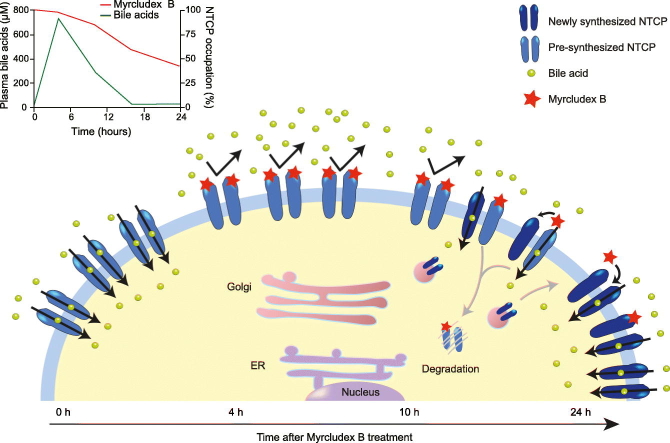
Abstract
Background & aims
The sodium taurocholate co-transporting polypeptide (NTCP) is the entry receptor for the hepatitis B and delta virus (HBV/HDV) and the main hepatic uptake transporter of conjugated bile acids. Myrcludex B, a synthetic peptide mimicking the NTCP-binding domain of HBV, blocks HBV/HDV infection and inhibits NTCP-mediated bile acid uptake. In humans this increases systemic bile acid levels, which remain elevated for hours even after Myrcludex B is cleared from the circulation. Here, we investigated the dynamics of Myrcludex B-induced NTCP-mediated bile acid transport inhibition in mice and if/how the duration of this effect relates to NTCP protein turnover.
Methods
Plasma bile acids were determined in Myrcludex B-treated OATP1a/1b-deficient mice. In vitro, plasma membrane-resident NTCP was labeled with biotin or fluorescein isothiocyanate (FITC)-labeled Myrcludex B and traced in time using hNTCP-overexpressing U2OS cells. Förster resonance energy transfer by fluorescent lifetime imaging microscopy was used to investigate whether Myrcludex B can transfer to newly synthesized NTCP.
Results
Conjugated bile salt levels in plasma peaked 4 h after subcutaneous Myrcludex B administration. After 24 h, plasma bile salt levels were completely normalized, in line with restored NTCP-mediated bile acid transport in vitro. Biotin-labeled NTCP disappeared faster than Myrcludex B-FITC, with almost 40% of FITC signal remaining after 24 h. FITC fluorescence lifetime was strongly decreased upon expression of DY547-labeled acyl carrier protein-tagged NTCP, demonstrating transfer of pre-bound Myrcludex B-FITC to newly formed NTCP.
Conclusions
The dynamics of NTCP protein turnover and Myrcludex B-induced plasma bile salt elevations are similar, suggesting that the Myrcludex B:NTCP interaction is very long-lived. Nevertheless, Myrcludex B is not completely degraded together with NTCP and can transfer to newly synthesized NTCP.
Lay summary
The experimental drug Myrcludex B binds the sodium taurocholate co-transporting polypeptide (NTCP), the viral entry receptor for the hepatitis B and D virus (HBV/HDV), and thereby prevents infection, but also inhibits hepatic bile salt uptake leading to transiently elevated bile salt levels. This study describes that while the normalization of plasma bile salt levels likely depends on the protein turnover rate of NTCP, Myrcludex B partly escapes co-degradation with NTCP by transferring from one NTCP molecule to another. This is of importance to the HBV/HDV research field as it provides a potential explanation for the distinct kinetics and dose-dependence of Myrcludex B’s effects on viral infection versus bile salt transport.
Key words: Bile acid, NTCP, OATP, Myrcludex B, hepatitis B virus, transporter, hepatitis Delta
Graphical Abstract
Highlights
-
•
Myrcludex B-induced plasma bile salt elevations coincide with NTCP protein turnover.
-
•
NTCP-bound Myrcludex B can transfer from one NTCP molecule to another.
-
•
Transfer to newly synthesized NTCP might extend the inhibitory potential of Myrcludex B.
-
•
50% occupation of NTCP by Myrcludex B is not enough to block bile acid transport.
Introduction
The sodium taurocholate co-transporting polypeptide (NTCP, SLC10A1) is exclusively expressed in the liver and plays a pivotal role in the enterohepatic circulation of bile salts as the main uptake transporter of conjugated bile salts from the (portal) blood into the liver.1 Furthermore, NTCP functions as the entry receptor for the hepatitis B and delta virus (HBV/HDV).2,3 The NTCP-binding myristoylated preS1-domain of the HBV envelope L-protein is mimicked by the synthetic peptide Myrcludex B, which effectively reduces HBV/HDV infection in vitro.4 Myrcludex B is being tested in phase III clinical trials and showed a strong transient dampening of HDV RNA serum levels and alanine aminotransferase normalization in HBV and HDV co-infected patients in the phase II studies.5,6 Myrcludex B also inhibits NTCP-mediated bile salt transport,2,5,7 leading to a temporary elevation of plasma bile salt concentrations, but also to hepatoprotection during cholestasis in mice.8,9 In wild-type mice, hepatic uptake of conjugated bile acids is efficiently performed not only by NTCP but also by members of the organic anion transporting polypeptide (OATP) family,7 which were previously believed to mediate only the transport of unconjugated bile acid species.1,10 Therefore, significant bile salt elevations following Myrcludex B administration in mice are only detected when gene expression of Oatp1a1 is absent or downregulated.7,9 After injection, Myrcludex B rapidly accumulated in the liver where it stays for more than 24 hours (half-life of approximately 16 h in mice).11 So, the relationship between Myrcludex B and conjugated bile salt levels in plasma follows a counterclockwise hysteresis loop where Myrcludex B is already largely cleared from the circulation while plasma bile salt levels continue to rise.8 Here, we aimed to further understand the relationship between Myrcludex B and bile acid levels in plasma by investigating the NTCP-Myrcludex B binding complex and its turnover.
Materials and methods
Chemicals and reagents
Myrcludex B and Myrcludex B labeled with fluorescein isothiocyanate (FITC) were custom synthesized by Pepscan (Lelystad, The Netherlands), with the FITC modification located at the side-chain of the C-term lysine. Hoechst 33342 was obtained from Merck Millipore (Darmstadt, Germany). [3H]Taurocholic acid (1mCi/ml) were purchased from PerkinElmer (Groningen, The Netherlands). Acyl carrier protein (ACP) synthase (P9301) and CoA-DY547 (S9249S) were obtained from New England Biolabs (Ipswich, USA). The following antibodies and beads were used: anti-hemagglutinin (HA) agarose beads (Sigma Aldrich, A2095), anti-HA horse radish peroxidase (HRP) (Sigma Aldrich, H6533), anti-FLAG® (Sigma Aldrich, F1804), anti-transferrin receptor (Invitrogen, 13-6890), anti-mouse-HRP (DAKO, P0447), also shown in the supplementary CTAT table.
Animals
Adult male OATP1a/1b-deficient mice (FVB background, Slco1a/1b cluster: slco1a1, slco1a4, slco1a5, slco1a6, slco1b2)10 were obtained from Taconic, Denmark. Animals were co-housed with 3-4 animals and kept on a 12 h light:12 h dark continuous cycle (7:00-19:00) with ad libitum access to food and water. Mice were treated with a single subcutaneous injection with Myrcludex B (2.5 mg/kg) and ~20 μl blood was drawn to measure plasma bile acid levels. In total 17 mice were used to evaluate bile acid levels over time. Experiments were performed at the Amsterdam University Medical Centers, location AMC. The study design and animal care and handling were approved by the Institutional Animal Care and Use Committee of the University of Amsterdam.
Plasma bile acid measurement
Total plasma bile acid and composition at time points 0 h, 4 h, 10 h, and 16 h were determined by reverse-phase high performance liquid chromatography (HPLC) and total plasma bile acid levels at time points 1.5 h and 24 h were measured by the Total Bile Acid Assay kit (Diazyme Laboratories, Poway, USA) as described previously.12
Cell culture
U2OS cells (from ATCC, VA, USA) were grown in Dulbecco’s modified Eagle’s medium (DMEM, Sigma Aldrich), supplemented with 10% FCS (Gibco), 1% L-glutamine (Lonza), and 1% penicillin/streptomycin (Lonza). Medium of U2OS cells stably transfected with human NTCP (U2OS-HA-hNTCP)13 was supplemented with 400 μg/ml Geneticin (Invitrogen), respectively. Wild-type or inactive/Myrcludex B resistant3 S267F human NTCP N-terminally tagged with either ACP or FLAG was transiently transfected in U2OS cells using PEI (Brunschwig, Basel, Switzerland) as previously described.14 Cell lines were passaged twice a week at a confluence of 80% and incubated in a humidified atmosphere of 37°C +5% CO2.
NTCP pulse-chase experiment
Cell surface biotinylation of confluent U2OS cells in 6-well format was performed as described,15 with slight adaptations. After washing with PBS-CM (PBS containing 1 mM MgCl2, 0.5 mM CaCl2, pH 8.0), cells were re-incubated with fresh complete DMEM for indicated chase periods. If applicable, cells were treated with control medium or 200 nM Myrcludex B prior to chase. After chase, lysis was performed in RIPA buffer (150 mM NaCl, 50 mM Tris PH 7.4, 0,1% SDS, 1% Nonidet P40) supplemented with protease inhibitors (Roche) and precipitation was performed overnight with high capacity neutravidin beads. Samples were subjected to immunoblotting as described previously.15 To detect NTCP, blots were incubated with anti-HA-HRP (1:2,000). Equal loading was ensured by reprobing the membrane with an anti-transferrin receptor antibody (1:2,000) which was visualized using anti-mouse-HRP (1:10,000). Quantification was performed using image J where the NTCP in the precipitated fraction was calculated as a percentage of the lysate fraction and results were plotted as a percentage of NTCP present after 0 h incubation.
Co-immunoprecipitation
Parental and U2OS cells stably expressing HA-hNTCP were seeded in 10 cm culture dishes and transfected with FLAG tagged hNTCP-S267F.Two days after transfection, cells were washed with PBS and lysed in RIPA buffer (150 mM NaCl, 25 mM Tris PH 7,4, 0.1% (w/v) SDS, 1% (v/v) Nonidet P40) supplemented with protease inhibitors. Lysates were incubated with monoclonal anti-HA antibody immobilized on agarose beads (Sigma 9568) for 16 h at 4°C as described previously.16 Beads were washed with RIPA buffer and samples were subsequently subjected to immunoblotting as described previously.15 HA-NTCP was visualized with anti-HA-HRP (1:2,000). To detect NTCP-S267F, blots were incubated with anti-FLAG and subsequently visualized using anti-mouse-HRP (1:10,000).
Myrcludex B pulse-chase experiment
U2OS cells of 80% confluency in 96-well format were incubated with 200 nM Myrcludex B-FITC for 30 min at 37°C. Subsequently, cells were washed 3 times with PBS and complete medium was added for the indicated chase period. After incubation, cells were washed 3 times with PBS and fluorescence intensity was measured using a Clariostar microplate reader (BMG Labtech GmbH, Offenburg) at λex / λem = 483-14 nm/ 530-30 nm. The option orbital averaging was set at a diameter of 3 mm. Background fluorescence, the Myrcludex B-FITC signal obtained in parental U2OS cells, was subtracted from the HA-hNTCP expressing U2OS cells and results were plotted as percentage of 0 h incubation.
Myrcludex B competitive binding
U2OS cells of 80% confluency in 96-well format were incubated with 200 nM Myrcludex B-FITC for 1 h at 37°C followed by 1 h incubation with 0, 200, 500 or 1,000 nM of unlabeled Myrcludex B. Cells were washed 3 times with PBS and fluorescence intensity was measured using a Clariostar microplate reader as described above.
Bile acid transport studies
Bile acid transport studies, with tritium labeled taurocholate ([3H]Taurocholic acid (1mCi/ml), PerkinElmer, Groningen), were performed with U2OS parental and U2OS-HA-hNTCP-overexpressing cells in a 24-well format as described previously.14 Cells were incubated with Myrcludex B for 10 min prior to uptake or for 1 h, 24 h before uptake.
Confocal microscopy
Preparation of U2OS-HA-hNTCP cells for confocal microscopy was performed as described.14 Live cell fluorescent imaging was performed using a Leica SP8X-SMD confocal microscope with LAS X software and fully enclosed incubation at 37°C. A 63x oil objective was used and images were captured while the cells were consecutively excited at 490 nm (FITC) and 350 nm (Hoechst).
Förster resonance energy transfer using fluorescence lifetime imaging as readout
U2OS parental or U2OS-HA-hNTCP-overexpressing cells were seeded in a sterile 8-well coverslip bottomed chamber slide (Thermo Fisher Scientific, 155411). After 24 h, medium was aspirated and cells were washed once with 300 μl Leibovitz’s L-15 Medium (Invitrogen), followed by a 1 h incubation with 200 nM Myrcludex B-FITC. Subsequently, cells were washed 3 times with PBS and transfected with ACP-hNTCP or ACP-hNTCP-S267F. After 24 h, ACP was labeled with CoA-DY547 using ACP synthase according to manufacturer’s protocol. After labeling and washing, 300 μl Leibovitz’s L-15 Medium was added to each well.
Fluorescence lifetime imaging (FLIM) was performed using Myrcludex B-FITC and DY547-labeled ACP-fused to NTCP as a fluorophore pair suitable for Förster resonance energy transfer (FRET). FRET is highly restricted by the distance between donor (FITC) and acceptor (DY547) and absent when these fluorophores are more than ~10 nm apart, and thus only detectable when Myrcludex B has transferred from a (non-ACP-tagged) NTCP to a newly synthesized ACP-tagged NTCP. A decrease in FITC fluorescence lifetime is one of the most reliable indicators of FRET, as this is independent of the FITC intensity. Data was acquired using a Leica inverted microscope with confocal detection head where Myrcludex B-FITC (the donor fluorophore) was excited using a 488 nm 20 MHz pulsed white-light laser. The photon arrival times were recorded by a Picoharp 300 time-correlated single-photon counting system (Picoquant, Berlin, Germany) and analyzed in SymPhoTime 5.13 software (Picoquant, Berlin, Germany). For each region of interest, the fluorescence decay curve was fitted using a double-exponential reconvolution fit, including the estimated instrument response function, according to the manufacturer’s instructions (http://www.picoquant.com). The FITC half-life (τD) in the absence of FRET was initially calculated on the samples lacking the acceptor fluorophore (HA-hNTCP cells without ACP-NTCP) labeled with Myrcludex B-FITC just prior to imaging. As a control for maximal FRET we used U2OS cells transiently expressing ACP-hNTCP wild-type (and not stably expressing HA-hNTCP) with the expressed ACP tag covalently labeled with DY547 and incubated with Myrcludex B-FITC just prior to imaging. In this condition all FITC molecules are in close proximity to the DY547-labeled ACP-NTCP, yielding maximal FRET (and thus the lowest FITC Td).
Statistical analysis
Data are provided as the mean ± standard error (in vivo experiments) or standard deviation (in vitro experiments) of the mean. Group sizes for animal experiments were calculated using nQuery software with power set at 80%, α = 0.05 and a common standard deviation of 15%. One-way ANOVA with Bonferroni post hoc analysis was used for multiple group comparisons. Statistical significance was considered at p ≪0.05, and calculations and graphs were generated using GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, USA).
Results
Plasma bile acid dynamics after Myrcludex B treatment in mice
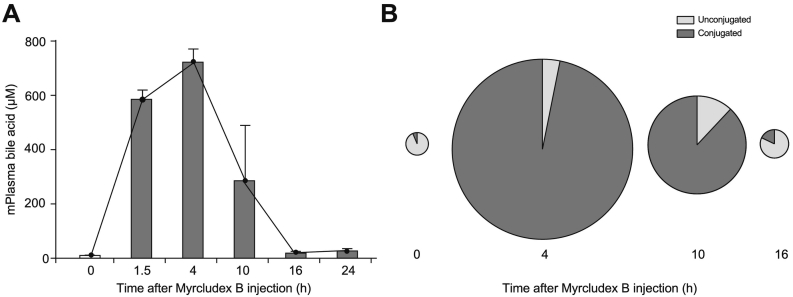
Plasma bile acid dynamics upon NTCP inhibition by Myrcludex B were investigated in OATP1a/1b-deficient mice that received a single subcutaneous injection of Myrcludex B (2.5 mg/kg). Peak plasma bile salt concentrations were reached 4 h after Myrcludex B administration (Fig. 1A), when most of the Myrcludex B had been cleared from the circulation,11,12 in line with the dynamics observed in humans8 and suggestive of a stable NTCP:Myrcludex B interaction. Bile salt levels remain elevated for 10 h, and this increase is dominated by conjugated plasma bile acids (Fig. 1B) with the main species being taurocholic acid and tauro-β-muricholic acid (Table 1). After 24 h, a complete normalization of plasma bile salt levels is seen in mice (Fig. 1A) and humans.8
Fig. 1.
Conjugated plasma bile acid levels increase upon Myrcludex B treatment in mice. (A-B) Total plasma bile acid levels (A) and the distribution in conjugated and unconjugated bile acid species (B) of Oatp1a/1b KO mice treated with Myrcludex B (2.5 mg/kg, subcutaneous injection). Blood was drawn at indicated time-points (n = 3–8), circle size represents the total amount of bile acids. Data is represented as mean ± SEM. KO, knockout.
Table 1.
Bile acid species measured by HPLC at indicated time points after myrcludex B treatment in Oatp1a/1b KO mice.
| Hours after Myrcludex B injection |
||||
|---|---|---|---|---|
| Bile acid species | 0 hours | 4 hours | 10 hours | 16 hours |
| Conjugated | ||||
| TMCα | 0.23 ± 0.07 | 3.36 ± 0.10 | 3.04 ± 0.50 | 0.81 ± 0.15 |
| TMCβ | 0.34 ± 0.11 | 25.45 ± 0.61 | 14.78 ± 3.37 | 3.56 ± 0.39 |
| TUDC | 0.21 ± 0.06 | 0.79 ± 0.28 | 9.66 ± 0.99 | 0.10 ± 0.02 |
| TC | 2.89 ± 0.63 | 63.41 ± 0.82 | 49.24 ± 2.07 | 12.01 ± 2.03 |
| GUDC | 0.12 ± 0.01 | 0.01 ± 0.00 | 0.00 | 0.14 ± 0.00 |
| GC | 0.11 ± 0.03 | 0.06 ± 0.01 | 0.00 | 0.54 ± 0.24 |
| TCDC | 0.37 ± 0.09 | 2.47 ± 0.18 | 0.28 ± 0.09 | 0.16 ± 0.07 |
| TDC | 0.72 ± 0.22 | 2.56 ± 0.34 | 4.52 ± 0.81 | 0.15 ± 0.05 |
| Unconjugated | ||||
| ωMC | 6.17 ± 0.82 | 0.09 ± 0.02 | 1.34 ± 0.70 | 5.24 ± 0.22 |
| αMC | 3.37 ± 1.45 | 0.00 | 0.28 ± 0.11 | 0.19 ±0.05 |
| βMC | 4.74 ± 0.44 | 0.32 ± 0.07 | 0.47 ± 0.20 | 7.78 ± 2.63 |
| GCDC | 0.59 ± 0.19 | 0.00 | 0.00 | 0.90 ± 0.29 |
| GDC | 0.65 ± 0.13 | 0.00 | 0.00 | 0.94 ± 0.26 |
| CA | 7.15 ± 1.47 | 1.35 ± 0.18 | 12.57 ± 4.65 | 19.28 ± 5.70 |
| UDC | 0.26 ± 0.05 | 0.09 ± 0.01 | 0.00 | 0.27 ± 0.12 |
| CDC | 28.40 ± 0.72 | 0.02 ± 0.01 | 0.77 ± 0.34 | 22.68 ± 3.31 |
| DC | 43.68 ± 2.07 | 0.00 | 3.06 ± 1.18 | 25.24 ± 4.24 |
Values are shown as mean ± SEM for n = 3–8 mice per time point. HPLC, high-performance liquid chromatography; KO, knockout.
The time-dependent disappearance of biotin-labeled NTCP and Myrcludex B-FITC are different
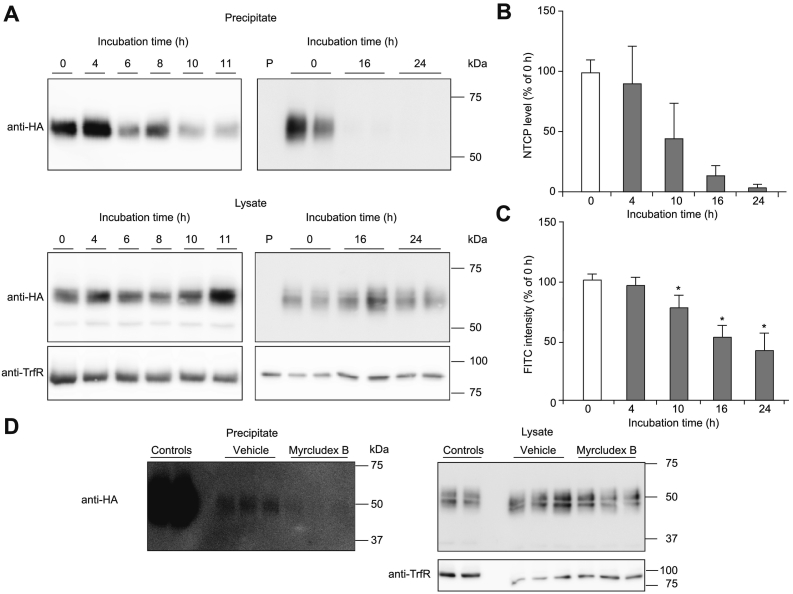
To further understand the effect of Myrcludex B on plasma bile acid levels, we studied the turnover of NTCP. Pulse-chase experiments were performed in which plasma membrane-resident NTCP was labeled by cell surface biotinylation (pulse). Cells were placed back at 37°C for various amounts of time (chase) and the percentage of the biotin-labeled NTCP was quantified by immunoblot analysis. As shown in Fig. 2A and B, the labeled NTCP gradually disappears in a time-dependent manner with 50% of the initial cell surface NTCP still present after 10 h, which almost completely disappears after 24 h (complete blots are shown in Fig. S1A). NTCP signals in the lysates were similar at all time points, indicating that all covalently labeled NTCP was replaced by a complete new set of NTCP molecules. We also labeled NTCP at the plasma membrane with Myrcludex B-FITC and confirmed specific binding of Myrcludex B-FITC to wild-type NTCP, yet not to a genetic variant of NTCP with a serine to phenylalanine substitution at position 267 (S267F) that is known to reduce both bile acid transport and the susceptibility to HBV and HDV infection3,17 (Fig. S2A-B). Myrcludex B-FITC fluorescence slowly decreased and 50% of the Myrcludex B-FITC initially bound to NTCP on the plasma membrane was still detectable after 24 h (Fig. 2C), which is similar to the half-life of Myrcludex B in mice, where 34% of radiolabeled Myrcludex B is still detected in the liver 24 h post injection.11 We excluded that the turnover of NTCP was slowed by Myrcludex B binding in a separate pulse-chase experiment with biotinylated NTCP (Figs. 2D and S1B-C). Together this shows that Myrcludex B binds NTCP at the plasma membrane, but is not co-degraded with the transporter at the same rate.
Fig. 2.
Disappearance rate and internalization of Myrcludex B. (A-B) Representative western blots (A) and quantification (B) of NTCP protein expression in a pulse-chase experiment using biotin in parental U2OS (P) and U2OS-HA-hNTCP cells. Western blots are representative of 5 independent experiments. Full uncropped blots are shown in Fig. S1. (C) Fluorescence intensity of Myrcludex B-FITC on U2OS-HA-hNTCP cells measured by Clariostar. (D) NTCP protein expression in U2OS-HA-hNTCP cells treated with vehicle or 200 nM Myrcludex B prior to the pulse chase experiment. Western blots are representative of 2 independent experiments. All data are represented as mean ± SD, *p ≪0.05 compared to the control determined by one-way ANOVA with Bonferroni post hoc analysis. FITC, fluorescein isothiocyanate; HA, hemagglutinin; NTCP, sodium taurocholate co-transporting polypeptide.
Myrcludex B binds in a strong, though non-covalent way to NTCP
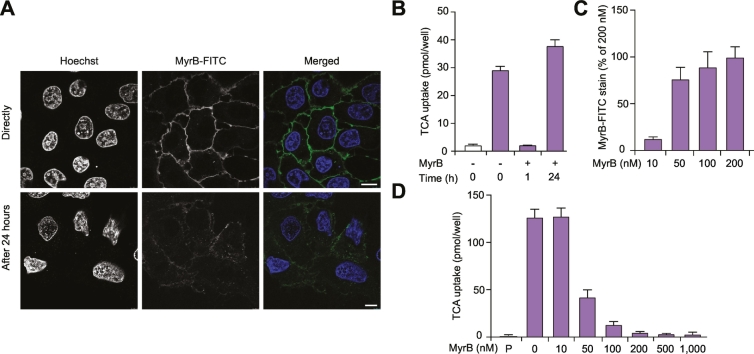
As the disappearance rate of Myrcludex B-FITC is slower than the disappearance rate of biotin-labeled NTCP, we performed confocal microscopy to track the Myrcludex B-FITC signal. Directly after Myrcludex B-FITC treatment on NTCP-overexpressing U2OS cells, Myrcludex B-FITC is exclusively detected at the plasma membrane, while after 24 h, Myrcludex B-FITC is also detected intracellularly (Fig. 3A). Furthermore, the difference in intensity of Myrcludex B-FITC directly and 24 h after treatment resembles our observations in Fig. 2C.
Fig. 3.
Time- and dose-dependent Myrcludex B binding to NTCP. (A) Fluorescence intensity of Myrcludex B-FITC on U2OS-HA-hNTCP cells measured by confocal microscopy directly or 24 h after treatment (scale bar 10 μm). (B) Taurocholate uptake assay in U2OS-HA-hNTCP cells 0 h, 1 h, and 24 h after Myrcludex B treatment. (C) Fluorescence intensity of concentration range of Myrcludex B-FITC on U2OS-HA-hNTCP cells measured by Clariostar. (D) Taurocholate uptake assay in U2OS-HA-hNTCP cells pre-incubated with the indicated concentrations of Myrcludex B for 10 min. (B-D) n = 8 from 2 or 3 independent experiments. All data are represented as mean ± SD. FITC, fluorescein isothiocyanate; HA, hemagglutinin; NTCP, sodium taurocholate co-transporting polypeptide.
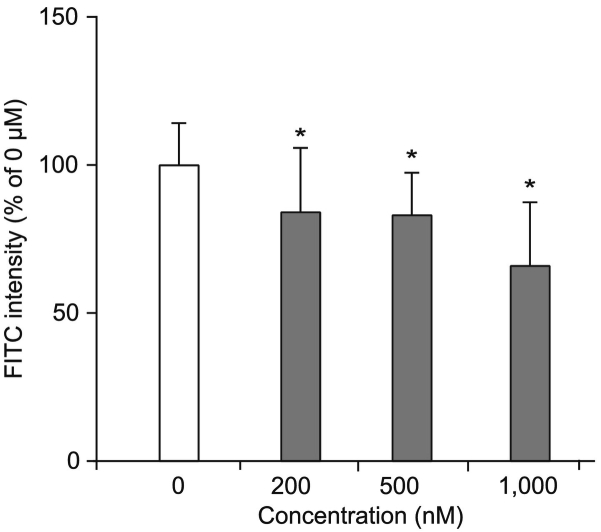
This suggests that the interaction between NTCP and Myrcludex B is non-covalent and that the Myrcludex B peptide can move away from NTCP molecules targeted for protein degradation. It is also possible that some of the Myrcludex B peptides transfer to newly synthesized NTCP molecules, thereby avoiding degradation. Interestingly, the amount of Myrcludex B-FITC that remains at the plasma membrane after 24 h is insufficient to block NTCP-mediated bile acid transport (Fig. 3B), suggesting that the threshold for Myrcludex B-mediated inhibition of NTCP is not met after 24 h. We confirmed a dose-dependent effect of Myrcludex B on NTCP-mediated bile acid transport and show that a low dose (10 nM) of Myrcludex B that gives a signal intensity of 15% compared to the saturating dose is not enough to block NTCP-mediated bile acid transport (Fig. 3C-D), while higher doses of 50–200 nM are able to reduce NTCP-mediated bile acid transport.
To specifically address whether Myrcludex B can dissociate from NTCP, NTCP-overexpressing cells were saturated with Myrcludex B-FITC and subsequently incubated with non-fluorescently labeled Myrcludex B. Myrcludex B-mediated inhibition of NTCP-dependent bile acid uptake saturated at 200 nM (Fig. 3D), a dose also previously published.14,18 Competition of non-fluorescently labeled Myrcludex B (up to a 5-fold excess) decreases the amount of bound Myrcludex B-FITC by ~40% in a dose-dependent fashion within 60 min (Fig. 4). This indicates a strong though reversible interaction between Myrcludex B and NTCP.
Fig. 4.
Pre-bound Myrcludex B can dissociate from NTCP. Competitive binding of unlabeled Myrcludex B to U2OS-HA-hNTCP cells initially labeled with Myrcludex B-FITC. Fluorescence intensity was measured after 1 h competition. n = 8 from 2 independent experiments. All data are represented as mean ± SD, *p ≪0.05 compared to the control determined by one-way ANOVA with Bonferroni post hoc analysis. FITC, fluorescein isothiocyanate; HA, hemagglutinin; NTCP, sodium taurocholate co-transporting polypeptide.
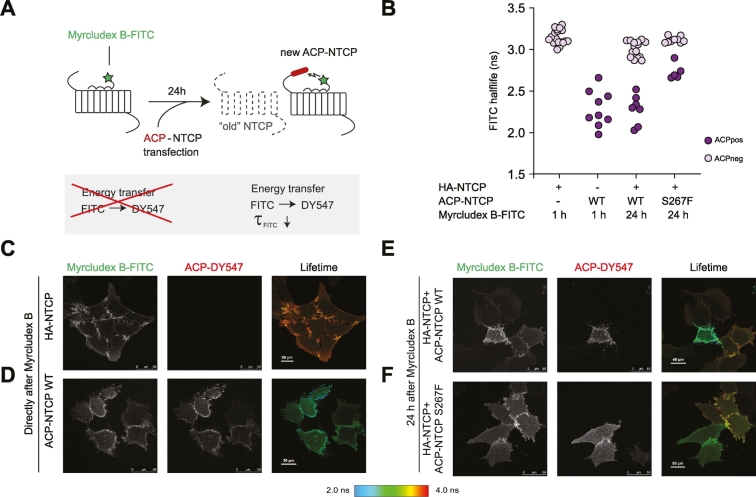
Pre-bound Myrcludex B can transfer to newly formed NTCP
To visualize the transfer of Myrcludex B to newly formed NTCP molecules, we saturated HA-NTCP-overexpressing cells for 1 h with Myrcludex B-FITC, followed by several washing steps and subsequent transfection of the cells with ACP-labeled NTCP wild-type or S267F. After 24 h, the ACP tag was covalently linked to DY547, a red fluorophore, and cells were imaged. To determine whether the Myrcludex B-FITC had transferred to a newly synthesized ACP-tagged NTCP molecule we measured FRET using FLIM as a readout. FRET is the non-radiative transfer of energy of a donor fluorophore (here Myrcludex B-FITC) to an acceptor fluorophore (here DY547-labeled ACP-NTCP). FRET is extremely restricted in distance between fluorophores and only occurs if the fluorescently labeled molecules are within less than 10 nm of live cells19,20 (schematic overview shown in Fig. 5A). FRET can be detected as a decrease of the donor fluorescence lifetime when the acceptor is in close proximity, independent of FITC concentration and sample geometry. In this situation FRET can only occur when the pre-bound Myrcludex B-FITC dissociates from the HA-NTCP and binds to DY547 labeled ACP-NTCP. Of note, no ACP-NTCP molecules were present at the time of labeling with Myrcludex B-FITC, as the transfection with the ACP-NTCP encoding construct was performed subsequently. HA-NTCP-expressing cells incubated with Myrcludex B-FITC directly before imaging, but without any acceptor fluorophore present, were used as controls without FRET (maximal FITC lifetime of approximately 3.2 ns (Fig. 5B and in pseudo-colored scale in Fig. 5C). Maximum FRET was shown in U2OS parental cells expressing DY547-labeled ACP-NTCP wild-type and incubated with Myrcludex B-FITC directly before imaging. In this situation Myrcludex B-FITC can only bind to ACP-NTCP which will result in maximal FRET, detectable as a strongly reduced lifetime of approximately 2.3 ns (Fig. 5B and D). HA-NTCP-overexpressing cells, incubated with Myrcludex B-FITC 24 h prior to imaging and not expressing ACP-NTCP, have a lifetime comparable to the untransfected control, but the lifetime is significantly decreased in the same microscopy view in neighboring cells that do express DY547-labeled ACP-NTCP (Fig. 5B and E), as a consequence of FRET from the Myrcludex B-FITC to the ACP-labeled NTCP. This indicates that after 24 h, part of the pre-bound Myrcludex B-FITC has dissociated from the original HA-NTCP and bound to the newly synthesized ACP-labeled NTCP. Interestingly, a similar experimental set-up where the ACP-labeled S267F NTCP was transfected showed only a slight decrease in lifetime, and thus a lower amount of energy transfer (Fig. 5B and F). As Myrcludex B-FITC cannot directly bind to the NTCP-S267F mutant (Fig. S2A), we investigated whether the low, but detectable FRET in this situation was a consequence of a close interaction between HA-NTCP (occupied with Myrcludex B-FITC) and the newly introduced ACP-NTCP-S267F, since NTCP operates as a dimer.13 A physical interaction between wild-type and S267F NTCP was demonstrated as FLAG-NTCP-S267F co-immunoprecipitated with HA-NTCP (Figs. 6 and S3). Omission of HA-NTCP prevented precipitation of NTCP-S267F, demonstrating the specificity of co-immunoprecipitation. This suggests that the occurrence of (a low level of) FRET between Myrcludex B-FITC bound to NTCP and ACP-labeled NTCP-S267F in Fig. 5E can be explained by the formation of (dimeric) complexes between wild-type and mutant NTCP.
Fig. 5.
Myrcludex B-FITC transfers to newly synthesized NTCP molecules. (A) Schematic overview of the FRET-FLIM assay with Myrcludex B-FITC and DY547-labeled ACP-NTCP as the FRET donor and acceptor respectively. (B) FITC fluorescence lifetime in individual cells. Red dots represent cells DY547-ACP-NTCP positive cells and green dots are acceptor-negative cells. Data was obtained in 3 independent experiments. (C-F) Representative images of the fluorescence intensity of Myrcludex B-FITC (left) and DY547-labeled ACP-NTCP (middle) and FITC fluorescence depicted in pseudocolor as indicated on the right. Images were taken 1 h (C,D) or 24 h after Myrcludex B- FITC labeling (E, F). ACP, acyl carrier protein; FITC, fluorescein isothiocyanate; FLIM, fluorescence lifetime imaging; FRET, Förster resonance energy transfer; NTCP, sodium taurocholate co-transporting polypeptide.
Fig. 6.
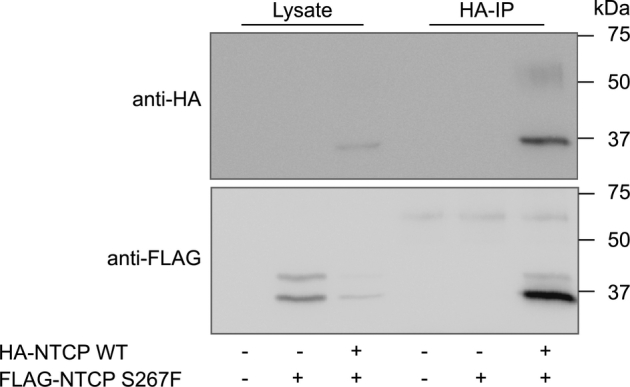
Dimerization of NTCP wild-type and S267F NTCP. Co-immunoprecipitation of U2OS parental or HA-NTCP-overexpressing U2OS cells transfected with FLAG®-NTCP-S267F. The immunoprecipitation is shown on the left and the lysate controls are shown on the right. Blot is representative of 2 independent experiments. NTCP, sodium taurocholate co-transporting polypeptide.
Discussion
Here, we show that the duration of the bile salt elevation is likely governed by NTCP turnover and repopulation of the plasma membrane with newly synthesized NTCP. Nevertheless, we also demonstrate that although the NTCP-Myrcludex B interaction is strong, Myrcludex B can transfer to newly synthesized NTCP, thereby prolonging its presence on NTCP-expressing cells to at least 24 h in vitro. In primary hepatocytes Myrcludex B fluorescence at the plasma membrane was detectable 20 h after labeling, with a calculated half-life of 11 h.18 This turnover rate is slightly faster than we observed here, likely explained by the virtually complete downregulation of NTCP within 24 h in primary hepatocytes. In line with Myrcludex B in vivo binding kinetic data of Schieck et al. (2013) where they could still detect ~34% of injected radiolabeled Myrcludex B in the livers of mice 24 h later,11 we observed that ~50% of the initial dose of FITC-labeled Myrcludex B applied to our hNTCP-overexpressing cells was still present 24 h after treatment. As the initial pool of NTCP molecules was completely degraded from the plasma membrane after 24 h, we postulated that Myrcludex B can partially escape internalization with NTCP by transferring to newly synthesized NTCP molecules; we used FRET-FLIM to demonstrate this. Still, occupation of ~50% of the pool of NTCP molecules at the plasma membrane is not enough to fully block bile acid transport. Since NTCP operates as a dimer it is likely that the ‘free’ NTCP molecules can compensate for Myrcludex B-occupied ones. This closely follows the research of Bijsmans et al., in which no effect on NTCP-mediated bile acid transport for dimers with 1 wild-type and 1 inactive mutant was shown.13 This probably means that more than 1 Myrcludex B molecule needs to bind the NTCP dimer to inhibit bile acid uptake. However, prevention of HBV/HDV viral entry by Myrcludex B does not necessarily require a similar level of receptor occupancy, which is also suggested by the 50-fold difference in IC50 value for Myrcludex B to block bile acid transport (IC50 of 52.5 nM) or HBV infection (IC50 of 669 pM for HBsAg/83 pM for HBeAg) in primary human hepatocytes.21 Indeed recent research by Fukano et al. suggests that NTCP oligomerization is indispensable for viral entry, as small NTCP fragments or the small molecule troglitazone that bind NTCP prevent NTCP oligomerization and subsequently interfere with internalization and infection of HBV.22 Interestingly, Fukano et al. also demonstrated that the presence of a myristoylated preS1 peptide (similar to Myrcludex B) seems to promote NTCP oligomerization.22 The size of the HBV particle, approximately 42 nm,23,24 is a lot larger than that of a single NTCP molecule. This could imply that multiple wild-type NTCP molecules are needed for particle internalization, and even a substoichiometric Myrcludex B-occupancy of NTCP molecules can interfere with viral entry. This is also in line with the inverse association between the S267F variant of NTCP and HBV progression to cirrhosis and hepatocellular carcinoma.25 Furthermore, in cell culture conditions it is already described that Myrcludex B still lowers HBV infection 5–7 days post HBV infection.26 In conclusion, we show that the remarkable discrepancy between rapid Myrcludex B clearance from plasma and prolonged elevations of conjugated bile salts in plasma is likely a consequence of the strong association between NTCP and this peptidic anti-HBV/HDV drug. Furthermore, we demonstrate that Myrcludex B can transfer from 1 NTCP molecule to another. This possibly extends Myrcludex B viral entry blocking efficacy which could be relevant for HBV/HDV treatment.
Financial support
SFJvdG is supported by the Netherlands Organization for Scientific Research (VIDI 91713319) and the European Research Council (Starting grant 337479).
Conflict of interest
All authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
JD, MA and SvdG carried out experiments. Study concept and design: JD, MA, SvdG. Drafting and initial review of the manuscript: JD, MA, SvdG. SvdG obtained funding. All authors were involved in analysis and interpretation of data and have read and approved the manuscript.
Acknowledgements
The authors thank Winona B. de Rooij, and Giustiniano V.A. van Kerster for helping with the cell culture experiments, Reinout L.P. Roscam Abbing for his contribution to the in vivo data, Dirk Rudi de Waart for helping with the HPLC, and Ron Hoebe for helping with the FLIM experiments.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.07.006.
Supplementary data
Supplementary figures
Supplementary material 1
Supplementary material 2
References•
- 1.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Yan H, Peng B, Liu Y, Xu G, He W, Ren B. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol. 2014;88:3273–3284. doi: 10.1128/JVI.03478-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulze A, Schieck A, Ni Y, Mier W, Urban S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J Virol. 2010;84:1989–2000. doi: 10.1128/JVI.01902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65:483–489. doi: 10.1016/j.jhep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490–498. doi: 10.1016/j.jhep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Slijepcevic D, Roscam Abbing RLP, Katafuchi T, Blank A, Donkers JM, van Hoppe S. Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology. 2017;66:1631–1643. doi: 10.1002/hep.29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blank A, Eidam A, Haag M, Hohmann N, Burhenne J, Schwab M. The NTCP-inhibitor Myrcludex B: Effects on Bile Acid Disposition and Tenofovir Pharmacokinetics. Clin Pharmacol Ther. 2018;103:341–348. doi: 10.1002/cpt.744. [DOI] [PubMed] [Google Scholar]
- 9.Slijepcevic D, Roscam Abbing RLP, Fuchs CD, Haazen LCM, Beuers U, Trauner M. Na+ -taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice. Hepatology. 2018;63(3):1057–1069. doi: 10.1002/hep.29888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120:2942–2952. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schieck A, Schulze A, Gahler C, Muller T, Haberkorn U, Alexandrov A. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013;58:43–53. doi: 10.1002/hep.26211. [DOI] [PubMed] [Google Scholar]
- 12.Slijepcevic D, Kaufman C, Wichers CG, Gilglioni EH, Lempp FA, Duijst S. Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in Na+ -taurocholate cotransporting polypeptide knockout mice. Hepatology. 2015;62:207–219. doi: 10.1002/hep.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijsmans IT, Bouwmeester RA, Geyer J, Faber KN, van de Graaf SF. Homo- and hetero-dimeric architecture of the human liver Na+-dependent taurocholate co-transporting protein. Biochem J. 2012;441:1007–1015. doi: 10.1042/BJ20111234. [DOI] [PubMed] [Google Scholar]
- 14.Donkers JM, Zehnder B, van Westen GJP, Kwakkenbos MJ, AP IJ, Oude Elferink RPJ. Reduced hepatitis B and D viral entry using clinically applied drugs as novel inhibitors of the bile acid transporter NTCP. Sci Rep. 2017;7 doi: 10.1038/s41598-017-15338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelman MD, Chakraborty A, Protzer U, McKeating JA, van de Graaf SF. N-Glycosylation of the Na+-Taurocholate Cotransporting Polypeptide (NTCP) Determines Its Trafficking and Stability and Is Required for Hepatitis B Virus Infection. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [•16].Robin MJD, Appelman MD, Vos HR, van Es RM, Paton JC, Paton AW. Calnexin Depletion by Endoplasmic Reticulum Stress During Cholestasis Inhibits the Na(+)-Taurocholate Cotransporting Polypeptide. Hepatol Commun. 2018;2:1550–1566. doi: 10.1002/hep4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binh MT, Hoan NX, Van Tong H, Sy BT, Trung NT, Bock CT. NTCP S267F variant associates with decreased susceptibility to HBV and HDV infection and decelerated progression of related liver diseases. Int J Infect Dis. 2019;80:147–152. doi: 10.1016/j.ijid.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Meier A, Mehrle S, Weiss TS, Mier W, Urban S. Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology. 2013;58:31–42. doi: 10.1002/hep.26181. [DOI] [PubMed] [Google Scholar]
- 19.Margineanu A, Chan JJ, Kelly DJ, Warren SC, Flatters D, Kumar S. Screening for protein-protein interactions using Forster resonance energy transfer (FRET) and fluorescence lifetime imaging microscopy (FLIM) Sci Rep. 2016;6 doi: 10.1038/srep28186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Förster T. New York: Modern Quantum Chemistry: Istanbul Lectures. Part III, Action of Light and Organic Crystals. 1965. Delocalized excitation and excitation transfer; pp. 93–137. [Google Scholar]
- 21.Nkongolo S, Ni Y, Lempp FA, Kaufman C, Lindner T, Esser-Nobis K. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J Hepatol. 2014;60:723–731. doi: 10.1016/j.jhep.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Fukano K, Tsukuda S, Oshima M, Suzuki R, Aizaki H, Ohki M. Troglitazone Impedes the Oligomerization of Sodium Taurocholate Cotransporting Polypeptide and Entry of Hepatitis B Virus Into Hepatocytes. Front Microbiol. 2018;9:3257. doi: 10.3389/fmicb.2018.03257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11:685–706. doi: 10.1016/j.cld.2007.08.002. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elnaggar YS. Multifaceted applications of bile salts in pharmacy: an emphasis on nanomedicine. Int J Nanomedicine. 2015;10:3955–3971. doi: 10.2147/IJN.S82558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu HH, Liu J, Lin YL, Luo WS, Chu YJ, Chang CL. The rs2296651 (S267F) variant on NTCP (SLC10A1) is inversely associated with chronic hepatitis B and progression to cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B. Gut. 2016;65:1514–1521. doi: 10.1136/gutjnl-2015-310686. [DOI] [PubMed] [Google Scholar]
- 26.Tu T, Budzinska MA, Vondran FWR, Shackel NA, Urban S. Hepatitis B virus DNA integration occurs early in the viral life cycle in an in vitro infection model via NTCP-dependent uptake of enveloped virus particles. J Virol. 2018 doi: 10.1128/JVI.02007-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
Supplementary material 1
Supplementary material 2