Background & Aims
In 2016, the World Health Assembly passed a resolution to eliminate viral hepatitis as a public health threat by 2030. We aimed to examine the status of the global viral hepatitis response.
Methods
In 2017, the World Health Organization (WHO) asked the Ministries of Health in all 194 Member States to complete a Country Profile on Viral Hepatitis policy uptake indicators, covering national plans/funding, engagement of civil society, testing guidance, access to treatment, and strategic information.
Results
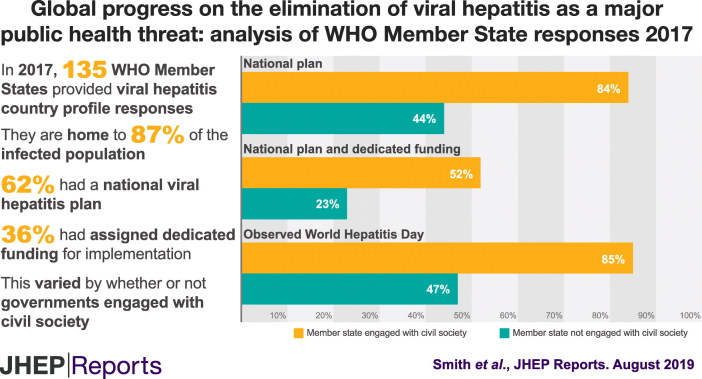
Of 194 Member States, 135 (70%) responded, accounting for 87% of the global population infected with hepatitis B virus (HBV) and/or C virus (HCV). Of those responding, 84 (62%) had developed a national plan, of which, 49 (58%) had dedicated funding, and 62 (46%) had engaged with civil society; those engaged with civil society were more likely to have a funded plan than others (52% vs. 23%, p = 0.001). Guidance on testing pregnant women (for HBV) and people who inject drugs (for HCV) was available in 70% and 46% of Member States, respectively; 59% and 38% of Member States reported universal access to optimal therapies for HBV and HCV, respectively.
Conclusions
Most people living with hepatitis B and C reside in a country with a national hepatitis strategy. Governments who engaged with civil society were more advanced in their response. Member States need to finance these national strategies and ensure that those affected have access to hepatitis services as part of efforts to achieve universal health coverage.
Lay summary
The World Health Organization’s goal to eliminate viral hepatitis as a public health threat by 2030 requires global action. Our results indicate that progress is being made by countries to scale-up national planning efforts; however, our results also highlight important gaps in current policies.
Key words: Public health, global health, viral hepatitis, universal health coverage, HBV, HCV, direct-acting antivirals
Graphical abstract
Highlights
-
•
Countries are making progress across all WHO regions in responding to viral hepatitis
-
•
Governments engaged with Civil Society are more advanced in their national planning efforts
-
•
Financing remains an issue with a minority of countries with a national plan having some dedicated funding
-
•
Stronger surveillance and monitoring systems are needed to direct hepatitis elimination plans
Introduction
The World Health Organization (WHO) estimates that, in 2015, viral hepatitis affected around 325 million people worldwide, with an estimated 257 million cases of chronic hepatitis B virus (HBV) and 71 million of chronic hepatitis C virus (HCV).[1], [2], [3] The WHO also estimated that, in 2015, viral hepatitis (principally HBV and HCV) caused 1.34 million deaths, mostly from cirrhosis and hepatocellular carcinoma.1 This death toll is comparable to that of tuberculosis (1.37 million deaths), and surpasses that of human immunodeficiency virus (HIV, 1.06 million deaths) and malaria (0.44 million deaths). While deaths related to HIV, malaria, and tuberculosis have all declined over the past decade or more, mortality related to viral hepatitis continues to rise (22% increase since 2000).1
In May 2016, the 69th World Health Assembly unanimously endorsed the first Global Health Sector Strategy (GHSS) on viral hepatitis.4 The GHSS sets a goal to eliminate viral hepatitis as a major public health threat, defined as a reduction in i) hepatitis-related deaths by 65% and ii) new chronic HBV and HCV infections by 90%, by 2030. Modelling work highlights that major scale-up of interventions to prevent transmission, as well as testing and treatment to prevent the consequences of infection, are needed to come close to reaching these ambitious targets and eliminating viral hepatitis.[4], [5], [6] Treatment is highly effective against HBV and curative for HCV. However, a comprehensive public health response is needed to improve access to these interventions if these targets are to be achieved.[6], [7], [8]
The GHSS lists priority actions for countries to progress their response to viral hepatitis, including assessment of their epidemiological situation, the development of a national plan with dedicated funding and the setting of targets on intervention coverage and impact.4 The WHO published a monitoring and evaluation framework, including 10 core indicators that focus on programmatic outcomes, such as coverage of prevention, diagnosis and treatment.9 To monitor progress on these outcomes, countries will need to invest in the establishment of information systems to deliver on these data.10 Meanwhile, we aimed to describe the early status of WHO Member States in their viral hepatitis response, focusing on priority actions, plans and processes in place to deliver on the GHSS. Reporting on interim progress (e.g., to the World Health Assembly) can be an important lever for countries to move towards elimination.
Materials and methods
Data collection
The WHO contacted Ministries of Health in all 194 Member States through regional and country offices, requesting them to complete an online Country Profile on Viral Hepatitis. The WHO developed the country profile tool in consultation with the World Hepatitis Alliance and piloted it with focal points from 3 countries (Morocco, Moldova and Tajikistan). Key items covered the establishment of national processes and plans on viral hepatitis (specifically a focal point, a strategic and technical advisory group [STAG], a published or drafted national plan, and dedicated funding), engagement with civil society groups (either as a member of the STAG or through other consultations), development of national testing guidance, access to therapies, and availability of official working estimates on impact and intervention coverage targets. We translated the data collection instrument into the 6 official United Nations languages. We uploaded the tool in “EU Survey”,11 a secure online survey management system. Data collection began in December 2016 and concluded in November 2017. During this period, the WHO regional and country offices sent reminders electronically. WHO staff also encouraged and supported participation at relevant viral hepatitis country and regional meetings.
Data analysis
We tabulated responses according to WHO region (African Region, Region of the Americas, South-East Asia Region, European Region, Eastern Mediterranean Region, and Western Pacific Region) and World Bank income classification (high-income, upper middle-income, lower middle-income, and low-income). We compared Member States who reported engagement with civil society groups with those who had not, in terms of the status of their national plan and dedicated funding using the Pearson Chi-square test with Yates’ continuity correction for categorical variables. We compared Member States with and without official working estimates on viral hepatitis mortality in terms of their report of having set numerical targets for reducing morbidity and mortality. We compared Member States with and without official working estimates on treatment coverage in terms of numerical targets on testing and treatment activities. We estimated the proportion of the global prevalence of HBV and HCV infection relating to WHO Member States’ responses to the country profiles using published country level estimates of the number of people chronically infected with HBV (257 million) and HCV (71 million) in 2015.[1], [2], [3] We also compared WHO Member States’ official HCV testing guidance for people who inject drugs (PWID) by regional groupings from a recent systematic review on PWID prevalence (East and Southeast Asia, Eastern Europe, North America, Latin American, Sub-Saharan Africa, South Asia, Western Europe, Middle East and North Africa, Central Asia, Australasia, Caribbean, Pacific Island States and Territories) according to the estimated number of PWID in each region.12 We analysed data using Microsoft Excel 2010 and STATA 13.0.
For further details regarding the materials used, please refer to the supplementary CTAT table.
Results
Response
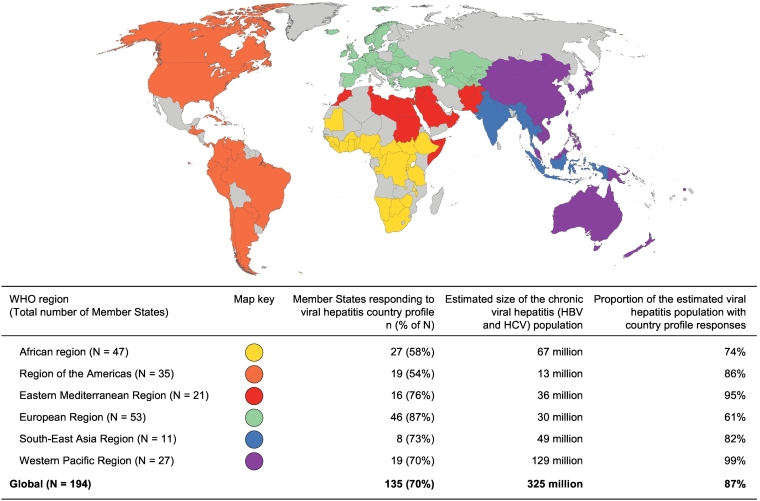
Between December 2016 and November 2017, 135 Member States (70%) completed their WHO viral hepatitis country profiles, accounting for 87% of those infected with HBV or HCV globally (Fig. 1). Responses ranged from 54% in the Region of the Americas to 87% in the European Region. The proportion of the regional burden of viral hepatitis accounted for by participating Member States exceeded the regional response proportion in all WHO regions, except in Europe (87% of response, relating to only 61% of the regional burden, Fig. 1). There was a relatively even distribution of responses (range 65–79%) according to income category (Table 1).
Fig. 1.
WHO Member States providing viral hepatitis country profile responses, 2016-2017. (n = 135, 70% of all WHO Member States and 87% of the 325 million persons infected with viral hepatitis globally). 1Univariate comparisons using the Pearson Chi-square test with Yates’ continuity correction for categorial variables. WHO, World Health Organization.
Table 1.
Viral hepatitis national planning indicators reported by WHO Member States, by WHO region, income, and estimated number of people living with viral hepatitis.
| All MS N1 (%) |
MS responding to viral hepatitis country profiles N2 (% of N1) |
MS with viral hepatitis focal point N3 (% of N2) |
MS with STAG1 established N4 (% of N2) |
MS engaged with CS N5 (% of N2) |
MS with national plan developed N6 (% of N2) |
MS with dedicated funding for plan N7 (% of N2) |
MS with national impact targets set3 N8 (% of N2) |
MS with national service coverage targets4 set N9 (% of N2) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | Yes | Yes2 | CS is member of STAG | Yes5 | Published Plan | Yes | Yes | Yes | |||
| Total number | 194 (100%) | 135 (70%) | 102 (75%) | 71 (52%) | 62 (46%) | 40 (30%) | 84 (62%) | 48 (36%) | 49 (36%) | 36 (27%) | 65 (48%) |
| By WHO region: | |||||||||||
| Eastern Mediterranean | 21 (11%) | 16 (76%) | 14 (88%) | 12 (75%) | 9 (56%) | 4 (25%) | 14 (88%) | 6 (38%) | 7 (44%) | 6 (38%) | 10 (63%) |
| Western Pacific | 27 (14%) | 19 (70%) | 17 (90%) | 12 (63%) | 9 (47%) | 7 (37%) | 12 (63%) | 7 (37%) | 7 (37%) | 6 (32%) | 10 (53%) |
| Americas | 35 (18%) | 19 (54%) | 13 (68%) | 10 (53%) | 12 (63%) | 4 (21%) | 10 (53%) | 5 (26%) | 7 (37%) | 6 (32%) | 8 (42%) |
| South-East Asia | 11 (6%) | 8 (73%) | 6 (75%) | 4 (50%) | 5 (63%) | 1 (13%) | 4 (50%) | 4 (50%) | 3 (38%) | 2 (25%) | 5 (63%) |
| European | 53 (27%) | 46 (87%) | 34 (74%) | 23 (50%) | 24 (52%) | 14 (30%) | 30 (65%) | 21 (46%) | 19 (41%) | 12 (26%) | 24 (52%) |
| African | 47 (24%) | 27 (58%) | 18 (67%) | 10 (37%) | 13 (48%) | 10 (37%) | 14 (52%) | 5 (19%) | 6 (22%) | 4 (15%) | 8 (30%) |
| By income classification: | |||||||||||
| High | 52 (27%) | 41 (79%) | 33 (81%) | 23 (56%) | 22 (54%) | 14 (34%) | 29 (71%) | 19 (46%) | 17 (42%) | 15 (37%) | 24 (58%) |
| Upper-middle | 49 (25%) | 32 (65%) | 25 (78%) | 23 (72%) | 12 (38%) | 9 (28%) | 21 (66%) | 11 (34%) | 10 (31%) | 8 (25%) | 16 (50%) |
| Lower-middle | 50 (26%) | 35 (70%) | 30 (86%) | 16 (46%) | 18 (51%) | 9 (26%) | 22 (63%) | 15 (43%) | 15 (43%) | 10 (29%) | 17 (49%) |
| Low | 34 (18%) | 24 (71%) | 11 (46%) | 7 (29%) | 10 (42%) | 8 (33%) | 11 (46%) | 3 (13%) | 6 (25%) | 2 (8%) | 6 (25%) |
| n.a. | 9 (5%) | 3 (33%) | 3 (100%) | 2 (67%) | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 1 (33%) | 1 (33%) | 2 (67%) |
| Estimated number of people living with chronic viral hepatitis in millions | 325 (100%) | 284 (87%) | 241 (74%) | 106 (32%) | 110 (34%) | 66 (20%) | 229 (71%) | 76 (23%) | 58 (18%) | 68 (21%) | 220 (68%) |
CV, civil society; HBV, hepatitis B virus; HCV, hepatitis C virus; MS, Member States; WHO, World Health Organization.
Strategic and technical advisory group (STAG) is responsible for assessing the hepatitis burden in their country and using evidence to inform their recommendations.
Civil society is either an official member of the STAG or has otherwise been consulted.
Numerical targets set for reducing morbidity (incidence and/or prevalence) or mortality (death) attributable to HBV and/or HCV.
Numerical targets set for activities to prevent or treat HBV and/or HCV.
Publised or drafted plan.
National plan and dedicated funding
Most responding Member States (63%) had developed a national viral hepatitis plan, which was in drafted (27%) or published (36%) form (Table 1). These Member States accounted for 71% of individuals infected with HBV or HCV. The proportion of Member States who had developed a plan ranged from 50% within the South-East Asia region to 88% in the Eastern Mediterranean region, and from 46% within low-income settings to 71% in high-income settings.
While the majority of WHO Member States had developed a plan (62%), only 37% of respondents had dedicated funding for implementation (58% of those with a plan developed). The 49 countries dedicating funding to implementation accounted for 18% of individuals infected with HBV or HCV worldwide. The proportion of WHO Member States reporting dedicated funding ranged from 22% within the African region to 44% in the Eastern Mediterranean region, and from 25% within low-income settings to 42-43% in lower middle to high-income settings.
Engagement with civil society
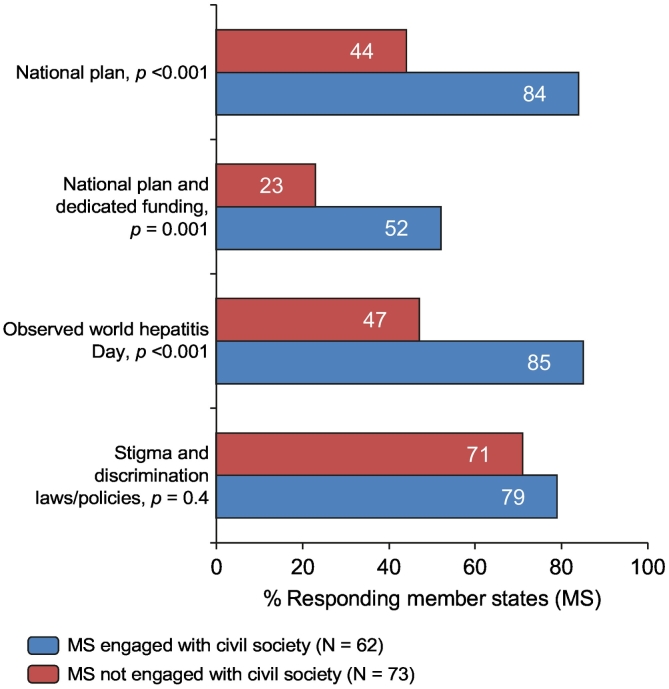
Of WHO Member States, 46% reported engagement with civil society, either as an official member of the government’s STAG or other official working group (30%) or through wider consultation on their response to viral hepatitis (16%, Table 1). The proportion of Member States reporting engagement with civil society ranged from 47% within the Western Pacific Region to 63% in the Americas and South-East Asia regions, and from 38% within upper middle-income settings to 54% in high-income settings. Compared with others, Member States that engaged civil society were more likely to have national viral hepatitis plans (84% vs. 44%, p < 0.001), to have assigned dedicated funding (52% vs. 23%, p = 0.001), and to have officially observed World Hepatitis Day in 2016 (85% vs. 47%, p < 0.001). Member States engaged with civil society were slightly more likely to have laws or policies on stigma and discrimination, compared to other Member States (79% vs. 71%, p = 0.4, Fig. 2).
Fig. 2.
Association between viral hepatitis indicators (including having a national plan, a plan with dedicated funding, observed World Hepatitis Day, and laws/policies on stigma and discrimination) and engagement with civil society as reported by responding Member States1.1Univariate comparisons using the Pearson Chi-square test with Yates’ continuity correction for categorial variables. MS, Member States.
Monitoring and targets
Few Member States reported having official working estimates on key monitoring and evaluation indicators for HBV and HCV as of 2017. For either HBV or HCV infection, 24–25% of responding Member States reported having official working estimates on mortality and 31–34% on incidence of new infection (i.e. measures of impact). Eight to 23% of responding Member States reported having official working estimates on either extent of diagnosed infection, treatment uptake or response to therapy for either HBV or HCV infection. Approximately half of responding Member States reported having official working estimates on prevalence of infection for HBV (56%) and HCV (51%). The availability of working estimates on key monitoring and evaluation indicators varied by WHO region and income classification (Appendix 1.1). Most Member States in the African, Americas and South-East Asia regions reported that they lacked working estimates on impact and intervention coverage indicators.
Around a quarter (27%) of responding Member States reported having numerical targets for reducing morbidity (incidence and/or prevalence) or mortality (death) attributable to HBV and/or HCV (Table 1). However, only 50% and 56% of those Member States that reported having set a target on morbidity/mortality also reported having an official working estimate on mortality attributable to either HBV or HCV, respectively (Appendix 1.2). While 48% of responding Member States reported having numerical targets for activities to prevent or treat HBV and/or HCV (Table 1), few of these Member States reported also having official working estimates on, for example, treatment coverage for HBV (25%) and HCV (39%, Appendix 1.3).
Testing guidance
Most responding Member States had official guidance on HBV testing, which included guidance on the diagnostic test to use (69%), testing of pregnant women (70%), and a protocol for referral to treatment and care following diagnosis (64%). HBV testing guidance varied greatly by WHO region and income classification (Table 2). The proportion of responding Member States reporting guidance for testing pregnant women for HBV ranged from 33% in low-income to 93% in high-income settings (p < 0.001, Table 2). Overall, we estimated that 65% of people living with chronic HBV infection (166 million) resided in a country with guidance on testing pregnant women for HBV (Table 2).
Table 2.
Proportion of responding Member States reporting having HBV testing guidance (covering diagnostic tests, pregnant women, and referral to treatment/care) and the use of optimal therapies in first-line treatment for HBV.
| HBV testing guidance which covers the following: |
Tenofovir or entecavir considered first-line treatment for the following: |
|||||
|---|---|---|---|---|---|---|
| HBV diagnostic test, n (% of N) | HBV testing of pregnant women, n (% of N) | Referral to treatment/care, n (% of N) | All patients, n (% of N) | For select patients according to prioritization1, n (% of N) | Not first line, n (% of N) | |
| Total responding Member States (N = 135) |
93 (69%) | 95 (70%) | 87 (64%) | 80 (59%) | 29 (22%) | 26 (19%) |
| By WHO region: | ||||||
| Eastern Mediterranean (16) | 14 (88%) | 9 (56%) | 9 (56%) | 10 (63%) | 2 (13%) | 4 (25%) |
| Western Pacific (19) | 14 (75%) | 13 (68%) | 12 (63%) | 12 (63%) | 3 (16%) | 4 (21%) |
| Americas (19) | 15 (79%) | 14 (74%) | 14 (74%) | 14 (74%) | 3 (16%) | 2 (11%) |
| South-East Asia (8) | 5 (63%) | 6 (75%) | 5 (63%) | 4 (50%) | 4 (50%) | 0 (0%) |
| European (46) | 33 (72%) | 40 (87%) | 36 (78%) | 28 (61%) | 11(24%) | 7 (15%) |
| African (27) | 12 (44%) | 13 (48%) | 11 (41%) | 12 (44%) | 6 (22%) | 9 (33%) |
| By income classification: | ||||||
| High (41) | 31 (76%) | 38 (93%) | 32 (78%) | 30 (73%) | 7 (17%) | 4 (10%) |
| Upper-middle (32) | 29 (91%) | 25 (78%) | 27 (84%) | 17 (53%) | 9 (28%) | 6 (19%) |
| Lower-middle (35) | 21 (60%) | 21 (60%) | 19 (54%) | 22 (63%) | 6 (17%) | 7 (20%) |
| Low (24) | 10 (42%) | 8 (33%) | 6 (25%) | 9 (38%) | 6 (25%) | 9 (38%) |
| n.a. (3) | 2 (67%) | 3 (100%) | 3 (100%) | 2 (67%) | 1 (33%) | 0 (0%) |
| Estimated number of people living with chronic HBV in millions (257) | 147 (57%) | 166 (65%) | 58 (22%) | 109 (74%) | 21 (8%) | 13 (5%) |
HBV, hepatitis B virus; WHO, World Health Organization.
e.g. restrictions on who receives treatment based on disease stage or risk group.
The majority of responding Member States had official guidance on HCV testing (64%) and protocols for referral to treatment and care (62%). However, only 46% of responding Member States had official guidance on testing PWID for HCV (Table 3); ranging from 11% in the African region to 72% in the European Region, and from 13% in low-income to 68% in high-income settings. In 2 regions with particularly high numbers of PWID, Latin America (1.8 million) and Sub-Saharan Africa (1.4 million), less than 20% of WHO Member States had guidance on testing PWID for HCV (Table 4).
Table 3.
Proportion of responding Member States reporting having HCV testing guidance (covering diagnostic tests, PWID, and referral to treatment/care) and the use of optimal therapies in first-line treatment for HCV.
| HCV Testing guidance which covers the following: |
Interferon-free direct-acting antiviral regimens considered the first line of treatment for the following: |
|||||
|---|---|---|---|---|---|---|
| HCV diagnostic test, n (% of N) | HCV testing of PWID, n (% of N) | Referral to treatment/care, n (% of N) | All patients, n (% of N) | For selected patients according to prioritization1, n (% of N) | No, n (% of N) | |
| Total responding Member States (N = 135) |
87 (64%) | 62 (46%) | 84 (62%) | 51 (38%) | 40 (30%) | 44 (33%) |
| By WHO region: | ||||||
| Eastern Mediterranean (16) | 15 (93%) | 10 (63%) | 12 (75%) | 9 (56%) | 3 (19%) | 4 (25%) |
| Western Pacific (19) | 12 (63%) | 8 (42%) | 11 (58%) | 7 (37%) | 2 (11%) | 10 (53%) |
| Americas (19) | 12 (63%) | 4 (21%) | 11 (58%) | 6 (32%) | 3 (16%) | 10 (53%) |
| South-East Asia (8) | 5 (75%) | 4 (50%) | 5 (63%) | 4 (50%) | 3 (38%) | 1 (13%) |
| European (46) | 33 (72%) | 33 (72%) | 38 (83%) | 18 (39%) | 22 (48%) | 6 (13%) |
| African (27) | 9 (33%) | 3(11%) | 7 (26%) | 7(26%) | 7 (26%) | 13 (48%) |
| By income classification: | ||||||
| High (41) | 30 (73%) | 28 (68%) | 33 (81%) | 17 (42%) | 21 (51%) | 3 (7%) |
| Upper-middle (32) | 26 (81%) | 13 (41%) | 24 (75%) | 10 (31%) | 9 (28%) | 13 (41%) |
| Lower-middle (35) | 19 (54%) | 15 (43%) | 19 (54%) | 16 (46%) | 4 (11%) | 15 (43%) |
| Low (24) | 10 (42%) | 3 (13%) | 5 (21%) | 7 (29%) | 5 (21%) | 12 (50%) |
| n.a. (3) | 2 (67%) | 3(100%) | 3 (100%) | 1 (33%) | 1 (33%) | 1 (33%) |
| Estimated number of people living with chronic HCV in millions (71) | 46 (64%) | 38 (53%) | 48 (68%) | 35 (49%) | 11 (16%) | 11 (16%) |
HCV, hepatitis C virus; PWID, people who inject drugs; WHO, World Health Organization.
e.g. restrictions on who receives treatment based on disease stage or risk group.
Table 4.
Responding Member States with HCV testing guidance for PWID, by region and ranked according to estimated number of PWID.1
|
Region (N1 = number of countries within region)1 |
Estimated number of PWID (95% UI)1 |
Population prevalence of PWID (95% UI)1 |
Responding Member States N2 (% of N1) |
Responding Member States with testing guidance for PWID N3 (% of N2) |
|---|---|---|---|---|
| East and Southeast Asia (16) | 3,989,000 (3,041,000–4,955,000) | 0.25% (0.19–0.31) | 15 (94%) | 8 (53%) |
| Eastern Europe (17) | 3,020,000 (1,653,500–5,008,000) | 1.30% (0.71–2.15) | 14 (82%) | 10 (71%) |
| North America (2) | 2,557,000 (1,498,500–4,428,000) | 1.06% (0.62–1.83) | 2 (100%) | 2 (100%) |
| Latin American (20) | 1,823,000 (1,392,000–2,380,000) | 0.46% (0.35–0.60) | 12 (60%) | 2 (17%) |
| Sub-Saharan Africa (47) | 1,378,000 (645,500–3,080,000) | 0.28% (0.13–0.62) | 27 (58%) | 3 (11%) |
| South Asia (9) | 1,023,500 (783,500–1,263,000) | 0.09% (0.07–0.11) | 6 (67%) | 4 (67%) |
| Western Europe (28) | 1,009,500 (686,500–1,386,500) | 0.34% (0.23–0.47) | 24 (86%) | 19 (79%) |
| Middle East and North Africa (21) | 349,500 (177,500–521,500) | 0.12% (0.06–0.18) | 17 (81%) | 9 (53%) |
| Central Asia (5) | 281,500 (189,500–416,500) | 0.63% (0.43–0.94) | 5 (100%) | 3 (60%) |
| Australasia (2) | 115,500 (83,000–148,000) | 0.59% (0.42–0.75) | 2 (100%) | 2 (100%) |
| Caribbean (13) | 79,500 (53,000–118,000) | 0.44% (0.30–0.66) | 5 (39%) | 0 (0%) |
| Pacific Island States and Territories (12) | 22,500 (15,000–33,500) | 0.33% (0.22–0.49) | 6 (50%) | 0 (0%) |
| Global (194) | 15,648,000 (10,219,000–23,737,500) | 0.33% (0.21–0.49) | 135 (70%) | 62 (46%) |
HCV, hepatitis C virus; PWID, people who inject drugs; WHO, World Health Organization.
Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Heal. 2017. 5:e1192-207.
Access to treatment
The majority of responding WHO Member States (59%) reported tenofovir or entecavir to be the first-line treatment for eligible patients with chronic HBV monoinfection, which accounted for 74% of the global chronic HBV infected population. This varied from 44% in the African region to 74% in the American region, and from 38% in low-income to 73% in high-income settings. Access to tenofovir or entecavir was prioritized for select patients in 22% of Member States and not available at all as first-line treatment in 19% of Member States (Table 2).
A minority of responding Member States (38%) reported direct-acting antiviral (DAA) regimens as first-line treatment for all patients with chronic HCV, which accounted for 49% of the global chronic HCV infected population. This varied from 26% in the African region to 56% in the Eastern Mediterranean region, and from 29% in low-income to 46% in lower middle-income settings. DAA regimens were prioritized for select patients in 30% of Member States and not yet available as first-line treatment in 33% of Member States (Table 2).
Discussion
The country profiles generated for 135 Member States, home to 87% of the infected population, describe the status of the global response to viral hepatitis B and C. First, the number of countries with a national viral hepatitis plan reached 84 in 2017, though only 58% of countries with a plan had assigned dedicated funding for implementation. Second, findings that the majority of countries have guidance on diagnostic testing (69% for HBV and 64% for HCV) and on referral pathways for treatment and care (64% for HBV and 62% for HCV) suggest that countries are progressing with their national response to viral hepatitis. Third, the availability of data for decision-making remains limited with only a minority of Member States having working estimates on mortality (24% for HBV and 25% HCV) and transmission (34% for HBV and 31% for HCV). A review of the progress reported by governments and the gaps remaining in national responses highlight the next steps needed to deliver on the GHSS and its goal of viral hepatitis elimination.4
National plans for hepatitis constitute the necessary first step towards action. Compared with 2012 data13 the 2017 country profiles suggest a 5-fold increase in the number of countries with national plans. The following factors may explain this increase: (1) 3 World Health Assembly resolutions that consecutively called for (i) action against hepatitis (2010),14 (ii) consideration of elimination (2014),15 and (iii) global targets for elimination (2016);16 (2) WHO guidance on the formulation of national plans (2015);17 (3) the GHSS, which articulates the need for countries to develop evidenced-based national plans with a budget to achieve the goal of elimination;4 (4) international civil society partners, such as the World Hepatitis Alliance, that help to link policy vision with patient advocates within countries.18
The findings of this study highlight the importance of civil society involvement, as it shows that countries that actively involve civil society in their hepatitis response are more likely to have a national plan as well as dedicated domestic funding for implementation. However, financing remains a serious concern as dedicated national funding for implementation was only available in 58% of Member States with national plans (relating to an estimated 18% of the global infected population). Countries making the most progress towards elimination are ones that have national plans with dedicated funding.19 Additionally, GHSS emphasizes that the global hepatitis response should involve an enabling environment of policies and laws that aim to reduce stigma and discrimination.4 Our analysis showed that the majority (75%) of countries reported having laws or policies that addressed stigma and discrimination in general; although, few (7%) countries had laws or policies specific to viral hepatitis. A recent global survey of civil society organizations, which aimed to explore how stigma and discrimination affects those living with viral hepatitis, found that only 4% of 156 respondents across 72 countries felt that their government was satisfactorily addressing stigma and discrimination.18 Legal, institutional and other barriers can impede access to prevention, diagnosis and treatment services, particularly for those populations at heightened risk of hepatitis infection (including PWID, men who have sex with men, prisoners and sex workers).20 Countries need to reform laws, policies and regulations that impede equitable access to hepatitis services, and adapt models of service delivery to meet the needs of affected populations.[4], [5], [6]
Scaling up testing and treatment of HBV and HCV infection is fundamental to reducing mortality as part of the GHSS elimination goal.[4], [5], [6] WHO guidelines on treatment have been available since 2014 for HCV infection21 (with updates in 201622 and 201823; the latter recommended treatment for all those living with HCV infection [except pregnant women] regardless of age, risk group or disease stage) and 2015 for HBV infection,24 while viral hepatitis testing guidelines were published in 2017.25 The country profiles indicate that the majority of countries report having policies on testing, but these are not necessarily comprehensive. Important gaps were revealed in policies on testing of key populations, with (i) low-income countries less likely to have guidance for testing pregnant women for HBV (33%) compared to high-income countries (93%), and (ii) many countries not having specific guidance for testing PWID for HCV, both overall (73 of 135 countries, 54%) and in the 5 regions (45 of 70 countries, 64%) with the largest share (82%) of the globally estimated 15.6 million PWID. Routine testing of pregnant women for HBV, particularly in settings with intermediate or high HBV surface antigen seroprevalence, and PWID for HCV are integral to both primary and secondary prevention efforts, and thus countries need to recognise and integrate these population groups into their testing policies.[4], [5], [6], [24], [25]
Many countries reported limited access to optimal HBV and HCV therapies in 2017. For HBV, only 59% of countries reported access to tenofovir or entecavir as first-line treatment for all eligible patients, and for HCV, only 38% reported access to interferon-free DAA regimens as first-line treatment for all patients. The price of medicines has been an obstacle to treatment but that is becoming less of an issue. For example, the price for a year of tenofovir treatment for HBV infection using generic medicine was 32 USD in 2016.26 Further, starting in 2018, tenofovir is no longer protected by patents, meaning all countries can use generic medicines. In 2017, 62% of people infected with HCV lived in countries where generic medicines could be accessed for around 150 USD or less for a course of treatment.27 Globally, uptake of treatment among those infected remains low in 2017, at 1.8% for HBV (4.5 million of the estimated 257 million infected) and 2.5% for HCV (1.76 million treated among 71 million infected).1 The scaling up of hepatitis testing and treatment will require comprehensive service delivery models that include (1) simple and standardized algorithms across the continuum of care; (2) integration of hepatitis testing, care and treatment with other related services; (3) strategies to strengthen linkage from testing to care, treatment and prevention; (4) decentralized services, supported by task-sharing/shifting; (5) community engagement and peer support to address stigma and discrimination, and reach vulnerable or disadvantaged communities; (6) efficient procurement and supply management of medicines and diagnostics; (7) data systems to monitor the quality of individual care and the cascade of care.23
Most countries lacked both baseline estimates and targets in terms of incidence of infection or mortality reduction. The 2016 monitoring and evaluation framework for viral hepatitis B and C outlines the chain of information required, from (i) context and needs (i.e., mostly prevalence of infection) to (ii) input (i.e., infrastructure), (iii) output and outcomes (i.e., prevention indicators and indicators of the cascade of care) and (iv) impact (i.e., incidence and mortality).9 Data systems needed to inform this framework include: (1) surveillance for acute hepatitis, chronic infections and sequelae; (2) programme data documenting prevention and treatment, which for the latter includes the cascade of care.10 Collaborations between viral hepatitis and other health programmes (e.g., immunization, communicable/non-communicable disease control, infection control, harm reduction, HIV, tuberculosis, maternal and child health) will be needed at the strategic, policy, technical, implementation and data management level to ensure that strategic information can be collected, transmitted, analysed and used for action without creating new data systems.[10], [27]
Our report suffers from a number of limitations. First, it is a baseline analysis that is hard to compare with other sources of data. This limits our capacity to validate our results. Second, our analysis could not determine whether or not these plans covered all the necessary elements or are in line with the WHO framework.17 Third, some responses to the country profiles could have been made out of a desire to present an ideal situation. As a consequence of these limitations, our report may be overly optimistic. However, the increasing trend in the development of national plans is consistent with that previously reported by the WHO in 2016.[18], [28] In conclusion, countries are making progress with respect to national hepatitis planning, but financing remains an issue. Scale-up of testing and treatment has started, but it is still in its infancy in many countries. Finally, the availability of strategic information to assess the situation and guide elimination plans remains limited. Based on these conclusions, we suggest governments, in partnership with civil society and with support from WHO and other key stakeholders, build upon the work done to establish national policies and plans and: (1) estimate the resource needs to deliver a comprehensive response and increase investment through innovative financing27 (in the context of universal health coverage); (2) build a stronger case for testing and treatment, documenting the cost-effectiveness and savings of these interventions; (3) strengthen the strategic information needed to direct hepatitis elimination, in the context of integrated systems that serve the broader health system. In 2018, the WHO established a new Global Reporting System for Hepatitis that will continue to collect key policy uptake indicators, along with indicators of the cascade of care, so that we can follow-up on the future evolutions of these trends.30
Financial support
Health Protection Scotland provided financial support for the conduct of the research and preparation of the article. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Authors’ contributions
Shanley Smith, Sharon Hutchinson, Yvan Hutin, Hande Harmanci, Raquel Peck, and Charles Gore designed the data collection tool. Shanley Smith designed the online interface of the data collection tool. Shanley Smith, Sharon Hutchinson, and Hande Harmanci, coordinated the collation of survey responses. Shanley Smith, Bharat Rewari, Antons Mozalevskis, Messeret Shibeshi, Mutale Mumba, Linh-Vi Le, Naoko Ishikawa, Désiré Nolna, and Leandro Sereno coordinated the data collection process. Shanley Smith, Sharon Hutchinson, and Yvan Hutin prepared the first draft and finalized the draft on the basis of comments from other authors. Shanley Smith and Sharon Hutchinson analysed the data, created associated figures and tables, and finalized the figures and tables based on comments from other authors. Sarah Hess and Yvan Hutin provided the official WHO map. All authors reviewed results, provided guidance on methods, and provided critical feedback on the manuscript.
Conflicts of interest
Sharon Hutchinson received honoraria for general public health presentation from Gilead. All other authors declare no competing interests..
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
We are grateful to the regional and country WHO staff who supported the implementation and completion of the country profiles. These include P Chan; M Dara; J Hermez; H Lago,F Lesi; F Lule; M Ghidinelli; R Mihigo; M Alonso; F Ndenzako; N Walsh; N Razakasoa; S Mukta; A Stengaard; K Yeboue.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2019.04.002.
Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
References
- 1.World Health Organization . World Health Organization; Geneva: 2017. Global hepatitis report.http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1 [Google Scholar]
- 2.Global and country estimates of immunization coverage and chronic HBV infection. World Health Organization; Geneva: 2018. http://whohbsagdashboard.com/#global-strategies [Google Scholar]
- 3.Blach S, Zeuzem S, Manns M, Altraif I, Duberg AS, Muljono DH. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. (accessed March 5, 2018).
- 5.Heffernan A, Cooke G, Nayagam S. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319–1329. doi: 10.1016/S0140-6736(18)32277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayagam S, Thursz M, Sicuri E. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16:1399–1408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 7.Hutin YJ-F, Bulterys M, Hirnschall GO. How far are we from viral hepatitis elimination service coverage targets? J Int AIDS Soc. 2018 doi: 10.1002/jia2.25050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razavi H, Waked I, Sarrazin C. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21:34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization; Geneva: 2016. Monitoring and evaluation for viral hepatitis B and C: recommended indicators and framework: Technical report. [Google Scholar]
- 10.Hutin Y, Low-Beer D, Bergeri I. Viral Hepatitis Strategic Information to Achieve Elimination by 2030: Key Elements for HIV Program Managers. JMIR Public Heal Surveill. 2017;3 doi: 10.2196/publichealth.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EUSurvey. https://ec.europa.eu/eusurvey/home/welcome
- 12.Degenhardt L, Peacock A, Colledge S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Heal. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . World Health Organization; Geneva: 2013. Global policy report on the prevention and control of viral hepatitis in WHO Member States. [Google Scholar]
- 14.Sixty-third World Health Assembly. World Health Organization; Geneva: 2010. Resolution WHA63.18. Viral hepatitis.http://apps.who.int/gb/ebwha/pdf_files/WHA63-REC1/WHA63_REC1-en.pdf [Google Scholar]
- 15.Sixty-seventh World Health Assembly. World Health Organization; Geneva: 2014. Resolution WHA67.6. Viral hepatitis.http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R6-en.pdf [Google Scholar]
- 16.Sixty-ninth World Health Assembly. World Health Organization; Geneva: 2016. Resolution WHA69.32. Viral Hepatitis.http://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_32-en.pdf [Google Scholar]
- 17.World Health Organization . World Health Organization; Geneva: 2015. Manual for the development and assessment of national viral hepatitis plans.http://apps.who.int/iris/bitstream/handle/10665/183726/9789241509350_eng.pdf;jsessionid=98A96891BACC2E5A69CB1516E91F710A?sequence=1 [Google Scholar]
- 18.World Hepatitis Alliance . Global Findings Report. 2017. Holding Governments Accountable. World Hepatitis Alliance Civil Society Survey. [Google Scholar]
- 19.Hill AM, Nath S, Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad. 2017;3:117–123. [PMC free article] [PubMed] [Google Scholar]
- 20.Day E, Hellard M, Treloar C. Hepatitis C elimination among people who inject drugs: Challenges and recommendations for action within a health systems framework. Liv er Int. 2018:1–11. doi: 10.1111/liv.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . World Health Organization; Geneva: 2014. Guidelines for the screening, care and treatment of persons with hepatitis C infection. [PubMed] [Google Scholar]
- 22.World Health Organization . World Health Organization; Geneva: 2016. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection. Updated version, April 2016. [PubMed] [Google Scholar]
- 23.World Health Organization . World Health Organization; Geneva: 2018. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection. Updated version, July 2018. [Google Scholar]
- 24.World Health Organization . World Health Organization; Geneva: 2015. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. [PubMed] [Google Scholar]
- 25.World Health Organization . World Health Organization; Geneva: 2017. WHO Guidelines on Hepatitis B and C Testing. [Google Scholar]
- 26.WHO . World Health Organization; Geneva: 2018. Progress report on access to hepatitis C treatment. [Google Scholar]
- 27.Hutin Y, Desaib S, Bulterys M. Preventing hepatitis B virus infection: milestones and targets. Bull World Heal Organ. 2018;96 doi: 10.2471/BLT.18.215210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . World Health Organization; Geneva: 2016. Combating hepatitis B and C to reach elimination by 2030. [Google Scholar]
- 29.Global Reporting System for Hepatitis https://extranet.who.int/dhis2/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3