Background & Aims
Data on the prevalence of chronic hepatitis B (HBV) and hepatitis C (HCV) virus infections, including the proportion of individuals aware of infection, are scarce among migrants living in Europe. We estimated the prevalence of past and present HBV and HCV infection, along with their determinants and peoples’ awareness of infection status, among different groups of first-generation migrants and Dutch-origin residents of Amsterdam.
Methods
Cross-sectional data of 998 Surinamese (mostly South-Asian and African-Surinamese), 500 Ghanaian, 497 Turkish, 498 Moroccan and 500 Dutch-origin participants from the observational population-based HELIUS study were used. Blood samples of participants were tested for HBV and HCV infection. Infection awareness was determined using records from participants’ general practitioners.
Results
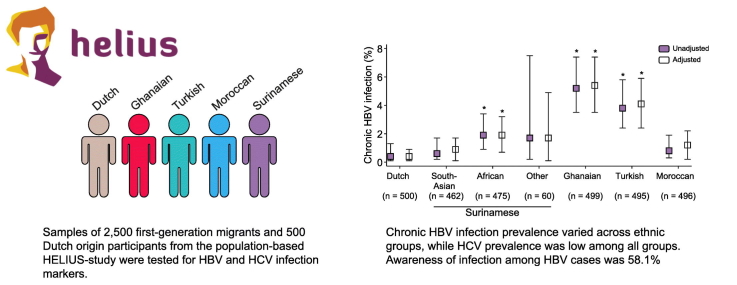
Age- and gender-adjusted chronic HBV prevalence was highest among Ghanaian participants (5.4%), followed by Turkish (4.1%), African-Surinamese (1.9%), Moroccan (1.2%), South-Asian Surinamese (0.9%) and Dutch (0.4%) participants. A total of 58.1% of the cases were aware of their infection. In multinomial logistic regression analyses, Ghanaian (adjusted odds ratio [aOR] 42.23; 95% confidence interval [CI] 9.29–192.01), African-Surinamese (aOR 6.16; 95% CI 1.27–29.79), and Turkish (aOR 13.44; 95% CI 2.94–61.39) participants were at increased risk of chronic HBV infection compared with those of Dutch origin. Older participants were also at increased risk (aOR 1.02 per year; 95% CI 1.00–1.05), whereas women were at lower risk (aOR 0.49; 95% CI 0.29–0.83). HCV prevalence was 0.4% (95% CI 0.1–1.3%) among Dutch and African-Surinamese and 0% (95% CI 0.0–0.5%) for each of the other groups; all cases with follow-up data were aware of their infection.
Conclusions
Ghanaian, Turkish and African-Surinamese first-generation migrants are at increased risk of chronic HBV infection and many are unaware of their infection, whereas HCV prevalence was low among all ethnic groups. Screening campaigns are urgently warranted and need to consider specific ethnic groups.
Lay summary
First-generation migrants of Ghanaian, Turkish and African-Surinamese origin were at increased risk of chronic hepatitis B infection, with most infections occurring in older individuals and males. Since over 40% of people were unaware of their chronic hepatitis B infection, screening of these migrant groups is urgently needed. The proportion of first-generation migrants chronically infected with hepatitis C virus was very low among all groups studied.
Keywords: Viral hepatitis, HBV, HCV, migrants, epidemiology, prevalence, population-based, HELIUS study
Graphical abstract
Highlights
-
•
We estimated the HBV and HCV prevalence among 6 ethnic groups living in Amsterdam.
-
•
HBV prevalence was highest among Ghanaian, Turkish and African-Surinamese migrants.
-
•
Many participants were unaware of their chronic HBV infection.
-
•
HBV screening programs for migrant groups are urgently needed.
-
•
HCV prevalence was low in all ethnic groups.
Introduction
Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are estimated to affect 4.7 million and 5.6 million people living in Europe, respectively.1 In the Netherlands, it is estimated that 49,000 people were living with chronic HBV infection in 2016 and 23,000 people had ever been chronically infected with HCV (0.34% and 0.16% of the adult population, respectively), with an estimated 500 deaths each year attributed to infection.[2], [3] Currently, effective treatment options to suppress HBV and cure HCV infection are available[4], [5] without restricted access in the Netherlands. Furthermore, the contacts of newly diagnosed HBV-infected individuals are notified and if still susceptible, are offered vaccination. However, the onset of HBV and HCV infection and initial development of liver damage in chronically infected patients is often asymptomatic,[6], [7], [8] which leaves many infected individuals unaware of their infection, delaying diagnosis and therefore treatment initiation. Reports from Western countries show that 21–55% and 43–50% of HBV and HCV-infected individuals, respectively, do not know their infection status.[9], [10], [11] However, data from the Netherlands is scarce. Data from the Dutch antenatal screening program suggest that up to 58% of HBV-infected individuals may be unaware of their infection12 and for HCV, it was estimated at 51% based on notification and laboratory data.13
Screening for HBV and HCV infections facilitates identifying undiagnosed infections, improving individual prognosis and limiting further transmission. For some risk groups in the Netherlands screening is part of existing health care programs (i.e., HIV care; HBV vaccination program and HIV pre-exposure prophylaxis [PrEP] for men who have sex with men [MSM] and sex workers; harm reduction programs for people who inject drugs [PWID][14], [15], [16], [17]). Furthermore, routine HBV screening is integrated into antenatal care in the Netherlands.12 Since 1989, newborns from HBV surface antigen (HBsAg)-positive mothers have been vaccinated immediately after birth and in 2011 universal HBV vaccination was introduced for all children regardless of ethnic origin.
First-generation migrants are estimated to account for a relatively large proportion of chronic HBV and HCV infections in the Netherlands (81% and 60% respectively) and many other Northern and Western European countries.18 The Health Council of the Netherlands and Dutch guidelines for general practitioners (GPs) advise to offer screening to migrants from HBV and/or HCV-endemic countries,[19], [20] but this is still not common practice. This may partly be due to the ethnic heterogeneity of migrant populations, each with an accompanying risk for infection and degree of infection status awareness.
Evaluating infection prevalence, associated risk factors and awareness of infection status across specific migrant groups is thus critical if screening and prevention programs are to effectively target individuals in need of screening. However, population-based studies on HBV and HCV prevalence among migrant subpopulations are limited, and those that have been conducted generally lack data regarding awareness of infection status.21 In order to inform the development of screening and preventive interventions for migrants, we studied the prevalence of HBV and HCV infection markers, along with their risk factors, using data from a large, population-based study including 5 large ethnic minority groups and individuals of Dutch origin living in Amsterdam, the Netherlands. We also evaluated infection status awareness among individuals identified with chronic infection.
Materials and methods
Study population and design
Participants were recruited from the HELIUS (HEalthy LIfe in an Urban Setting) study, a multi-ethnic population-based cohort study in Amsterdam. The design, methodology and response rate of the HELIUS study are described in detail elsewhere.[22], [23] In brief, the HELIUS study aimed to investigate mechanisms underlying the impact of ethnicity on communicable and non-communicable diseases. An invitation for HELIUS study participation was sent to individuals aged 18-70 years old, randomly sampled, stratified by ethnicity, from the municipality register of Amsterdam. People of Surinamese, Turkish, Moroccan, Ghanaian, and Dutch ethnic origin were included. Of the Surinamese living in the Netherlands, most are either of African or South-Asian origin. Participants underwent a physical examination during which biological samples were collected. Furthermore, a detailed questionnaire was administered in which questions were asked on sexual orientation, level of education, risk factors for HBV and/or HCV infection (having surgery in a non-Western country [i.e. all forms of surgery including dental surgery, cesarean section, cosmetic and keyhole surgery involving skin incisions, etc.], having a blood transfusion in a non-Western country or before 1990 in a Western country, ever-injecting drug use and lifetime number of sexual partners) and, except for those of Dutch origin, migration history (age at migration and reason for migration).
All participants provided written informed consent for participation in the study. In the written consent form, participants could opt out of having their biological samples stored and/or medical data obtained from patient records. Baseline data collection of the HELIUS study took place from 2011 to 2015. The HELIUS study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the AMC Ethical Review Board.
For the current study, HELIUS participants recruited between January 2011 and June 2014 were eligible for selection if they had completed the questionnaire, participated in the physical examination and had at least 4 stored tubes of EDTA plasma. We only included first-generation migrants for non-Dutch ethnic groups. We selected a random sample of 500 eligible participants for each ethnic group, while oversampling for Surinamese (n = 1,000) in order to include sufficient numbers of African and South-Asian Surinamese participants. This resulted in a total sample size of 3,000 people.
Ethnicity
Ethnicity was based on the country of birth of the participant and the country of birth of both parents, which is a widely accepted indicator of ethnic origin in the Netherlands.24 More specifically, a participant was considered of non-Dutch ethnic origin if he or she was (A) born outside the Netherlands and had at least 1 parent who was born outside the Netherlands (first-generation migrant) or (B) the participant was born in the Netherlands and both her/his parents were born outside the Netherlands (second-generation migrant). For the Dutch sample, we invited people who were born in the Netherlands and whose parents were born in the Netherlands. A limitation of the country-of-birth indicator for ethnicity is that people who are born in the same country might have a different ethnic background, which, in the Dutch context, is applicable to the Surinamese population.24 Therefore, participants of Surinamese ethnic origin were further classified according to self-reported ethnic origin (obtained by questionnaire) into ‘African’, ‘South-Asian’, ‘Javanese’ or ‘other/unknown’ Surinamese ethnic origin.23
Laboratory testing
Blood samples were centrifuged and stored at -20°C within 2 h of collection and transported to -80°C storage on the same day. Stored blood samples of the 3,000 selected participants obtained at HELIUS baseline data collection were tested for markers of past and present HBV and HCV infection in 2015 and 2016. For HBV, samples were first tested for antibodies to hepatitis B core antigen (anti-HBc total) using microparticle enzyme immunoassay (LIAISON® Anti-HBc, Diasorin, Italy). When positive or indeterminate, HBsAg and antibodies to HBsAg (anti-HBs) were determined (LIAISON® XL Murex HBsAg Quant Assay, Diasorin, Italy). Samples positive for HBsAg (≥ 0.05 IU/ml) were tested for hepatitis B-e-antigen (HBeAg) and antibodies to HBeAg (anti-HBe) (LIAISON® HBeAg and LIAISON® Anti-HBe respectively), and for the presence of HBV-DNA (COBAS® AmpliPrep/COBAS® TaqMan® HBV Test, v2.0, Roche Molecular Diagnostics, Pleasanton, USA). For HBV-DNA measurements, sera were diluted 1:10 with HBV-DNA negative serum, resulting in a quantitative detection limit of 165 IU/ml. HCV serological testing was done by third-generation commercial microparticle EIA system (AxSym HCV version 3.0; Abbott Laboratories, North Chicago, IL, USA). Samples with HCV antibody (anti-HCV) reactivity ≥ 1.0 were further tested for HCV-RNA using COBAS Ampliprep/COBAS TaqMan (CAP-CTM HCV test v2.0; Roche Diagnostics, Mannheim, Germany). Participants with low-reactive anti-HCV test results (reactivity between 1.0–1.8) who tested HCV-RNA negative were considered anti-HCV negative (non-exposed). From these results, we defined 3 infection groups for HBV: non-exposed, past-HBV infection (further differentiated as resolved-infection and isolated anti-HBc) and chronic HBV infection); and 3 infection groups for HCV: non-exposed, past (resolved) HCV infection and chronic HCV infection (Table 1).
Table 1.
Definition of HBV and HCV infection groups.
| HBV infection groups | Anti–HBc | HBsAg | Anti–HBs |
|---|---|---|---|
| Non–exposed a | Negative | Negativeb | n/a |
| Past infection: Resolved-HBV infection | Positive or indeterminate | Negative | positive |
| Past infection: Isolated anti–HBc | Positive or indeterminate | Negative | negative |
| Chronic HBV infection | Positive or indeterminate | Positive | negative |
| HCV infection groups | Anti–HCV | HCV–RNA | |
|---|---|---|---|
| Non–exposed | Negative | Negative c | |
| Low–reactived | Negative | ||
| Past infection: Resolved-HCV infection | Positive | Negative | |
| Chronic HCV infection | Positive | Positive |
Anti-HBc, antibodies to hepatitis B core antigen; anti-HBs, antibodies to HBsAg; anti-HCV, antibodies to HCV; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; n.a., not available
vaccination status unknown;
not tested but assumed negative when anti-HBc was negative;
not tested but assumed negative when anti-HCV was negative;
reactivity between 1.0–1.8.
Awareness of infection status
According to HELIUS study procedures, we informed GPs of participants with a chronic HBV or HCV infection by letter asking them to verify whether their patient was aware of his/her infection and if not, to inform their patient and to perform confirmatory testing. We collected data on the number of previously diagnosed infections and confirmatory results by sending a data collection form to GPs 6 weeks after the initial letter. GPs who did not respond were re-contacted and data were collected over telephone. Participants with no declared GP were sent a letter asking to contact the Public Health Service of Amsterdam for further information on their test results and repeat testing.
Statistical analyses
Demographic characteristics were compared between ethnic groups using Pearson’s χ2 or Fisher’s exact tests for categorical variables and Kruskal-Wallis tests for continuous variables. For each ethnic group, crude prevalences of HBV and HCV infection groups were estimated along with their 95% confidence interval (CI) using the Jeffreys method. For HBV, adjusted prevalence was also presented as average marginal predicted probabilities from a logistic regression model correcting for age and sex. Univariable and multivariable multinominal logistic regression models were used to assess determinants of past-HBV infection or chronic HBV infection when compared to HBV non-exposed. Variables associated with an overall p value ≤ 0.1 in univariable analyses were considered in a full multivariable model. A stepwise backward selection procedure was used whereby covariables with associated p values > 0.05 were removed to reach a final multivariable model. We evaluated the following variables: ethnicity, age, sex, level of education (i.e. less than higher vocational schooling or university versus vocational schooling or university), having surgery in a non-Western country, having a blood transfusion in a non-Western country or before 1990 in a Western country, and lifetime number of sexual partners. Interactions between ethnicity and other variables in the final model were tested. We also added age at migration and reason for migration separately to the final multivariable model while only including first-generation migrants. SPSS for Windows (version 21, Chicago, IL) and STATA Intercooled (version 13.1, College Station, TX) were used for statistical analyses and a p value of ≤ 0.05 was considered statistically significant.
Results
Study population
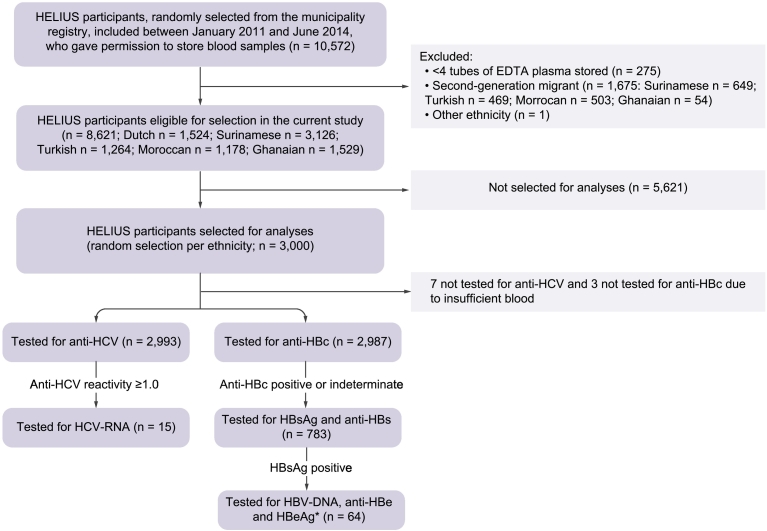
Of 10,572 HELIUS participants, 8,621 were eligible for selection in the current study (Fig. 1). Five hundred people of Dutch origin and 2,500 first-generation migrants (500 Turkish, 500 Moroccan, 500 Ghanaian and 1,000 Surinamese) were randomly selected, of whom 2,993 were tested for anti-HCV and 2,987 for anti-HBc. Table 2 summarizes demographic characteristics, migration history and presence of risk factors for HBV and/or HCV infection in the study population stratified by ethnic group. We found significant differences across ethnic groups regarding age (p < 0.001), sex (p = 0.003), educational level (p < 0.001), age at migration (p < 0.001), reason for migration (p < 0.001), having had surgery in a non-Western country (p < 0.001), having had a blood transfusion (p = 0.003), and number of lifetime sexual partners (p < 0.001).
Fig. 1.
Flowchart depicting the selection of participants and laboratory testing algorithm of the study on HBV and HCV prevalence and awareness of infection status within the HELIUS study, Amsterdam, the Netherlands, 2011-2014.
*1 individual was not tested for HBeAg due to insufficient blood.
Anti-HBc, antibodies to hepatitis B core antigen; anti-HBe, antibodies to HBe; anti-HBs, antibodies to HBsAg; anti-HCV, antibodies to HCV; HBeAg, hepatitis B-e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus.
Table 2.
Demographic characteristics, migration history and HBV/HCV risk factors.
| Dutch (n = 500) | Surinamese (n = 998) |
Ghanaian (n = 500) | Turkish (n = 497) | Moroccan (n = 498) | p value | |||
|---|---|---|---|---|---|---|---|---|
| South–Asian Surinamese (n = 463) | African-Surinamese (n = 475) | Other/Unknown a (n = 60) | ||||||
| Age (yr) | ||||||||
| Median [IQR] | 46 [35–57] | 51 [44–58] | 53 [45–58] | 52 [45–57] | 48 [41–54] | 46 [39–51] | 47 [39–53] | < 0.001b |
| Missing n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Sex & male sexual behavior status, n (%) | ||||||||
| Women | 261 (52.2) | 262 (56.6) | 295 (62.1) | 36 (60.0) | 288 (57.6) | 247 (49.7) | 288 (57.8) | 0.003c, d |
| Men | 239 (47.8) | 201 (43.4) | 180 (37.9) | 24 (40.0) | 212 (42.4) | 250 (50.3) | 210 (42.2) | |
| MSW | 200 (40.0) | 161 (34.8) | 155 (32.6) | 16 (26.7) | 177 (35.4) | 180 (36.2) | 151 (30.3) | |
| MSM | 27 (5.4) | 9 (1.9) | 7 (1.5) | 0 (0) | 5 (1.0) | 7 (1.4) | 5 (1.0) | |
| Men who never had sex | 7 (1.4) | 12 (2.6) | 6 (1.3) | 5 (8.3) | 8 (1.6) | 19 (3.8) | 28 (5.6) | |
| Missing data on sexual partners' gender | 5 (1.0) | 19 (4.1) | 12 (2.5) | 3 (5.0) | 22 (4.4) | 44 (8.9) | 26 (5.2) | |
| Highest level of education, n (%) | ||||||||
| Less than higher vocational schooling or university | 177 (35.4) | 370 (79.9) | 381 (80.2) | 44 (73.3) | 459 (91.8) | 441 (88.7) | 440 (88.4) | < 0.001c |
| Higher vocational schooling or university | 321 (64.2) | 90 (19.4) | 90 (18.9) | 13 (21.7) | 29 (5.8) | 54 (10.9) | 53 (10.6) | |
| Missing | 2 (0.4) | 3 (0.6) | 4 (0.8) | 3 (5.0) | 12 (2.4) | 2 (0.4) | 5 (1.0) | |
| Age at migration | ||||||||
| Median [IQR] | 18 [11–23] | 20 [12–27] | 23 [16–29] | 28 [23–33] | 17 [11–22] | 18 [10–24] | < 0.001b, e | |
| Missing, n (%) | – | 8 (1.7) | 11 (2.3) | 8 (13.3) | 11 (2.2) | 12 (2.4) | 25 (5.0) | |
| Reason for migration | ||||||||
| Work/study | – | 69 (14.9) | 99 (20.8) | 11 (18.3) | 153 (30.6) | 81 (16.3) | 79 (15.9) | < 0.001c, e |
| Family reunification/migrated with parents | – | 263 (56.8) | 243 (51.2) | 24 (40.0) | 227 (45.4) | 374 (75.3) | 374 (75.1) | |
| Political asylum seeker/social security/better future | – | 73 (15.8) | 60 (12.8) | 10 (16.7) | 45 (9.2) | 4 (0.8) | 7 (1.4) | |
| Other/more than one reason | – | 56 (12.1) | 65 (13.7) | 7 (11.7) | 62 (12.4) | 28 (5.6) | 27 (5.4) | |
| Missing | – | 2 (0.4) | 8 (1.7) | 8 (13.3) | 13 (2.6) | 10 (2.0) | 11 (2.2) | |
| Surgery in non–Western country, n (%) | ||||||||
| No | 494 (98.8) | 421 (90.9) | 409 (86.1) | 50 (83.3) | 429 (85.8) | 404 (81.3) | 465 (93.4) | < 0.001c |
| Yes | 5 (1.0) | 41 (8.9) | 61 (12.8) | 8 (13.3) | 55 (11.0) | 81 (16.3) | 29 (5.8) | |
| Missing | 1 (0.2) | 1 (0.2) | 5 (1.1) | 2 (3.3) | 16 (3.2) | 12 (2.4) | 4 (0.8) | |
| Blood transfusion, n (%) | ||||||||
| Never | 448 (89.6) | 388 (83.8) | 397 (83.6) | 49 (81.7) | 432 (86.4) | 438 (88.1) | 432 (86.7) | 0.003c, f |
| > 1990 in Western country | 9 (1.9) | 17 (3.7) | 19 (4.0) | 4 (6.7) | 15 (3.0) | 19 (3.8) | 24 (4.8) | |
| In non–Western country | 17 (3.4) | 29 (6.3) | 33 (6.9) | 2 (3.3) | 22 (4.4) | 13 (2.6) | 15 (3.0) | |
| Missing | 26 (5.2) | 29 (6.3) | 26 (5.5) | 5 (8.3) | 31 (6.2) | 27 (5.7) | 27 (5.4) | |
| Ever–injecting drug use, n (%) | ||||||||
| No | 494 (98.8) | 455 (98.3) | 470 (98.9) | 58 (96.7) | 482 (96.4) | 481 (96.8) | 481 (96.6) | 0.466g |
| Yes | 1 (0.2) | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 4 (0.8) | 2 (0.4) | |
| Missing | 5 (1.0) | 7 (1.5) | 5 (1.1) | 2 (3.3) | 17 (3.4) | 12 (2.4) | 15 (3.0) | |
| Lifetime sexual partners, n (%) | ||||||||
| 0–1 | 65 (13.0) | 196 (42.3) | 57 (12.0) | 13 (21.7 | 72 (14.4) | 310 (62.4) | 345 (69.3) | < 0.001c |
| 2 | 34 (6.8) | 72 (15.6) | 38 (8.0) | 11 (18.3) | 76 (15.2) | 30 (6.0) | 33 (6.6) | |
| 3–6 | 147 (29.4) | 81 (17.5) | 177 (37.3) | 18 (30.0) | 177 (35.4) | 34 (6.8) | 49 (9.8) | |
| > 7 | 247 (49.4) | 83 (17.9) | 164 (34.5) | 13 (21.7) | 108 (21.6) | 63 (12.7) | 39 (7.8) | |
| Missing | 7 (1.4) | 31 (6.7) | 39 (8.2) | 5 (8.3) | 67 (13.4) | 60 (12.1) | 32 (6.4) | |
Among 2,993 Dutch and first-generation migrant participants of the HELIUS study, Amsterdam, the Netherlands, 2011-2014. HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; MSM, men who have sex with men; MSW, men who have sex with women only.
Including 29 Javanese, 13 of mixed ethnicity, 4 other, 14 unknown;
Kruskal-Wallis test;
Pearson’s χ2 test;
p value for the difference in gender;
Dutch participants were excluded for the calculation of the p-value and the median and IQR for the total group (if applicable);
Surinamese other/unknown were excluded for the calculation of the p value due to the low numbers of participants in this group.
Fisher's exact test
Chronic HBV and HCV infection
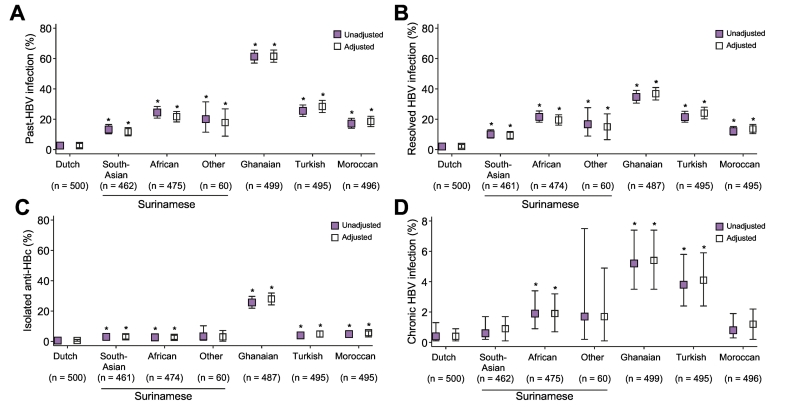
The age- and sex-adjusted prevalence of past and chronic HBV infection ranged from 2.6% (95% CI 1.2–4.0%) and 0.4% (95% CI ≤ 0.1–0.9%) among individuals of Dutch origin to 61.5% (95% CI 57.5–65.6%) and 5.4% (95% CI 3.5–7.4%) among Ghanaians, respectively (Fig. 2; Table S1). Results were comparable to crude estimates. Age- and sex-adjusted prevalence of past and chronic HBV infection significantly differed between ethnic groups (overall p < 0.001). The 3 Surinamese ethnic groups differed significantly from each other regarding past-HBV infection (p < 0.001) but not regarding chronic HBV infection (p = 0.19).
Fig. 2.
Prevalence of HBV infection among participants of the HELIUS study, 2011-2014. Prevalence of HBV status is given for each ethnic group recruited in the HELIUS study. Past-HBV infection (A) is separated into either resolved-HBV infection (B) or isolated anti-HBc positive infection (C). Chronic HBV infection is given in (D). Point estimates are represented by boxes and 95% confidence intervals by bars. Estimates in colored boxes are unadjusted whereas those in hollow boxes are adjusted for age and sex.
*p < 0.05 compared with participants of Dutch origin.
Anti-HBc, antibodies to hepatitis B core antigen; HBV, hepatitis B virus.
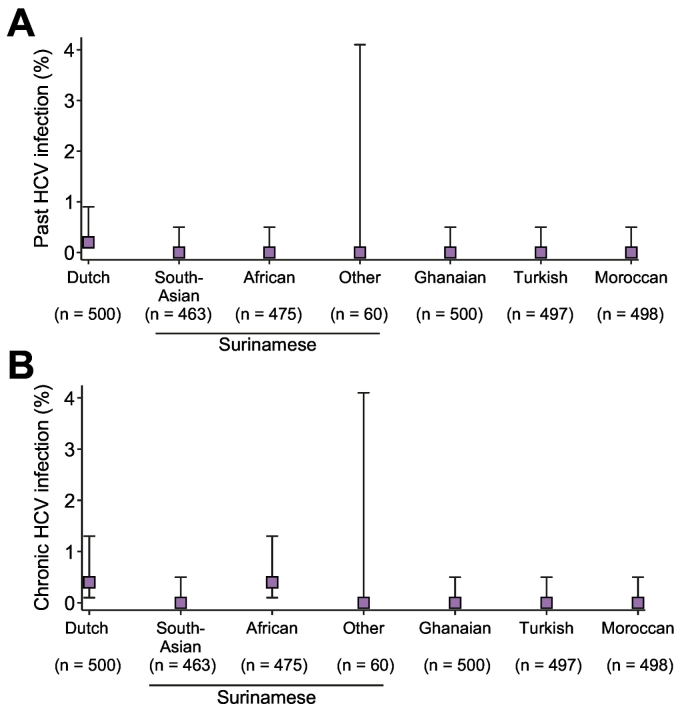
The prevalence of chronic HCV infection per ethnic group ranged from 0% (95% CI 0.0–0.5%) among South-Asian Surinamese, Ghanaian, Turks and Moroccan to 0.4% (95% CI 0.1–1.3%) among people of Dutch and African-Surinamese origin (Fig. 3, Table S1).
Fig 3.
Prevalence of HCV infection among participants of the HELIUS study, 2011-2014. Prevalence of HCV status is given for each ethnic group recruited in the HELIUS study. Point estimates of unadjusted past-HCV infection (A) and chronic HCV infection (B) are represented by boxes and 95% confidence intervals by bars.
HCV, hepatitis C virus.
Awareness of HBV and HCV infection status
Data regarding awareness of infection status and follow-up test results were obtained for 41/64 (64.1%) people with chronic HBV infection. For 23 people these data were not obtained for the following reasons: no consent for retrieval of medical information (n = 7), did not show-up for re-testing at the GP (n = 5), no registration at a GP and did not show-up for re-testing at the Public Health Service (n = 5), no data obtained from GP (n = 4), person moved abroad and went to foreign GP for re-testing (n = 1); person was deceased (n = 1). In total, 75.6% (n = 31/41) had a confirmed chronic HBV infection either from re-testing or by their GP. Of 31 confirmed chronically infected individuals, 18 (58.1%; 95% CI 40.6–74.1%) were already aware of their infection status. We found differences between ethnic groups and men and women regarding awareness of infection status, although not statistically significant. When stratified by ethnicity, the proportion of people aware of their chronic HBV infection status were 33.3% (4/12; Ghanaian), 50.0% (1/2; Moroccan), 63.6% (7/11; Turkish), and 100% (1/1 [Dutch]; 4/4 [African-Surinamese]; 1/1 [South-Asian Surinamese]) (p = 0.13). When stratified by gender, women were more likely to be aware of their chronic HBV infection status than men (75% [9/12] vs. 47.4% [9/19]), although the difference was not statistically significant (p = 0.16).
Data regarding awareness of HCV infection status were available for 3 of 4 people chronically infected with HCV. All 3 had been previously diagnosed and were in specialized care. The GP of the fourth chronically infected person was uncertain whether the patient had been previously diagnosed; however, this person was referred to specialized care after our notification letter was received.
Markers of HBV infection and immunity
Among all people with past-HBV infection for whom an anti-HBs test result (positive/negative) was available, 71.4% (503/704; 95% CI 68.0–74.7%) tested anti-HBs positive while 28.6% (201/704; 95% CI 25.3–32.0%) had no anti-HBs. The prevalence of isolated anti-HBc positivity differed significantly between ethnic groups (Dutch: 23.1% [3/13]; South-Asian Surinamese: 23.3% [14/60]; African-Surinamese: 11.3% [13/115]; Ghanaian: 42.5% [125/294]; Turkish: 15.9% [20/126]; Moroccan: 28.6% [24/85]; p < 0.001).
Among people with chronic HBV infection, 3.2% (2/63; 95% CI 0.7–9.8%) were HBeAg positive and 91.9% (57/62; 95% CI 83.2–96.9%) were anti-HBe positive (1/64 had insufficient blood available for HBeAg testing and 2/64 had indeterminate anti-HBe test results). In total, 3.1% (2/64; 95% CI 0.7–9.6%) of chronically infected participants had HBV-DNA levels of 20,000 IU/ml or higher; 12.5% (8/64; 95% CI 6.1–22.2%) had HBV-DNA levels between 2,000–19,999 IU/ml; 25% (16/64; 95% CI 15.7–36.5%) had HBV-DNA levels between 220–1,999 IU/ml; whereas in 59.4% (38/64; 95% CI 47.1–70.8%) of participants HBV-DNA was below 220 IU/ml or not detected.
All individuals newly diagnosed with chronic HBV infection as a result of our study (n = 13) were HBeAg negative and anti-HBe positive. Of those, 15.4% (2/13; 95% CI 3.34–40.90%) had HBV-DNA levels between 2,000–19,999 IU/ml; 46.2% (6/13; 95% CI 22.11–71.71%) between 220–2,000 IU/ml and in 38.5% (5/13; 95% CI 16.5–65.0%) HBV-DNA was below 220 IU/ml or not detected.
Determinants of infection
In multivariable analyses, ethnicity, age, sex and educational level were significantly associated with HBV infection status (Table 3). Participants of South-Asian Surinamese (adjusted odds ratio [aOR] 4.12; 95% CI 2.19–7.75), African-Surinamese (aOR 9.28; 95% CI 5.06–17.03), Ghanaian (aOR 66.10; 95% CI 35.76–122.17), Turkish (aOR 13.19; 95% CI 7.13–24.39) and Moroccan (aOR 7.06; 95% CI 3.79–13.18) origin were at increased risk of past-HBV infection compared with those of Dutch ethnicity. Participants of African-Surinamese (aOR 6.16; 95% CI 1.27–29.79), Ghanaian (aOR 42.23, 95% CI 9.29–291.01) and Turkish (aOR 13.44; 95% CI 2.94–61.39) origin were at increased risk of chronic HBV infection compared with those of Dutch origin, whereas those of South-Asian Surinamese (aOR 1.70; 95% CI 0.27–10.56) and Moroccan (aOR 2.50; 95% CI 0.43–14.35) origin were not.
Table 3.
Multinominal univariable and multivariable logistic regression analyses of determinants associated with past-HBV infection and chronic HBV infection (vs. non-exposed). Among 2,987 Dutch and first-generation migrant participants of the HELIUS study, Amsterdam, the Netherlands, 2011-2014.
| Past-HBV exposure |
Chronic HBV infection |
p valuea | |||
|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | ||
| Ethnicityb | < 0.001 | ||||
| Dutch | 1 | 1 | 1 | 1 | |
| South-Asian Surinamese | 5.72 (3.10–10.56) | 4.12 (2.19–7.75) | 1.83 (0.30–10.99) | 1.70 (0.27–10.56) | |
| African Surinamese | 12.36 (6.86–22.29) | 9.28 (5.06–17.03) | 6.24 (1.34–29.04) | 6.16 (1.27–29.79) | |
| Ghanaian | 68.36 (38.19–122.36) | 66.10 (35.76–122.17) | 37.75 (8.87–160.78) | 42.23 (9.29–192.01) | |
| Turkish | 13.43 (7.47–24.16) | 13.19 (7.13–24.39) | 13.16 (3.05–56.88) | 13.44 (2.94–61.39) | |
| Moroccan | 7.79 (4.28–14.17) | 7.06 (3.79–13.18) | 2.38 (0.43–13.08) | 2.50 (0.43–14.35) | |
| Age (per year) | 1.04 (1.03–1.05) | 1.06 (1.05–1.07) | 1.01 (0.99–1.04) | 1.02 (1.00–1.05) | < 0.001 |
| Sex | 0.009 | ||||
| Men | 1 | 1 | 1 | 1 | |
| Women | 0.86 (0.73–1.02) | 0.83 (0.68–1.01) | 0.54 (0.33–0.90) | 0.49 (0.29–0.83) | |
| Highest level of education | 0.005 | ||||
| Less than higher vocational schooling or university | 1 | 1 | 1 | 1 | |
| Higher vocational schooling or university | 0.24 (0.18–0.32) | 0.59 (0.42–0.82) | 0.39 (0.19–0.82) | 0.92 (0.42–2.05) | |
| Surgery in non–Western country | |||||
| No | 1 | 1 | |||
| Yes | 1.40 (1.06–1.84) | 1.29 (0.58–2.87) | |||
| Blood transfusion | |||||
| Never or > 1990 in Western country | 1 | 1 | |||
| Before 1990 or > 1990 in non–Western country | 1.08 (0.72–1.61) | 1.10 (0.34–3.58) | |||
| Lifetime sexual partners | |||||
| 0–1 | 1 | 1 | |||
| 2 | 1.34 (0.99–1.81) | 1.29 (0.57–2.92) | |||
| 3–6 | 1.51 (1.20–1.88) | 1.29 (0.69–2.40) | |||
| > 7 | 0.91 (0.72–1.16) | 0.72 (0.36–1.45) | |||
In multivariable analysis, the following variables were excluded because their overall p values were no longer below the pre-specified threshold: surgery in non-Western country (p = 0.545) and lifetime sexual partners (p = 0.495).
aOR, adjusted odds ratio; HBV, hepatitis B virus; OR, odds ratio.
Overall p value of a log-likelihood ratio test comparing full model to a model with individual covariable excluded.
Surinamese of other/unknown ethnicity were not included in the analyses because of a low number of participants.
Older age was significantly associated with past-HBV infection (aOR 1.06 per year; 95% CI 1.05–1.07) and tended to be associated with chronic HBV infection (aOR 1.02 per year; 95% CI 1.00–1.05). Women were at significantly lower risk of chronic HBV infection (aOR 0.49; 95% CI 0.29–0.83) compared with men, and a similar, but non-significant, sex difference was observed for past-HBV infection (aOR 0.83; 95% CI 0.68–1.01). Those with higher educational level (aOR 0.59; 95% CI 0.42–0.82) were less likely to have past-HBV infection compared to less well educated participants.
Among non-Dutch participants, higher age at migration was significantly associated with past-HBV infection (aOR 1.02 per year; 95% CI 1.00–1.03; p = 0.01) but not with chronic HBV infection (aOR 1.02 per year; 95% CI 0.99–1.06; p = 0.20)(overall p = 0.02). The reason for migration was not significantly associated with HBV infection status (p = 0.9; data not shown).
The few participants infected with HCV precluded multivariable analysis. All 4 HCV-RNA-positive people were aged ≥ 50 years; 2 were of Dutch origin and 2 of African-Surinamese origin; 3 were men. Two individuals reported to be HIV-positive, 1 person reported ever-injecting drug use, and 1 person of African-Surinamese origin did not report any risk factors for HCV infection. None of the 4 participants were co-infected with HBV.
Discussion
In this population-based cross-sectional study, conducted in a country with low prevalence of HBV and HCV infection in the general population, we found a higher prevalence of chronic HBV infection among first-generation migrants of Ghanaian, Turkish and African-Surinamese ethnicity compared to the host population, whereas the prevalence of chronic HCV infection was low among all ethnic groups. All HCV-infected individuals for whom this information was available were aware of their infections, whereas a substantial proportion of people with chronic HBV infection were in fact unaware.
The age- and sex-adjusted chronic HBV infection prevalence in our study among first-generation migrants from Turkey (4.1%) and Morocco (1.2%) is comparable to previous estimates among first-generation migrants from these countries25 including those of an Amsterdam study among a small group of first-generation migrants,26 as well as others reporting country-of-origin estimates.[25], [27] Slightly lower chronic HBV infection prevalence was found in several screening pilot programs for first-generation migrants of Turkish origin in the Netherlands, ranging from 1.7% to 3.1%.[28], [29], [30] These programs, however, most likely do not attract individuals who are already aware of their infection, which may explain their lower prevalence.
For first-generation migrants from Suriname, we found a higher prevalence of past and chronic HBV infection among the African-Surinamese than among the South-Asian Surinamese, although the latter difference was not statistically significant. The difference in HBV infection prevalence between Surinamese ethnic groups has also been demonstrated in a study conducted in Suriname, where prevalence among the South-Asian Surinamese was similar to our findings and prevalence among the African-Surinamese was somewhat higher.31 Interestingly, as the African-Surinamese are mostly descendants of enslaved people from Western Africa (a high-prevalence region for HBV), whereas the South-Asian Surinamese are descendants of contract workers from India (a country of much lower HBV prevalence),27 the difference in prevalence could be explained by confined interactions within sub-ethnicities over centuries, which then carried over when immigrating to the Netherlands. Nevertheless, our findings illustrate the need to consider ethnic subgroups within countries of origin when studying prevalence and identifying people in need of HBV screening.
The prevalence of chronic HBV infection among first-generation migrants of Ghanaian origin (5.4%) was much lower compared with estimates in Ghana derived from 2 large meta-analyses (12.9%; 95% CI 12.4–13.4%27 and 12.3%; 95% CI 11.3–13.4%32) and a study among recent (< 5 years) migrants of Ghanaian ethnicity in Spain (15/92; 16.3%; 95% CI 9.8–24.8%).33 The lower prevalence in our study may be explained by shorter exposure time to HBV in their home country compared to those who did not migrate, the salmon bias (i.e., severely ill migrants with HBV infection tend to return to die in their home country) and/or the healthy migrant hypothesis (i.e., a selection of healthier individuals opt for migration).
It is of concern that over 40% of the participants chronically infected with HBV were not aware of their infection status. Unawareness of infection status was much lower for those identified with HCV infection. Men, who are at higher risk of chronic HBV infection, were less likely to be aware of their HBV infection status than women (although not statistically significant). Women may be more likely to be aware of their infection as a result of routine HBV screening in prenatal care programs in the Netherlands to prevent mother-to-child transmission, which was implemented in 1989. Our findings indicate that additional screening programs are needed to identify undiagnosed HBV infections among both men and women of Ghanaian and Turkish origin in order to prevent liver-related disease and onward transmission.
A large proportion of those of non-Dutch ethnicity had past-HBV infection, with the proportions per ethnic group reflecting endemic patterns of their countries of origin. This indicates that many migrants acquired immunity to HBV infection. We did, however, find relatively high levels of isolated-anti-HBc serologic status, especially among those of Ghanaian origin. Isolated-anti-HBc may result from different scenarios: resolved infection acquired many years ago where detectable anti-HBs has been lost, acute HBV infection, false-positive anti-HBc test, or occult HBV infection where patients with undetectable HBsAg have detectable HBV-DNA. Patients originating from high HBV-endemic countries are known to be more likely to develop occult HBV infection compared with those from low-endemic countries.34 However, even in high-endemic regions for HBV, occult HBV infection usually is not very common, and estimated at around 6% in anti-HBc positive, HBsAg negative blood donors.35 Hence, we believe that the high levels of isolated anti-HBc among non-Dutch ethnic groups are more likely to result from resolved infections that were acquired during early childhood and adolescence. As a large proportion of first-generation migrants are immune to HBV, the primary requirement for HBV prevention is screening to identify chronic infections.
Although migrants are estimated to account for many chronic HCV infections in the Netherlands,3 as well as in many other European countries,[18], [21] we found only 4 chronic HCV infections among 2,993 people tested. For first-generation migrants of Turkish and Moroccan ethnicity, we did not expect an anti-HCV antibody prevalence higher than among those of Dutch origin, since country-of-origin estimates are low36 and the prevalence among these groups in previous studies in the Netherlands was also low.37 For migrants from Ghana and Suriname, we expected a higher prevalence based on previous studies in the country of origin and/or the Netherlands.[37], [38], [39], [40] The low HCV infection prevalence found in our study may be due to the same reasons as mentioned for HBV infection. Alternatively, for some countries of origin, there are vast differences in HCV prevalence across regions (Ghana38) and in sub-ethnicities (Javanese-Surinamese40), which might not be reflected in our study.
Although HCV screening for the ethnic groups in our study does not seem justified, combined screening for HBV and HCV may still be cost-effective as the additional costs of simultaneous screening for HCV are small, especially when considering the cost-effectiveness of this strategy for countries of origin with chronic HBV and HCV prevalence ≥ 0.41% and ≥ 0.22%, respectively.41 This suggests that combined HBV and HCV screening of first-generation migrants of Ghanaian and Surinamese origin may be cost-effective despite the low HCV prevalence found in our study.
This study has several limitations. First, although HELIUS participants aged 18–70 years were recruited via an ethnicity stratified random selection of the municipal registry of Amsterdam and our study includes a random selection of HELIUS participants, there may be selection bias. Undocumented people and those aged > 70 years who may be at higher risk of infection are not included. Furthermore, HELIUS participants may be more concerned about their health compared with non-participants. Notwithstanding the low response rate in the HELIUS study (28%),23 non-response was not linked to socioeconomic differences. Second, with 500 participants per ethnic group, comparisons in HBsAg and HCV prevalence could be underpowered. A posteriori sample size calculation revealed that based on the unadjusted HBsAg prevalence of 0.4% in Dutch-origin participants as reference group, a minimum HBsAg prevalence of 1.6% would have been required in another ethnic group to find a significant difference with 80% power.42 In particular, we did observe a notably higher prevalence of chronic HBV infection for participants of South-Asian Surinamese, Surinamese other/unknown and Moroccan origin than those of Dutch origin, but the aORs were likely not significant because of insufficient power. Third, because the numbers of MSM were small in the non-Dutch ethnic origin groups, sexual behavior status was not included in the multivariable regression analyses assessing factors associated with HBV infection. Fourth, 10 HBsAg-positive individuals tested HBsAg negative after re-testing at their GP. We found that 8 of those had low levels of HBsAg (< 0.1) in the initial test and undetectable or low levels (< 220 IU/ml) of HBV-DNA. This suggests the presence of low-level HBsAg, a false-positive primary test result, or clearance of HBsAg between HELIUS data collection and re-testing. Finally, we did not determine anti-HBs among those who tested anti-HBc negative, hence we were unable to assess the proportion of our population vaccinated against HBV infection.
In conclusion, the prevalence of chronic HBV infection among first-generation migrants of Ghanaian, Turkish and African-Surinamese origin living in Amsterdam is relatively high compared with the Dutch-origin population, and many are unaware of their infection status. Increased case-finding efforts by GPs and HBV screening programs targeting these migrant groups, as recommended by the Dutch Health Council,20 are needed to identify undiagnosed infections and link infected people to care to decrease HBV-related morbidity and mortality. Importantly, as emphasized by the World Health Organization,43 screening and treatment programs for migrant populations should provide accessible options for timely screening and treatment, as there are a number of barriers to detection and treatment of infectious diseases among migrants.44 Programs should address factors influencing participation in HBV screening, such as stigma and fatalism[45], [46], [47] and integrate strategies to facilitate access, such as outreach awareness raising.44 HCV screening for the large ethnic groups in Amsterdam seems unjustified, but combined HBV and HCV screening might still be cost-effective if offered to first-generation migrants of Ghanaian and Surinamese origin.
Financial support
The HELIUS study is conducted by the Academic Medical Center Amsterdam and the Public Health Service of Amsterdam. Both organizations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation (2010T084), the Netherlands Organization for Health Research and Development (ZonMw) (200500003), the European Union (FP-7) (278901), and the European Fund for the Integration of non-EU immigrants (EIF) (2013EIF013). The current study was additionally funded by the R&D Fund of the Public Health Service of Amsterdam, the Regional Support Programme of the RIVM Centre for Infectious Disease Control, and via an unrestricted grant from Gilead Sciences. In addition, Roche Diagnostics provided test kits for HBV-DNA testing (unrestricted grant). Gilead Sciences and Roche Diagnostics did not have involvement in study design, data collection, analysis and interpretation, nor in the writing and publication of this article.
Conflicts of interest
FZ worked on projects (partly) funded or sponsored by non-restricted grants from Abbvie, Gilead Sciences, Janssen-Cilag, MSD, Orasure Technologies and Roche Diagnostics. JB has worked on several research projects for which her institute received (restricted and unrestricted) grants from Gilead Sciences. MV: no conflicts of interest. MS: no conflicts of interest. AB: no conflicts of interest. PB: no conflicts of interest. GS: no conflicts of interest. JS serves as a scientific advisor for Gilead Sciences, and received non-restricted research grants from Gilead Sciences, Abbvie, MSD, Roche Diagnostics, paid to her institute. MP obtained unrestricted research grants and speaker’s fees from Gilead Sciences, Roche, Abbvie and MSD, paid to her institute.
Authors’ contributions
FZ coordinated the current study and drafted the manuscript; JB performed data management and the statistical analyses and coordinated the collection of clinical follow-up data; MV performed data management and analyses during her internship; MS coordinated the HELIUS study (data collection and data management) and contributed to the study design of the current study; AB contributed to the statistical analyses; PB and JS coordinated the laboratory testing; JS and GS contributed to the study design; MP conceived and supervised the current study. All authors contributed to the interpretation of the results and the writing of the manuscript.
Acknowledgements
The authors would like to acknowledge the HELIUS participants for their contribution; the HELIUS team for data collection and management; Annemarie Teitsma-Jansen for sending notification letters and data collection forms to GPs; Sylvia Bruisten and Nienke Alberts for coordinating the sample selection and transportation; Gini van Rijckevorsel for advice on the algorithm for HBV testing; and the funders for financial support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.04.003.
Supplementary data
HBV and HCV prevalence among Dutch and first-generation migrant participants of the HELIUS study, Amsterdam, the Netherlands, 2011-2014
Supplementary material 1
Supplementary material 2
References
- 1.European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2016. Systematic review on hepatitis B and C prevalence in the EU/EEA. [Google Scholar]
- 2.Hofman R, Nusselder WJ, Veldhuijzen IK, Richardus JH. Mortality due to chronic viral hepatitis B and C infections in the Netherlands [in Dutch] Ned Tijdschr Geneeskd. 2016;160:D511. [PubMed] [Google Scholar]
- 3.Koopsen J, van Steenbergen JE, Richardus JH, Prins M, Op de Coul ELM, Croes EA. Chronic hepatitis B and C infections in the Netherlands: estimated prevalence in risk groups and the general population. Epidemiol Infect. 2019;147 doi: 10.1017/S0950268819000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadziyannis SJ. Update on Hepatitis B Virus Infection: Focus on Treatment. J Clin Transl Hepatol. 2014;2:285–291. doi: 10.14218/JCTH.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memon MI, Memon MA. Hepatitis C: an epidemiological review. J Viral Hepat. 2002;9:84–100. doi: 10.1046/j.1365-2893.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 7.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 8.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 9.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047–1055. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meffre C, Le Strat Y, Delarocque-Astagneau E, Dubois F, Antona D, Lemasson JM. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: social factors are important predictors after adjusting for known risk factors. J Med Virol. 2010;82:546–555. doi: 10.1002/jmv.21734. [DOI] [PubMed] [Google Scholar]
- 12.van der Ploeg C, Schönbeck Y, Hirschberg H. TNO; Leiden: 2015. Prenatal screening for infectious diseases and erythrocytes immunisation [in Dutch] [PubMed] [Google Scholar]
- 13.Willemse SB, Razavi-Shearer D, Zuure FR, Veldhuijzen IK, Croes EA, van der Meer AJ. The estimated future disease burden of hepatitis C virus in the Netherlands with different treatment paradigms. Neth J Med. 2015;73:417–431. [PubMed] [Google Scholar]
- 14.Dutch Association of HIV-Treating Physicians (NVHB) HIV treatment guidelines for the Netherlands [in Dutch] http://richtlijnhiv.nvhb.nl/index.php/Inhoud Accessed August 2018 at.
- 15.Loth C, Wits E, de Jong C, van de Mheen D. Resultaat Scoren; Amersfoort: 2012. Guideline for opiate maintenance treatment, revised version. [in Dutch] [Google Scholar]
- 16.van Rijckevorsel GWJ, Kretzschmar M, Siedenburg E, Sonder G, Geskus R, Coutinho R, van den Hoek A. Targeted vaccination programme successvul in reducing acute hepatitis B in men having sex with men in Amsterdam, The Netherlands. J Hepatol. 2013;59:1177–1183. doi: 10.1016/j.jhep.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Hoornenborg E, Rijnders B. Dutch Association of HIV-Treating Physicians (NVHB); 2016. HIV Pre-expositie profylaxe (PrEP) guidelines Netherlands. [in Dutch] [Google Scholar]
- 18.European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2016. Epidemiological assessment of hepatitis B and C among migrants in the EU/EEA. [Google Scholar]
- 19.Dutch College of General Practitioners (NHG) NHG; 2016. NHG Guideline Viral Hepatitis and other liver diseases (third revision) [in Dutch] [Google Scholar]
- 20.Health Council of the Netherlands . Health Council of the Netherlands; The Hague: 2016. Screening risk groups for hepatitis B and C. [Google Scholar]
- 21.Hahne SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar M. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181. doi: 10.1186/1471-2334-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stronks K, Snijder MB, Peters RJ, Prins M, Schene AH, Zwinderman AH. Unravelling the impact of ethnicity on health in Europe: the HELIUS study. BMC Public Health. 2013;13:402. doi: 10.1186/1471-2458-13-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snijder MB, Galenkamp H, Prins M, Derks EM, Peters RJG, Zwinderman AH. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stronks K, Kulu-Glasgow I, Agyemang C. The utility of 'country of birth' for the classification of ethnic groups in health research: the Dutch experience. Ethn Health. 2009;14:255–269. doi: 10.1080/13557850802509206. [DOI] [PubMed] [Google Scholar]
- 25.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–433. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 26.Baaten GG, Sonder GJ, Dukers NH, Coutinho RA, Van den Hoek JA. Population-based study on the seroprevalence of hepatitis A, B, and C virus infection in Amsterdam, 2004. J Med Virol. 2007;79:1802–1810. doi: 10.1002/jmv.21009. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 28.van der Veen YJ, van Empelen P, de Zwart O, Visser H, Mackenbach JP, Richardus JH. Cultural tailoring to promote hepatitis B screening in Turkish Dutch: a randomized control study. Health Promot Int. 2014;29:692–704. doi: 10.1093/heapro/dat020. [DOI] [PubMed] [Google Scholar]
- 29.Richter C, Beest GT, Sancak I, Aydinly R, Bulbul K, Laetemia-Tomata F. Hepatitis B prevalence in the Turkish population of Arnhem: implications for national screening policy? Epidemiol Infect. 2012;140:724–730. doi: 10.1017/S0950268811001270. [DOI] [PubMed] [Google Scholar]
- 30.Niessen W, Benne R, van Zeijl J, van der Have J, Broer J. Inviting migrants for hepatitis B testing by letter. Is it effective? [in Dutch] Inf Bulletin. 2013;24:107–112. [Google Scholar]
- 31.Mac Donald-Ottevanger MS, van de Laar T, Zijlmans W, Prins M, Brinkman K, Roosblad J. Prevalence, determinants of chronic hepatitis B infection and hepatitis B genotypes in multi-ethnic Suriname. Abstracts of The International Liver Congress 2017. J Hepatol. 2017;66:S252–S253. [Google Scholar]
- 32.Ofori-Asenso R, Agyeman AA. Hepatitis B in Ghana: a systematic review & meta-analysis of prevalence studies (1995-2015) BMC Infect Dis. 2016;16:130. doi: 10.1186/s12879-016-1467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzardo C, Trevino B, Gomez i, Prat J, Cabezos J, Mongui E, Claveria I. Communicable diseases in the immigrant population attended to in a tropical medicine unit: epidemiological aspects and public health issues. Travel Med Infect Dis. 2008;6:4–11. doi: 10.1016/j.tmaid.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Chemin I, Trepo C. Clinical impact of occult HBV infections. J Clin Virol. 2005;34:S15–S21. doi: 10.1016/s1386-6532(05)80005-8. [DOI] [PubMed] [Google Scholar]
- 35.Said ZN. An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1927–1938. doi: 10.3748/wjg.v17.i15.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polaris Observatory, HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 37.Urbanus AT, van de Laar TJ, van den Hoek A, Zuure FR, Speksnijder AG, Baaten GG. Hepatitis C in the general population of various ethnic origins living in the Netherlands: should non-Western migrants be screened? J Hepatol. 2011;55:1207–1214. doi: 10.1016/j.jhep.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Agyeman AA, Ofori-Asenso R, Mprah A, Ashiagbor G. Epidemiology of hepatitis C virus in Ghana: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:391. doi: 10.1186/s12879-016-1708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 40.Mac Donald-Ottevanger MS, Vreden S, van der Helm JJ, van de Laar T, Molenkamp R, Dams E. Prevalence, determinants and genetic diversity of hepatitis C virus in the multi-ethnic population living in Suriname. Virology. 2016;499:114–120. doi: 10.1016/j.virol.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Suijkerbuijk AWM, van Hoek AJ, Koopsen J, de Man RA, Mangen MJ, de Melker HE. Cost-effectiveness of screening for chronic hepatitis B and C among migrant populations in a low endemic country. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26:3385–3397. doi: 10.1002/sim.2771. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization (WHO) WHO; Geneva: 2017. Global Hepatitis Report 2017. [Google Scholar]
- 44.Seedat F, Hargreaves S, Nellums LB, Ouyang J, Brown M, Friedland JS. How effective are approaches to migrant screening for infectious diseases in Europe? A systematic review. Lancet Infect Dis. 2018;18:e259–e271. doi: 10.1016/S1473-3099(18)30117-8. [DOI] [PubMed] [Google Scholar]
- 45.Hamdiui N, Stein ML, Timen A, Timmermans D, Wong A, van den Muijsenbergh M. Hepatitis B in Moroccan-Dutch: a quantitative study into determinants of screening participation. BMC Med. 2018;16:47. doi: 10.1186/s12916-018-1034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Veen YJ, van Empelen P, Looman CW, Richardus JH. Social-cognitive and socio-cultural predictors of hepatitis B virus-screening in Turkish migrants, the Netherlands. J Immigr Minor Health. 2014;16:811–821. doi: 10.1007/s10903-013-9872-y. [DOI] [PubMed] [Google Scholar]
- 47.Blanas DA, Nichols K, Bekele M, Shankar H, Bekele S, Jandorf L. Adapting the Andersen model to a francophone West African immigrant population: hepatitis B screening and linkage to care in New York City. J Community Health. 2015;40:175–184. doi: 10.1007/s10900-014-9916-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HBV and HCV prevalence among Dutch and first-generation migrant participants of the HELIUS study, Amsterdam, the Netherlands, 2011-2014
Supplementary material 1
Supplementary material 2