Summary
Cholangiocarcinoma (CCA) represents a heterogeneous group of epithelial tumours that are classified according to anatomical location as intrahepatic (iCCA), perihilar (pCCA), or distal (dCCA). Although surgical resection and liver transplantation following neoadjuvant therapy are potentially curative options for a subset of patients with early-stage disease, the currently available medical therapies for CCA have limited efficacy. Immunotherapeutic strategies such as immune checkpoint blockade (ICB) harness the host immune system to unleash an effective and durable antitumour response in a subset of patients with a variety of malignancies. However, response to ICB monotherapy has been relatively disappointing in CCA. CCAs are desmoplastic tumours with an abundant tumour immune microenvironment (TIME) that contains immunosuppressive innate immune cells such as tumour-associated macrophages and myeloid-derived suppressor cells. A subset of CCAs may be classified as immune ‘hot’ tumours with a high density of CD8+ T cells and enhanced expression of immune checkpoint molecules. Immune ‘hot’ tumour types are associated with higher response rates to ICB. However, the suboptimal response rates to ICB monotherapy in human clinical trials of CCA imply that the preponderance of CCAs are immune ‘cold’ tumours with a non-T cell infiltrated TIME. An enhanced comprehension of the immunobiology of CCA, particularly the innate immune response to CCA, is essential in the effort to develop effective combination immunotherapeutic strategies that can target a larger subset of CCAs.
Keywords: Distal cholangiocarcinoma, immune checkpoint blockade, immune cold CCA, immune hot CCA, intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma
Key points
Infiltration of immunosuppressive innate immune cells such as TAMs and MDSCs in CCA is associated with poor patient outcomes.
T cell-infiltrated or immune ‘hot’ CCAs have increased CD8+ T cell infiltration with enhanced interferon γ and granzyme B activity, increased expression of immune checkpoint molecules such as PD-1 and its ligand PD-L1, and enhanced responsiveness to ICB.
Non-T cell-infiltrated or immune ‘cold’ CCAs are devoid of CD8+ T cells and have a preponderance of immunosuppressive cells such as TAMs, MDSCs, and tolerogenic DCs.
Conventional chemotherapy has limited efficacy in metastatic cholangiocarcinoma, prompting interest in immunotherapy approaches. The only FDA-approved immunotherapy in cholangiocarcinoma is pembrolizumab, an anti-PD-1 antibody, which received tissue-agnostic approval for solid tumours with microsatellite instability or mismatch repair deficiency, including cholangiocarcinoma.
The response rate to PD-1 blockade monotherapy is low in unselected cases of advanced cholangiocarcinoma, underscoring the need for biomarkers of response, novel immunotherapies, and combination therapies.
Immune-mediated approaches currently under investigation include combining immune checkpoint blockade with molecularly targeted therapy, local ablative therapy, chemotherapy, and other agents. Cell-based therapies, cancer vaccines, and agents targeting novel immune checkpoints, cytokines, colony stimulating factors, and the tumour microenvironment are also under development.
Alt-text: Unlabelled Box
Introduction
Cholangiocarcinoma (CCA) is the most common biliary malignancy and the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC). CCAs are heterogeneous biliary epithelial tumours that are classified into intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) subtypes based on their anatomic location within the biliary tree. The overall incidence of CCA, particularly iCCA, has increased over recent decades. Unfortunately, the 5-year overall survival (OS) for CCA remains less than 10%.[1], [2] Surgical resection or liver transplantation following neoadjuvant chemoradiation are potentially curative treatment options for the subset of patients who present with early-stage disease.2 However, diagnosing CCA at an early stage remains a significant challenge, and the majority of patients present with advanced stage disease.[2], [3], [4], [5]
Advances in our understanding of the immunobiology of the tumour immune microenvironment (TIME) have resulted in the advent of cancer immunotherapies that modulate the host immune response against tumours.6 Tumours can escape the host immune attack by induction of immune checkpoints such as programmed death-1 (PD-1) and its ligand PD-L1, as well as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Accordingly, antibody-based therapies targeting these mediators, so called immune checkpoint blockade (ICB), unleash pre-existing immunity and have become the major focus of anticancer therapeutic interventions. ICB therapies have demonstrated durable responses in a subset of patients.7 ICB response is associated with the TIME phenotype, with a T cell-infiltrated TIME having a higher response to ICB compared to a non-T cell infiltrated TIME.[6], [8] A T cell-infiltrated TIME displays spontaneous immune activation and is characterized by the presence of high infiltration of CD8+ T cells, high expression of PD-L1, chemokines and other factors implicated in T cell recruitment. A non-T cell infiltrated TIME displays immune exclusion and lacks T cells due to the absence of chemokines and activation factors involved in T cell recruitment.[6], [8] The latter phenotype also lacks T cell priming, likely due to the absence of upstream innate immune activation.
CCAs are desmoplastic tumours with a dense TIME populated by cancer-associated fibroblasts as well as immunosuppressive innate immune cells such as tumour-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). These stromal elements are essential in promoting an immunosuppressive TIME and foster CCA progression by producting cytokines and chemokines. Herein, we review the innate and adaptive host immune response to CCA and emerging immunotherapies modulating the immune system.9
The innate immune system in cholangiocarcinoma
The liver has ample unique immunological features including the ability to induce immune tolerance as well as a robust innate immunity.10 The liver is constantly exposed to intestinal microbial products and must have the ability to suppress inappropriate inflammatory responses while remaining alert to potential harmful stimuli such as infectious agents or cancer cells.10 The liver’s distinct immune environment includes the largest population of resident macrophages (80–90% of total body population) referred to as Kupffer cells (KCs) and an abundance of natural killer (NK) cells.[10], [11] KCs are key mediators of induction of immunological tolerance in the liver. The tolerogenic capability of the liver may be important in tumour biology as cancers may co-opt this machinery to promote immune tolerance, facilitating tumour progression. Hence, elucidating the innate immune response to CCA is essential in the effort to uncover effective immunotherapies.12
Macrophages in cholangiocarcinoma
Macrophages are phagocytic innate immune cells which are extremely heterogeneous, and play an essential role in hepatobiliary malignancy.[11], [13] They represent the first line of defence against damage-associated molecular patterns expressed by cancer cells, or pathogen-associated molecular patterns.14 Hepatic macrophages may be categorized by ontogeny as resident or recruited macrophages. Resident macrophages include yolk-sac derived KCs which have the capacity to self-renew, and a recently described population of liver capsular macrophages which are replenished from blood monocytes.15 Recruited hepatic macrophages include circulating monocytes that differentiate into macrophages, and a reservoir of peritoneal macrophages which traffic through the capsule into the liver parenchyma.[11], [16] Polarization refers to the functional activation of macrophages. In tumour biology, TAMs are an essential component of the TIME, and are implicated in tumour immune escape.[17], [18] The terminology for TAM polarization is complex, and includes an immunosuppressive, alternatively activated, pro-tumour ‘M2-like’ phenotype and an antitumour, classically activated ‘M1-like’ phenotype.18 TIME factors contributing to TAM plasticity include cytokines, as well as hypoxia and cancer cell-derived extracellular vesicles (EVs).[20], [21], [22], [23], [17], [19] Several studies have demonstrated an association between the presence of TAMs and patient outcomes in CCA.24 In a cohort of 39 patients with iCCA, TAM infiltration was associated with angiogenesis, increased infiltration of regulatory T cells (Treg), and poor disease-free survival.24 The authors also demonstrated that CCA cells induce an M2-like phenotype via signal transducer and activator of transcription 3 activation.[17], [24] Similarly, a retrospective analysis employing immunohistochemistry (IHC) in 114 patients with CCA demonstrated a positive correlation between tumour-infiltrating neutrophils, TAMs, and Tregs.25 Moreover, the presence of these immunosuppressive immune cell populations was significantly associated with poor recurrence-free survival.25 Conflicting results have been obtained from studies assessing a link between TAM localization within the tumour and patient outcomes; one study demonstrated worse outcomes (47 patients with pCCA) and another demonstrated improved OS (88 patients with iCCA) with high infiltration of TAMs in the tumour invasive front.[26], [27], [28], [29], [30]
Studies investigating the mechanism of TAM-mediated CCA progression are limited. Yuan et al. have demonstrated that chronic liver injury induces mitochondrial dysfunction, resulting in oxidative stress and the recruitment of KCs.31 Moreover, tumor necrosis factor (TNF) derived from KCs promotes JNK-mediated CCA proliferation and oncogenic transformation, depletion of KCs has been shown to reduce pre-malignant CCA lesions.31 Canonical WNT signalling drives cell proliferation, and is activated in CCA.32 Alternatively activated macrophages activate WNT signalling in CCA with consequent CCA progression.32 Macrophage depletion in preclinical models results in inhibition of WNT signalling, and reduction in tumour growth.32 As TAM infiltration has been associated with poor patient outcomes, it has been postulated that CCA cells may modulate the surrounding stroma to a tumour supportive immune niche. Cellular spheroids generated from CCA cells molded macrophages to a TAM phenotype with high invasive capacity.33 TAMs isolated from resected human CCA specimens recapitulated the phenotype of the in vitro macrophages educated by CCA cells.33 Although these studies have explored the mechanisms underlying TAM-mediated CCA progression, further work is needed to elucidate the mechanisms behind the pro-tumour role of macrophages in CCA.
Myeloid-derived suppressor cells in cholangiocarcinoma
MDSCs are a subset of immature myeloid cells with potent immunosuppressive function.34 In a variety of malignancies, MDSCs accumulate in the bone marrow, peripheral blood, lymphoid tissues, and the tumour microenvironment with resultant augmentation of tumour immune evasion and immunotherapy resistance.35 MDSCs are not an independent lineage of myeloid cells. Instead, they comprise immature myeloid cells that are pathologically activated in the setting of chronic inflammation. MDSCs inhibit cytotoxic T cells (CTLs), NK cells and other subsets via multiple antigen-specific and non-specific mechanisms, including production of arginase, inducible nitric oxide synthase (iNOS), indoleamine 2,3-dioxygenase (IDO), reactive oxygen species (superoxide, myeloperoxidase, hydroxyl peroxide, and peroxynitrite) and immunosuppressive cytokines (including transforming growth factor-beta [TGF-β] and interleukin [IL]-10).[36], [37], [38] MDSC are subdivided into monocytic and granulocytic or polymorphonuclear (PMN) subsets (M-MDSC and PMN-MDSC), that are phenotypically similar to macrophages and neutrophils, respectively, albeit biochemically and functionally distinct.39 M-MDSC can differentiate into TAMs in the tumour immune microenvironment, while short-lived PMN-MDSCs likely overlap with immunosuppressive tumour-associated neutrophils.40
The majority of data regarding the role of MDSCs in hepatobiliary cancers come from HCC. Clinically, an increase in M-MDSC in peripheral blood is prognostic, and has been associated with decreased OS in HCC.41 In murine models of HCC, MDSCs accumulate in the liver and polarize Kupffer cells to an immunosuppressive phenotype.[42], [43] MDSC-mediated effects on lymphocytes include fostering Treg development, promoting CD8+ T cell anergy, and inhibiting NK cell cytotoxicity.[44], [45], [46] Murine models of HCC suggest that depletion of PMN-MDSC may increase sensitivity to PD-L1 checkpoint inhibitor therapy.47 However, MDSCs have been relatively unexplored in CCA. A single publication documented a significant increase in the percentage of circulating M-MDSC (CD11b+/CD14+/HLA-DR−) in whole blood from 17 patients with CCA compared to healthy controls. However, further characterization or functional confirmation was not carried out.48 Therefore, additional studies are needed to characterize the contribution of MDSCs to CCA and explore their potential as a viable immunotherapeutic target.
Natural killer cells in cholangiocarcinoma
Preclinical and clinical studies have demonstrated that NK cell deficiency or impaired NK cell function is linked to increased incidence of a variety of malignancies. NK cells are ‘ready to kill’; indeed, NK cells are able to identify and spontaneously eliminate abnormal cells such as cancer cells without prior sensitization.49 Activated NK cells mediate tumour immunosurveillance and modulate the immune response via secretion of a large spectrum of cytokines and chemokines. NK cells also play an essential role in cancer immunoediting via secretion of interferon-gamma which induces activation of M1-like macrophages.50 Nonetheless, it has been postulated that the predominant role of NK cells in tumour immunosurveillance might be prevention of metastasis, as NK cells are abundant in the circulation but relatively scarce in solid tumours. NK cells comprise approximately 30-40% of the total hepatic lymphocyte population; a liver resident NK cell subset with adaptive immune properties that originates from hepatic stem cells has been described.[51], [52]
Natural killer group 2D (NKG2D), an activating NK cell receptor, is involved in NK cell-mediated killing of tumour cells. Genetic variants of the NKG2D receptor impair the cytotoxic function of NK cells. Accordingly, NKG2D receptor variants have been linked to CCA development in patients with primary sclerosing cholangitis.53 In contrast, high expression of NKG2D ligands in human CCA are associated with improved disease-free and overall patient survival, implying that treatment strategies that encourage interaction between NKG2D and its ligand may be a promising therapeutic approach in CCA.54 Preclinical data from studies assessing therapeutic strategies that augment NK cell activity in CCA are encouraging, albeit limited. Co-culture of CCA cells with the epidermal growth factor receptor monoclonal antibody, cetuximab, and NK cells significantly enhanced CCA cell death by potentiating antibody-dependent cellular cytotoxicity.55 Similarly, infusion of ex vivo expanded human NK cells into CCA xenograft mice resulted in inhibition of tumour growth.56 Although these findings hold promise, further work is needed to investigate NK cell-based therapies in CCA.
Dendritic cells in cholangiocarcinoma
Dendritic cells (DCs) are antigen presenting cells (APCs) which are essential in activation of the adaptive immune response.57 DCs are categorized broadly as classical DCs (cDCs) and plasmacytoid DCs (pDC). cDCs are highly phagocytic APCs which are replenished from bone marrow precursors.58 cDCs initiate adaptive immune responses in secondary lymphoid organs following their interaction with antigens in peripheral tissues.58 pDCs, although developmentally related to cDCs, are not phagocytic, and are ineffective at presenting exogenous antigens to CD4+ T cells. Following activation, pDCs acquire typical DC morphology and release interferon-gamma.59
Compared to healthy controls, patients with CCA have a significant decrease in the absolute number of peripheral blood cDCs as well as a decline in the TNFα-producing cDCs.60 Immunohistochemical analysis has demonstrated a correlation between CD83+ (mature) cDCs and CD4+/CD8+ T cell infiltration at the invasive margin of cancer. Moreover, patients with an increased number of CD83+ cDCs at the tumour invasive margin had a lower incidence of lymph node metastasis and overall better outcomes compared to patients with a paucity of CD83+ cDCs.61 As the presence of DCs confers a better patient outcome, the therapeutic potential of DC-based immunotherapies has been explored in limited preclinical and clinical studies of CCA. DCs loaded with aspartate-β-hydroxylase (ASPH), a tumour-associated cell surface protein present in a number of malignancies, induced suppression of tumour growth and metastasis, as well as increased CD3+ lymphocyte infiltration in an orthotopic rat model of iCCA.62 Interestingly, the remaining tumour cells still expressed ASPH. This latter finding implies that “escape mutants” mediating tumour evasion had not developed, and additional immunizations may be necessary for optimal antitumour activity.62 Overall, the role of DCs and various DC subsets in CCA needs to be further delineated.
Adaptive immune response in cholangiocarcinoma
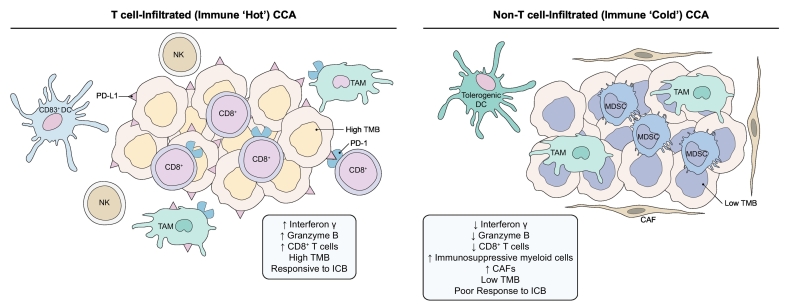
Tumour-infiltrating lymphocytes (TILs) are a highly heterogeneous population that includes CD8+ cytotoxic T cells, CD4+ T helper cells, Tregs and B lymphocytes.[63], [64], [65] TILs are essential in cancer immune surveillance and in the elimination of tumour cells. Adaptive immune response components decrease with CCA progression.66 Conversely, an increase in CD8+ TILs is associated with improved overall patient survival.[66], [67], [68], [69] Intratumoural CD4+/CD8+ TILs are found in 57–68% of CCA.[70], [71] Based on immunohistochemical analyses, CD8+ TILs appear to be the primary TILs within the tumour tissue whereas CD4+ cells are the predominant lymphocyte population in the peritumoural area.[61], [72] The presence of mature DCs at the invasive margin of CCAs correlates significantly with CD8+ and CD4+ T cell infiltration in the tumour region and improved patient survival.61 However, the role of CD4+ TILs in the tumour immune response is controversial. Although, CD4+ TILs can suppress tumour growth through cytokine secretion, a low CD4/CD8 ratio is associated with a better prognosis in colorectal carcinoma, suggesting an immunosuppressive effect of CD4+ TILs.[73], [74] However, in a cohort of 306 resected human CCA specimens immunohistochemical analysis demonstrated that an increase in tumour-infiltrating CD4+ T cells was associated with longer patient survival.66 Interplay between components of the innate immune system and TILs can impact the antitumour immune response. Patients with CCA and high tumour tissue expression of CD15, a carbohydrate epitope expressed on neutrophils, have shorter OS and disease-free survival.75 Accordingly, an elevated neutrophil-lymphocyte ratio (NLR) is associated with a higher percentage of PD-1+ TILs and lower percentage of IFN-γ+ TILs.76 In a cohort of 102 patients with iCCA, who had undergone surgical resection, an elevated NLR was an independent predictor for poor OS and recurrence-free survival.76 A subset of CCAs are immune ‘hot’ tumours with increased infiltration of TILs and high PD-L1 expression (Fig. 1). Immunohistochemical analysis of human resected specimens (n = 99, 58 iCCA and 41 pCCA) demonstrated a significant correlation between PD-L1 expression and a higher density of CD3+ TILs.70 PD-L1 expression in CCA varies between different series, and has been reported to be 55–72%.[70], [77], [78] Moreover, PD-L1 is expressed predominantly on immune cells in CCA (46–63%)[70], [78] rather than cancer cells (9–23%).[70], [71], [79]
Fig. 1.
The evolving tumour immune microenvironment of CCA.
T cell-infiltrated or immune ‘hot’ CCAs have increased CD8+ T cell infiltration with enhanced interferon γ and granzyme B activity, antitumour DCs and NK cells, increased immune checkpoint molecules such as PD-1 and its ligand PD-L1, and enhanced responsiveness to ICB. Non-T cell-infiltrated or immune ‘cold’ CCAs are devoid of CD8+ T cells and have a preponderance of immunosuppressive cells such as M2-like TAMs, MDSCs, and tolerogenic DCs. These tumours are generally poorly responsive to ICB. CAF, cancer-associated fibroblast; CCA, cholangiocarcinoma; DC, dendritic cell; ICB, immune checkpoint blockade; MDSC, myeloid-derived suppressor cell; NK, natural killer; PD-1, programmed death-1; PD-L1, programmed death ligand 1; TAM, tumour-associated macrophage; TMB, tumour mutational burden.
High PD-L1 expression has been linked to an increase in apoptotic TILs.80 FoxP3+ TILs have also been implicated in CD8+ T cell apoptosis and consequent tumour immune escape in CCA.81 Indeed, downregulation of FoxP3 results in the attenuation of CCA proliferation and invasion, as well as enhanced tumour cell apoptosis.81 Although CD20+ B cells represent a minor proportion of the total TIL population in CCA, their presence has been linked to a favourable prognosis in CCA.[66], [72] Collectively, our knowledge of the adaptive immune system in CCA is based primarily on small retrospective studies utilizing immunohistochemical analysis. Future studies should employ sophisticated immunoprofiling techniques such as mass cytometry to elucidate the role of the adaptive immune system in CCA.
Immunotherapy clinical trials in cholangiocarcinoma
Standard of care
The standard first-line treatment for advanced biliary tract cancers (BTCs), including cholangiocarcinoma, is gemcitabine and cisplatin combination chemotherapy. This regimen modestly increased survival, with a median OS of 11.7 months in patients treated with gemcitabine/cisplatin, compared to 8.1 months for those treated with gemcitabine alone in a phase III trial.82 The use of other gemcitabine or fluoropyrimidine-based chemotherapy regimens is supported by phase II trials. An open-label, single-arm, phase II clinical trial demonstrated prolonged median progression free survival (PFS) (11.8 months) and median OS (19.2 months) in patients with advanced BTC (n = 62) treated with nab-paclitaxel plus gemcitabine-cisplatin compared to historical controls treated with gemcitabine-cisplatin alone.83 However, given the limited efficacy of these regimens, there is a pressing need to develop additional therapeutic approaches.
Immune checkpoint blockade
Immune checkpoint inhibitors are designed to overcome inhibitory receptors on CTLs to promote an antitumour immune response. Agents targeting the PD-1 and CTLA-4 pathways have been approved in multiple malignancies, and in some cases provide durable responses. However, even among immunogenically ‘hot’ tumour types such as melanoma and non-small cell lung cancer, response to ICB is variable. Current research priorities in CCA and other tumour types include determining biomarkers of response, and developing combination strategies to improve response rates and circumvent immunologic tolerance and resistance.84 One established predictor of response to ICB is the neo-antigen burden of a tumour, which may be secondary to carcinogen exposure, oncoviral integration, APOBEC gene expression, microsatellite instability due to mismatch repair deficiency (MSI-high/MMR-deficient), or other factors. In a comprehensive molecular analysis, approximately 6% of biliary cancers were hypermutated (with a tumour mutation rate ≫11.13/Mb), including 2% that were MMR-deficient.85 This suggests that a subset of CCAs may be primed to respond to ICB (Fig. 2). Indeed, ICB has shown promise in patients with MSI-high/MMR-deficient CCA. The efficacy of pembrolizumab, a monoclonal anti-PD-1 antibody, was evaluated in a prospective manner in 86 patients with advanced MSI-high/MMR-deficient cancers including CCA (n = 4).86 The disease control rate (DCR) in patients with CCA was 100%; 1 patient had complete response (CR) and 3 had stable disease.86 An analysis of 5 uncontrolled, single-arm, open-label trials of pembrolizumab (KEYNOTE-012, 016, 028, 158, and 164) included 149 patients with MSI-high/MMR-deficient tumours (90 metastatic colorectal cancers and 59 other tumour types, including 11 BTCs). The objective response rate (ORR, sum of CR and partial response [PR]) was 39.6%, with 78% of those having a duration of response of 6 months or longer. Out of 11 patients with BTC, 3 (27%) had a response, with duration of response ranging from 11.6 to 19.6 months.87 These encouraging results led to the accelerated food and drug administration (FDA) approval of pembrolizumab for the first tissue/site agnostic indication in MSI-high/MMR-deficient tumours (www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm). Taken together, these analyses suggest that PD-1 blockade may be effective for advanced MSI-high/MMR-deficient CCA that has progressed on standard therapies.
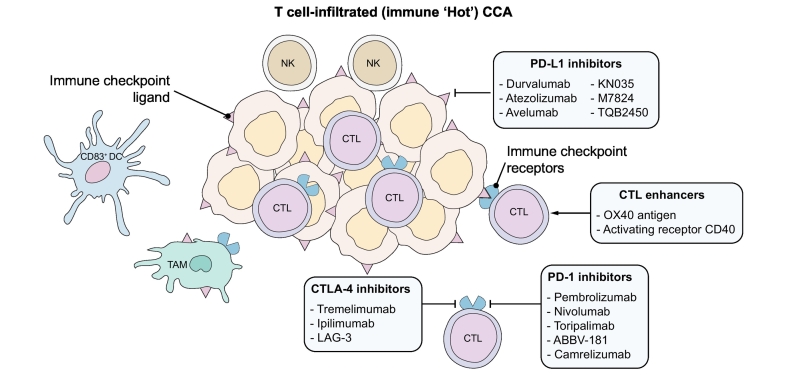
Fig. 2.
Schematic representation of therapeutic strategies for immune ‘hot’ CCA.
The targets of immunotherapies currently under investigation in CCA that may be beneficial in immune ‘hot’ CCA are represented schematically. CCA, cholangiocarcinoma; CTL, cytotoxic T lymphocyte; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DC, dendritic cell; NK, natural killer; PD-1, programmed death-1; PD-L1, programmed death ligand 1; TAM, tumour-associated macrophage.
Tumour expression of PD-L1 is a biomarker for response to ICB in other tumour types, but has not been fully explored in CCA. KEYNOTE-028 (NCT02054806) was an open-label, phase I basket trial with 20 different solid tumour cohorts, including BTCs.77 All selected patients had positive PD-L1 expression, defined by expression on ≫1% of tumour cells by IHC-based assay. The BTC cohort included 23 patients, with an ORR of 17% (4 of 23), including 1 CR and 3 PR; an additional 3 patients had stable disease. Median PFS was 1.8 months, and median OS was 6.2 months. Multiple biomarkers were evaluated, including expression of an 18-gene T cell-inflamed profile, PD-L1 expression, and tumour mutational burden (TMB). All 3 were correlated with higher response rates in the study population overall, and the highest response rates were found among patients with both elevated TMB and a second biomarker (PD-L1 expression or T cell-inflamed gene expression profile).77 Although biomarker data was not presented for the biliary cancer cohort specifically, these results suggest that a combination of biomarkers can identify patients most likely to respond to ICB.
These encouraging results led to a follow-up trial with an expansion cohort, KEYNOTE-158 (NCT02628067), the largest study to date of ICB in BTCs. This is an ongoing phase II, single-arm, open-label trial of pembrolizumab in multiple advanced cancers. An interim report was presented in 2018 with data from 104 patients with BTC.88 The ORR was only 5.8%, including 6 PR and zero CR, with an additional 17 patients (16%) having stable disease. The duration of response (DoR) ranged from 6.2 to ≫15 months (in 2 patients), with the median DoR not reached. Interestingly, PD-L1 expression was not predictive of response. The cohort contained 61 patients with PD-L1 positive tumours, and 31 patients without PD-L1 expression. Although ORR was slightly higher in the PDL-1 positive group (6.6% vs. 2.9%), there were no significant differences in median PFS (1.9 vs. 2.1 months) or OS (7.2 vs. 9.6 months).88 In this cohort, none of the evaluated patients had MSI-high tumours. This limited response to ICB monotherapy in an unselected cohort of advanced biliary cancer further emphasizes the need for biomarkers (that identify patients likely to respond), and combinatorial treatment strategies (that overcome limited antitumour responses in CCA).
Immune checkpoint blockade – combination therapies
Given the limitations of ICB monotherapy, there is tremendous interest in developing combination immunotherapy strategies. Such strategies include dual ICB as well as ICB combined with another immunomodulatory agent, molecular targeted therapy, cytotoxic chemotherapy, or local therapy (Table 1, Table 2).89 The premise of dual ICB is that blocking a single checkpoint may not be sufficient to activate CTLs. Although combining CTLA-4 and PD-1 blockade has increased efficacy, it has also increased adverse events (AE) in melanoma.[90], [91] At least 2 early phase trials of dual CTLA-4 and PD-1 blockade are ongoing in advanced solid tumours, including CCAs (NCT02834013 and NCT01938612). Interim results from an open-label phase I/II study of durvalumab plus tremelimumab in patients with HCC and BTC who had progressed on prior therapy (NCT02821754) were relatively disappointing for BTC. None of the patients in the BTC cohort had PR or CR, and only 5 (42%) had stable disease. The median PFS was 3.1 months and OS was 5.5 months, while multiple grade 3/4 treatment-related AEs were reported.92 Efficacy in this study may have been hampered by inclusion of an unselected BTC population. However, given the increased risk for adverse events and limited efficacy of combination PD-1/CTLA-4 blockade, there is substantial interest in investigating alternative combination immunotherapies.
Table 1.
Ongoing ICB-based clinical trials in cholangiocarcinoma.
| Intervention | Trial type | Population (# participants, estimated enrollment) | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Immune checkpoint blockade monotherapy | |||
| Pembrolizumab (anti-PD-1 antibody) | Phase II, single arm, open label; Phase II, non-randomized, open label; Phase Ib, single arm, open label; Phase II, single arm, open label; Prospective observational cohort |
Advanced, refractory BTC (33 pts); Microsatellite unstable cancers, including BTC (171 pts); PD-L1 positive cancers, including BTC (477 pts); Advanced, refractory cancers, including BTC (1,350 pts); HCC or BTC (100 pts) |
NCT03110328; NCT01876511; NCT02054806; NCT02628067; NCT03695952 |
| Nivolumab (anti-PD-1 antibody) | Phase II, single arm, open label; Phase II, non-randomized, open label Prospective observational cohort |
Advanced, refractory BTC (52 pts); Advanced, refractory cancers with MMR deficiency (6,452 pts); HCC or BTC (100 pts) |
NCT02829918; NCT02465060; NCT03695952 |
| Durvalumab (anti-PD-L1 antibody) | Phase I, non-randomized, open label | Advanced solid tumours, including BTC (269 pts) | NCT01938612 |
| Toripalimab (anti-PD-1 antibody) | Phase Ib/II, single arm, open label | HCC or iCCA, eligible for resection (20 pts) | NCT03867370 |
| Atezolizumab (anti-PD-L1 antibody) | Phase II, Non-randomized, open label | Advanced, refractory solid tumours, including BTC, elevated tTMB (765 pts) | NCT02091141 |
| Dual immune checkpoint blockade | |||
| Nivolumab + Ipilimumab (anti-CTLA-4 antibody) | Phase II, single arm, open label; Phase II, randomized, open label |
Advanced, refractory solid tumours including BTC (707 pts); Unresectable, untreated BTC (64 pts) |
NCT02834013; NCT03101566 |
| Durvalumab + Tremelimumab (anti-CTLA-4 antibody) | Phase I, non-randomized, open label | Advanced, refractory, biopsiable solid tumours, including BTC (269 pts) | NCT01938612 |
| Immune checkpoint blockade plus local ablative therapy | |||
| Durvalumab + Tremelimumab + TACE, RFA, or cryoablation | Phase II, non-randomized, open label | Unresectable, refractory HCC or BTC (90 pts) | NCT02821754 |
| Tremelimumab + RFA | Phase I, non-randomized, open label | Unresectable, refractory HCC or BTC, eligible for RFA (61 pts) | NCT01853618 |
| Pembrolizumab + SBRT vs. GEMCIS chemotherapy | Phase II, randomized, open label | Unresectable, untreated iCCA, eligible for radiotherapy (184 pts) | NCT03898895 |
| Durvalumab + Tremelimumab + radiation therapy | Phase II, single arm, open label | Unresectable HCC or BTC (70 pts) | NCT03482102 |
| Immune checkpoint blockade plus chemotherapy | |||
| Durvalumab + Tremelimumab + GEM or GEMCIS vs. GEMCIS chemotherapy | Phase II, randomized, open label | Untreated BTC (63 pts) | NCT03473574 |
| Durvalumab + Tremelimumab + GEMCIS chemotherapy | Phase II, single arm, open label | unresectable, untreated BTC (31 pts) | NCT03046862 |
| Durvalumab + Tremelimumab + Paclitaxel | Phase II, randomized, open label | Recurrent or advanced, refractory BTC (102 pts) | NCT03704480 |
| Durvalumab + GEMCIS vs. GEMCIS chemotherapy | Phase III, randomized, double-blind, placebo-controlled | Unresectable, untreated BTC (474 pts) | NCT03875235 |
| Durvalumab + Guadecitabine | Phase Ib, single arm, open label | Unresectable, refractory HCC, PDAC, or BTC excluding ampullary (90 pts) | NCT03257761 |
| Camrelizumab (anti-PD-1 antibody) + GEMOX chemotherapy | Phase II, single arm, open label | Advanced CCA (38 pts) | NCT03486678 |
| Camrelizumab + Apatinib (VEGFR2 inhibitor), FOLFOX4 or GEMOX chemotherapy | Phase II, non-randomized, open label | Advanced, untreated HCC or BTC (152 pts) | NCT03092895 |
| Pembrolizumab + CAPOX chemotherapy | Phase II, single arm, open label | Unresectable, refractory, biopsiable BTC (19 pts) | NCT03111732 |
| Pembrolizumab + GEMCIS | Phase II, single arm, open label | Unresectable, untreated BTC (50 pts) | NCT03260712 |
| Toripalimab + Gemcitabine | Phase II, single arm, open label | Advanced BTC (40 pts) | NCT03796429 |
| Nivolumab + GEMCIS | Phase I/II, single arm, open label; Phase II, randomized, open label |
Unresectable BTC (30 pts); Unresectable, untreated BTC (64 pts) |
NCT03311789; NCT03101566 |
| nivolumab + nal-irinotecan + 5-fluorouracil + leucovorin | Phase I/II, single arm, open label | Unresectable, refractory BTC (40 pts) | NCT03785873 |
| KN035 (anti-PD-L1 antibody) + GEMOX vs. GEMOX chemotherapy | Phase III, randomized, open label | Unresectable, untreated BTC (390 pts) | NCT03478488 |
| Immune checkpoint blockade plus molecularly targeted therapy | |||
| Pembrolizumab + pemigatinib (FGFR1-3 inhibitor) | Phase I/II, single arm, open label | Advanced, refractory solid tumours, including CCA, with genetic alteration of FGF or FGFR genes (325 pts) | NCT02393248 |
| Nivolumab + FT-2102 (mutant IDH1 inhibitor) | Phase I/II, non-randomized, open label | Selected solid tumours, including BTC, with IDH1 mutations (200 pts) | NCT03684811 |
| Atezolizumab + cobimetinib (MEK inhibitor) | Phase II, randomized, open label | Unresectable, refractory BTC (82 pts) | NCT03201458 |
| Durvalumab + tremelimumab + selumetinib (MEK inhibitor) | Phase I, non-randomized, open label | Advanced, refractory solid tumours, including BTC (58 pts) | NCT02586987 |
| Nivolumab + rucaparib (PARP inhibitor) | Phase II, single arm, open label | Advanced, refractory BTC (35 pts) | NCT03639935 |
| TPST-1120 (PPARα antagonist) + Nivolumab, docetaxel chemotherapy or cetuximab (anti-EGFR antibody) | Phase I, non-randomized, open label | Advanced solid tumours, including CCA (338 pts) | NCT03829436 |
| Pembrolizumab + XL888 (Hsp90 inhibitor) | Phase I, single arm, open label | Advanced, refractory GI cancers, including CCA (50 pts) | NCT03095781 |
| Atezolizumab + DKN-01 (anti-Dickkopf-1 antibody) | Phase I, single arm for BTC, open label | Non-operable, refractory oesophageal and BTC (123 pts) | NCT03818997 |
| Nivolumab, pembrolizumab or chemotherapy + TRK-950 (monoclonal antibody targeting a proprietary tumour antigen) | Phase I, non-randomized, open label | Advanced solid cancers, including CCA (36 pts) | NCT03872947 |
Ongoing clinical trials were identified by searching ClinicalTrials.gov using the terms "Biliary Cancer," "cholangiocarcinoma," "biliary carcinoma," "bile duct," or "biliary tract" and manually curated for inclusion of an immunotherapy arm. Trials were included with status of "Recruiting," "Not yet recruiting," "Active, not recruiting," "Completed," or "Enrolling by invitation." Trials of general solid tumours were excluded unless a BTC arm or inclusion was specified. Search was updated as of 4/1/19. BTC, biliary tract cancer; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; ICB, immune checkpoint blockade; MMR, mismatch repair; PDAC, pancreatic ductal adenocarcinoma; RFA, radio frequency ablation; SBRT, Stereotactic body radiation therapy; TACE, transarterial chemoembolization; TMB, tumor mutational burden.
Table 2.
Ongoing immunotherapy clinical trials targeting the immune microenvironment in cholangiocarcinoma.
| Intervention | Trial type | Population (# participants, estimated enrolment) | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Immune microenvironment targeted therapy | |||
| Interferon alpha + G-CSF + fluorouracil + hydroxyurea | Phase II, single arm, open label | Unresectable GI cancers, including BTC (60 pts) | NCT00019474 |
| INCB001158 (arginase inhibitor) + FOLFOX, GEMCIS or paclitaxel chemotherapy | Phase I/II, non-randomized, open label | Advanced solid tumours including BTC (249 pts) | NCT03314935 |
| Recombinant interleukin-12 + trastuzumab (anti-HER2 antibody) | Phase I, single arm, open label | Advanced, refractory, HER2-expressing solid tumours, including BTC (15 pts) | NCT00004074 |
| CDX-1140 (CD40 agonist antibody) +/- CDX-301 (dendritic cell growth factor) | Phase I, non-randomized, open label | Advanced, refractory, biopsiable cancers, including CCA (180 pts) | NCT03329950 |
| ABBV-368 (OX40 agonist antibody) +/- ABBV-181 (anti-PD-1 antibody) | Phase I, non-randomized, open label | Advanced solid cancers, including CCA (170 pts) | NCT03071757 |
| Immune microenvironment targeted therapy plus immune checkpoint blockade | |||
| Peginterferon alpha-2b + Pembrolizumab | Phase II, single-arm, open label | Advanced, refractory, biopsiable CCA (44 pts) | NCT02982720 |
| Sargramostim (GM-CSF) + Pembrolizumab | Phase II, single arm, open label | Advanced BTC (42 pts) | NCT02703714 |
| Cabiralizumab (anti-CSF1R antibody) + Nivolumab | Phase II, randomized, open label | Resectable, biopsiable BTC (16 pts) | NCT03768531 |
| M7824 (anti-PD-L1/TGFbetaRII fusion protein) | Phase II, single arm, open label | Advanced, refractory BTC (141 pts) | NCT03833661 |
| Entinostat (histone deacetylase inhibitor) + Nivolumab | Phase II, non-randomized, open label | Advanced, untreated CCA or PDAC (54 pts) | NCT03250273 |
| Ramucirumab (anti-VEGFR-2 antibody) + Pembrolizumab | Phase I, single arm, open label | Select advanced, refractory, biopsiable cancers, including BTC (155 pts) | NCT02443324 |
| Lenvatinib (VEGFR2 inhibitor) + Pembrolizumab | Phase II, single arm, open label; Phase II, single arm, open label |
Advanced, refractory, primary liver cancer or BTC (50 pts); Selected advanced, refractory solid tumours, including BTC (180 pts) |
NCT03895970; NCT03797326 |
| Anlotinib hydrochloride (multi-RTK and VEGFR2-3 inhibitor) + TQB2450 (Anti-PD-L1 antibody) | Phase Ib/II, single arm, open label | Advanced, refractory BTC or HCC (60 pts) | NCT03825705 |
| Avelumab (anti-PD-L1 antibody) with Regorafenib (multi-RTK and VEGFR 2/3 inhibitor) | Phase I/II, non-randomized, open label | Advanced, refractory digestive tumours, not MMR-deficient (212 pts) | NCT03475953 |
| pegylated recombinant human hyaluronidase PH20 + atezolizumab + GEMCIS chemotherapy | Phase I, randomized, open label | Advanced, untreated BTC (70 pts) | NCT03267940 |
Ongoing clinical trials were identified by searching ClinicalTrials.gov using the terms "Biliary Cancer," "cholangiocarcinoma," "biliary carcinoma," "bile duct," or "biliary tract" and manually curated for inclusion of an immunotherapy arm. Trials were included with status of "Recruiting," "Not yet recruiting," "Active, not recruiting," "Completed," or "Enrolling by invitation." Trials of general solid tumours were excluded unless a BTC arm or inclusion was specified. Search was updated as of 4/1/19. BTC, biliary tract cancer; GI, gastrointestinal; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; MMR, mismatch repair; PDAC, pancreatic ductal adenocarcinoma.
Beyond CTLA-4 and PD-1, there are a host of additional immune checkpoints which may be modulated to promote an antitumour immune response, including the inhibitory receptors LAG-3, TIM-3, TIGIT, and VISTA, and activating receptors OX40, GITR, 4-1BB, and CD40 ligand.89 Preclinical and clinical data regarding these checkpoints are far less mature, but there is early data from melanoma and renal cell carcinoma suggesting some clinical effect of LAG-3 inhibitors.[93], [94] Clinical trials are currently underway targeting CD40 (NCT03329950) and OX40 (NCT03071757) as single agent or combination therapies in advanced cancers including CCA.
Several cytokine-targeted therapies have been combined with ICB. Granulocyte–macrophage colony-stimulating factor (GM-CSF), encoded by the CSF2 gene, is a cytokine that can increase antigen presentation and cytotoxic T cell function. Systemic administration of GM-CSF in combination with ICB prolonged OS compared to ICB alone in a phase II trial in melanoma.95 Interim analysis of an open-label, single-arm, phase II trial of pembrolizumab plus GM-CSF in CCA (n = 27; 70% iCCA) showed an ORR of 21% (5 of 24 patients), including PR in 4 patients with microsatellite stable (MSS) tumours. Two additional MSS patients had durable declines in CA19-9 for ≫11 months.96
Interferon-alpha-2 is a cytokine that increases antigen presentation via upregulation of host MHC class I and II molecules, leading to increased tumour infiltration of dendritic and T cells.97 Systemic administration of pegylated interferon-alpha-2b is approved for use in the adjuvant setting for treatment of melanoma.98 In CCA, clinical trials evaluating interferon-alpha-2 with pembrolizumab (NCT02982720) or in combination with chemotherapy (NCT00019474) are currently ongoing. Other ongoing trials in CCA featuring immunomodulators include a fusion protein designed to inhibit PD-L1 and TGF-β, an immunosuppressive cytokine (NCT03825705),99 and recombinant IL-12, a pro-inflammatory cytokine, combined with HER2 targeted therapy (NCT00004074; Table 2).
Combination of immune checkpoint blockade and microenvironment-directed therapy
Agents targeting the tumour microenvironment have also been combined with ICB. The vascular endothelial growth factor (VEGF) pathway mediates tumour angiogenesis, growth, and metastasis. VEGF receptor (VEGFR) inhibitors are approved for treatment of multiple cancers, including HCC, but have shown limited activity as monotherapy in CCA.[100], [101] KEYNOTE-098 (NCT02443324) is an open-label, phase I trial of pembrolizumab combined with ramucirumab, an anti-VEGFR-2 antibody, which recruited 26 patients with biliary cancer (42% iCCA).102 Of 24 evaluable patients, only 1 patient had PR (4%) while an additional 9 patients had stable disease, and no patients had CR. Median PFS was 1.6 months, and median OS 6.4 months. Overall, there was no response to this combination compared to historical controls. Of note, PD-L1 positive patients had no change in PFS, but had improved OS compared to patients with PD-L1 negative disease (11.3 vs. 6.1 months). Although these data support PD-L1 as a biomarker for ICB response, the small sample size and the lack of significant difference in outcomes in PD-L1 positive patients reported for KEYNOTE-158 suggests that further studies are necessary. At least 3 additional ongoing trials are evaluating the combination of VEGFR blockade and ICB (Table 2).
CCAs are characterized by a desmoplastic stroma with dense extracellular matrix (ECM). Hyaluronidase is an enzyme that breaks down hyaluronic acid in the ECM. In pancreatic cancer, which is similarly desmoplastic, an interim analysis of a phase II trial showed that PEGylated recombinant human hyaluronidase (PEGPH20) increased ORR and PFS.103 This agent is now being evaluated in combination with ICB and chemotherapy in CCA (NCT03267940).
Combination of immune checkpoint blockade and molecular targeted therapy
Integrative genomic analysis of BTCs has identified recurrent genetic alterations which may be amenable to targeted therapy.104 A tantalizing rationale for combining targeted therapies and immunotherapies is that molecular targeted therapies can produce significant but often short-lived responses in susceptible tumours, which could theoretically be prolonged with induction of an effective antitumour immune response. A pan-cancer analysis of tumour mutational burden and specific targetable mutations in The Cancer Genome Atlas dataset suggested that 9% of cancers could be amenable to combined molecular and immune-targeting therapy.105 There are several ongoing human clinical trials evaluating the combination of ICB with a variety of targeted therapies including inhibitors of FGFR, mutant IDH, MEK, PARP, PPAR-alpha, and HSP90 (summarized in Table 1).
Combination of immune checkpoint blockade and local ablative therapy
Radiation and other local ablative techniques are tumouricidal. Thus, they potentially increase presentation and immune recognition of tumour neoantigens that are released by cell death, providing a rationale for the combination with ICB. NCT01853618 was an open-label, phase I study of tremelimumab (anti-CTLA4 antibody) with radiofrequency ablation in 20 patients with advanced biliary cancer. Among 16 patients with evaluable disease, 2 (13%) had PR lasting 8.0 and 18.1 months, respectively and 5 (31%) had stable disease. The median PFS was 3.4 months, and the median OS was 6.0 months. Interestingly, this study included assays for the assessment of effective tumouricidal immune responses, including expansion of CD8+ T cells with an activated phenotype and expansion of the T cell repertoire. Although conclusions are limited from this small study, there was some correlation between markers of immune activation and clinical response (HLA-DR+).106
Combination of immune checkpoint blockade and cytotoxic chemotherapy
Chemotherapy can also increase tumour neo-antigen release by direct tumour cell killing, and alters TIME through cytotoxicity of immune subsets. MDSCs can be eliminated by chemotherapy, providing a rationale for combining cytotoxic chemotherapy with ICB or other immunotherapy.[107], [108] At least 14 clinical trials are ongoing in this category, none have yet reported interim results (Table 1).
Macrophage and myeloid-directed immunotherapies
Given the importance of macrophages and MDSCs in shaping tumour immunity, there is great interest in targeting these cell types, particularly in combination with ICB.[109], [110] TAMs depend upon trophic support from macrophage colony-stimulating factor (M-CSF), encoded by the CSF1 gene, which signals through the myeloid CSF1 receptor (CSF1R). Inhibition of the CSF1/CSF1R axis leads to TAM depletion, enhanced CTL function and improved tumour response to chemotherapy or ICB in multiple preclinical models, although this has not been investigated in CCA specifically.[111], [112] Initial results of the phase I, first-in-human study of combination CSF1R and PD-1 blockade in pancreatic cancer (NCT02526017), another highly desmoplastic tumour type with low ICB monotherapy response rates, showed a promising ORR of 10%. However, 43% of patients had grade 3-5 treatment-related AEs attributed to cabiralizumab.113 Based on these results, combined treatment with cabiralizumab and nivolumab is currently being assessed for BTC in a phase II trial (NCT03768531). Alternative therapeutic strategies target the recruitment, polarization, and activity of TAMs and MDSCs. Inhibiting chemokine receptors (CCR2, CCR5, and CXCR2 etc.) to prevent recruitment of TAMs and MDSCs to the TIME is under investigation in pancreatic cancer and other tumour types, but has not yet been explored in BTC. CD40 agonists under clinical investigation in CCA (NCT03329950) are known to modify macrophage polarization in addition to their effects on adaptive immunity.[114], [115] The histone deacetylase inhibitor entinostat was shown to inhibit MDSC activity and increase the efficacy of PD-1 blockade in preclinical models of lung and renal cell cancer.116 This combination is currently under investigation in a phase II clinical trial of advanced CCA and pancreatic cancer (NCT03250273). INCB001158 is an arginase inhibitor designed to inhibit the activity of MDSCs,117 and is currently being evaluated in combination with chemotherapy (FOLFOX, gemcitabine/cisplatin, or paclitaxel) in a phase I/II study of advanced solid tumours including CCA (NCT03314935).
Adoptive cell therapy
As the response to ICB in CCA has been subpar, it is possible that these are immunologically “cold” tumours that lack a substantial tumour-reactive T cell population. Adoptive cell therapy using CTLs or NK cells attempts to overcome this limitation (Fig. 3), with encouraging early results. In a single patient with metastatic CCA, adoptive transfer of TILs enriched for a CD4+ T helper 1 population of cells that recognized a tumour-specific mutation resulted in PR lasting 13 months.118 A single patient with metastatic CCA was treated with subsequent infusions of CAR T cells targeting EGFR and CD113, with partial responses to each infusion (8.5 and 4.5 months respectively, although complicated by toxicities).119 DC-based adjuvant immunotherapy was investigated in a small study of 62 iCCA undergoing surgical resection. Patients who received autologous tumour lysate pulsed DCs plus ex vivo activated T cell transfer following surgery had improved median PFS and OS (18.3 and 31.9 months, respectively) compared to patients who underwent surgery alone (7.7 and 17.4 months, respectively).120 These results indicate that adoptive cell therapy could generate durable antitumour responses. Multiple clinical trials assessing the antitumour efficacy of adoptive T cell transfer in solid organ malignancies including CCA are ongoing (Table 3).
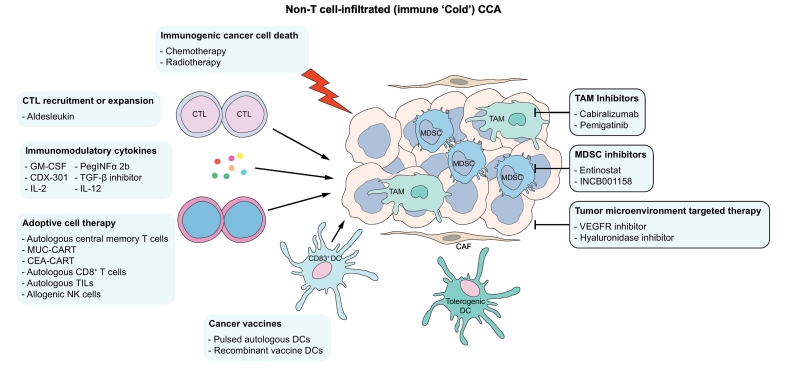
Fig. 3.
Schematic representation of therapeutic strategies for immune ‘cold’ CCA.
The targets of immunotherapies currently under investigation in CCA that may be beneficial in immune ‘cold’ CCA are represented schematically. CAF, cancer-associated fibroblast; CCA, cholangiocarcinoma; CTL, cytotoxic T lymphocyte; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin-2; IL-12, interleukin-12; MDSC, myeloid-derived suppressor cell; NK, natural killer; PEG-INFγ, PEG-interferon γ; TAM, tumour-associated macrophage; TGF-β, transforming growth factor-β; VEGFR, vascular endothelial factor receptor.
Table 3.
Ongoing cell-based and vaccine immunotherapy clinical trials in cholangiocarcinoma.
| Intervention | Trial type | Population (# participants, estimated enrolment) | ClinicalTrials.gov Identifier |
|---|---|---|---|
| Adoptive cell therapy | |||
| Autologous central memory T cell therapy + radiotherapy or chemotherapy | Phase II, randomized, open label | iCCA after radical resection with CR (20 pts) | NCT03820310 |
| Autologous CD8+ T-cell therapy + pembrolizumab | Phase I, single arm, open label | Advanced GI malignancies, including CCA (40 pts) | NCT02757391 |
| Autologous tumour infiltrating lymphocytes (TIL) | Phase II, single arm, open label | Unresectable, refractory BTC (59 pts) | NCT03801083 |
| Autologous TIL + pembrolizumab + adesleukin (recombinant IL-2) + conditioning chemotherapy | Phase II, non-randomized, open label | Selected metastatic, refractory cancers including CCA (332 pts) | NCT01174121 |
| Autologous MUC-1 CAR T-cell therapy + fludarabine/cyclophosphamide | Phase I/II, single arm, open label | MUC-1 positive iCCA (9 pts) | NCT03633773 |
| Autologous Anti-CEA CAR T-cell therapy | Phase I, single arm, open label | Advanced, refractory, CEA+ cancer including BTC (not specified) | NCT00004178 |
| Allogeneic NK cell therapy | Phase I, single arm, open label | Advanced, refractory BTC (9 pts) | NCT03358849 |
| Autologous cytokine-induced NK cells + RFA vs. RFA | Phase II/III, non-randomized, single blind | Unresected CCA, without extrahepatic metastasis (50 pts) | NCT02482454 |
| Vaccine and DC-based therapies | |||
| Autologous dendritic cells pulsed with CEA RNA | Phase I, single arm, open label | Metastatic, refractory, CEA-expressing cancers, including BTC (24 pts) | NCT00004604 |
| Autologous dendritic cells infected with fowlpox vector encoding CEA and costimulatory molecules (fowlpox-CEA-TRICOM) | Phase I, single arm, open label | Advanced, CEA-expressing cancers, including BTC (14 pts) | NCT00027534 |
| Recombinant fowlpox-CEA-TRICOM vaccine + sargramostim (GM-CSF) or recombinant fowlpox-GM-CSF vaccine | Phase I, single arm, open label | Advanced, CEA-expressing cancers, including BTC (48 pts) | NCT00028496 |
| Oral vaccine V3-X (pooled, inactivated CCA antigens) | Phase I/II, single arm, open label | CCA with elevated CA19-9 (20 pts) | NCT03042182 |
| Attenuated oncolytic vaccinia virus encoding RUC-GFP | Phase I, single arm, open label | Advanced solid cancers, including CCA (36 pts) | NCT02714374 |
| DNA vector encoding E-PRA and E-PSM peptides | Phase I, single arm, open label | Advanced solid cancers, including BTC (12 pts) | NCT00423254 |
| Other viral and bacterial vectors | |||
| Oncolytic adenovirus encoding immunostimulatory TMZ-CD40L and 4-1BBL | Phase I/II, single arm, open label | Selected advanced solid tumours, including BTC (50 pts) | NCT03225989 |
| Attenuated Salmonella Typhimurium expressing IL-2 | Phase I, single arm, open label | Any solid tumour, including BTC, with liver involvement or metastasis (22 pts) | NCT01099631 |
Ongoing clinical trials were identified by searching ClinicalTrials.gov using the terms "Biliary Cancer," "cholangiocarcinoma," "biliary carcinoma," "bile duct," or "biliary tract" and manually curated for inclusion of an immunotherapy arm. Trials were included with status of "Recruiting," "Not yet recruiting," "Active, not recruiting," "Completed," or "Enrolling by invitation." Trials of general solid tumours were excluded unless a BTC arm or inclusion was specified. Search was updated as of 4/1/19. BTC, biliary tract cancer; CAR, chimeric antigen receptor; CR, complete response; GI, gastrointestinal; iCCA, intrahepatic cholangiocarcinoma.
Other immunotherapeutic strategies
Other immunotherapeutic strategies of interest in CCA include peptide or DC-based vaccines, oncolytic viruses, and attenuated bacteria-based therapies. Peptide and DC-based vaccines are designed to increase antigen presentation and T cell priming in immunologically “cold” tumours, while viral and bacterial vectors simultaneously lyse tumour cells, increase antigen presentation, and stimulate a T cell response (Fig. 3). Peptide-based cancer vaccines have been designed to target immunogenic and tumour-associated antigens.121 Several small, early phase studies of peptide vaccines targeting proteins such as Wilms tumour 1 (WT1) and mucin 1 (MUC1) have shown limited clinical efficacy in CCA to date.[122], [123], [124], [125] DC-based therapy was FDA-approved in metastatic prostate cancer after showing a modest benefit in OS.126 DC-based therapies have been investigated in CCA.127 A retrospective analysis of 65 patients with BTC treated with DCs pulsed with peptides from WT1, MUC1 or both, showed adequate safety and a median survival of 7.2 months following vaccination.128
Talimogene laherparepvec is an oncolytic viral therapy that is FDA-approved for metastatic melanoma. It consists of a modified HSV with tumour-selective replication and GM-CSF overexpression, and it is delivered intratumourally.129 In CCA, preclinical studies have attempted to identify viral vectors capable of tumour-cell selective replication and lysis.[130], [131], [132] An oncolytic adenovirus encoding immunostimulatory transgenes is currently being assessed in a clinical trial in solid tumours including CCA (NCT03225989; Table 3). There is an intriguing preclinical rationale behind the use of live, attenuated bacterial vectors, as they exhibit tropism for the hypoxic tumour microenvironment and the ability to stimulate innate and adaptive immune responses. However, there are significant safety concerns with this approach, and no therapies are currently FDA-approved.133 NCT01099631 is an ongoing trial in patients with metastatic liver cancer including biliary cancer, treated with attenuated salmonella expressing IL-2.
Future perspectives
The prevailing knowledge on the immunobiology of CCA is based primarily on IHC analyses. Future studies should employ sophisticated techniques such as mass cytometry and single cell transcriptomics to delineate the role of innate and adaptive immune cell subsets in CCA progression. In view of the subpar response rates to ICB monotherapy in CCA, effective combination immunotherapeutic strategies which harness the innate as well as the adaptive immune response to CCA are required (Fig. 2, Fig. 3). Such strategies would couple ICB with immunotherapies targeting immunosuppressive immune cells in CCA. Consequently, a greater understanding of the immunobiology of CCA will direct development of combination immunotherapeutic strategies. Although a T cell-inflamed TIME, high TMB, and even PD-L1 expression may correlate with response in other tumour types, the utility of these biomarkers in CCA is unknown. Accordingly, investigative efforts should also be directed towards development of biomarkers which predict response to immunotherapy in CCA.
Financial support
The work of the authors is supported by the Mayo Center for Cell Signaling in Gastroenterology (Pilot & Feasibility Award P30DK084567), American Gastroenterology Association Research Scholar Award, the Cholangiocarcinoma Foundation, the Satter Family Liver Cancer Award, the Mayo Hepatobiliary Cancer SPORE (P50 CA210964) Career Enhancement Program, and the Mayo Foundation.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors' contributions
All authors contributed significantly to this manuscript and reviewed the final version.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.06.003.
Supplementary data
Supplementary material
References
- 1.Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol. 2016;23:235–243. doi: 10.1245/s10434-015-4642-9. [DOI] [PubMed] [Google Scholar]
- 4.Ilyas SI, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 6.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 8.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 9.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 10.Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 12.Berg M, Wingender G, Djandji D, et al. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur J Immunol. 2006;36:2960–2970. doi: 10.1002/eji.200636033. [DOI] [PubMed] [Google Scholar]
- 13.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Tang D, Kang R, Coyne CB, et al. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierro F, Evrard M, Rizzetto S, et al. A Liver Capsular Network of Monocyte-Derived Macrophages Restricts Hepatic Dissemination of Intraperitoneal Bacteria by Neutrophil Recruitment. Immunity. 2017;47:374–388.e6. doi: 10.1016/j.immuni.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016;165:668–678. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 20.Saha B, Momen-Heravi F, Kodys K, et al. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J Biol Chem. 2016;291:149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridder K, Sevko A, Heide J, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4 doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasita H, Komohara Y, Okabe H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitano Y, Okabe H, Yamashita YI, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118:171–180. doi: 10.1038/bjc.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atanasov G, Dietel C, Feldbrugge L, et al. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1331806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atanasov G, Hau HM, Dietel C, et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. 2015;15:790. doi: 10.1186/s12885-015-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartneck M, Schrammen PL, Mockel D, et al. The CCR2(+) Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell Mol Gastroenterol Hepatol. 2019;7:371–390. doi: 10.1016/j.jcmgh.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q, Zhou W, Yin S, et al. Blocking TREM-1(+) Tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-PD-L1 resistance in liver cancer. Hepatology. 2019 doi: 10.1002/hep.30593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Li J, Salcedo R, et al. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72:3977–3986. doi: 10.1158/0008-5472.CAN-12-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan D, Huang S, Berger E, et al. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell. 2017;31:771–789.e6. doi: 10.1016/j.ccell.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulter L, Guest RV, Kendall TJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raggi C, Correnti M, Sica A, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Highfill SL, Cui Y, Giles AJ, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava MK, Sinha P, Clements VK, et al. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condamine T, Dominguez GA, Youn J-I, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar V, Cheng P, Condamine T, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44:303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Ma X, Zhu C, et al. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilkovitch D, Lopez DM. The Liver Is a Site for Tumor-Induced Myeloid-Derived Suppressor Cell Accumulation and Immunosuppression. Cancer Res. 2009;69:5514. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lacotte S, Slits F, Orci LA, et al. Impact of myeloid-derived suppressor cell on Kupffer cells from mouse livers with hepatocellular carcinoma. Oncoimmunology. 2016;5:e1234565. doi: 10.1080/2162402X.2016.1234565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Kalathil S, Lugade AA, Miller A, et al. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Liu M, Sun H, et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67:931–944. doi: 10.1136/gutjnl-2017-314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu XD, Hu J, Wang M, et al. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2016;15:99–105. doi: 10.1016/s1499-3872(15)60413-1. [DOI] [PubMed] [Google Scholar]
- 49.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 50.O'Sullivan T, Saddawi-Konefka R, Vermi W, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209:1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 52.Peng H, Jiang X, Chen Y, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melum E, Karlsen TH, Schrumpf E, et al. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology. 2008;47:90–96. doi: 10.1002/hep.21964. [DOI] [PubMed] [Google Scholar]
- 54.Tsukagoshi M, Wada S, Yokobori T, et al. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci. 2016;107:116–122. doi: 10.1111/cas.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morisaki T, Umebayashi M, Kiyota A, et al. Combining Cetuximab with Killer Lymphocytes Synergistically Inhibits Human Cholangiocarcinoma Cells In Vitro. Anticancer Res. 2012;32:2249–2256. [PubMed] [Google Scholar]
- 56.Jung IH, Kim DH, Yoo DK, et al. In Vivo Study of Natural Killer (NK) Cell Cytotoxicity Against Cholangiocarcinoma in a Nude Mouse Model. In Vivo. 2018;32:771–781. doi: 10.21873/invivo.112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jonuleit H, Schmitt E, Schuler G, et al. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satpathy AT, Wu X, Albring JC, et al. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 60.Martin-Sierra C, Martins R, Laranjeira P, et al. Functional Impairment of Circulating FcepsilonRI(+) Monocytes and Myeloid Dendritic Cells in Hepatocellular Carcinoma and Cholangiocarcinoma Patients. Cytometry B Clin Cytom. 2019 doi: 10.1002/cyto.b.21777. [DOI] [PubMed] [Google Scholar]
- 61.Takagi S, Miyagawa S, Ichikawa E, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol. 2004;35:881–886. doi: 10.1016/j.humpath.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Noda T, Shimoda M, Ortiz V, et al. Immunization with aspartate-beta-hydroxylase-loaded dendritic cells produces antitumour effects in a rat model of intrahepatic cholangiocarcinoma. Hepatology. 2012;55:86–97. doi: 10.1002/hep.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 64.Reichert TE, Day R, Wagner EM, et al. Absent or low expression of the zeta chain in T cells at the tumor site correlates with poor survival in patients with oral carcinoma. Cancer Res. 1998;58:5344–5347. [PubMed] [Google Scholar]
- 65.Yasunaga M, Tabira Y, Nakano K, et al. Accelerated growth signals and low tumor-infiltrating lymphocyte levels predict poor outcome in T4 esophageal squamous cell carcinoma. Ann Thorac Surg. 2000;70:1634–1640. doi: 10.1016/s0003-4975(00)01915-9. [DOI] [PubMed] [Google Scholar]
- 66.Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109:2665–2674. doi: 10.1038/bjc.2013.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miura T, Yoshizawa T, Hirai H, et al. Prognostic Impact of CD163+ Macrophages in Tumor Stroma and CD8+ T-Cells in Cancer Cell Nests in Invasive Extrahepatic Bile Duct Cancer. Anticancer Res. 2017;37:183–190. doi: 10.21873/anticanres.11304. [DOI] [PubMed] [Google Scholar]
- 68.Oshikiri T, Miyamoto M, Shichinohe T, et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol. 2003;84:224–228. doi: 10.1002/jso.10321. [DOI] [PubMed] [Google Scholar]
- 69.Lim YJ, Koh J, Kim K, et al. High ratio of programmed cell death protein 1 (PD-1)(+)/CD8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother Oncol. 2015;117:165–170. doi: 10.1016/j.radonc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Fontugne J, Augustin J, Pujals A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:24644–24651. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim R, Coppola D, Wang E, et al. Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget. 2018;9:23366–23372. doi: 10.18632/oncotarget.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasper HU, Drebber U, Stippel DL, et al. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol. 2009;15:5053–5057. doi: 10.3748/wjg.15.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diederichsen AC, Hjelmborg J, Christensen PB, et al. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–428. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol. 2006;12:7233–7238. doi: 10.3748/wjg.v12.i45.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mao ZY, Zhu GQ, Xiong M, et al. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J Gastroenterol. 2015;21:4961–4968. doi: 10.3748/wjg.v21.i16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin G, Liu Y, Li S, et al. Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget. 2016;7:50963–50971. doi: 10.18632/oncotarget.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ott PA, Bang YJ, Piha-Paul SA, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol. 2019;37:318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 78.Gani F, Nagarajan N, Kim Y, et al. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients With Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2016;23:2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Y, Wang XY, Zhang Y, et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8(+) T-cell immune responses. Cancer Manag Res. 2018;10:4113–4123. doi: 10.2147/CMAR.S172719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye Y, Zhou L, Xie X, et al. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–504. doi: 10.1002/jso.21376. [DOI] [PubMed] [Google Scholar]
- 81.Ma C, Peng C, Lu X, et al. Downregulation of FOXP3 inhibits invasion and immune escape in cholangiocarcinoma. Biochem Biophys Res Commun. 2015;458:234–239. doi: 10.1016/j.bbrc.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 82.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 83.Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824–830. doi: 10.1001/jamaoncol.2019.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 86.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Merck, Co I. 2017. Keytruda (pembrolizumab) injection for intravenous use prescribing information. [Google Scholar]
- 88.Ueno M. ESMO 2018 Congress, Munich, Germany, October 19, 2018. 2018. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase 2 KEYNOTE-158 study. [Google Scholar]
- 89.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest. 2018;128:3209–3218. doi: 10.1172/JCI120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolchok JD, Rollin L, Larkin J. Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:2503–2504. doi: 10.1056/NEJMc1714339. [DOI] [PubMed] [Google Scholar]
- 91.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Floudas CS, Xie C, Brar G, et al. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC) J Clin Oncol. 2019;37:336. [Google Scholar]
- 93.Ascierto PA, Melero I, Bhatia S, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J Clin Oncol. 2017;35:9520. [Google Scholar]
- 94.Brignone C, Escudier B, Grygar C, et al. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 95.Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312:1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelley RK, Mitchell E, Behr S, et al. Phase II trial of pembrolizumab (PEM) plus granulocyte macrophage colony stimulating factor (GM-CSF) in advanced biliary cancers (ABC) J Clin Oncol. 2018;36:386. [Google Scholar]
- 97.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 98.Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 99.Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumour activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- 100.Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102:68–72. doi: 10.1038/sj.bjc.6605458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mizrahi J, Javle MM, Xiao L, et al. A phase II study of ramucirumab for advanced, pre-treated biliary cancers. J Clin Oncol. 2018;36:4081. [Google Scholar]
- 102.Arkenau HT, Martin-Liberal J, Calvo E, et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF) Oncologist. 2018;23:1407–e136. doi: 10.1634/theoncologist.2018-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hingorani SR, Harris WP, Seery TE, et al. Interim results of a randomized phase II study of PEGPH20 added to nab-paclitaxel/gemcitabine in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol. 2016;34:439. [Google Scholar]
- 104.Ilyas SI, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2017;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colli LM, Machiela MJ, Zhang H, et al. Landscape of Combination Immunotherapy and Targeted Therapy to Improve Cancer Management. Cancer Res. 2017;77:3666–3671. doi: 10.1158/0008-5472.CAN-16-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie C, Duffy AG, Mabry-Hrones D, et al. Tremelimumab in Combination With Microwave Ablation in Patients With Refractory Biliary Tract Cancer. Hepatology. 2019;69:2048–2060. doi: 10.1002/hep.30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumour immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 108.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumour immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 109.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fleming V, Hu X, Weber R, et al. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wainberg ZAea. 32nd Annual Meeting and Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2017): Late-Breaking Abstracts. J ImmunoTher Cancer. 2017;5:89. [Google Scholar]
- 114.Wiehagen KR, Girgis NM, Yamada DH, et al. Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumour Immunity. Cancer Immunol Res. 2017;5:1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 115.Hoves S, Ooi CH, Wolter C, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med. 2018;215:859–876. doi: 10.1084/jem.20171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Orillion A, Hashimoto A, Damayanti N, et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumour Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin Cancer Res. 2017;23:5187–5201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steggerda SM, Bennett MK, Chen J, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5:101. doi: 10.1186/s40425-017-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng KC, Guo YL, Liu Y, et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. doi: 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shimizu K, Kotera Y, Aruga A, et al. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:171–178. doi: 10.1007/s00534-011-0437-y. [DOI] [PubMed] [Google Scholar]
- 121.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaida M, Morita-Hoshi Y, Soeda A, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011;34:92–99. doi: 10.1097/CJI.0b013e3181fb65b9. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto K, Ueno T, Kawaoka T, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25:3575–3579. [PubMed] [Google Scholar]
- 124.Aruga A, Takeshita N, Kotera Y, et al. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med. 2014;12:61. doi: 10.1186/1479-5876-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshitomi M, Yutani S, Matsueda S, et al. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med. 2012;3:463–469. doi: 10.3892/etm.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 127.Lepisto AJ, Moser AJ, Zeh H, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- 128.Kobayashi M, Sakabe T, Abe H, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–1617. doi: 10.1007/s11605-013-2286-2. [DOI] [PubMed] [Google Scholar]
- 129.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 130.Jarnagin WR, Zager JS, Hezel M, et al. Treatment of cholangiocarcinoma with oncolytic herpes simplex virus combined with external beam radiation therapy. Cancer Gene Ther. 2006;13:326–334. doi: 10.1038/sj.cgt.7700890. [DOI] [PubMed] [Google Scholar]
- 131.Pugalenthi A, Mojica K, Ady JW, et al. Recombinant vaccinia virus GLV-1h68 is a promising oncolytic vector in the treatment of cholangiocarcinoma. Cancer Gene Ther. 2015;22:591–596. doi: 10.1038/cgt.2015.60. [DOI] [PubMed] [Google Scholar]
- 132.Zhu ZB, Chen Y, Makhija SK, et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int J Oncol. 2006;29:1319–1329. [PubMed] [Google Scholar]
- 133.Forbes NS, Coffin RS, Deng L, et al. White paper on microbial anti-cancer therapy and prevention. J Immunother Cancer. 2018;6:78. doi: 10.1186/s40425-018-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material