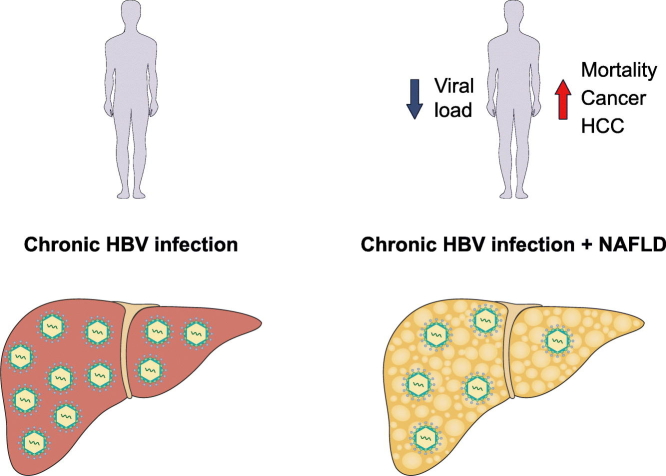
Background & Aims
Liver steatosis may occur concomitantly in patients with chronic hepatitis B infection (CHB) and is implicated in increased morbidity and mortality. Hepatitis B virus (HBV) viral load is a marker for disease progression and long-term outcomes in CHB. We investigated the association between liver steatosis and HBV viral load and their individual effects on all-cause mortality and the development of cancer in patients with CHB and liver steatosis.
Methods
This retrospective study included 524 treatment-naïve patients with CHB, with a mean follow-up of 6 years. Liver biopsy was available for 170 patients and liver steatosis was validated by at least 3 ultrasonographic examinations.
Results
A total of 241/524 (46%) patients with CHB had liver steatosis, with a strong correlation between the degree of liver steatosis as assessed by ultrasonography or by liver biopsy (r = 0.9, p < 0.001). Although liver steatosis was not significantly associated with advanced fibrosis, a multivariate analysis showed that liver steatosis was associated with a 4-fold increased risk of all-cause mortality and cancer (hazard ratio 4.35; 95% CI 1.69–8.99; p < 0.001), irrespective of other major metabolic factors. However, baseline HBV viral load was not significantly associated with this composite outcome (hazard ratio 1.65; p = 0.29). In addition, liver steatosis was inversely associated with HBV viral load.
Conclusion
Patients with CHB and liver steatosis have an increased risk of all-cause mortality and cancer development compared to patients with CHB without liver steatosis, regardless of their baseline HBV viral load. Although tending to have a lower baseline viral load, patients with CHB and liver steatosis should be closely monitored irrespective of viral load.
Lay summary
Patients with chronic hepatitis B infection (CHB) may have liver steatosis at the same time. Here we show that in patients with CHB, liver steatosis is significantly associated with all-cause mortality and cancer, irrespective of other major metabolic factors, and the effect of liver steatosis on mortality and cancer is stronger than the effect of hepatitis B viral load on these outcomes. Thus, patients with CHB and liver steatosis should be closely monitored, irrespective of their viral load.
Keywords: Non-alcoholic fatty liver disease, viremia, prognosis
Graphical abstract
Highlights
-
•
HBV viral load is an important predictor of adverse outcomes in patients with chronic HBV (CHB).
-
•
Liver steatosis may co-occur with CHB but its effect on all-cause mortality and cancer has not been determined.
-
•
Liver steatosis is significantly associated with all-cause mortality and cancer in patients with CHB.
-
•
The effect of liver steatosis on mortality and cancer is stronger than the effect of HBV viral load.
-
•
Patients with CHB and liver steatosis should be closely monitored, irrespective of their viral load.
Introduction
Chronic hepatitis B infection (CHB) is one of the most prevalent liver diseases worldwide1 and is a leading cause of death, mainly due to the development of end-stage liver disease and liver cancer (hepatocellular carcinoma, [HCC]).[2], [3] Elevated viral load (VL) is a strong predictor for liver cirrhosis, HCC and liver-related mortality,[4], [5], [6], [7], [8] underscoring the importance of long-term treatment of viremic patients with drugs that efficiently suppress viral replication.9
It is estimated that at least 30% of patients with CHB have non-alcoholic fatty liver disease with liver steatosis (LS),[10], [11] a condition associated with the metabolic syndrome and a major risk factor by itself for liver and non-liver-related morbidity and mortality.[12], [13], [14], [15], [16] Previous studies have recognized the metabolic syndrome as a risk factor for progression to cirrhosis in patients with CHB infection, independent of VL and alanine aminotransferase levels.[17], [18] In addition, elevated body mass index (BMI) proved to be an independent risk factor for liver-related mortality in this group of patients.19
Although both hepatitis B virus (HBV) VL and LS are implicated in long-term unfavorable outcomes, interactions between LS and VL, and their relative contributions to long-term outcomes in these patients, have yet to be determined. In this study, we investigated possible interactions between LS and HBV replication and their distinctive contributions to major clinical endpoints, including all-cause mortality and the development of cancer.
Patients and methods
Patients
Patients presenting at our liver clinic from January 2007 to December 2017, older than 18 years and positive for hepatitis B surface antigen (HBsAg), were eligible for the study. Patients with other concomitant liver diseases, including hepatitis C, alcohol-related liver disease or alcohol consumption, drug-related liver disease, liver transplantation, previous liver surgery regardless of type and cause, a diagnosis of any type of cancer (including HCC), known HIV infection or pregnancy were excluded. Patients who already had been on antiviral treatment for CHB were excluded. The study was approved by the local institutional review board of the Rabin Medical Center according to the local regulations (RMC-17-0530) and was conducted according to ethical guidelines of the 1975 Declaration of Helsinki.
Assessments and outcome measures
A comprehensive assessment of patients’ demographic, clinical and laboratory data, and a systematic review of patients’ electronic records were performed. Patients were assigned a diagnosis of type 2 DM in cases of documented use of oral hypoglycemic drugs or insulin, or if the general practitioner (GP) had made this diagnosis. Patients were considered as having hypertension if the GP had made this diagnosis according to established guidelines or if they were using antihypertensive drug(s). The duration of CHB was defined from the first HBsAg-positivity known in the patients’ electronic records. Laboratory assessment included basic liver biochemistries, creatinine, albumin, bilirubin, alpha-fetoprotein, complete blood count, international normalized ratio, triglycerides, total cholesterol, hemoglobin A1C and serum HBV DNA. Liver fibrosis was assessed by aspartate aminotransferase to platelet ratio index (APRI) score and fibrosis-4 (FIB-4) score with well-established cut-offs. All patients included in the study underwent at least 3 ultrasonographic (US) studies with repeated evaluation of LS in each exam. Patients with less than 3 US studies or conflicting results of LS status were excluded. The sonographic appearance of LS was ranked as mild, moderate or severe by the examiners as recorded in the medical records. In cases where liver biopsies had been performed, the histological results were recorded from the patients’ files. Only biopsies that took place in the first 6 months after presentation were included in the study. The level of steatosis was assessed and graded on a scale from 1 to 3 (1 = up to 30% of hepatocytes affected, 2 = 30%–60% of hepatocytes affected, 3 = more than 70% of hepatocytes affected). The degree of fibrosis was reported using the Metavir score, and Metavir scores of F3-F4 were considered as advanced fibrosis.
Time at risk was defined as time from the date of first visit in the liver clinic to the date of outcome or to the last day of follow-up. The primary end point of the study was the composite endpoint of all-cause mortality and development of cancer. Secondary endpoints included all-cause mortality, malignancy of any type, and the development of HCC. According to well established guidelines, HBV VL of 2,000 IU/ml is a threshold for antiviral treatment in HBV e antigen (HBeAg) negative patients with hepatitis.20 Our cohort of patients is composed primarily of HBeAg negative patients, and according to that, we defined a high level of HBV VL as > 2,000 IU/ml, and a low level of HBV VL as ≤ 2,000 IU/ml.
Statistical analysis
Characteristics of study patients were compared using the Student’s t test, Chi-square, or Fisher’s exact tests, as appropriate. The probability of the composite endpoint of all-cause mortality and cancer by LS for each VL group was graphically displayed according to the Kaplan-Meier method, with comparison of cumulative events by the log-rank test.
Correlation between LS assessed by US and liver biopsy was evaluated by the Pearson correlation coefficient. A step-wise cox proportional hazards regression analysis was conducted to identify independent variables associated with the primary endpoint, and predictors with p value < 0.1 were included in the first model. We forced HBV VL, type 2 DM and BMI as a part of the cox regression analysis in order to find the contribution of both HBV VL and these metabolic factors to the primary outcome, and to compare their effect to the effect of LS on the primary outcome. The final multivariate analysis model included the following variables: age, albumin, alpha-fetoprotein, LS type 2 DM, BMI and HBV VL. Variables with missing values in more than 20% of the patients were not included in the statistical analysis. In order to confirm the robustness of our statistical analysis and in order to avoid potential biases we also conducted sensitivity analyses. In addition, the potential synergistic effect between LS and CHB was assessed by comparison to a historic cohort of 153 patients with biopsy-proven non-alcoholic fatty liver disease (NAFLD) who were monitored in our facilities. The patients included in this historic cohort underwent liver biopsy as part of their evaluation between 2006 to 2012, and were monitored until 2017 with a mean follow-up of 8 years as previously described.21 Statistical analysis was performed using SPSS version 25 (SPSS Inc., Chicago, IL). A 2-sided p value of less than 0.05 was considered statistically significant.
Results
Patients’ baseline characteristics
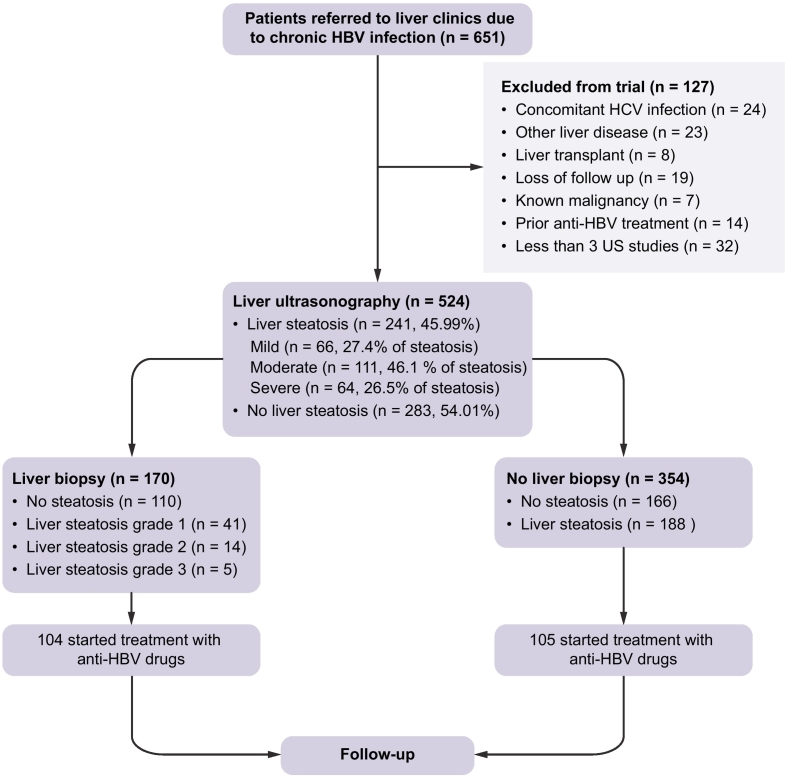
A total of 651 patients presented to the liver clinic due to CHB infection, of which 524 treatment-naïve patients were included in the study, as depicted in Fig. 1. According to liver ultrasonography, 241 patients (46% of the study population) had LS while 283 did not.
Fig. 1.
Study flow chart.
HBV, hepatitis B virus. HCV, hepatitis C virus.
One-hundred and seventy patients had undergone liver biopsy at the discretion of their physician. We found a strong correlation between the level of LS, as assessed by US and the actual biopsy-proven liver steatosis (Pearson’s r = 0.9, p < 0.001).
The baseline characteristics of the study population, according to LS status, are presented in Table 1. Patients with LS were significantly older than patients without LS. As expected, patients with LS had higher BMI, triglyceride levels and total cholesterol levels than patients without LS. In addition, type 2 DM and hypertension were significantly more common in the LS population (p < 0.05 for all). Patients with LS had lower APRI score (0.53, compared to 0.69 for patients without LS, p = 0.051), and the 2 groups did not significantly differ in their baseline FIB-4 score. In addition, the 2 groups did not differ in the levels of serum liver enzymes and duration of CHB infection. Only 7.44% of the study population were positive for HBeAg, and most of them did not have LS (12.8% vs. 1.2%, p < 0.001).
Table 1.
Characteristics of study population according to baseline liver steatosis status as reported by ultrasound.
| Liver steatosis (n = 241) | No liver steatosis (n = 283) | p value | |
|---|---|---|---|
| Gender male, % (n) | 58.90 (142) | 61.10 (173) | 0.612 |
| Age (range) | 50.54 (23.16–83.96) | 42.32 (18.50–83.50) | < 0.001 |
| BMI (range) | 27.16 (19.53–41.00) | 24.63 (18.17–41.00) | < 0.001 |
| HTN diagnosis, % | 28.60 | 10.20 | < 0.001 |
| DM2 % | 26.60 | 11.30 | < 0.001 |
| Hemoglobin A1C (%) (range) | 6.33 (4.40–13.60) | 5.61 (3.60–10.20) | < 0.001 |
| Total cholesterol (mg/dl) (range) | 179.20 (92–367) | 170.09 (33–320) | < 0.001 |
| Triglycerides (mg/dl) (range) | 126.14 (46–486) | 100.27 (38–366) | 0.010 |
| Creatinine (mg/dl) (range) | 0.90 (0.4–6.00) | 0.89 (0.4–12.98) | 0.916 |
| Hemoglobin (g/dl) (range) | 13.61 (5–18) | 13.95 (8–15) | 0.931 |
| Platelets (range) | 219.87 (40–474) | 204.27 (125–480) | 0.010 |
| INR (range) | 1.03 (0.8–3.22) | 1.05 (0.75–1.7) | 0.148 |
| Albumin (g/dl) (range) | 4.21 (3–5.1) | 4.20 (3–5) | 0.718 |
| Bilirubin (mg/dl) (range) | 0.73 (0.20–2.30) | 0.74 (0.28–4) | 0.010 |
| Alpha feto protein (range) | 5.16 (0.61–148) | 3.66 (0.63–41) | 0.096 |
| AST (U/L) (range) | 32.02 (9–345) | 38.48 (10–254) | 0.355 |
| ALT (U/L) (range) | 39.02 (10–500) | 45.50 (10–255) | 0.210 |
| ALP (mg/dl) (range) | 81.55 (26–251) | 79.39 (36–250) | 0.561 |
| GGT (U/L) (range) | 42.14 (9–321) | 36.47 (8–300) | 0.134 |
| Log HBV DNA (± SD) | 2.09 (± 0.94) | 4.83 (± 1.87) | < 0.001 |
| HBeAg positive % | 1.20 | 12.80 | < 0.001 |
| HDV Ab positive % | 5.50 | 6.20 | 0.881 |
| HBV duration, years (range) | 11.16 (0.66–28.16) | 10.96 (0.91–28.16) | 0.732 |
| APRI score | 0.53 | 0.69 | 0.051 |
| FIB-4 score | 1.51 | 1.62 | 0.516 |
The data for continuous variables include mean and range.
ALP, alkaline phosphatase; APRI, AST to platelet ratio index; BMI, body mass index; DM2 = type 2 diabetes mellitus; FIB-4, fibrosis 4 score; GGT, gamma glutamyltransferase; HBV, hepatitis B virus; HBeAg, HBV e antigen; HDV, hepatitis D virus; HTN, hypertension; INR, international normalized ratio.
HBV DNA lower limit of detection is 1.3 log IU/ml (20 IU/ml); SD = standard deviation
Major clinical outcome during follow-up
The cohort of patients included in the study had a mean follow-up period of 73.62 months (median 70 months, range 1.79–168.04). Table 2 outlines major clinical outcomes of the study population during the follow-up period according to baseline LS status. The presence of LS was significantly associated with the development of the composite primary outcome (15.4% compared to 4.6% in patients without LS, p < 0.001). LS was also associated with each component of the primary outcome – all-cause mortality (6.6% vs. 1.4%, p = 0.01), the development of any type of cancer (13.3% vs. 3.2%, p < 0.001) and HCC (5.4% vs. 1.4%, p = 0.01). Patients with LS were more likely to be hospitalized during the follow-up period (1.48 vs. 0.81 admissions, p < 0.001) and had longer hospitalization stays (1.4 vs. 0.8 days, p = 0.01) compared to patients without LS. In addition, patients without LS were more likely to start anti-HBV treatment during the follow-up period (48.8% vs. 29.5%, p < 0.001), most probably due to their higher baseline VL.
Table 2.
Major clinical outcomes of study population during the follow-up period according to baseline liver steatosis status.
|
Liver steatosis (n = 241) |
No liver steatosis (n = 283) |
p value | |
|---|---|---|---|
| Length of follow up (months) (range) | 72.4 (0.96–168.48) | 80.2 (1.44–168) | 0.085 |
| Composite outcome of all-cause mortality and cancer, % (n) | 15.4 (37) | 4.6 (13) | < 0.001 |
| All-cause mortality, % (n) | 6.6 (16) | 1.4 (4) | 0.010 |
| Malignancy (all types), % (n) | 13.7 (33) | 3.2 (9) | < 0.001 |
| Hepatocellular carcinoma, % (n) | 5.8 (14) | 1.4 (4) | 0.010 |
| Extra-hepatic malignancies, % (n) | 9.9 (19) | 1.8 (5) | 0.010 |
| Initiation of anti-HBV treatment, % (n) | 29.5 (71) | 48.8 (138) | < 0.001 |
Twenty mortality events were recorded during follow-up, of which 8 (40%) were attributed to cirrhosis and its complications, 8 (40%) were attributed to infections and 4 (20%) were attributed to cardiovascular diseases.
A newly diagnosed cancer occurred in 42 patients (8% of the study population) during follow-up. Of them, 18 patients (42.85%) had HCC, 5 patients (11.90%) had breast cancer, 4 patients (9.52%) had colon cancer, 4 patients (9.52%) had lymphoma and 2 patients (4.76%) had prostate cancer. Nine other patients had (1 case each): carcinoid, cholangiocarcinoma, esophageal cancer, gastric cancer, glioblastoma multiforme, multiple myeloma, renal cell carcinoma, sarcoma or transitional cell carcinoma.
A total of 209 patients (39.88%) started antiviral therapy during the follow-up period; 188 patients (35.87% of the study population) had started antiviral therapy within the first 12 months of the follow-up and another 21 patients, who were not eligible for treatment at the first year of follow-up, have become eligible for treatment during follow-up thereafter. Treatment regimens include tenofovir (82 patients, 39.23% of treated patients), entecavir (65 patients, 31.10% of treated patients), lamivudine (45 patients, 21.53% of treated patients) and pegylated interferon (17 patients, 8.13% of treated patients).
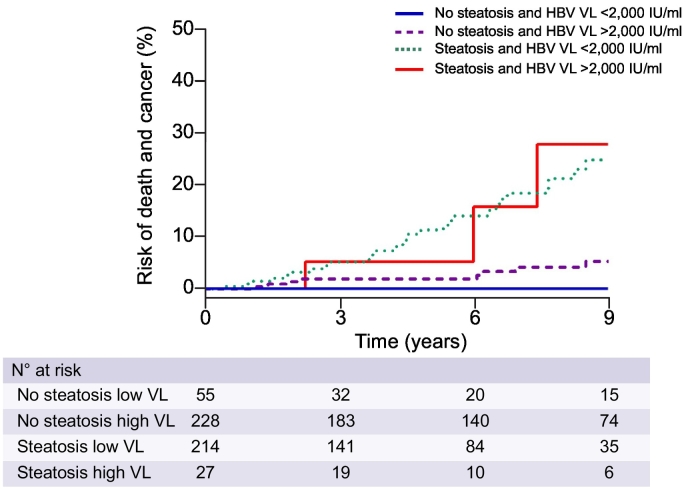
Next, a Kaplan-Meier analysis of the composite endpoint of mortality and cancers during the follow-up period, as a function of both baseline VL and LS status, was performed (Fig. 2). As expected, patients without LS and with a low baseline VL (< 2,000 IU/ml) had the most favorable prognosis during the follow-up period, whereas patients with LS had bad prognosis regardless of HBV VL. Most importantly, patients with high VL but without LS did better than those with LS but with low VL, suggesting that LS has a greater impact on long-term prognosis than VL in patients with CHB.
Fig. 2.
Cumulative rates of mortality and all types of cancer (primary composite outcome) according to the status of HBV viral load and liver steatosis. HBV, hepatitis B virus; VL, viral load.
Table S1 presents variables associated with the composite outcome of mortality or development of cancer among patients. By multivariate analysis, baseline LS was associated with a 4-fold increased risk of the composite outcome, even after adjusting for other major metabolic factors as shown in Table 3. Other independent predictors of the primary outcome included old age, elevated alpha-fetoprotein and low albumin levels. Interestingly, high HBV VL, type 2 DM and BMI were not found to be significantly associated with the composite outcome of mortality and cancer.
Table 3.
Multivariate analysis: Predictors of the composite endpoint of all-cause mortality and cancer.
| HR (95% CI) | p value | |
|---|---|---|
| Baseline liver steatosis | 4.35 (1.69–8.99) | < 0.001 |
| HBV VL > 2,000 IU/ml | 1.65 (0.65–4.20) | 0.298 |
| Age | 1.04 (1.01–1.06) | < 0.001 |
| Albumin | 0.42 (0.20–0.81) | 0.010 |
| Alpha-fetoprotein | 1.02 (1.01–1.03) | 0.010 |
| BMI | 0.97 (0.91–1.05) | 0.454 |
| Type 2 DM | 1.56 (0.83–2.99) | 0.177 |
DM, Diabetes mellitus; HBV, hepatitis B virus; VL, viral load; HBV DNA lower limit of detection 1.3 log IU/ml (20 IU/ml).
The interaction between HBV viral load and liver steatosis
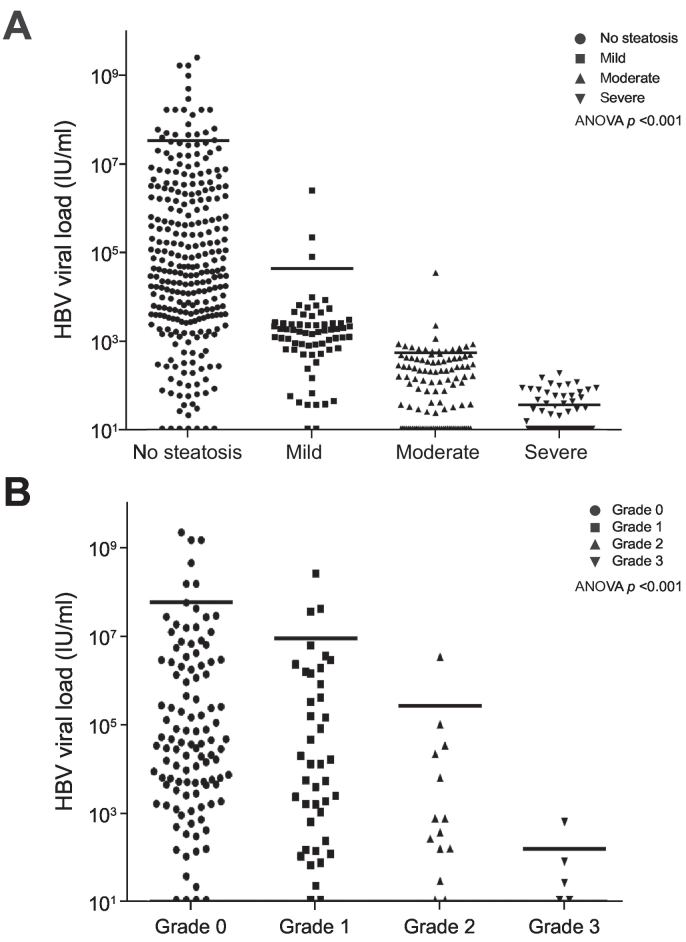
Untreated patients with CHB and LS had a significantly lower average baseline HBV VL compared to patients without LS (Log HBV DNA 2.09 ± 0.94 vs. 4.83 ± 1.87, p < 0.001) (Table 1). The distribution of HBV VL according to the degree of LS, as determined by US and by liver biopsies, is shown in Fig. 3A and 3B, respectively. An inverse association was observed between the degree of LS, evaluated either by US or by liver biopsy, and HBV VL (ANOVA p < 0.001 for both).
Fig. 3.
HBV viral load according to the status of liver steatosis. Assessed by (A) ultrasonography, or by (B) liver biopsy. HBV, hepatitis B virus.
Fibrosis and major clinical outcomes
Out of 170 patients that underwent liver biopsy as part of their evaluation, 27 (19.28%) had advanced fibrosis. The presence of advanced fibrosis was significantly associated with all-cause mortality and the development of cancer (p = 0.01, Table S2-A). We also evaluated the association between the non-invasive fibrosis markers APRI and FIB-4 scores and major clinical outcomes, as reported in Table S2B and S2C.
Sensitivity analyses
In order to test the robustness of our analysis and to remove the potential bias related to the lower rates of antiviral therapy in patients with LS, a phenomenon that might explain the higher risk of all-cause mortality and cancer in these patients, we have conducted several sensitivity analyses. First, we evaluated the rates of the primary outcome only in patients who received antiviral therapy during follow-up (Table S3). Even in this population of high baseline VL, which ultimately led to antiviral therapy initiation, LS was significantly associated with the development of the composite primary outcome (36.6% compared to 6.5% in patients without LS, p < 0.001).
Next, in order to assess the specific influence of the initiation of antiviral therapy on the primary outcome, we forced it to the multivariate analysis. This did not significantly change the effect of LS on the primary outcome (hazard ratio [HR] 4.96; 95% CI 1.84–13.33; p = 0.002, Table S4). In addition, we assessed the impact of advanced fibrosis markers on the primary outcome by adding APRI and FIB-4 scores to the multivariate analysis. This also did not change the influence of LS on the primary outcome as shown in Table S5. We further evaluated the effect of LS on a different composite outcome of all-cause mortality and the development of HCC specifically (Table S6), showing that the effect of LS on this specific outcome (HR 4.29; 95% CI 1.18–15.61; p = 0.029) was similar to its effect on the primary composite outcome of mortality and cancer.
Finally, we evaluated the potential synergism between LS and CHB using a well-established historic cohort of 153 patients with NAFLD. As this latter cohort included only patients that underwent liver biopsy, we used our cohort of patients with CHB who underwent liver biopsy (n = 170) for comparison. A Kaplan-Meier analysis of the composite endpoint of mortality and cancers as a function of the basic liver pathology was performed and depicted in Fig. S1. Patients with LS and CHB had significantly higher rates of the primary outcome compared to patients with NAFLD or patients with CHB without liver steatosis (log-rank p < 0.001). In a multivariate analysis, the presence of LS and CHB were significantly associated with the primary outcome (HR 21.15; 95% CI 4.90–90.09; p < 0.001) (Table S7).
Discussion
In this large single-center retrospective cohort study, the presence of LS among treatment-naïve patients with CHB was a major risk factor for the development of all types of cancer, both hepatic and extrahepatic, in addition to all-cause mortality, whereas HBV VL was not significantly associated with these poor outcomes. Importantly, about a third of the patients had an available liver biopsy, and a good correlation between US and liver biopsy in assessing LS status was found. In addition, we found that LS and its degree were inversely associated with HBV VL.
The main question in our study focused on the association of major clinical outcomes among patients with CHB and co-existent LS. The association between CHB infection and the development of HCC and all-cause mortality has been previously studied,[22], [23] although the association of LS with these outcomes in this particular population of patients with CHB had not been thoroughly investigated. Previous studies have shown the connection between LS and the metabolic syndrome, a major risk factor by itself for liver and non-liver-related morbidity and mortality. We believe that this observation stands in the basis of our findings. In addition, we identified a synergistic effect between LS and CHB compared to patients with biopsy-proven NAFLD that may explain our results.
In contrast to NAFLD, the majority of mortality events in our cohort resulted from complications related to cirrhosis and infections. Mortality due to cardiovascular events occurred in only 20% of deceased patients and was the third leading cause of death. Although previous studies suggested that cardiovascular, rather than liver-related, complications are the leading cause of mortality in patients with NAFLD,24 we believe that the synergistic effect of LS with CHB resulted in a more rapid development of advanced liver disease and its complications, compared to patients with NAFLD but without CHB. However, further studies directly comparing long-term outcomes of patients with CHB and LS to patients with NAFLD without CHB are needed to confirm this hypothesis.
After a mean follow-up period of 73.62 months, 39.88% of the study population started anti-HBV treatment, most of them with tenofovir or entecavir. These rates are similar to reported treatment eligibility rates of patients with CHB, which range from 16% to 50%,[25], [26] with patients undergoing evaluation in community gastroenterology clinics or by primary care physicians being less likely to receive treatment.27 In our study, patients without LS were more likely to be eligible for treatment, most probably due to a higher baseline VL associated with the absence of LS. Nonetheless, even when evaluated in the subgroup of patients that were treated with anti-HBV drugs, LS was still significantly associated with all-cause mortality and cancer, and its hazardous effect was statistically significant even when adjusting for initiation of anti-HBV therapy.
In our study, we also observed an inverse association between HBV VL and LS. Previous studies have reported this inverse association[28], [29] but have not used liver biopsies as the gold standard for the assessment of LS. In our cohort, the inverse association between HBV VL and LS was observed both in the entire cohort and in the subgroup of 170 patients who underwent a liver biopsy. The mechanisms underlying the inverse association between LS and HBV replication are not clear. The cross-talk between HBV gene expression and replication and the metabolic milieu of the liver has been shown in several studies in the past.[30], [31], [32], [33] Moreover, few studies have postulated that fat deposition in HBV-infected hepatocytes may reduce HBV replication directly, or alternatively, by inducing hepatocyte apoptosis.[34], [35] In this regard, in our study we did not observe any significant differences in serum alanine aminotransferase levels between patients with CHB, with or without LS, arguing against the possibility of massive hepatocyte damage in these patients.
In line with our results obtained in patients with CHB, a study by Hu et al.36 has shown that the presence of LS attenuates HBV replication in hydrodynamically HBV DNA injected immunocompetent mice. Further in vitro and in vivo studies are needed in order to elucidate whether the presence of LS creates a local micro-environment that is suboptimal for HBV replication, or alternatively the systemic effect implicated by the metabolic derangements associated with fatty liver is the cause for HBV replication reduction.
This study has several strengths. It is based on a large cohort of treatment-naïve patients at baseline and in addition, a large portion of these patients underwent liver biopsy and therefore information about the grade of their LS was available. Nonetheless, this study is retrospective in nature and based on data of a single tertiary center. In addition, waist circumference, smoking status, high-density lipoprotein and HBsAg quantitative plasma levels were not available for more than 20% of the study population and hence were not a part of the statistical analysis.
In our cohort, patients with LS did not have a higher baseline fibrosis stage as assessed by APRI or FIB-4 scores, compared to patients without LS. However, assessment or liver fibrosis by biopsy or elastography was not available for most of our study cohort and we are well aware of the limitation of both APRI and FIB-4 as non-invasive tools for assessing liver fibrosis in patients with CHB.37
In conclusion, LS, although associated with a lower HBV VL, is a major risk factor for all-cause mortality and the development of cancer in patients with CHB. Therefore, this subgroup of patients should be closely monitored and screened for both hepatic as well extrahepatic malignancies, irrespective of their VL. Further studies are needed to address possible mechanisms underlying the inverse association between HBV VL and the presence of LS.
Financial support
The authors received no financial support to produce this manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Noam Peleg – Conceptualization, data collection and analysis, writing the first draft and final draft. Assaf Issachar – Data collection analysis and interpretation, reviewing/editing. Orly Sneh Arbib – Data collection analysis and interpretation, reviewing/editing. Michal Cohen-Naftaly – Data collection analysis and interpretation, reviewing/editing. Marius Braun- Data collection analysis and interpretation, reviewing/editing. Alon Barsheshet – Statistical analysis and reviewing/editing the paper. Moshe Leshno – Statistical analysis and reviewing/editing the paper. Amir Shlomai – Conceptualization, data collection and analysis, writing first draft and final draft, supervising the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2019.02.002.
Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
References
- 1.MacLachlan JH, Cowie BC. Hepatitis B Virus Epidemiology. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gish RG, Given BD, Lai C-L, Locarnini SA, Lau JYN, Lewis DL. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47–58. doi: 10.1016/j.antiviral.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 6.Iloeje Uchenna H, Yang Hwai‐ I, Chen CJ. Natural history of chronic hepatitis B: what exactly has REVEAL Revealed? Liver Int. 2012;32:1333–1341. doi: 10.1111/j.1478-3231.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee MH, Yang Hwai‐ I, Liu J, Batrla‐Utermann R, Jen CL, Iloeje Uchenna H. Prediction models of long‐term Cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology. 2013;58:546–554. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 8.Nakazawa T, Shibuya A, Takeuchi A, Shibata Y, Hidaka H, Okuwaki Y. Viral level is an indicator of long‐term outcome of hepatitis B virus e antigen‐negative carriers with persistently normal serum alanine aminotransferase levels. J Viral Hepat. 2011;18:e191–e199. doi: 10.1111/j.1365-2893.2010.01427.x. [DOI] [PubMed] [Google Scholar]
- 9.Lok ASF, McMahon BJ, Brown RS, Wong JB, Ahmed AT, Farah W. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63(1):284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 10.Machado Mariana V, Oliveira António G, Cortez‐Pinto H. Hepatic steatosis in hepatitis B virus infected patients: Meta‐analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361–1367. doi: 10.1111/j.1440-1746.2011.06801.x. [DOI] [PubMed] [Google Scholar]
- 11.Spradling PR, Bulkow L, Teshale EH, Negus S, Homan C, Simons B. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol. 2014;61:785–791. doi: 10.1016/j.jhep.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 13.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 14.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 15.Kim G-A, Lee HC, Choe J, Kim M-J, Lee MJ, Chang H-S. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017;68:140–146. doi: 10.1016/j.jhep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 17.Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B – a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883–893. doi: 10.1111/apt.12658. [DOI] [PubMed] [Google Scholar]
- 18.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 19.Yu M-W, Shih W-L, Lin C-L, Liu C-J, Jian J-W, Tsai K-S. Body-Mass Index and Progression of Hepatitis B: A Population-Based Cohort Study in Men. J Clin Oncol. 2008;26:5576–5582. doi: 10.1200/JCO.2008.16.1075. [DOI] [PubMed] [Google Scholar]
- 20.Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Peleg N, Sneh Arbib O, Issachar A, Cohen-Naftaly M, Braun M, Shlomai A. Noninvasive scoring systems predict hepatic and extra-hepatic cancers in patients with nonalcoholic fatty liver disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202393. e0202393-e0202393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim G-A, Lim Y-S, Han S, Choi J, Shim JH, Kim KM. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945. doi: 10.1136/gutjnl-2017-314904. [DOI] [PubMed] [Google Scholar]
- 23.Mortality GBD, Causes of Death C Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Jung Chin W, Tan J, Tan N, Kuo Melissa N, Ashok A, Eells Samantha J. Evidence for the insufficient evaluation and undertreatment of chronic hepatitis B infection in a predominantly low‐income and immigrant population. J Gastroenterol Hepatol. 2010;25:369–375. doi: 10.1111/j.1440-1746.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen VG, Wan K, Trinh HN, Li J, Zhang JQ, Nguyen MH. Chronic Hepatitis B Treatment Eligibility and Actual Treatment Rates in Patients in Community Gastroenterology and Primary Care Settings. J Clin Gastroenterol. 2015;49:145–149. doi: 10.1097/MCG.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 27.Vu VD, Do A, Nguyen NH, Kim LH, Trinh HN, Nguyen HA. Long-term follow-up and suboptimal treatment rates of treatment-eligible chronic hepatitis B patients in diverse practice settings: a gap in linkage to care. BMJ Open Gastroenterol. 2015;2 doi: 10.1136/bmjgast-2015-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rastogi A, Sakhuja P, Kumar A, Hissar S, Jain A, Gondal R. Steatosis in chronic hepatitis B: Prevalence and correlation with biochemical, histologic, viral, and metabolic parameters. Indian J Pathol Microbiol. 2011;54:454–459. doi: 10.4103/0377-4929.85074. [DOI] [PubMed] [Google Scholar]
- 29.Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J. Inverse relationship between hepatic steatosis and hepatitis B viremia: Results of a large case‐control study. J Viral Hepat. 2017;25:97–104. doi: 10.1111/jvh.12766. [DOI] [PubMed] [Google Scholar]
- 30.Bar-Yishay I, Shaul Y, Shlomai A. Hepatocyte metabolic signalling pathways and regulation of hepatitis B virus expression. Liver Int. 2011;31:282–290. doi: 10.1111/j.1478-3231.2010.02423.x. [DOI] [PubMed] [Google Scholar]
- 31.Oehler N, Volz T, Bhadra Oliver D, Kah J, Allweiss L, Giersch K. Binding of hepatitis B virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology. 2014;60:1483–1493. doi: 10.1002/hep.27159. [DOI] [PubMed] [Google Scholar]
- 32.Shlomai A, Paran N, Shaul Y. PGC-1 alpha controls hepatitis B virus through nutritional signals. Proc Natl Acad Sci U S A. 2006;103:16003–16008. doi: 10.1073/pnas.0607837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shlomai A, Shaul Y. The "metabolovirus" model of hepatitis B virus suggests nutritional therapy as an effective anti-viral weapon. Med Hypotheses. 2008;71:53–57. doi: 10.1016/j.mehy.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 2006;31:871. doi: 10.1038/sj.ijo.0803479. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Pan Q, Duan XY, Liu Q, Mo GY, Rao GR. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J Gastroenterol Hepatol. 2012;27:1858–1864. doi: 10.1111/j.1440-1746.2012.07268.x. [DOI] [PubMed] [Google Scholar]
- 36.Hu D, Wang H, Wang H, Wang Y, Wan X, Yan W. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int. 2018;12(5):438–446. doi: 10.1007/s12072-018-9877-7. [DOI] [PubMed] [Google Scholar]
- 37.Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773–780. doi: 10.1016/j.jhep.2015.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3