19
Impact HBV/HDV in progression to cirrhosis
Chronic hepatitis D promotes inflammatory cell infiltration and progressive fibrosis, leading to cirrhosis, as with other forms of chronic viral hepatitis.16 Indeed, liver histology from HDV-infected patients is usually no different from that observed in other forms of viral hepatitis.57 Although HDV has been reported to have direct cytolytic effects,58 it seems that liver damage in patients with HDV infection is mainly driven by an immune-mediated process. HDV infection is associated with high levels of cytotoxic CD4+ T cells, as seen in HBV and HCV infections.59 In contrast to HBV, HDV induces a strong innate immune response via IFN β/λ stimulation after being sensed by the pattern recognition receptor melanoma differentiation-associated protein 5 (MDA5), suggesting a different inflammatory profile.60 Notably, persistently high levels of aspartate aminotransferase and alanine aminotransferase have been described in HBV/HDV-coinfected patients, probably reflecting a high degree of inflammation in the liver and enhanced fibrosis formation.[61], [62]
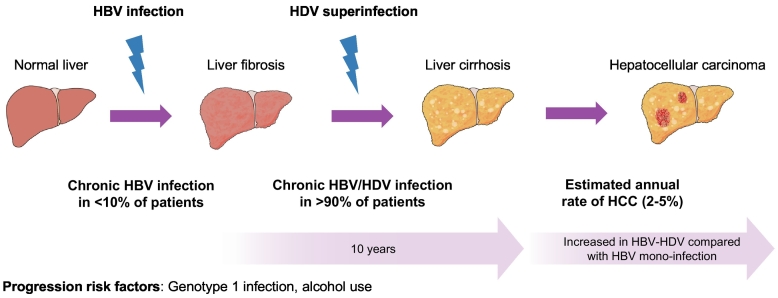
Cirrhosis and HCC are the final stages of chronic liver inflammation, including HDV-related liver disease.63 Cirrhosis underlies HCC in 70–80%64 of cases, and thus, to decipher the oncogenic role of HDV it is necessary to evaluate its impact on cirrhosis development. In viral hepatitis, progression from chronic infection to cirrhosis depends on numerous co-factors such as alcohol consumption, diabetes, or the existence of viral coinfection.[65], [66] HDV has repeatedly been included among these co-factors that enhance the risk of cirrhosis.67 The natural history of HDV infection is shown in Fig. 3. The delta antigen was initially discovered in patients with HBV infection who presented with a particularly severe disease course,[68], [70], [71], [72] including acute fulminant HBV hepatitis.69 Different studies have evaluated the potential role of HDV as a catalyst of liver fibrosis in patients with HBV coinfection. A summary of these studies is shown in Table 1. Additionally, a few studies have compared the risk of cirrhosis in HBV/HDV-coinfected patients to that in HBV-monoinfected patients. In a small study from Italy, progression to cirrhosis was observed in 77% of HDV-infected patients compared to 30% of HBV-monoinfected patients over a follow-up period of 1–15 years. Strikingly, in 70% of these patients progression to cirrhosis occurred in the first 2 years of follow-up.73 In another Japanese study with a median follow-up of 10 years, only 12% of 69 HBV/HDV-coinfected patients developed cirrhosis, but this was significantly higher than the 4% observed in HBV-monoinfected individuals.74 Conversely, in 2 studies performed in Taiwan, including 90 and 64 patients with HDV, there was no increase in the risk of cirrhosis development in HBV/HDV-coinfected patients compared to HBV-monoinfected patients.[75], [76] Cirrhosis has been reported in 51–75% of histological samples in 2 large series of HBV/HDV-coinfected patients from Italy.[77], [78] Furthermore, a retrospective multicentric European study including 200 HBV-cirrhotic patients showed that HDV-infected patients were younger (34 vs. 48 years) than those with HBV monoinfection.79 Recent data consistently show that patients with HBV/HDV coinfection have higher odds of developing cirrhosis than individuals with HBV monoinfection. For instance, a prospective study in Greece showed a higher rate of liver-related events during follow-up among HBV/HDV-coinfected than HBV-monoinfected individuals (20% vs. 8% at 4 years); the vast majority of events being cirrhosis development.81 Another report from Australia found a higher risk of cirrhosis progression in patients with HDV,82 while a very recent study in Vietnam showed that HDV-infected patients were more likely to be Child-Pugh B or C than Child-Pugh A, indicating a predisposition towards advanced liver disease in patients with HDV.62
Fig. 3.
Natural history of HDV infection. Commonly, HDV infects hepatocytes already infected by HBV (i.e. superinfection). After that, 90% of patients will develop a chronic HBV/HDV infection with a faster evolution to cirrhosis in 10 years. Some risk factors, such as alcohol consumption or genotype 1 infection may accelerate liver disease development. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HDV, hepatitis delta virus.
Table 1.
Studies assessing the clinical course of patients with HDV infection
|
Study |
Design |
Location |
HBV, n |
Age, mean ± SD or median (range), yr |
Follow-up, mean ± SD or median (range), yr |
Baseline cirrhosis, n (%) |
Disease progression, n (%) |
Limitations |
| HDV, n | ||||||||
|
Studies comparing HBV-monoinfected with HBV/HDV-coinfected patients | ||||||||
| Coghill, 201882 | Retrospective | Australia | 370 | 40 (16–86) | n.a. | 83 (22) | a54/287 (19) | Retrospective. Only 79 patients were tested for HDV RNA. Information about alcohol consumption not collected. No operational definition of cirrhosis. |
| 179 | 41 (16–69) | n.a. | 67 (37) | 55/112 (49)⁎ | ||||
| Manesis, 201381 | Ambispective | Greece | 1,997 | 47.5 (35.1–57.9) | 3.6 (3.3–3.9) | 145 (7) | a142/1,852 (8) | Ambispective. Only a subset of patients with HBV were tested for HDV, probably based on risk factors. HDV RNA was not tested. |
| 81 | 43.1 (31.4–53.1) | 4.2 (2.9–5.3) | 16 (20)⁎ | 12/65 (18) | ||||
| Cross, 200880 | Cross–sectional | England | 882 | 35 (29–46) | n.a. | 109 (12) | n.a. | Retrospective and cross-sectional. No HDV RNA was tested. Susceptible to selection bias, being King´s College a tertiary referral center. |
| 82 | 36 (30–47) | 22 (27)⁎ | n.a. | |||||
| Liaw, 200476 | Retrospective | Taiwan | 64 | n.a. | 12.3 ± 6.4 (1–20) | 0 | a11/64 (17) | Retrospective. Sample size. |
| 64 | n.a. | 8.2 ± 5.2 (1–21) | 0 | 12/64 (19) | ||||
| Huo, 200075 | Retrospective | Taiwan | 426 | 42 ± 15 | 5.7 ± 3.4 (1–17) | 0 | a56/426 (13) | Cirrhosis diagnosis was based on imaging features. Patients with acute decompensation that lead to death were excluded. Patients with a follow-up of less than 1 year were excluded. |
| 90 | 0 | 15/90 (17) | ||||||
| Fattovich, 200079 | Retrospective | Western Europe | 161 | 48 (11–78) | 6.7 (0.5–16.5) | 161 (100) | b31/161 (19) | Competing outcomes were censored. No HDV RNA was determined. HDV status was forced into the multivariate analysis. |
| 39 | 34 (13–28) | 31 (100) | 12/39 (30) | |||||
| Tamura, 199374 | Prospective | Japan | 1,058 | n.a. | 10.1 (3–18) | n.a. | a43/1,058 (4) | HDV RNA was not assessed. No multivariate analysis was performed. |
| 69 | n.a. | n.a. | 8/69 (12)⁎ | |||||
| Fattovich, 198773 | Retrospective | Italy | 128 | 36 ± 13 | 4.7 ± 2.7 | 29 | a19/69 (28) | No HDV RNA was determined. Some patients received corticosteroids during follow-up. Inclusion criteria included presence of transaminitis for 12 months. Patients with a follow-up of less than 1 year were excluded. |
| 18 | 26 ± 8 | 5 ± 3.6 | 5 | 10/13 (77)⁎ | ||||
| Colombo, 198377 | Retrospective | Italy | 142 | 37 (8–72) |
n.a. (1–5) | 31 (22) | a13/56 (23) | HDV diagnosis was based on liver biopsies. Some patients received steroids during follow-up. No multivariate analysis. |
| 50 | 21 (42)⁎ | 4/23 (17) | ||||||
| Studies in HBV/HDV coinfected patients | ||||||||
| Rosina, 199978 | Retrospective. | Italy | 159 | 34 ± 11 | 6.5 ± 4.9 | 73 (46) | n.a. | No control group available. Patients with follow-up of less than 6 months were excluded. |
| Govindarajan, 198672 | Not clear | USA | 23 | n.a. | 2.5 (0.5–11) | 7 (35) | 6/16 (38) | No other factors for progression were evaluated. Assessment for coinfection with HCV was not possible. |
| Rizzetto, 198370 | Retrospective | Italy | 137 | 34 (1–70) | n.a. (2–6) | 32 (23) | 31/75 (41) | Assessment for co–infection with HCV was not possible. |
| Rizzetto, 197968 | Retrospective | Italy Japan USA |
63 | 35 (20–66) | n.a. (1–4) | n.a. | 9/63 (14) | Included cases with both acute and chronic presentations. Assessment for coinfection with HCV was not possible. No other cofactors of progression of liver disease were assessed. |
Anti-HDV, antibodies against HDV; APRI, aspartate aminotransferase-to-platelet-ratio index; CAH, chronic active hepatitis; EIA, enzyme immunoassay; HBV, hepatitis B virus; HDAg, delta antigen; HDV, hepatitis delta virus; IF, immunofluorescence; n.a., not available; OR, odds ratio; RIA, radioimmunoassay; RR, relative risk.
Statistically significant difference between HBV-monoinfected and HBV/HDV-coinfected patients
Progression to cirrhosis;
Decompensation.
Altogether, these studies suggest that HDV is associated with a more aggressive liver disease, leading to accelerated progression and an increased risk of cirrhosis. However, the mechanisms by which HDV hastens the progression to end-stage liver disease have not been elucidated. Accordingly, it is plausible that HDV confers a higher risk of HCC development than HBV monoinfection. Whether this is a result of enhanced inflammation/fibrosis mediated by HDV or a direct oncogenic effect remains unknown.
Treatment of HDV infection
Treatment for HDV is currently limited to PegIFN alpha 2a-2b for 48 weeks. Antiviral efficacy is modest, with virologic response rates ranging between 17% and 47%. Furthermore, HDV relapse after treatment occurs in more than 50% of responders,83 highlighting the need for new treatments. For that purpose, surrogate markers that can be used to develop clinical endpoints have recently been proposed.84 Additionally, new drugs, such as inhibitors of viral entry into the hepatocyte, inhibitors of HBsAg secretion, and virus assembly inhibitors are currently under investigation.85
Preliminary evidence of HDV as a risk factor for HCC development
The studies that evaluated the association between HDV and HCC are generally small, retrospective and have suboptimal designs. Thus, the extent of HDV involvement in HCC development is controversial. The unfailing presence of HBV in all HDV-infected hepatocytes leads one to question how HDV modifies the carcinogenic risk already imposed by HBV. Patients with HBV can develop HCC in the absence of cirrhosis. This is due to direct oncogenic viral effects such as DNA integration into the host genome, prolonged expression of the HBx regulatory protein or epigenetic changes.30 Compared to other risk factors, HBV-related tumours have a different genetic profile, with a higher rate of chromosomal alterations and TP53 mutations.30 Apart from enhancing fibrosis and inflammation, there is no evidence to suggest a direct oncogenic mechanism of HDV.
Studies comparing HCC incidence between HBV/HDV-coinfected and HBV-monoinfected patients provide the best evidence from which to determine the oncogenic potential of HDV (Table 2). Evidence from these retrospective and cohort studies suggests that HBV/HDV coinfection accelerates progression to cirrhosis and is associated with an increased risk of HCC development compared to HBV monoinfection.[79], [80], [82], [86], [87], [88] This increased risk is difficult to estimate due to the nature of the data. A thorough European study including 200 patients with HBV-related cirrhosis showed that those with HBV/HDV coinfection had an estimated relative risk (RR) of HCC development of 3.2 compared to those with HBV monoinfection over a follow-up of 6.6 years.79 Similar studies reported an increased incidence of HCC from 3% to 9% in HBV/HDV-coinfected individuals compared to HBV-monoinfected individuals.74 The largest cohort published to date is a population-based study performed in Sweden, including 9,160 patients with HBV, 327 with chronic HDV infection and 323 with acute HDV-infection.86 After adjusting for age, the authors found a significantly higher risk of HCC in patients with acute (RR 6.1; 95% CI 2.8–11.7) or chronic (RR 3.9; 95% CI 1.6–7.2) HDV-infection.76 Another large study including 2,175 American veterans with HBV found a 2.9-fold higher incidence of HCC in individuals with HDV, after adjusting for cirrhosis and HCV infection.88 Conversely, in another study including 962 consecutive HBV-infected patients, those with HBV/HDV coinfection (n = 82) had a similar prevalence of HCC (9.7% vs. 7.8%) as those with HBV monoinfection80 A recent prospective study in a HIV cohort identified HDV as a strong predictor of HCC, with an RR of 9.3.87 Although results were described for all patients with HDV, only 73/116 (63%) were RNA positive, which can impact on disease progression.89 Finally, a case-control study in Australia reported no association between HDV infection and HCC in a cohort of 179 patients with HDV.82
Table 2.
Studies evaluating association between HDV and hepatocellular carcinoma.
| Authors & reference | Study type | Location | HBV, n |
Follow–up (range) | HCC incidence in HBV |
Main limitations |
|---|---|---|---|---|---|---|
| HDV, n (%) | HCC incidence in HBV/HDV | |||||
| Studies comparing HBV/HDV-coinfected vs. HBV-monoinfected | ||||||
| Coghill, 201882 |
Case–control | Australia | 4,407 | None | 5.4% | Case-control design. |
| 179 (3%) | 7.8% (RR 1.1; 95% CI 0.9–2.2; p = 0.17) | |||||
| Béguelin, 201787 | Prospective | Switzerland | 771 | 8.7 (IQR 5–13.8) years | – | HIV cohort. Only 73 (61%) patients presented HDV RNA+ |
| 119 (15%) | RR 9.3 (95% CI 3–28.6; p ≪0.001) | |||||
| Kushner, 201588 | Retrospective | United States | 2,175 | Not stated | – | Low HDV prevalence and very low HDV testing in the cohort (8%) |
| 73 (3%) | Adjusted OR 2.1 (95% CI 1.1–3.9; p = 0.025) | |||||
| Ji, 201286 | Retrospective | Sweden | 9,162 | None | – | Cross-sectional. Not clear definition of acute vs. chronic HDV infection. |
| 323/327 (4%) | Acute OR 6.11 (2.77– 11.65) / Chronic OR 3.90 (1.61–7.22) | |||||
| Cross, 200880 | Cross–sectional | England (London) | 962 | None | 7.8% | Cross–sectional (no follow-up, high risk of bias) |
| 82 (9%) | 9.7% (OR 1.34; 95% C.I. 0.62–2.91; p = ns) | |||||
| Fattovich, 200079 | Retrospective | Europe | 200 | 80 (6–198) months | 2–4% | Retrospective. Shows a trend without statistical significance |
| 39 (20%) | 13% (RR 3.2; 95% CI 1–10; p = 0.0523) | |||||
| Tamura, 199374 |
Prospective | Japan | 1,127 | 121 (36–216) months | 3% | Old study performed in a small Japanese region. |
| 69 (6%) | 9% (p ≪0.01) | |||||
| Studies evaluating HCC incidence in HBV/HDV-coinfected | ||||||
| Wranke, 201891 | Retrospective | Worldwide | – | None | – | Study design, no comparison |
| 1,576 | 1.9% | |||||
| Amougou, 201695 | Case–control | Cameroon | – | None | – | Study design, healthy controls |
| 24 | OR 29.3 (95% CI 4.1–1231) compared to controls | |||||
| Romeo, 201489 | Retrospective | Italy | – | 9.5 years | – | Study design, no comparison |
| 193 | OR 1.88 (95% CI 1.11–3.19; p = 0.019) in HDV–RNA+ | |||||
| Buti, 201194 | Retrospective | Spain | – | 6 years | – | Study design, no comparison |
| 158 | 3% | |||||
| Niro, 201092 | Retrospective | Italy | – | 8 years | – | Study design, no comparison |
| 126 | 1% HCC annual rate | |||||
| Romeo, 200990 | Retrospective | Italy | – | 28 years | – | Study design, no comparison |
| 299 | 2.8% HCC annual rate | |||||
| Gheorghe, 200593 | Retrospective | Romania | – | 10 years | – | Study design, no comparison |
| 166 | 12% | |||||
HDV, hepatitis delta virus.
There are also studies on HDV-related HCC that do not compare incidence rates in patients with HBV monoinfection. In a retrospective study from Italy, involving 299 HDV-infected patients over a follow-up of 28 years, 46 patients developed HCC, which represents an annual rate of 2.8%.90 HDV RNA status was not associated with HCC development in this study. Years later, the same authors reported an association between HDV RNA ≫600,000 copies/ml and progression to cirrhosis and HCC.89 The biggest descriptive cohort of HDV-infected patients published so far included 1,576 patients from 19 countries and reported a low HCC annual incidence of 1.9%.91 Smaller studies reported variable rates of HDV-related HCC ranging between 13%[92], [93] and 3%94 after a median follow-up of 5 to 10 years. Even considering the suboptimal quality of the evidence, it is reasonable to assume an association between HDV and HCC development, likely due to the more aggressive underlying liver disease imposed by HDV.
Conclusions and future directions
Many questions remain unanswered regarding the oncogenic role of HDV. It has been suggested that HDV accelerates the disease course, leading to cirrhosis and likely enhancing HCC development, compared to HBV monoinfection. However, studies evaluating HCC incidence in HBV/HDV-infected patients are discordant and they mostly provide low levels of evidence. Furthermore, the potential mechanisms underlying HDV-specific oncogenesis are poorly understood. Overall, well-designed prospective studies comparing cohorts of HBV/HDV-coinfected and HBV-monoinfected individuals will be key to determine the oncogenic capacity of HDV. A summary of the actual unmet needs in HDV research are shown in Box 1. Remarkably, in this context of unknown pathophysiological mechanisms, molecular studies evaluating HDV-infected HCC samples will be a great resource to understand the singularities of this condition and identify novel targets for therapies in HDV-infected patients.
Box 1. Unmet needs in HDV-related HCC research.
-
•
To catalogue the worldwide epidemiological distribution of HDV.
-
•
To standardize methods to detect and monitor HDV infection, such as the ones proposed by the WHO.
-
•
To understand the clinical course of HDV/HBV co-infection as opposed to HBV monoinfected patients, particularly in terms of their HCC risk.
-
•
To dissect the contribution of HDV to HCC development in well-designed, prospective studies adjusting for well-known HCC risk factors (e.g., sex, family history, smoking, HBV genotype, viral load, presence of basal core promoter and precore mutations, stage of liver disease, HIV or HCV co-infections, amongst others).
-
•
To perform a molecular characterization of HDV-related HCC, covering mutational landscape and signatures, DNA copy number alterations, gene expression de-regulation, immune profiling and epigenetic aberrations.
-
•
To develop new effective therapies to HDV.
HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HDV, hepatitis delta virus; WHO, World Health Organization.
Alt-text: Box 1
Financial support
M. Puigvehi received a scholarship grant from Asociación Española para el Estudio del Hígado (AEEH). A. Villanueva was supported by the DoD Translational Team Science Award (CA150272P3). J.M. Llovet was supported by NCI (P30-CA196521), DoD Translational Team Science Award (CA150272P1), European Commission (EC)/Horizon 2020 Program (HEPCAR, Ref. 667273-2), EIT Health (CRISH2, Ref. 18053), Accelerator Award (CRUCK, AEEC, AIRC) (HUNTER, Ref. C9380/A26813), Samuel Waxman Cancer Research Foundation, Spanish National Health Institute (SAF2016-76390), and the Generalitat de Catalunya/AGAUR (SGR-1358).
Conflicts of interest
Prof. Josep M. Llovet is receiving research support from Bayer HealthCare Pharmaceuticals, Eisai Inc, Bristol-Myers Squibb and Ipsen, and consulting fees from Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Eisai Inc, Celsion Corporation, Eli Lilly, Exelixis, Merck, Ipsen, Glycotest, Navigant, Leerink Swann LLC, Midatech Ltd, Fortress Biotech, Sprink Pharmaceuticals, Nucleix and CatFite. Dr. Villanueva reports personal fees from Health Advances LLC, personal fees from GroupH, personal fees from Gerson Lehrman Group, non-financial support from Champions Oncology, personal fees from Exelixis, personal fees from Exact Sciences, personal fees from Fujifilm Dako Diagnostics, personal fees from NGM Pharmaceuticals, personal fees from Nucleix, outside the submitted work. Marc Puigvehi and Carlos Moctezuma have no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
All authors made substantial contributions to each stage of the preparation of this manuscript for publication.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.05.001.
Contributor Information
Augusto Villanueva, Email: augusto.villanueva@mssm.edu.
Josep M. Llovet, Email: josep.llovet@mountsinai.org.
Supplementary data
Supplementary material
References
- 1.Rizzetto M, Canese MG, Arico S. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lempp FA, Ni Y, Urban S. Hepatitis delta virus: Insights into a peculiar pathogen and novel treatment options. Nat Rev Gastroenterol Hepatol. 2016;13:580–589. doi: 10.1038/nrgastro.2016.126. [DOI] [PubMed] [Google Scholar]
- 3.Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: Update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40. doi: 10.1038/nrgastro.2009.205. [DOI] [PubMed] [Google Scholar]
- 4.2018. https://monographs.iarc.fr/agents-classified-by-the-iarc
- 5.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85. doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Lok ASF, McMahon BJ. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . 2017. Global Hepatitis Report, 2017. doi:ISBN 978-92-4-156545-5. [Google Scholar]
- 8.Chen H-Y, Shen D-T, Ji D-Z. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. September 2018 doi: 10.1136/gutjnl-2018-316601. [DOI] [PubMed] [Google Scholar]
- 9.Stockdale AJ, Kreuels B, Henrion MR, Giorgi E, Kyomuhangi I, Geretti AM. Hepatitis D prevalence: problems with extrapolation to global population estimates. Gut. 2019 doi: 10.1136/gutjnl-2018-317874. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 10.Stockdale AJ, Chaponda M, Beloukas A. Prevalence of hepatitis D virus infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e992–e1003. doi: 10.1016/S2214-109X(17)30298-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarponi CFO, Silva RDND, Souza Filho JA, Guerra MRL, Pedrosa MAF, Mol MPG. Hepatitis Delta Prevalence in South America: A Systematic Review and Meta-Analysis. Rev Soc Bras Med Trop. 2019;52 doi: 10.1590/0037-8682-0289-2018. [DOI] [PubMed] [Google Scholar]
- 12.Rizzetto M. The adventure of delta. Liver Int. 2016;36:135–140. doi: 10.1111/liv.13018. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Oidovsambuu O, Liu P. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians. Hepatology. 2017;66:1739–1749. doi: 10.1002/hep.28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dény P. Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol. 2006;307:151–171. doi: 10.1007/3-540-29802-9_8. http://www.ncbi.nlm.nih.gov/pubmed/16903225 [DOI] [PubMed] [Google Scholar]
- 15.Le Gal F, Brichler S, Drugan T. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology. 2017;66:1826–1841. doi: 10.1002/hep.29574. [DOI] [PubMed] [Google Scholar]
- 16.Sureau C, Negro F. The hepatitis delta virus: Replication and pathogenesis. J Hepatol. 2016;64:S102–S116. doi: 10.1016/j.jhep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Rizzetto M, Ciancio A. Epidemiology of hepatitis D. Semin Liver Dis. 2012;32:211–219. doi: 10.1055/s-0032-1323626. [DOI] [PubMed] [Google Scholar]
- 18.Wu JC, Choo KB, Chen CM, Chen TZ, Huo TI, Lee SD. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet (London, England) 1995;346:939–941. doi: 10.1016/s0140-6736(95)91558-3. http://www.ncbi.nlm.nih.gov/pubmed/7564729 [DOI] [PubMed] [Google Scholar]
- 19.Su C, Huang Y, Huo T. Genotypes and Viremia of Hepatitis B and D Viruses Are Associated With Outcomes of Chronic Hepatitis D Patients. Gastroenterology. 2006;130:1625–1635. doi: 10.1053/j.gastro.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH. Gerin JL. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A. 1980;77:6124–6128. doi: 10.1073/pnas.77.10.6124. http://www.ncbi.nlm.nih.gov/pubmed/6934539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciancio A, Rizzetto M. Chronic hepatitis D at a standstill: Where do we go from here? Nat Rev Gastroenterol Hepatol. 2014;11:68–71. doi: 10.1038/nrgastro.2013.164. [DOI] [PubMed] [Google Scholar]
- 22.Yurdaydın C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepat. 2010;17:749–756. doi: 10.1111/j.1365-2893.2010.01353.x. [DOI] [PubMed] [Google Scholar]
- 23.Botelho-Souza LF, Vasconcelos MPA, Dos Santos ADO, Salcedo JMV, Vieira DS. Hepatitis delta: Virological and clinical aspects. Virol J. 2017;14:1–15. doi: 10.1186/s12985-017-0845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitas N, Cunha C, Menne S, Gudima SO. Envelope proteins derived from naturally integrated hepatitis B virus DNA support assembly and release of infectious hepatitis delta virus particles. J Virol. 2014;88:5742–5754. doi: 10.1128/JVI.00430-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor JM. Hepatitis delta virus. Virology. 2006;344:71–76. doi: 10.1016/j.virol.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Giersch K, Dandri M. Hepatitis B and Delta Virus: Advances on Studies about Interactions between the Two Viruses and the Infected Hepatocyte. J Clin Transl Hepatol. 2015;3:220–229. doi: 10.14218/JCTH.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romeo R, Petruzziello A, Pecheur EI. 2018. Epidemiology and Infection Hepatitis delta virus and hepatocellular carcinoma: an update. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majumdar A, Curley SA, Wu X. Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:530–538. doi: 10.1038/nrgastro.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SH, Jeong SH, Hwang SB. Large Hepatitis Delta Antigen Modulates Transforming Growth Factor-β Signaling Cascades: Implication of Hepatitis Delta Virus-Induced Liver Fibrosis. Gastroenterology. 2007;132:343–357. doi: 10.1053/j.gastro.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Murata M, Matsuzaki K, Yoshida K. Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-beta signaling from tumor suppression to oncogenesis in early chronic hepatitis B. Hepatology. 2009;49:1203–1217. doi: 10.1002/hep.22765. [DOI] [PubMed] [Google Scholar]
- 32.Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CY, Oh SH, Kang SM, Lim YS, Hwang SB. Hepatitis delta virus large antigen sensitizes to TNF-α-induced NF-κB signaling. Mol Cells. 2009;28:49–55. doi: 10.1007/s10059-009-0100-5. [DOI] [PubMed] [Google Scholar]
- 34.Williams V, Brichler S, Khan E. Large hepatitis delta antigen activates STAT-3 and NF-κB via oxidative stress. J Viral Hepat. 2012;19:744–753. doi: 10.1111/j.1365-2893.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 35.He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto T, Kato N, Ono-Nita SK. Large isoform of hepatitis delta antigen activates serum response factor-associated transcription. J Biol Chem. 2000;275:37311–37316. doi: 10.1074/jbc.M002947200. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, Du D, Zheng W. Small Hepatitis Delta Antigen Selectively Binds to Target mRNA in Hepatic Cells: A Potential Mechanism by Which Hepatitis D Virus Down-Regulates Glutathione S-Transferase P1 and Induces Liver Injury and Hepatocarcinogenesis. Biochem Cell Biol. August 2018 doi: 10.1139/bcb-2017-0321. [DOI] [PubMed] [Google Scholar]
- 38.Villanueva A, Portela A, Sayols S. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology. 2015;61:1945–1956. doi: 10.1002/hep.27732. [DOI] [PubMed] [Google Scholar]
- 39.Kang YK, Hong SW, Lee H, Kim WH. Overexpression of clusterin in human hepatocellular carcinoma. Hum Pathol. 2004;35:1340–1346. doi: 10.1016/j.humpath.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 40.Liao FT, Lee YJ, Ko JL, Tsai CC, Tseng CJ, Sheu GT. Hepatitis delta virus epigenetically enhances clusterin expression via histone acetylation in human hepatocellular carcinoma cells. J Gen Virol. 2009;90:1124–1134. doi: 10.1099/vir.0.007211-0. [DOI] [PubMed] [Google Scholar]
- 41.Benegiamo G, Vinciguerra M, Guarnieri V, Niro G, Andriulli A, Pazienza V. Hepatitis delta virus induces specific DNA methylation processes in Huh-7 liver cancer cells. FEBS Lett. 2013;587:1424–1428. doi: 10.1016/j.febslet.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Matsuura K, Kleiner DE, Zamboni F, Alter HJ, Farci P. Analysis of long noncoding RNA expression in hepatocellular carcinoma of different viral etiology. J Transl Med. 2016;14:1–11. doi: 10.1186/s12967-016-1085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beeharry Y, Goodrum G, Imperiale CJ, Pelchat M. The Hepatitis Delta Virus accumulation requires paraspeckle components and affects NEAT1 level and PSP1 localization. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-24500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giersch K, Allweiss L, Volz T. Hepatitis Delta coinfection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV monoinfection. J Hepatol. 2015;63:346–353. doi: 10.1016/j.jhep.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Mendes M, Pérez-Hernandez D, Vázquez J, Coelho AV, Cunha C. Proteomic changes in HEK-293 cells induced by hepatitis delta virus replication. J Proteomics. 2013;89:24–38. doi: 10.1016/j.jprot.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Mederacke I, Filmann N, Yurdaydin C. Rapid early HDV RNA decline in the peripheral blood but prolonged intrahepatic hepatitis delta antigen persistence after liver transplantation. J Hepatol. 2012;56:115–122. doi: 10.1016/j.jhep.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Giersch K, Bhadra OD, Volz T. Hepatitis delta virus persists during liver regeneration and is amplified through cell division both in vitro and in vivo. Gut. 2019;68:150–157. doi: 10.1136/gutjnl-2017-314713. [DOI] [PubMed] [Google Scholar]
- 48.Schaper M, Rodriguez-Frias F, Jardi R. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol. 2010;52:658–664. doi: 10.1016/j.jhep.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Raimondo G, Brunetto MR, Pontisso P. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology. 2006;43:100–107. doi: 10.1002/hep.20944. [DOI] [PubMed] [Google Scholar]
- 50.Williams V, Brichler S, Radjef N. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol. 2009;90:2759–2767. doi: 10.1099/vir.0.011239-0. [DOI] [PubMed] [Google Scholar]
- 51.Fernández M, Quiroga JA, Carreño V. Hepatitis B virus downregulates the human interferon-inducible MxA promoter through direct interaction of precore/core proteins. J Gen Virol. 2003;84:2073–2082. doi: 10.1099/vir.0.18966-0. [DOI] [PubMed] [Google Scholar]
- 52.Suárez-Amarán L, Usai C, Di Scala M. A new HDV mouse model identifies mitochondrial antiviral signaling protein (MAVS) as a key player in IFN-β induction. J Hepatol. 2017;67:669–679. doi: 10.1016/j.jhep.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Alfaiate D, Lucifora J, Abeywickrama-Samarakoon N. HDV RNA replication is associated with HBV repression and interferon-stimulated genes induction in super-infected hepatocytes. Antiviral Res. 2016;136:19–31. doi: 10.1016/j.antiviral.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Freitas N, Abe K, Cunha C, Menne S, Gudima SO. Support of the infectivity of hepatitis delta virus particles by the envelope proteins of different genotypes of hepatitis B virus. J Virol. 2014;88:6255–6267. doi: 10.1128/JVI.00346-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu S-C, Syu W-J, Sheen I-J, Liu H-T, Jeng K-S, Wu J-C. Varied assembly and RNA editing efficiencies between genotypes I and II hepatitis D virus and their implications. Hepatology. 2002;35:665–672. doi: 10.1053/jhep.2002.31777. [DOI] [PubMed] [Google Scholar]
- 56.Shih HH, Jeng K-S, Syu W-J. Hepatitis B Surface Antigen Levels and Sequences of Natural Hepatitis B Virus Variants Influence the Assembly and Secretion of Hepatitis D Virus. J Virol. 2008;82:2250–2264. doi: 10.1128/JVI.02155-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verme G, Amoroso P, Lettieri G. A histological study of hepatitis delta virus liver disease. Hepatology. 1986;6:1303–1307. doi: 10.1002/hep.1840060613. http://www.ncbi.nlm.nih.gov/pubmed/3793008 [DOI] [PubMed] [Google Scholar]
- 58.Nakano T, Shapiro CN, Hadler SC. Characterization of hepatitis D virus genotype III among Yucpa Indians in Venezuela. J Gen Virol. 2001;82:2183–2189. doi: 10.1099/0022-1317-82-9-2183. [DOI] [PubMed] [Google Scholar]
- 59.Aslan N, Yurdaydin C, Wiegand J. Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepat. 2006;13:505–514. doi: 10.1111/j.1365-2893.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, Filzmayer C, Ni Y. Hepatitis D virus replication is sensed by MDA5 and induces IFN-β/λ responses in hepatocytes. J Hepatol. 2018;69:25–35. doi: 10.1016/j.jhep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 61.Liao B, Zhang F, Lin S. Epidemiological, Clinical and Histological Characteristics of HBV/HDV Coinfection: A Retrospective Cross-Sectional Study in Guangdong, China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Binh MT, Hoan NX, Van Tong H. HDV infection rates in northern Vietnam. Sci Rep. 2018;8:1–7. doi: 10.1038/s41598-018-26446-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 64.Nault JC. Pathogenesis of hepatocellular carcinoma according to aetiology. Best Pract Res Clin Gastroenterol. 2014;28:937–947. doi: 10.1016/j.bpg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Sagnelli E, Coppola N, Scolastico C. Virologic and clinical expressions of reciprocal inhibitory effect of hepatitis B, C, and delta viruses in patients with chronic hepatitis. Hepatology. 2000;32:1106–1110. doi: 10.1053/jhep.2000.19288. [DOI] [PubMed] [Google Scholar]
- 66.Jardi R, Rodriguez F, Buti M. Role of hepatitis B, C, and D viruses in dual and triple infection: Influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34:404–410. doi: 10.1053/jhep.2001.26511. [DOI] [PubMed] [Google Scholar]
- 67.Wursthorn K, Manns MP, Wedemeyer H. Natural history: The importance of viral load, liver damage and HCC. Best Pract Res Clin Gastroenterol. 2008;22:1063–1079. doi: 10.1016/j.bpg.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Rizzetto M, Shih JW, Gocke DJ, Purcell RH, Verme G, Gerin JL. Incidence and significance of antibodies to delta antigen in hepatitis B virus infection. Lancet (London, England) 1979;2:986–990. doi: 10.1016/s0140-6736(79)92561-3. http://www.ncbi.nlm.nih.gov/pubmed/91776 [DOI] [PubMed] [Google Scholar]
- 69.Smedile A, Farci P, Verme G. Influence of delta infection on severity of hepatitis B. Lancet (London, England) 1982;2:945–947. doi: 10.1016/s0140-6736(82)90156-8. http://www.ncbi.nlm.nih.gov/pubmed/6127458 [DOI] [PubMed] [Google Scholar]
- 70.Rizzetto M, Verme G, Recchia S. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the delta antigen. An active and progressive disease unresponsive to immunosuppressive treatment. Ann Intern Med. 1983;98:437–441. doi: 10.7326/0003-4819-98-4-437. http://www.ncbi.nlm.nih.gov/pubmed/6340574 [DOI] [PubMed] [Google Scholar]
- 71.Saracco G, Rosina F, Brunetto MR. Rapidly progressive HBsAg-positive hepatitis in Italy. The role of hepatitis delta virus infection. J Hepatol. 1987;5:274–281. doi: 10.1016/s0168-8278(87)80032-6. http://www.ncbi.nlm.nih.gov/pubmed/3429834 [DOI] [PubMed] [Google Scholar]
- 72.Govindarajan S, De Cock KM, Redeker AG. Natural course of delta superinfection in chronic hepatitis B virus-infected patients: histopathologic study with multiple liver biopsies. Hepatology. 1986;6:640–644. doi: 10.1002/hep.1840060415. http://www.ncbi.nlm.nih.gov/pubmed/3525368 [DOI] [PubMed] [Google Scholar]
- 73.Fattovich G, Boscaro S, Noventa F. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis. 1987;155:931–935. doi: 10.1093/infdis/155.5.931. http://www.ncbi.nlm.nih.gov/pubmed/3559292 [DOI] [PubMed] [Google Scholar]
- 74.Tamura I, Kurimura O, Koda T. Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: a study from Kure, Japan. J Gastroenterol Hepatol. 1993;8:433–436. doi: 10.1111/j.1440-1746.1993.tb01543.x. http://www.ncbi.nlm.nih.gov/pubmed/8218990 [DOI] [PubMed] [Google Scholar]
- 75.Huo T, Wu JC, Hwang SJ. Factors predictive of liver cirrhosis in patients with chronic hepatitis B: a multivariate analysis in a longitudinal study. Eur J Gastroenterol Hepatol. 2000;12:687–693. doi: 10.1097/00042737-200012060-00019. http://www.ncbi.nlm.nih.gov/pubmed/10912490 [DOI] [PubMed] [Google Scholar]
- 76.Liaw Y-F, Chen Y-C, Sheen I-S, Chien R-N, Yeh C-T, Chu C-M. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024–1029. doi: 10.1053/j.gastro.2004.01.011. http://www.ncbi.nlm.nih.gov/pubmed/15057742 [DOI] [PubMed] [Google Scholar]
- 77.Colombo M, Cambieri R, Rumi MG, Ronchi G, Del Ninno E, De Franchis R. Long-term delta superinfection in hepatitis B surface antigen carriers and its relationship to the course of chronic hepatitis. Gastroenterology. 1983;85:235–239. http://www.ncbi.nlm.nih.gov/pubmed/6345255 [PubMed] [Google Scholar]
- 78.Rosina F, Conoscitore P, Cuppone R. Changing pattern of chronic hepatitis D in Southern Europe. Gastroenterology. 1999;117:161–166. doi: 10.1016/s0016-5085(99)70563-9. http://www.ncbi.nlm.nih.gov/pubmed/10381923 [DOI] [PubMed] [Google Scholar]
- 79.Fattovich G, Giustina G, Christensen E. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. Gut. 2000 doi: 10.1136/gut.46.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cross TJS, Rizzi P, Horner M. The Increasing Prevalence of Hepatitis Delta Virus (HDV) Infection in South London. Vol 80. 2008. www.interscience.wiley.com [DOI] [PubMed]
- 81.Manesis EK, Vourli G, Dalekos G. Prevalence and clinical course of hepatitis delta infection in Greece: A 13-year prospective study. J Hepatol. 2013;59:949–956. doi: 10.1016/j.jhep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Coghill S, McNamara J, Woods M, Hajkowicz K. Epidemiology and clinical outcomes of hepatitis delta (D) virus infection in Queensland, Australia. Int J Infect Dis. 2018;74:123–127. doi: 10.1016/j.ijid.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 83.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 84.Yurdaydin C, Abbas Z, Buti M. Clinical Trial Watch Treating chronic hepatitis delta : The need for surrogate markers of treatment efficacy. J Hepatol. 2019;70:1008–1015. doi: 10.1016/j.jhep.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 85.Koh C, Heller T, Glenn JS. Pathogenesis of and New Therapies for Hepatitis D. Gastroenterology. 2019;156:461–476.e1. doi: 10.1053/j.gastro.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ji J, Sundquist K, Sundquist J. A Population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:790–792. doi: 10.1093/jnci/djs168. [DOI] [PubMed] [Google Scholar]
- 87.Beguelin C, Moradpour D, Sahli R. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol. 2017;66:297–303. doi: 10.1016/j.jhep.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol. 2015;63:586–592. doi: 10.1016/j.jhep.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romeo R, Foglieni B, Casazza G, Spreafico M, Colombo M, Prati D. High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romeo R, Del Ninno E, Rumi M. A 28-Year Study of the Course of Hepatitis Δ Infection: A Risk Factor for Cirrhosis and Hepatocellular Carcinoma. Gastroenterology. 2009;136:1629–1638. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 91.Wranke A, Pinheiro Borzacov LM, Parana R. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: The Hepatitis Delta International Network (HDIN) Liver Int. 2018;38:842–850. doi: 10.1111/liv.13604. [DOI] [PubMed] [Google Scholar]
- 92.Niro GA, Smedile A, Ippolito AM. Outcome of chronic delta hepatitis in Italy: A long-term cohort study. J Hepatol. 2010;53:834–840. doi: 10.1016/j.jhep.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Gheorghe L, Iacob S, Simionov I. Natural history of compensated viral B and D cirrhosis. Rom J Gastroenterol. 2005;14:329–335. [PubMed] [Google Scholar]
- 94.Buti M, Homs M, Rodriguez-Frias F. Clinical outcome of acute and chronic hepatitis delta over time: A long-term follow-up study. J Viral Hepat. 2011;18:434–442. doi: 10.1111/j.1365-2893.2010.01324.x. [DOI] [PubMed] [Google Scholar]
- 95.Amougou MA, Noah DN, Moundipa PF, Pineau P, Njouom R. A prominent role of Hepatitis D Virus in liver cancers documented in Central Africa. BMC Infect Dis. 2016;16:647. doi: 10.1186/s12879-016-1992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material