Background & Aims
Acute-on-chronic liver failure (ACLF) is a recently (re)defined syndrome of acute decompensation of cirrhosis that presents with extrahepatic organ failure(s) and poor outcome. Given the prominent role of inflammation and activation of coagulation in ACLF, we hypothesized that ACLF might be characterized by the generation of neutrophil extracellular traps (NETs), that could drive both activation of coagulation and progression of organ failure.
Methods
We measured markers of circulating DNA, activation of coagulation, inflammation, and oxidative stress in 52 patients with acute decompensation (AD) of cirrhosis and 57 patients with ACLF on admission, and compared levels with 40 healthy controls.
Results
All analytes were higher in patients compared to controls. Plasma levels of cell-free DNA, but not of the specific NET marker myeloperoxidase-DNA complexes were higher in patients with ACLF compared to AD cirrhosis. In addition, TAT complexes (coagulation), IL-6 (inflammation), and TBARS (oxidative stress) were higher in ACLF compared to AD. Markers for activation of coagulation were not associated with circulating DNA, IL-6, or TBARS. In contrast, levels of circulating DNA, IL-6, and TBARS were higher in patients with more severe disease, higher in patients with organ failure, and higher in patients that died within 30 days of admission. Importantly, myeloperoxidase-DNA levels did not differ between patients with complications and poor outcome.
Conclusions
Collectively, we show that cell-free DNA, inflammation, and oxidative stress are associated with outcomes in AD and ACLF, but not with activation of coagulation. Our data argue against a role of NETs in activation of coagulation and in progression of organ failure in patients with AD and ACLF.
Lay summary
Acute-on-chronic liver failure is a devastating syndrome that can follow acute decompensation of chronic liver disease. Herein, we demonstrate that these patients accumulate DNA released from dying cells in their blood, and that the quantity of this DNA is related to the outcome of disease. We also show that outcome of disease is not related to recently described neutrophil extracellular traps, which have been shown in animal models to play vital roles in the progression of liver diseases.
Keywords: cirrhosis, liver, acute-on-chronic liver disease, neutrophil extracellular trap, coagulation, sepsis, mortality, inflammation, oxidative stress
Graphical abstract
Highlights
-
•
Levels of circulating DNA, IL6, and TBARS are higher in patients with ACLF than in patients with AD
-
•
Circulating DNA, IL6, and TBARS are higher in patients with organ failure and those who died
-
•
Circulating DNA, IL6, and TBARS are not correlated with markers of activation of coagulation
-
•
The neutrophil extracellular trap marker MPO-DNA was not related to coagulation or outcome
-
•
DNA- or NET-related coagulation does not appear to drive ACLF progression
Introduction
Acute-on-chronic liver failure (ACLF) is a recently (re)defined syndrome characterized by acute decompensation (AD) of cirrhosis (development of ascites, hemorrhage, encephalopathy and/or bacterial infection), organ failure (liver, kidney, brain, coagulation, respiration, circulation), and high short-term mortality.[1], [2], [3] Patients with ACLF exhibit a very dynamic disease course. Patients may improve (50%) or worsen (20%) within a short period of time, and prognosis depends on the early clinical course.4 The pathogenesis underlying the ACLF syndrome have not been well elucidated, but a systemic inflammatory response appears to play a central role. It not known precisely which factors induce this inflammatory response. Sepsis, acute alcoholic hepatitis, and reactivation of chronic viral hepatitis are precipitating factors for ACLF in some patients, but up to 40–50% of cases have no identifiable trigger.1
Patients with AD and ACLF have complex changes in their hemostatic system, with both hypo- and hypercoagulable features.[5], [6] Indeed, both bleeding and thrombotic complications are common in these patients7, although the incidence and severity of these hemostatic complications have not been systematically documented in large series. Local or systemic activation of hemostasis may result in macrovascular thrombotic complications such as deep vein thrombosis or portal vein thrombosis. In addition, microvascular thrombotic events may occur and precipitate or worsen organ failure, a phenomenon that has been well established in the context of sepsis without underlying liver disease8 and in patients with disseminated intravascular coagulation.9 Since activation of the hemostatic system is accompanied by inflammatory responses and vice versa,[10], [11] a role of the complex reciprocal interactions between hemostasis and inflammation in macro- and microvascular thrombotic complications is likely.
Neutrophil extracellular traps (NETs) have recently been described as a link between inflammation and coagulation.[12], [13] NETs are a web-like structure composed of neutrophil DNA complexed to various neutrophil-derived proteins and are generated following activation of neutrophils by various stimuli. NETs were originally described as a mechanism to enable neutrophils to entrap and kill pathogens.14 Subsequently, it was demonstrated that NETs can be generated in the absence of pathogens, and NETs are now implicated as drivers of a variety of non-infectious diseases including diabetes, atherosclerosis, cancer, and trauma.15 In addition, NETs have been shown to be potent activators of hemostasis, and have been implicated in thrombotic disease.[16], [17], [18]
We have recently reported generation of NETs during liver transplantation in humans.19 Although there is clear activation of coagulation during liver transplantation, we found no evidence of a role for NETs in driving this process. Rather, the release of cell-free DNA, likely from hepatocytes damaged by ischemia-reperfusion injury, appeared to drive activation of coagulation. However, NET formation was assessed in systemic circulation, and it may be that NETs do drive activation of coagulation locally.
Given the role of systemic inflammation in ACLF,4 the established role of NETs in infection and sepsis,20 the role of NETs in various types of liver injury,21 and the hypercoagulable features of the hemostatic system in ACLF,5 we hypothesized that NETs are generated in AD, but particularly in ACLF, and that NETs drive activation of coagulation with subsequent worsening of organ failure.
Materials and methods
Patients
Between February 2018 and September 2018, adult patients with acutely decompensated cirrhosis (AD, n = 52) or acute-on-chronic liver failure (ACLF, n = 57) consecutively admitted to King's College Hospital London (United Kingdom) and Hospital Clinic Barcelona (Spain) who gave written informed consent were included in this study. The NRES Committee London-Westminster (12/LO/1417) and the medical ethical committee of Hospital Clinic Barcelona (2017/0948) approved the study protocol which was in accordance with the Helsinki Declaration of 1975. Informed consent was obtained from participants or from personal consultees when the patients were incapacitated and unable to provide informed consent. Exclusion criteria for this study were acute liver failure, known congenital coagulation disorders, the use of anticoagulants or platelet function inhibitors, pregnancy, serological evidence of the HIV, or evidence of disseminated malignancy. Cirrhosis was defined by the presence of 2 or more of: i) histological evidence of cirrhosis on liver biopsy, ii) laboratory abnormalities consistent with cirrhosis or iii) radiological findings consistent with cirrhosis and portal hypertension. AD of chronic liver disease and ACLF were defined and graded according to number of organ failures in concordance with criteria reported in the CANONIC study,1 see http://www.efclif.com/scientific-activity/score-calculators for online score calculator. Patients were followed-up until 30 days after discharge, death or transplant, whatever happened first. Healthy controls aged ≫18 years (n = 40) were enrolled to establish reference values for the various laboratory tests performed, and excluded those with a personal history of thrombotic or liver-related diseases, medical conditions known to be associated with hemostatic alterations, or current use of anticoagulants, platelet function inhibitors, or oral contraceptives.
Data collection
We collected data on patient demographics, comorbidities, biochemistry and illness severity scores. The severity of liver disease was evaluated with sequential organ failure assessment (SOFA), Chronic Liver Failure Consortium (CLIF)-AD, CLIF-ACLF, model of end-stage liver disease (MELD), and Child-Pugh scores. Sepsis syndrome was defined according to the SEPSIS-3 guidelines.
Blood samples
Blood samples were collected in sodium citrate-containing vacutainer tubes (0.129 M) from an arterial line, central venous catheter, or by standard peripheral venous phlebotomy within the first 2 days of admission or after the development of ACLF. Samples were obtained prior to the administration of blood products, anticoagulants, or platelet function inhibitors. Within 2 h after the blood draw, the sample was centrifuged at 2.000 g and 10.000 g respectively for 10 min at ambient temperature. Plasma was stored at -80°C until it was used for analyses.
Assessment of cell-free DNA, coagulation activation, inflammatory markers and oxidative stress
The concentration of cell-free DNA (cfDNA) was measured using the Quant-iT PicoGreen double strand DNA assay kit (Fisher Scientific, Landsmeer, the Netherlands). In short, 10 μl of plasma was added to 90 μl of Tris-EDTA (TE) buffer (10mM Tris-HCl, 1mM EDTA, pH 7.5) in a 96-well microtiter plate. Next, 100 μl of PicoGreen solution (diluted 1:200 in TE buffer) was added to the wells. After 10 minutes of incubation in the dark at room temperature, fluorescence was measured at 480 nm excitation and 520 nm emission using a Victor3 (PerkinElmer, Groningen, the Netherlands) spectrophotometer. The concentration of nucleosomes in the plasma samples was determined using the cell death detection ELISA (Sigma-Aldrich, Zwijndrecht, The Netherlands). The test was performed according to the manufacturer’s instructions. Complexes of myeloperoxidase (MPO) and DNA – a selective marker for neutrophil extracellular traps in plasma – were quantified by ELISA as previously described22 using a commercially available monoclonal antibody (clone 4A4) to MPO (Sanbio, Uden, the Netherlands) and the detection antibody derived from the cell death detection ELISA (Sigma-Aldrich, Zwijndrecht, The Netherlands). To assess activation of coagulation the concentration of thrombin/antithrombin complex (TAT), which is an established marker for in vivo thrombin generation, was determined. Quantification of TAT was done with the use of Enzygnost TAT micro ELISA kit (Siemens Healthcare Diagnostics, the Hague, the Netherlands), according to the manufacturers’ protocol. Levels of D-dimers were measured on the ACL 300 top using the HemosIL D-Dimer HS500 assay which was obtained from Werfen (Breda, The Netherlands). Interleukin 6 (IL-6) levels were determined using a commercially available ELISA from R&D Systems (Minneapolis, MN, USA). Thiobarbituric acid-reactive substances (TBARS) were measured as a marker for oxidative stress as previously described.23
Statistical analyses
Statistical analyses were performed using SPSS statistics, version 23 (IBM inc., Chicago Illinois, USA) and GraphPad Prism (San Diego, USA). Data were presented as means with standard deviation or medians and interquartile ranges (IQRs) for continuous variables as appropriate, and as percentages for categorical variables. Means of 2 groups were compared by Mann-Whitney U test as all analytes were not normally distributed. Multiple groups were compared Kruskal-Wallis H test (with Dunn’s post hoc test). Pearson’s correlation coefficient was used to assess the correlation between variables. A p value ≪0.05 was considered significant.
Results
Patient characteristics
We have studied levels of cfDNA, markers for activation of coagulation, markers for oxidative stress and inflammatory markers in the plasma of 52 adult patients with AD cirrhosis and 57 adult patients with ACLF. We compared these levels with plasma concentrations in 40 healthy controls. Patient demographics, clinical and laboratory data are presented in Table 1. Healthy controls were younger than patients (35 [28-43] years, and 45% were male). Among patients with ACLF, 27 (47%) had ACLF grade 1 (23 of those had renal failure only), 13 (23%) ACLF grade 2 and 17 (30%) ACLF grade 3, at inclusion. Infection was present in 7 (14%) and 26 (46%) patients with AD and ACLF, respectively, at inclusion.
Table 1.
Demographic and laboratory data of the study population.
| AD n = 52 |
ACLF n = 57 |
|
|---|---|---|
| Age, years | 58 (50–67) | 59 (49–66) |
| Male | 29 | 40 |
| Etiology | ||
| Alcohol | 32 | 33 |
| Viral | 0 | 11 |
| NASH | 10 | 6 |
| Biliary | 3 | 2 |
| Other | 7 | 5 |
| SOFA score | 4 (3–6) | 8 (6–11) |
| CLIF–SOFA score | 60 (47–108) | 87 (74–97) |
| MELD | 15 (11–21) | 27 (23–35) |
| Child-Pugh | 9 (7–10) | 10 (8–12) |
| Haemoglobin, g/dl | 8.6 (8.0–11.0) | 8.5 (7.7–9.9) |
| mmol/L | 5.0 (5.3–6.8) | 5.3 (4.8–6.1) |
| Leucocytes, x109/L | 5.5 (3.6–6.5) | 8.2 (3.6–13.7) |
| Neutrophils, x109/L | 2.9 (2.1–3.9) | 3.6 (1.7–9.1) |
| Na, mmol/L | 136 (132–139) | 135 (132–141) |
| Urea, mg/dl | 14 (8–22) | 22 (11–25) |
| mmol/L | 5 (3–8) | 8 (4–9) |
| Creatinine, mg/dl | 0.83 (0.61–1.23) | 2.39 (1.52–3.19) |
| μmol/L | 73 (54–109) | 211 (134–282) |
| Bilirubin, mg/dl | 2.3 (1.6–5.9) | 5.8 (2.3–22.3) |
| μmol/L | 39 (28–101) | 100 (40–381) |
| Gamma glutamyltransferase, IU/L | 77 (43–145) | 70 (42–1589 |
| Alkaline phosphatase, IU/L | 120 (82–181) | 116 (80–142) |
| Aspartate aminotransferase, IU/L | 62 (44–100) | 65 (40–108) |
| Albumin, g/L | 29 (26–33) | 29 (24–33) |
| Platelets, x109 | 88 (62–127) | 70 (38–107) |
| Fibrinogen, g/L | 2.2 (1.4–2.9) | 1.8 (1.1–2.5) |
| INR | 1.4 (1.3–1.8) | 1.7 (1.4–2.6) |
| APTT, seconds | 36 (30–43) | 41 (33–56) |
APTT, activated partial thromboplastin time; CLIF, Chronic Liver Failure Consortium; INR, international normalized ratio; MELD, model of end-stage liver disease; NASH, non-alcoholic steatohepatitis; SOFA, sequential organ failure assessment.
DNA levels in systemic circulation in patients with AD cirrhosis and ACLF
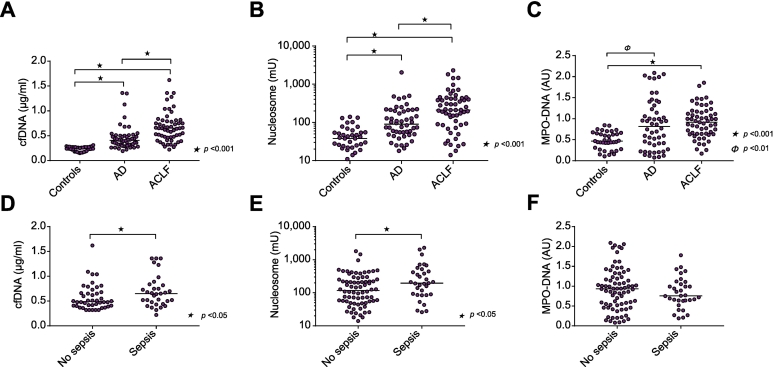
We quantified plasma levels of cfDNA, nucleosomes, and MPO-DNA complexes in samples from patients with AD cirrhosis and ACLF (Fig. 1). Plasma levels of cfDNA, nucleosomes, and MPO-DNA complexes were significantly higher in the AD and ACLF cohorts, compared to levels in healthy controls. Additionally, levels of cfDNA and nucleosomes were significantly higher in patients with ACLF compared to levels in patients with AD cirrhosis. MPO-DNA levels were not significantly different between the AD and ACLF cohorts. cfDNA and nucleosome levels were higher in patients with sepsis compared to patients without sepsis, whereas MPO-DNA levels were similar in patients with and without sepsis. Levels of cfDNA and nucleosomes remained significantly higher in patients with ACLF compared to those with AD when analyses were repeated without the patients with sepsis (median cfDNA 0.37 μg/ml [0.30–0.49] in AD vs. 0.58 [0.40–0.78] in ACLF p ≪0.001; nucleosomes 96 mU [52–206] in AD vs. 211 [49–400] in ACLF p = 0.044). In contrast, MPO-DNA levels were similar between AD and ACLF when patients with sepsis were excluded from the analysis (0.86 AU [0.41–1.35] in AD vs. 0.99 [0.62–1.19] in ACLF). In a subset of 32 patients with ACLF, we took a second sample 72 h after inclusion in the study. We observed a small, although statistically significant, change in cfDNA levels over the 72 h (0.67 μg/ml [0.55–0.82] at baseline vs. 0.58 [0.46–0.84] at 72 h, p ≪0.01), consistent with little change in other laboratory parameters over this short period of time (C-reactive protein 2.8 mg/L (1.4–6.3) at baseline vs. 3 (1.4–6.0) at 72 h, not significant; lactate 18 mmol/L (13–27) at baseline vs. 15 (13–24) at 72 h, p = 0.03, leukocyte count 5.2 x 109/L (2.6–9.6) at baseline vs. 5.1 (3.4–9.3) at 72 h, not significant).
Fig. 1.
Elevated levels of circulating DNA in acutely ill patients with cirrhosis. Plasma levels of (A) cfDNA, (B) nucleosomes, and (C) complexes of MPO and DNA (MPO-DNA), were determined in patients with AD, ACLF, and healthy controls. Plasma levels of (D) cfDNA, (E) nucleosomes, and (F) complexes of MPO and DNA (MPO-DNA), were determined in patients with and without sepsis. Horizontal lines indicate medians. A, B, C: Kruskal-Wallis H test with Dunn’s post hoc test; D, E, F: Mann-Whitney U test. AD, acute decompensation; ACLF, acute-on-chronic liver failure; cfDNA, cell-free DNA; MPO, myeloperoxidase.
Activation of coagulation in patients with AD and ACLF
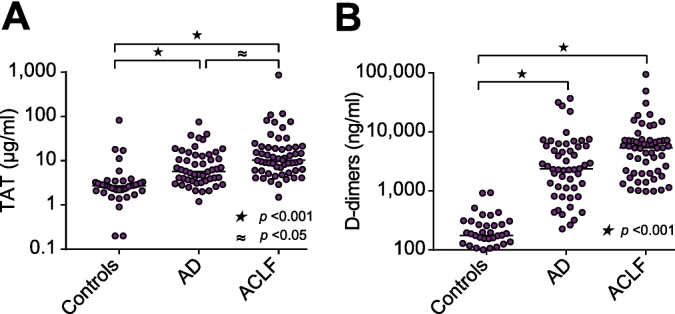
Activation of coagulation was assessed by plasma levels of TAT complexes and D-dimers (Fig. 2). Substantially and significantly increased levels of TAT complexes and D-dimers were observed in patients with AD and ACLF, compared to healthy controls. Furthermore, TAT levels were significantly higher in patients with ACLF compared to patients with AD.
Fig. 2.
Activation of coagulation in acutely ill patients with cirrhosis. (A) Plasma levels of TAT and (B) D-dimers, were determined in patients with AD, ACLF, and healthy controls. Horizontal lines indicate medians. Comparisons by Kruskal-Wallis H test with Dunn’s post hoc test. AD, acute decompensation; ACLF, acute-on-chronic liver failure; TAT, thrombin-antithrombin complexes.
Systemic inflammation and oxidative stress in patients with AD and ACLF
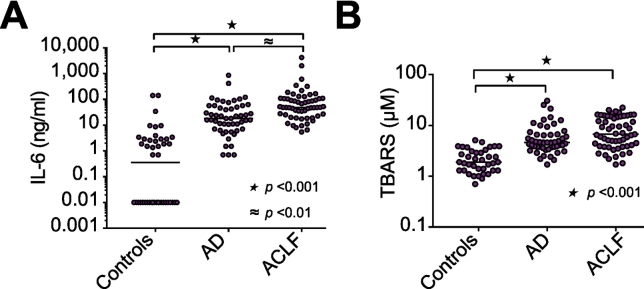
Systemic inflammation and oxidative stress were assessed by plasma levels of IL-6 and TBARS, respectively (Fig. 3). Both IL-6 and plasma TBARS were higher in patients compared to controls. In addition, IL-6 was higher in patients with ACLF compared to patients with AD, and within the ACLF cohort, levels of TBARS were higher in patients with ACLF grades 2 and 3 compared to patients with grade 1 ACLF (13.0 μM (8.1–15. 8) vs. 3.9 μM (2.8–5.1), p ≪0.001).
Fig. 3.
Inflammation and oxidative stress in acutely ill patients with cirrhosis. Plasma levels of (A) IL-6, and (B) TBARS, were determined in patients with AD, ACLF, and healthy controls. Horizontal lines indicate medians. Comparisons by Kruskal-Wallis H test with Dunn’s post hoc test. AD, acute decompensation; ACLF, acute-on-chronic liver failure; IL-6, interleukin 6; TBARS, thiobarbituric acid-reactive substances.
Lack of association between activation of coagulation and circulating DNA, inflammation, and oxidative stress
Given the established roles of circulating DNA, inflammation, and oxidative stress in activation of coagulation, we assessed linear correlations between TAT complexes and D-dimer levels with levels of circulating DNA, IL-6, and TBARS (Table 2). In all patients combined, we did not find any linear relations between TAT or D-dimer levels and levels of circulating DNA, IL-6, or TBARS. Also, when these analyses were repeated in patients with AD and ACLF separately, no linear relations were detected (data not shown).
Table 2.
Correlation between markers for activation of coagulation and circulating DNA, MPO-DNA complexes, nucleosomes, IL-6, and TBARS.
| cfDNA | MPO-DNA | Nucleosomes | IL-6 | TBARS | |
|---|---|---|---|---|---|
| TAT | r2= 0.006 | r2= 0.003 | r2= 0.001 | r2 = 0.0003 | r2= 0.0002 |
| p = 0.401 | p = 0.482 | p = 0.663 | p = 0.857 | p = 0.865 | |
| D–dimer | r2= 0.003 | r2= 0.015 | r2= 0.047 | r2 = 0.002 | r2= 0.001 |
| p = 0.561 | p = 0.199 | p = 0.023 | p = 0.582 | p = 0.665 |
Linear correlation coefficients (Pearson’s) with corresponding p values between the indicated analytes are presented for all patients combined. cfDNA, cell-free DNA; IL-6, interleukin 6; MPO, myeloperoxidase; TAT, thrombin-antithrombin complexes; TBARS, thiobarbituric acid-reactive substances.
Circulating DNA, inflammation, and oxidative stress in relation to organ failure
We stratified patients according to overall clinical scores (Table 3). In patients with a SOFA score below the median, plasma levels of cfDNA, IL-6, TBARS, and D-dimer were significantly lower compared to patients with a SOFA score above the median. cfDNA, nucleosome, Il6, TBARS and D-dimer levels were significantly lower in patients with CLIF-AD scores below the median, whereas cfDNA and TBARS were lower in patients with CLIF-ACLF below the median. cfDNA, IL-6 levels, TBARS, and D-dimers were lower in patients with Child-Pugh scores below the median.
Table 3.
Levels of circulating DNA, MPO-DNA complexes, nucleosomes, IL-6, and TBARS, TAT, and D-dimers.
| SOFA (median value 6) | |||
| Low (≤6), n = 61 | High (>6), n = 48 | p | |
| cfDNA, μg/ml | 0.46 (0.35–0.55) | 0.72 (0.47–0.95) | ≪0.001 |
| MPO-DNA, AU | 0.81 (0.41–1.11) | 0.94 (0.63–1.30) | n.s. |
| Nucleosomes, mU | 125 (54– 252) | 193 (71– 382) | n.s. |
| IL-6, ng/ml | 22 (10– 54) | 50 (25– 109) | ≪0.001 |
| TBARS, μM | 4.5 (3.3– 6.5) | 8.9 (4.9–15.2) | ≪0.001 |
| TAT, μg/ml | 6.4 (4.0–15.5) | 9.5 (5.9– 18.7) | n.s. |
| D-dimers, ng/ml | 2,391 (1,085–5,501) | 6,301 (2,220–12,056) | ≪0.001 |
| CLIF-AD (median value 51) | |||
| Low (≤ 51), n = 25 | High (≫51), n = 27 | p | |
| cfDNA, μg/ml | 0.36 (0.29–0.42) | 0.48 (0.37–0.68) | ≪0.01 |
| MPO-DNA, AU | 0.69 (0.20–1.23) | 0.92 (0.42–1.31) | n.s. |
| Nucleosomes, mU | 53 (39–126) | 118 (71–255) | ≪0.01 |
| IL-6, ng/ml | 11 (4–16) | 39 (22–90) | ≪0.001 |
| TBARS, μM | 4.1 (3.3–4.9) | 5.5 (3.8– 11.2) | 0.02 |
| TAT, μg/ml | 5.0 (3.2–6.9) | 8.5 (3.9–15.4) | n.s. |
| D-dimers, ng/ml | 1,588 (650–2,317) | 4,751 (2,391–7,182) | ≪0.001 |
| CLIF-ACLF (median value 53) | |||
| Low (≤ 53), n = 29 | High (≫87), n = 28 | p | |
| cfDNA, μg/ml | 0.49 (0.37–0.63) | 0.75 (0.65–1.06) | ≪0.001 |
| MPO-DNA, AU | 0.94 (0.66–1.16) | 0.83 (0.58–1.15) | n.s. |
| Nucleosomes, mU | 216 (50–382) | 211 (154–485) | n.s. |
| IL-6, ng/ml | 48 (20– 100) | 77 (34–175) | n.s. |
| TBARS, μM | 4.7 (3.0– 7.0) | 8.8 (4.9–15.1) | ≪0.001 |
| TAT, μg/ml | 10.6 (6.5–19.3) | 10.1 (5.3–20.5 | n.s. |
| D-dimers, ng/ml | 5,327 (1,603–7,137) | 6,076 (2,853–8,363) | n.s. |
| Child-Pugh (median value 9) | |||
| Low (≤ 9), n = 55 | High (≫9), n = 54 | p | |
| cfDNA, μg/ml | 0.45 (0.35–0.56) | 0.67 (0.47–0.89) | ≪0.001 |
| MPO-DNA, AU | 0.86 (0.53–1.02) | 0.98 (0.59–1.39) | n.s. |
| Nucleosomes, mU | 102 (53–250) | 193 (74–377) | n.s. |
| IL-6, ng/ml | 22 (11– 62) | 47 (18– 92) | 0.04 |
| TBARS, μM | 3.9 (3.1– 4.8) | 10.4 (6.0– 15.4) | ≪0.001 |
| TAT, μg/ml | 7.0 (4.1–14.4) | 9.8 (5.0–17.2) | n.s. |
| D-dimers, ng/ml | 2,215 (1,013–4,751) | 6,268 (3,310–7,450) | ≪0.001 |
| Liver failure (bilirubin ≫205 μmol/L) | |||
| No, n = 80 | Yes, n = 29 | p | |
| cfDNA, μg/ml | 0.46 (0.35–0.56) | 0.86 (0.66–1.24) | ≪0.001 |
| MPO-DNA, AU | 0.88 (0.52–1.1) | 0.86 (0.64– 1.25) | n.s. |
| Nucleosomes, mU | 125 (53– 254) | 211 (89– 485) | 0.01 |
| IL-6, ng/ml | 25 (11– 74) | 48 (22–101) | n.s. |
| TBARS, μM | 4.5 (3.6– 6.4) | 15.3 (9.8– 16.8) | ≪0.001 |
| TAT, μg/ml | 8.9 (4.9–18.7) | 11.1 (4.2–15.3) | n.s. |
| D-dimers, ng/ml | 4,031 (1,412–7,122) | 4,427 (2,962–6,991) | n.s. |
| Renal failure (creatinine ≫176 μmol/L) | |||
| No, n = 65 | Yes, n = 44 | p | |
| cfDNA, μg/ml | 0.45 (0.35–0.54) | 0.81 (0.67–1.15) | ≪0.03 |
| MPO-DNA, AU | 0.74 (0.41–1.14) | 0.99 (0.73–1.18) | 0.03 |
| Nucleosomes, mU | 109 (57–221) | 211 (57–418) | n.s. |
| IL-6, ng/ml | 23 (10–68) | 48 (22– 101) | ≪0.01 |
| TBARS, μM | 4.9 (3.8–10.4) | 6.0 (3.8– 12.1) | n.s. |
| TAT, μg/ml | 6.5 (3.8–15.3) | 9.8 (5.5–19.7) | n.s. |
| D-dimers, ng/ml | 3,219 (1,351–6,887) | 5,304 (1,699–7,107) | 0.03 |
| Coagulation failure (INR ≫2.5) | |||
| No, n = 90 | Yes, n = 19 | p | |
| cfDNA, μg/ml | 0.47 (0.35 0.62) | 0.81 (0.66–1.13) | ≪0.001 |
| MPO-DNA, AU | 0.86 (0.52–1.18) | 0.64 (0.99 1.18) | n.s. |
| Nucleosomes, mU | 127 (54–300) | 191 (93–352) | n.s. |
| IL-6, ng/ml | 28.6 (12.8–84.2) | 48.4 (14.2– 109.5) | n.s. |
| TBARS, μM | 4.6 (3.6–7.9) | 11.7 (6.9– 16.1) | ≪0.001 |
| TAT, μg/ml | 8.4 (4.1–18.0) | 9.6 (6.0–12.7) | n.s. |
| D-dimers, ng/ml | 2,923 (1,334–6,995) | 6,076 (3,859–7,122) | 0.04 |
| Hemodynamic failure (MAP 80 mmHg with vasoactive support) | |||
| No, n = 88 | Yes, n = 21 | p | |
| cfDNA, μg/ml | 0.48 (0.36–0.65) | 0.80 (0.65–1.07) | ≪0.001 |
| MPO-DNA, AU | 0.90 (0.52–1.21) | 0.83 (0.62–1.06) | n.s. |
| Nucleosomes, mU | 121 (55–289) | 204 (130– 414) | n.s. |
| IL-6, ng/ml | 25.3 (11.1–66.6) | 81.3 (41.7–111.1) | ≪0.01 |
| TBARS, μM | 4.9 (3.8–9.2) | 9.5 (6.0–16.1) | 0.02 |
| TAT, μg/ml | 7.6 (4.1–15.2) | 10.3 (6.0–20.6) | n.s. |
| D-dimers, ng/ml | 3,028 (1,326–6,594) | 6,076 (3,172–1,1761) | 0.01 |
| Respiratory failure (PaO2/FiO2≪200) | |||
| No, n = 98 | Yes, n = 11 | p | |
| cfDNA, μg/ml | 0.48 (0.36–0. 68) | 1.02 (0.80–1.40) | ≪0.001 |
| MPO-DNA, AU | 0.90 (0.52–1.19) | 0.68 (0.63–0.99) | n.s. |
| Nucleosomes, mU | 133 (55–297) | 196 (90–728) | n.s. |
| IL-6, ng/ml | 27.2 (11.4–69.6) | 109.5 (77.1–603.9) | ≪0.001 |
| TBARS, μM | 4.9 (3.7–9.5) | 15.1 (6.9–18.9) | ≪0.01 |
| TAT, μg/ml | 8.2 (4.1–15.4) | 10.1 (8.7–19.9) | n.s. |
| D-dimers, ng/ml | 3,380 (1,397–6,995) | 6,293 (4,427–9,505) | 0.03 |
| Neurologic failure (encephalopathy moderate-severe) | |||
| No, n = 76 | Yes, n = 33 | p | |
| cfDNA, μg/ml | 0.47 (0.35–0.64) | 0.72 (0.49–0.99) | ≪0.001 |
| MPO-DNA, AU | 0.94 (0.58–1.32) | 0.68 (0.43–1.00) | n.s. |
| Nucleosomes, mU | 121 (57–220) | 255 (70–506) | 0.02 |
| IL-6, ng/ml | 26.6 (12.0–76.5) | 47.4 (18. 9– 109.5) | n.s. |
| TBARS, μM | 4.6 (3.5–7.3) | 9.3 (5.3–15.5) | ≪0.001 |
| TAT, μg/ml | 6.4 (4.0–14.3) | 10.3 (7.1–18.3) | ≪0.01 |
| D-dimers, ng/ml | 2,624 (1,345–6,169) | 6,207 (3,700–8,518) | ≪0.01 |
Stratified by low and high SOFA, CLIF-AD, CLIF-ACLF, and Child-Pugh scores and by the presence / absence of liver, renal, coagulation, hemodynamic, respiratory and neurological failure. Median and interquartile ranges are shown with corresponding p values. Comparisons by Kruskal-Wallis H test with Dunn’s post hoc test.
AD, acute decompensation; ACLF, acute-on-chronic liver failure; cfDNA, cell-free DNA; CLIF OF, Chronic Liver Failure Consortium organ failure score; IL-6, interleukin 6; MPO, myeloperoxidase; MAP, mean arterial pressure; SOFA, sequential organ failure assessment; TAT, thrombin-antithrombin complexes; TBARS, thiobarbituric acid-reactive substances. Comparisons by Mann Whitney U test.
We subsequently assessed plasma levels of these analytes per organ failure. Levels of cfDNA, nucleosomes, Il6, and TBARS were higher in patients that had failure of any organ system (although not all comparisons reached statistical significance), whereas D-dimer levels were higher with any organ failure except liver failure. In contrast, MPO-DNA levels were only clearly increased in patients with renal failure.
Association of cfDNA, plasma levels of IL-6 and TBARS with short-term mortality
Seventeen (15%) patients (15 ACLF and 2 AD) died within 30 days after hospital admission. Baseline levels of cfDNA, nucleosome, D-dimer, IL-6 and TBARS were higher in patients that died within 30 days, compared to 30-day survivors (Table 4), although the difference did not reach statistical significance for nucleosome levels (p = 0.177). Baseline levels of other analytes were not different between survivors and non-survivors.
Table 4.
Plasma level of markers for activation of coagulation, oxidative stress and DNA in patients that survived beyond 30 days of admission and those who did not.
| Survived (n = 89) | Died (n = 17) | p | |
|---|---|---|---|
| cfDNA, μg/ml | 0.47 (0.36–0.65) | 0.82 (0.70–1.31) | ≪0.001 |
| MPO-DNA complex, AU | 0.86 (0.49–1.14) | 0.99 (0.67– 1.28) | n.s. |
| Nucleosome, mU | 125 (51–302) | 204 (132–403) | n.s. |
| TAT, ug/ml | 8.3 (4.1–16.3) | 9.6 (7.1–20.6.) | n.s. |
| D-dimers, ng/ml | 2,962 (151–6,303) | 6,310 (4,420–8,363) | 0.01 |
| IL-6, ng/ml | 25.6 (11.6–74.7) | 77.1 (41.7–137.0) | 0.01 |
| TBARS. μM | 4.6 (3.7–8.1) | 14.6 (6.8–16.2) | ≪0.001 |
Shown are medians and interquartile ranges. Comparisons by Mann Whitney U test.
cfDNA, cell-free DNA; IL-6, interleukin 6; MPO, myeloperoxidase; TAT, thrombin-antithrombin complexes; TBARS, thiobarbituric acid-reactive substances.
Discussion
In this study, we found elevated plasma levels of circulating DNA, and elevated plasma levels of markers for activation of coagulation, systemic inflammation and oxidative stress in acutely ill patients with cirrhosis. All analytes were higher in patients with ACLF than in patients with AD with the notable exception of levels of MPO-DNA complexes, which is an established marker for NETs. We found no evidence for a role of circulating DNA, inflammatory stress, or oxidative stress in activation of coagulation in these patients. cfDNA, Il6, and TBARS much better discriminated between patients with organ failure than MPO-DNA levels. Finally, we found levels of circulating DNA, and markers for inflammatory and oxidative stress to be substantially higher in those patients with poor outcome.
Experimental studies have established that NETs are important contributors to micro- and macrovascular thrombus formation as infusion of DNAse,16 which dismantles NETs, or a genetic defect in NET formation, by PAD4 deficiency for example,24 reduces thrombus formation. In addition, NETs have been detected in human thrombi,[17], [25] and plasma levels of circulating DNA are elevated in patients with thrombotic disease.26 However, whether NETs, components of NETs, or free circulating DNA indeed contribute to activation of hemostasis in humans or whether elevated levels of these components are simply a consequence of the thrombotic event has not been established. Importantly, despite convincing evidence for a role of NETs in thrombotic events in experimental models, it remains controversial whether NETs27 or cfDNA28 can activate coagulation to a relevant extent in the human setting.
A previous study from our group showed that plasma levels of cfDNA, but not of MPO-DNA as a marker for NET formation, were associated with activation of coagulation in patients undergoing liver transplantation.19 As we demonstrated NET formation within the liver, we hypothesized that NETs might contribute to activation of coagulation locally. Our present data extended these studies with the notion that a systemic inflammatory response with an increased neutrophil count is central in the ACLF syndrome.[29], [30] However, although we confirm an elevated inflammatory response in patients with ACLF, as evidenced by increased IL-6 levels, we did not detect increased NETosis, as MPO-DNA levels were similar between patients with AD and ACLF. It may be that the concept of immuneparalysis[31], [32] (i.e., leukocyte dysfunction in cirrhosis) results in declining capacity to form NETs with increasing disease severity, and interestingly the patients that became septic had numerically lower levels of MPO-DNA complexes than patients without sepsis. Alternatively, NETosis might occur locally, without massive release of MPO-DNA complexes into systemic circulation, and the assessment of local NETosis with potential local thrombotic consequences, although of definitive interest in these patients, is complex as it would require tissue biopsies at the time of severe disease.
Our present data suggest substantial activation of coagulation in acutely ill patients with cirrhosis, with an exaggerated response in ACLF compared to AD. Although these results are in line with our previous finding of enhanced thrombin generating capacity in patients with AD and ACLF,512 it is important to stress that elevated levels of D-dimer and TAT may indeed reflect enhanced coagulation activation, but may also, in part, be explained by a decreased capacity of the diseased liver to clear these markers.33 We did not find evidence for a role of circulating DNA in activation of coagulation in these patients, and future studies should assess whether the enhanced activation of coagulation is real (and not an artifact related to defective clearance), and if so, what the trigger of coagulation activation in these patients might be. Animal experiments have suggested a key role for activation of tissue factor on ‘stressed’ hepatic cells in activation of coagulation.34 If intrahepatic activation of coagulation would indeed drive elevated D-dimer and TAT levels in AD and ACLF, one expects a relation between the extent of intrahepatic fibrin deposition and levels of D-dimer and TAT, but again such analyses would require tissue biopsies. However, we found that D-dimers were clearly increased in patients with organ failure, with the notable exception of liver failure. Indeed D-dimers were not different between patients with high or low AD- or ACLF-CLIF scores. These findings argue against substantial intrahepatic activation of coagulation but rather suggest that elevated D-dimer levels are driven by extrahepatic activation of coagulation, which could be related to extrahepatic organ injury. We do not have a clear explanation for why the injured liver would not, but all other failing organs would contribute to coagulation activation. Also, the fact that D-dimer, but not TAT levels are related to organ injury and outcome suggest that the balance between activation of coagulation and activation of fibrinolysis, rather than activation of coagulation alone, is key in determining intra-organ fibrin accumulation.
ACLF is characterized by high short-term mortality. These patients have a narrow window for liver transplantation and the supportive care may be futile and expensive.35 Distinguishing between ACLF and traditional AD also requires biomarkers that we currently lack, which means that the management strategy is similar for patients with ACLF and those with traditional AD. Differences in the intensity of systemic inflammation and organ dysfunction between AD and ACLF may be exploited for this purpose. Studies in critically ill patients without underlying liver disease have demonstrated that severe systemic inflammation may lead to organ dysfunction and failure through direct cellular injury, leading to the release of nuclear or mitochondrial DNA that drive organ failure.36 Therefore, levels of circulating cfDNA may be helpful predictors of outcome. Indeed, plasma levels of cfDNA strongly predict intensive care unit mortality in patients with severe sepsis.[37], [38] In line with these findings, in our cohort, plasma levels of cfDNA were substantially higher in patients who died within 30 days of admission. In addition, levels of IL-6 and TBARS were substantially higher in patients that died within 30 days. Collectively, these results suggest that inflammation and oxidative stress with accompanying cellular death and release of cfDNA, rather than neutrophil activation with NETosis and NETosis-associated activation of coagulation and intra-organ microthrombosis, drives development and/or progression of ACLF. As plasma levels of cfDNA were higher in patients with all types of organ failure (Table 3), it is likely that the cfDNA is not only liver-derived, but is a result of cell death within all affected organs. This conclusion is different from that of a previous study in which the cell death markers caspase-cleaved keratin 18 and keratin 18 were quantified in plasma from patients with AD and ACLF.39 This study concluded that these cell death markers were primarily of hepatic origin in patients with AD and ACLF. Whether release of cfDNA is distinct from that of cleaved keratin 18 and keratin 18, or whether the different conclusions between the studies are explained by patient characteristics would require additional study.
ACLF is a very heterogenous disease entity. Analyzing this heterogeneous group as a whole is a limitation of our study, which was too small to allow for meaningful subgroup analyses. Future larger studies should separately analyze patients based on underlying etiology and the severity of ACLF. Also, future studies should take the precipitating event for decompensation into account. Notably infection as a precipitating event must be studied separately, given the role of NETs in infection and the inflammatory component of ACLF.
The finding that NETosis does not appear to be an important driver of outcome and that NETosis was not increased in patients with AD/ACLF that developed sepsis, compared to those that did not develop sepsis, challenges some of the recently outlined concepts regarding the role of NETs in liver diseases,21 and the role of NETs in driving organ failure in sepsis.40 Importantly, many of these concepts were developed primarily using data from experimental animal studies, which reinforces the need to validate such concepts in human studies.
In the present study, blood samples were taken at a single time point. Given the dynamic clinical course of acutely ill patients with cirrhosis, and the fact that the clinical course of these patients is a more accurate predictor of short-term outcomes than the initial disease severity, this is a limitation of our study. The analysis of serial samples would add valuable information about the outcome of this patient population.
In conclusion, we found levels of circulating DNA, and markers for inflammatory and oxidative stress to be elevated in patients with AD and ACLF with a clear relation to development of organ failure and short-term mortality. In addition, D-dimer levels were related to organ failure and mortality, but not with severity of liver disease. We have provided evidence that NETosis, inflammation, and oxidative stress are not involved in activation of coagulation in patients with AD and ACLF, which argues against a role of NETosis in the progression of multiorgan failure in acutely ill patients with cirrhosis.
Financial support
This study was supported by departmental funds from TL.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Conception and design: AB, VCP, JF, WB, TL; Data acquisition: AB, VCP, JA, SA, FA; Data interpretation: AB, VCP, JF, WB, TL; Manuscript drafting: AB, TL; Revision of manuscript: all; statistical analysis: AB, TL; obtained funding: TL; technical support: JA, SA, FA; study supervision: JF, WB, TL
Acknowledgements
AB acknowledges Dr Graciela Martinez-Pallí and colleagues of the digestive disease anesthesia section Hospital Clinic Barcelona for taking over clinical duties during the period this research was performed in the UMCG in The Netherlands.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.06.002.
Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Mahmud N, Kaplan DE, Taddei TH, Goldberg DS. Incidence and mortality of acute on chronic liver failure using two definitions in patients with compensated cirrhosis. Hepatology. 2019;69:2150–2163. doi: 10.1002/hep.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernaez R, Kramer JR, Liu Y, Tansel A, Natarajan Y, Hussain KB. Prevalence and short-term mortality of acute-on-chronic liver failure: A national cohort study from the USA. J Hepatol. 2019;70:639–647. doi: 10.1016/j.jhep.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Hernaez R, Sola E, Moreau R, Gines P. Acute-on-chronic liver failure: An update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher C, Patel VC, Stoy SH, Singanayagam A, Adelmeijer J, Wendon J. Balanced haemostasis with both hypo- and hyper-coagulable features in critically ill patients with acute-on-chronic-liver failure. J Crit Care. 2017;43:54–60. doi: 10.1016/j.jcrc.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 6.Blasi A, Calvo A, Prado V, Reverter E, Reverter JC, Hernandez-Tejero M. Coagulation failure in patients with acute-on-chronic liver failure and decompensated cirrhosis: Beyond the international normalized ratio. Hepatology. 2018;68:2325–2337. doi: 10.1002/hep.30103. [DOI] [PubMed] [Google Scholar]
- 7.Lisman T, Bernal W. Management of hemostatic disorders in patients with advanced liver disease admitted to an intensive care unit. Transfus Med Rev. 2017;31:245–251. doi: 10.1016/j.tmrv.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Levi M, van der Poll T, Schultz M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin Immunopathol. 2012;34:167–179. doi: 10.1007/s00281-011-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 11.van der Poll T, de Boer JD, Levi M. The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis. 2011;24:273–278. doi: 10.1097/QCO.0b013e328344c078. [DOI] [PubMed] [Google Scholar]
- 12.Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2017;371:567–576. doi: 10.1007/s00441-017-2727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 15.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD., Jr. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, Ten Berg JM. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter european study: A report of the prevention of late stent thrombosis by an interdisciplinary global european effort consortium. Eur Heart J. 2016;37:1538–1549. doi: 10.1093/eurheartj/ehv419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Meijenfeldt FA, Burlage LC, Bos S, Adelmeijer J, Porte RJ, Lisman T. Elevated plasma levels of cell-free DNA during liver transplantation are associated with activation of coagulation. Liver Transpl. 2018;24:1716–1725. doi: 10.1002/lt.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien XM, Biron BM, Reichner JS. Consequences of extracellular trap formation in sepsis. Curr Opin Hematol. 2017;24:66–71. doi: 10.1097/MOH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15:206–221. doi: 10.1038/nrgastro.2017.183. [DOI] [PubMed] [Google Scholar]
- 22.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karimian G, Kirschbaum M, Veldhuis ZJ, Bomfati F, Porte RJ, Lisman T. Vitamin E attenuates the progression of non-alcoholic fatty liver disease caused by partial hepatectomy in mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laridan E, Denorme F, Desender L, Francois O, Andersson T, Deckmyn H. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Alcazar M, Kim N, Fuchs TA. Circulating extracellular DNA: Cause or consequence of thrombosis? Semin Thromb Hemost. 2017;43:553–561. doi: 10.1055/s-0036-1597284. [DOI] [PubMed] [Google Scholar]
- 27.Noubouossie DF, Whelihan MF, Yu YB, Sparkenbaugh E, Pawlinski R, Monroe DM. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SA, Baker CJ, Gajsiewicz JM, Morrissey JH. Silica particles contribute to the procoagulant activity of DNA and polyphosphate isolated using commercial kits. Blood. 2017;130:88–91. doi: 10.1182/blood-2017-03-772848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claria J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–1264. doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 30.Agiasotelli D, Alexopoulou A, Vasilieva L, Kalpakou G, Papadaki S, Dourakis SP. Evaluation of neutrophil/leukocyte ratio and organ failure score as predictors of reversibility and survival following an acute-on-chronic liver failure event. Hepatol Res. 2016;46:514–520. doi: 10.1111/hepr.12582. [DOI] [PubMed] [Google Scholar]
- 31.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, Vidacek D, Siewert E. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Stoy S, Patel VC, Sturgeon JP, Manakkat Vijay GK, Lisman T, Bernal W. Platelet-leucocyte aggregation is augmented in cirrhosis and further increased by platelet transfusion. Aliment Pharmacol Ther. 2018;47:1375–1386. doi: 10.1111/apt.14600. [DOI] [PubMed] [Google Scholar]
- 33.Hughes RD, Lane DA, Ireland H, Langley PG, Gimson AE, Williams R. Fibrinogen derivatives and platelet activation products in acute and chronic liver disease. Clin Sci (Lond) 1985;68:701–707. doi: 10.1042/cs0680701. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan BP, Kopec AK, Joshi N, Cline H, Brown JA, Bishop SC. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood. 2013;121:1868–1874. doi: 10.1182/blood-2012-09-455436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McPhail MJ, Auzinger G, Bernal W, Wendon JA. Decisions on futility in patients with cirrhosis and organ failure. Hepatology. 2016;64:986. doi: 10.1002/hep.28539. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: Derivation and validation. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001577. discussion e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dwivedi DJ, Toltl LJ, Swystun LL, Pogue J, Liaw KL, Weitz JI. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care. 2012;16:R151. doi: 10.1186/cc11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macdonald S, Andreola F, Bachtiger P, Amoros A, Pavesi M, Mookerjee R. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology. 2018;67:989–1002. doi: 10.1002/hep.29581. [DOI] [PubMed] [Google Scholar]
- 40.Czaikoski PG, Mota JM, Nascimento DC, Sonego F, Castanheira FV, Melo PH. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2