Background & Aims
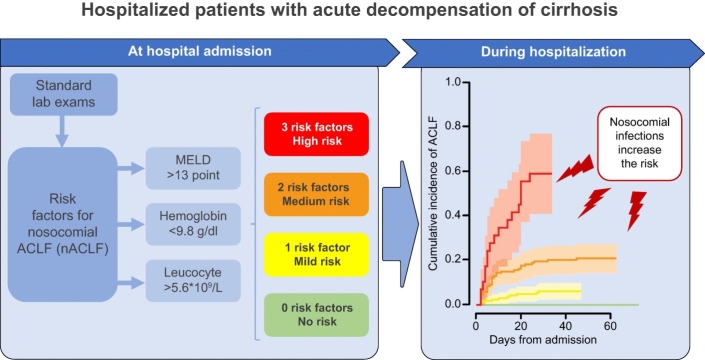
Nosocomial acute-on-chronic liver failure (nACLF) develops in at least 10% of patients with cirrhosis hospitalized for acute decompensation (AD), greatly worsening their prognosis. In this prospective observational study, we aimed to identify rapidly obtainable predictors at admission, which allow for the early recognition and stratification of patients at risk of nACLF.
Methods
A total of 516 consecutive patients hospitalized for AD of cirrhosis were screened: those who did not present ACLF at admission (410) were enrolled and surveilled for the development of nACLF.
Results
Fifty-nine (14%) patients developed nALCF after a median of 7 (IQR 4–18) days. At admission, they presented a more severe disease and higher degrees of systemic inflammation and anemia than those (351; 86%) who remained free from nACLF. Competing risk multivariable regression analysis showed that baseline MELD score (sub-distribution hazard ratio [sHR] 1.15; 95% CI 1.10–1.21; p ≪0.001), hemoglobin level (sHR 0.81; 95% CI 0.68–0.96; p = 0.018), and leukocyte count (sHR 1.11; 95% CI 1.06–1.16; p ≪0.001) independently predicted nACLF. Their optimal cut-off points, determined by receiver-operating characteristic curve analysis, were: 13 points for MELD score, 9.8 g/dl for hemoglobin, and 5.6x109/L for leukocyte count. These thresholds were used to stratify patients according to the cumulative incidence of nACLF, being 0, 6, 21 and 59% in the presence of 0, 1, 2 or 3 risk factors (p ≪0.001). Nosocomial bacterial infections only increased the probability of developing nACLF in patients with at least 1 risk factor, rising from 3% to 29%, 16% to 50% and 52% to 83% in patients with 1, 2 or 3 risk factors, respectively.
Conclusions
Easily available laboratory parameters, related to disease severity, systemic inflammation, and anemia, can be used to identify, at admission, hospitalized patients with AD at increased risk of developing nACLF.
Lay summary
More than 10% of patients with cirrhosis hospitalized because of an acute decompensation develop acute-on-chronic liver failure, which is associated with high short-term mortality, during their hospital stay. We found that the combination of 3 easily obtainable variables (model for end-stage liver disease score, leukocyte count and hemoglobin level) help to identify and stratify patients according to their risk of developing nosocomial acute-on-chronic liver failure, from nil to 59%. Moreover, if a nosocomial bacterial infection occurs, such an incidence proportionally increases from nil to 83%. This simple approach helps to identify patients at risk of developing nosocomial acute-on-chronic liver failure at admission to hospital, enabling clinicians to put in place preventive measures.
Keywords: Anemia, Systemic inflammation, MELD score, Liver cirrhosis, Hospitalizations, Organ failures, Nosocomial infections
Graphical Abstract
Highlights
-
•
ACLF complicates more than 10% of hospitalizations for acute decompensation of cirrhosis.
-
•
Easily available predictors of nosocomial ACLF are fundamental to implement appropriate preventive strategies.
-
•
MELD score, leucocyte count and hemoglobin can stratify patients according to their risk of developing nosocomial ACLF.
-
•
Further studies are required to validate these results and provide additional insights.
Introduction
The clinical course of decompensated cirrhosis seldom follows a steady downward path. Indeed, episodes of acute decompensation (AD) of the disease requiring hospitalization can develop due to precipitating events.1 In this setting, the patients become at risk of acute-on-chronic liver failure (ACLF), a distinct clinical syndrome characterized by the development of one or more extrahepatic organ or system failure and burdened by a high short-term mortality despite its potential reversibility.2 In hospitalized patients with AD of cirrhosis, ACLF is already present at admission in about one-fourth of cases, but further develops during the hospital stay in 11% of cases.3
Because of its grave prognosis, the prevention of ACLF, whenever possible, would assume great relevance. Effective prevention can only ensue if predicting factors are known and easily identifiable. Unfortunately, this matter is still ill-defined in patients who develop ACLF. Independent predictors for the development of this syndrome have recently been identified in outpatients with cirrhosis,4 but scanty information is available in those admitted to hospital because of AD who subsequently develop ACLF3.
In order to shed more light on this matter, we carried out a prospective observational study in hospitalized patients with cirrhosis and AD with the aim of identifying simple and rapidly acquirable predictors of nosocomial ACLF (nACLF) able to stratify patients according to their risk of developing this complication.
Patients and methods
Study design and population
The study population included individuals enrolled in a prospective observational study performed from January 2014 to March 2016 in 2 Italian hospitals, the S. Orsola-Malpighi University Hospital in Bologna and the Infermi Hospital in Rimini. The study protocol was approved by the local institutional review boards. Written informed consent was obtained from patients or from legal surrogates before enrolment, according to the 1975 Declaration of Helsinki.
All consecutive patients with liver cirrhosis admitted to the participating hospitals were screened for enrolment within 36 h from admission. Inclusion criteria were: a) cirrhosis diagnosed by a composite of clinical signs, laboratory tests, endoscopy, and imaging; b) admission because of an episode of AD; c) age ≫18. Exclusion criteria were: a) admission for a scheduled procedure; b) hepatocellular carcinoma beyond the Milan criteria5; c) severe chronic extrahepatic diseases; d) extrahepatic malignancy; e) previous liver transplantation.
Patient management and follow-up
Patients fulfilling inclusion and exclusion criteria were enrolled and those who did not present ACLF at admission were then monitored during the entire hospital stay for the potential development of nACLF. The follow-up was interrupted in case of hospital discharge, liver transplantation (LT) or death. All patients were managed by the attending physicians according to international and local guidelines.
Data collection
Data were collected using an online electronic case report form and their integrity was systematically checked before being entered into the database. The following data were collected at the time of enrolment: demographic characteristics, etiology of cirrhosis, laboratory and clinical features including the presence of hepatocellular carcinoma (HCC) and/or other co-morbidities assessed by the Charlson score.6 Based on the collected data, the model for end-stage liver disease (MELD),7 MELD-sodium,8 Child-Pugh,9 Chronic Liver Failure Consortium (CLIF-C) organ failure (CLIF-C OF),10 CLIF-C acute decompensation (CLIF-C AD)11 and CLIF-C ACLF10 scores were calculated. During hospitalization, patients were assessed daily for the occurrence of bacterial infections and ACLF.
Definitions
AD of cirrhosis was defined by: a) acute onset of grade 2 or 3 ascites, according to the International Club of Ascites classification12; b) new episode of hepatic encephalopathy (HE) graded according to the West-Haven criteria13 in patient with previous normal consciousness and no evidence of an acute neurologic disease; c) upper or lower gastrointestinal bleeding; d) bacterial infection; e) any combination of these events.
ACLF was diagnosed and graded as described by Moreau et al.3 nACLF was defined as any occurrence of ACLF after 48 h from hospital admission.
Active alcoholism was defined as the assumption of more than 14 drinks per week in women and more than 21 drinks per week in men within the previous 3 months.3 Bacterial infections were diagnosed according to international[14], [15], [16] and local guidelines. Each event of infection was revised by an infectious disease specialist for consistency and accuracy. Nosocomial infections were considered if infection signs and/or symptoms started more than 48 h after hospital admission.
Statistical analysis
The sample size was estimated under the assumption that about 10% of patients with cirrhosis and acute decompensation, not fulfilling diagnostic criteria for ACLF at hospital admission, will develop the syndrome during hospitalization.3 Thus, in order to develop a prediction model based on 4 clinical or laboratory data available at admission, at least 40 nACLF events need to be recorded.
For all continuous parameters the normality of distribution and homogeneity of variance were evaluated by the Kolmogorov-Smirnov and Levene tests, then variables were reported as mean and standard deviation or median and interquartile range (IQR) as appropriate. Accordingly, comparisons between groups were performed by means of Student’s t test or Mann-Whitney U test when appropriate. Categorical parameters were reported as frequency and percentage and compared by the χ2 square test.
A multivariable logistic regression (LR) model with backward elimination (p ≫0.05) was fitted in order to identify predictors of ACLF among parameters available at hospital admission and significantly associated to nACLF (p ≪0.05). For each parameter included in the final model, the odds ratio (OR) and 95% CI are reported. In order to account for death, LT and discharge as events able to influence the probability of nACLF, the parameters significantly associated with nACLF at univariate analysis were also included in a multivariable competing risk regression (CRR) model with backward elimination (p ≫0.05), which was performed according to the Fine and Gray method. For each parameter included in the final model the sub-distribution hazard ratio (sHR) with the 95% CI is reported. The disease severity score with the highest area under the curve (AUROC) at receiver-operating characteristics (ROC) curve analysis was included in the LR and CRR models, while its components were excluded to avoid multicolinearity. Following regression analysis each predictor was categorized according to the value associated to the highest Youden Index at ROC curve analysis, then nACLF cumulative incidence curves for patients presenting none, 1, 2 or 3 risk factors were calculated considering death, LT and discharge as competing events, and compared by the Gray’s test. The association between risk factors and 28- and 90-day mortality was evaluated by a CRR analysis in which LT was considered a competing event, while predictors of in-hospital mortality were analyzed by CRR analysis in which LT and hospital discharge were considered competing events. No imputation for missing data was done. All tests were 2-sided and p values ≪0.05 were considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS version 25, IBM corp) and the cmprsk package on R statistical software (cmprsk http://www.R-project.org/)
Results
Study population
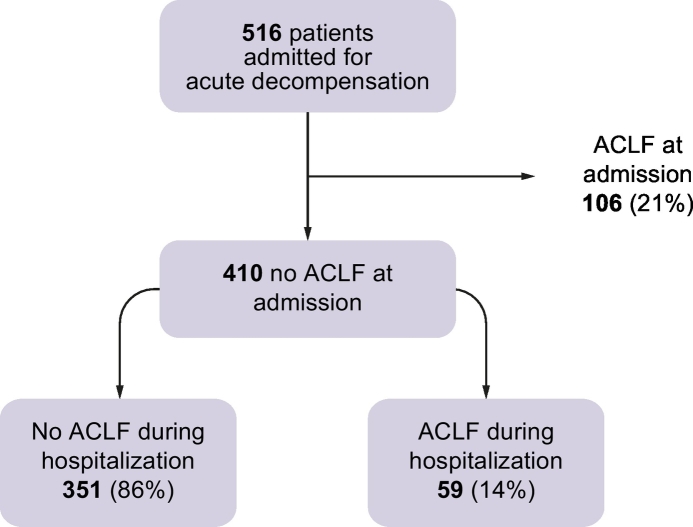
Five hundred and sixteen patients with cirrhosis and AD consecutively admitted to hospital were enrolled in the study. ACLF was present at the time of hospitalization in 106 (21%) cases. Thus, the study cohort included 410 (79%) patients who did not meet the criteria for ACLF diagnosis at the time of hospital admission. The median length of hospital stay of the entire study population was 9 (IQR 5–14; min 1, max 73) days. Overall, 59 (14%) patients developed ACLF during hospitalization (nACLF) after a median of 7 (IQR 4–18; min 2, max 45) days (Fig. 1).
Fig. 1.
Patients with an AD of cirrhosis included in the analysis divided according to the presence of ACLF at study inclusion or the development of ACLF during hospital stay. ACLF, acute-on-chronic liver failure; AD, acute decompensation.
Baseline characteristics of patients without ACLF at hospital admission
Baseline demographic, biochemical and clinical data of patients who remained free from ACLF until hospital discharge or developed nACLF are reported in Table 1. No differences were seen between the 2 groups regarding age, gender distribution, etiology of cirrhosis, history of prior AD and active alcoholism. Overall, patients who developed nACLF had a more severe liver disease. Indeed, they presented higher values of the prognostic scores (Child-Pugh, MELD, MELD-Na and CLIF-C AD), lower hemoglobin levels, more severe alterations of the parameters of liver function (serum bilirubin, serum albumin, and international normalized ratio [INR] values), and higher serum creatinine concentration. Ascites was also more frequent among individuals developing nACLF. Furthermore, markers of systemic inflammation, such as leukocyte count and serum C-reactive protein (CRP) were significantly higher in this group of patients. Finally, bacterial infections at admission were similarly distributed between the 2 groups; however, a significantly greater prevalence of community and healthcare-related infections was observed in the subgroup of patients developing nACLF unrelated to nosocomial infection (21% vs. 44%, p = 0.002).
Table 1.
Demographic, biochemical and clinical characteristics of patients without ACLF at hospital admission, divided according to the development of ACLF during hospitalization.
| No ACLF during the study | ACLF during hospitalization | p value | |
|---|---|---|---|
| N | 351 | 59 | |
| Demographics | |||
| Age (years) | 63 (51–73) | 60 (52–67) | 0.130 |
| Male sex | 213 (61) | 36 (61) | 0.961 |
| Etiology of cirrhosis | |||
| Viral | 156 (44) | 24 (41) | 0.590 |
| Alcohol | 68 (19) | 11 (19) | 0.895 |
| NASH | 18 (5) | 3 (5) | 0.989 |
| Mixed etiology | 57 (16) | 11 (19) | 0.646 |
| Other | 52 (15) | 10 (17) | 0.672 |
| AD at admission | |||
| Ascites | 179 (36) | 40 (68) | 0.017 |
| Encephalopathy grade III/IV | 43 (12) | 4 (7) | 0.222 |
| Renal dysfunction | 21 (6) | 16 (27) | ≪0.001 |
| GI bleeding | 25 (7) | 2 (3) | 0.285 |
| Bacterial infection | 70 (20) | 17 (29) | 0.123 |
| Clinical history | |||
| Admitted ≪24 h from AD | 248 (79) | 47 (80) | 0.906 |
| No prior AD⁎ | 140 (42) | 18 (31) | 0.086 |
| Active alcoholism⁎ | 61 (18) | 8 (14) | 0.367 |
| Biochemical and hemodynamic data | |||
| Hemoglobin (g/dl) | 10.8 (9.5–12.2) | 9.7 (9.0–11.6) | 0.009 |
| Leukocyte (109/L) | 5.1 (3.5–7.5) | 6.4 (5.1–9.6) | ≪0.001 |
| CRP (mg/dl)§ | 1.0 (0.3–2.9) | 3.0 (1.2–5.4) | ≪0.001 |
| Platelets (109/L) | 92 (58–144) | 94 (57–139) | 0.475 |
| Sodium (mmol/L) | 137 (134–140) | 136 (133–139) | 0.100 |
| Bilirubin (mg/dl) | 1.8 (1.0–3.3) | 3.9 (2.0–6.9) | ≪0.001 |
| Creatinine (mg/dl) | 0.9 (0.7–1.1) | 1.1 (0.9–1.5) | ≪0.001 |
| Albumin (g/dl) | 3.2 (2.9–3.6) | 2.9 (2.5–3.3) | 0.001 |
| INR | 1.4 (1.2–1.5) | 1.5 (1.4–1.8) | ≪0.001 |
| MAP (mmHg) | 87 (78–93) | 83 (77–90) | 0.103 |
| HR (bpm) | 75 (66–84) | 82 (71–90) | 0.002 |
| Prognostic scores | |||
| Child-Pugh score | 8 (6–9) | 10(8–11) | ≪0.001 |
| Child-Pugh Class | |||
| Class A | 103 (29) | 5 (9) | 0.001 |
| Class B | 181 (52) | 24 (41) | 0.122 |
| Class C | 67 (19) | 30 (51) | ≪0.001 |
| MELD | 13 (10–16) | 19 (15–23) | ≪0.001 |
| MELD-Na | 15 (12–19) | 22 (17–25) | ≪0.001 |
| CLIF-C AD | 49 (43–54) | 55 (51–60) | ≪0.001 |
| Concomitant medications | |||
| PPI | 226 (64) | 35 (59) | 0.454 |
| Beta-blockers | 157 (45) | 27 (46) | 0.883 |
| Rifaximin | 94 (27) | 17 (30) | 0.611 |
| Quinolone | 6 (2) | 2 (3) | 0.390 |
| Comorbidities | |||
| CCI | 6.0 (4.9–7.2) | 6.1 (4.9–7.5) | 0.515 |
| HCC | 92 (26) | 13 (22) | 0.489 |
| Diabetes | 124 (35) | 18 (31) | 0.472 |
Data are presented as frequencies [n (%)] or mean (±SD)/median (IQR) according to their distribution.
ACLF, acute-on-chronic liver failure; CCI, Charlson comorbidity index; CLIF-C AD, Chronic Liver Failure Consortium acute decompensation score; CRP, C-reactive protein; GI, gastrointestinal; HCC, hepatocellular carcinoma; HR, heart rate; INR, international normalized ratio; MAP, mean arterial pressure; MELD, model for end-stage liver disease; MELD-Na, MELD incorporating serum sodium; NASH, non-alcoholic steatohepatitis; PPI, proton pump inhibitor.
Available in 390 patients.
Available in 387 patients.
Clinical characteristics of nACLF
Among patients who developed nACLF, 37 (63%) developed grade 1, 15 (25%) grade 2 and 7 (12%) grade 3 ACLF. The median CLIF-OF score was 8 (7–9), with renal failure occurring in 30 (51%), liver failure in 15 (25%), coagulation failure in 16 (27%), brain failure in 15 (25%), respiratory failure in 9 (15%) and circulatory failure in 3 (5%) patients.
Bacterial infections were the most frequent precipitating event of nACLF. Indeed, in 34% of patients, nACLF was related to the development of nosocomial infection, while an infection was already present at admission in an additional 19% of individuals. Thus, in 53% of cases nACLF was related to a bacterial infection. The other precipitating events were gastrointestinal hemorrhage in 7% of cases, transjugular intrahepatic porto-systemic shunting in 3% of cases and active alcohol abuse in 3% of cases. Finally, in 34% of patients no precipitating events were identified.
Risk factors for nACLF at admission
The baseline parameters significantly associated with the development of nACLF at univariate analysis (Table 1) were entered into a multivariable LR analysis to identify the independent predictors of nACLF. Variables included in the regression model were ascites at admission, hemoglobin, leukocyte count, CRP, albumin, heart rate, and MELD score. Serum creatinine and renal dysfunction (serum creatinine above 1.5 mg/dl) were excluded from the model to avoid multicollinearity with the MELD score.
Among disease severity scores, MELD has been selected since it showed the highest AUROC for nACLF (MELD: AUROC 0.775, 95% CI 0.709–0.840; MELD-Na: AUROC 0.764, 95% CI 0.700–0.828; CLIF-C AD: AUROC 0.743, 95% CI 0.682–0.803; Child-Pugh: AUROC 0.714, 95% CI 0.646–0.783).
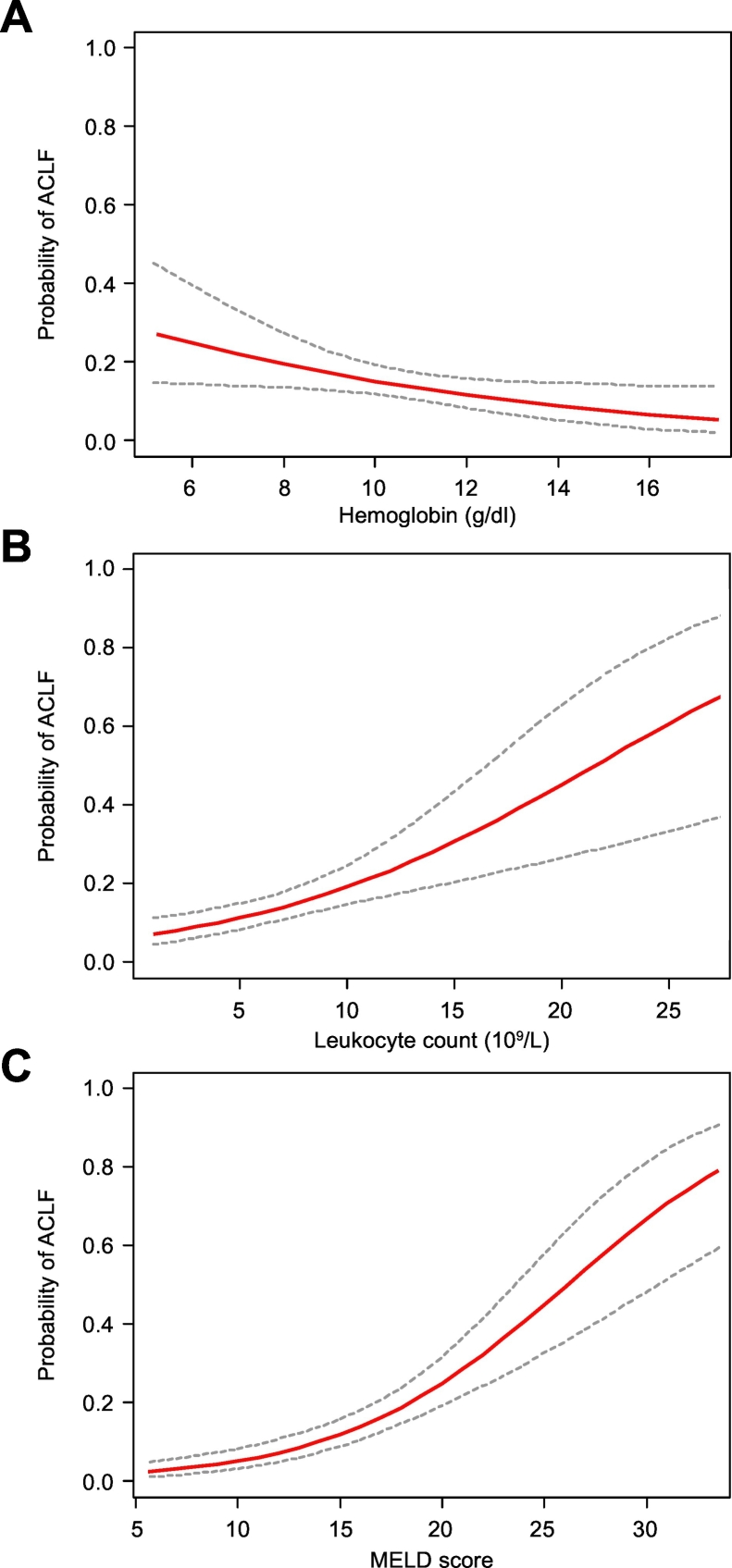
Among these parameters, lower hemoglobin level, higher leukocyte count, and higher MELD score were identified as independent risk factors for nACLF (Table 2). CRR analysis considering LT, death and hospital discharge as competing events, further supported this association, confirming that these 3 parameters were independent predictors of nACLF (Table 3). Interestingly, the probability of developing nosocomial ACLF progressively increased in parallel with the worsening of each risk factor (Fig. 2).
Table 2.
Multivariable logistic regression with backward selection of factors associated with the development of ACLF during hospital stay.
| Parameter | OR (95% CI) | p value |
|---|---|---|
| MELD score (1-point increase) | 1.19 (1.12–1.27) | ≪0.001 |
| Hemoglobin (1 g/dl increase) | 0.81 (0.68–0.97) | 0.022 |
| Leukocyte (109/L increase) | 1.11 (1.03–1.19) | 0.008 |
Data are presented as odds ratio and 95% CI. Variables entered in the first step also included ascites, serum albumin, C-reactive protein and heart rate at hospital admission. The disease severity score to be included in the analysis was selected by the receiver-operating characteristic analysis.
ACLF, acute-on-chronic liver failure; MELD, model for end-stage liver disease.
Table 3.
Multivariable CRR according to the Fine and Gray method with backward selection of factors associated to the development of ACLF during hospitalization.
| Parameter | sHR (95% CI) | p value |
|---|---|---|
| MELD score (1-point increase) | 1.15 (1.10–1.21) | ≪0.001 |
| Hemoglobin (1 g/dl increase) | 0.81 (0.68–0.96) | 0.018 |
| Leukocyte (109/L increase) | 1.11 (1.06–1.16) | ≪0.001 |
In-hospital mortality, liver transplantation and hospital discharge were considered as competing events. Data are presented as sub-distribution hazard ratio and 95% CI. Variable entered in the first step included also ascites, serum albumin, C-reactive protein and heart rate at hospital admission. The disease severity score to be included in the analysis was selected by the receiver-operating characteristic analysis.
ACLF, acute-on-chronic liver failure; CRR, competing risk regression; MELD, model for end-stage liver disease.
Fig. 2.
Estimated probability of developing ACLF during hospitalization. Probability of ACLF during hospitalization (solid line) and 95% CI (dotted lines) according to hemoglobin level (A), leukocyte count (B), and MELD score (C) at hospital admission. ACLF, acute-on-chronic liver failure; MELD, model for end-stage liver disease.
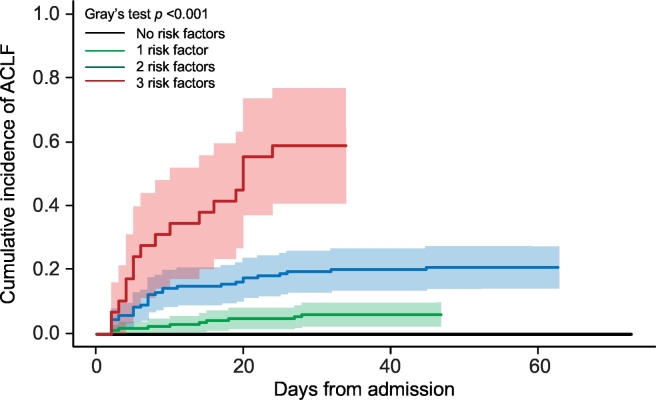
The identified risk factors were then categorized according to the optimal cut-off value associated to the highest Youden index at ROC curve analysis: 9.8 g/dl for hemoglobin, 5.6 x 109/L for leukocyte count and 13 points for MELD score. These values were used to estimate the cumulative incidence of nACLF in patients presenting none, 1, 2 or 3 risk factors considering death, LT and hospital discharge as competing events (Fig. 3). Interestingly, while all the patients who did not present any risk factor remained free from nACLF, the cumulative incidence of nACLF significantly increased in parallel with the number of risk factors, being 6% (95% CI 3–11), 21% (95% CI 15–28) and 59% (95% CI 38–75) in patients with 1, 2 or 3 risk factors, respectively (Fig. 3).
Fig. 3.
Cumulative incidence of ACLF during hospitalization in patients presenting 0, 1, 2 or 3 risk factors at hospital admission. Risk factors were hemoglobin level below 9.8 g/dl, leukocyte count and MELD score above 5.59x109/L and 13 points, respectively. ACLF, acute-on-chronic liver failure; MELD, model for end-stage liver disease.
Moreover, in CRR analysis, considering LT as competing events, MELD scores higher than 13 points and a leukocyte count above 5.59 109/L predicted 28-day mortality, while all 3 parameters were significantly associated with an increased hazard for 90-day mortality (Fig. S1).
Impact of hospital-acquired bacterial infections on the development of nACLF
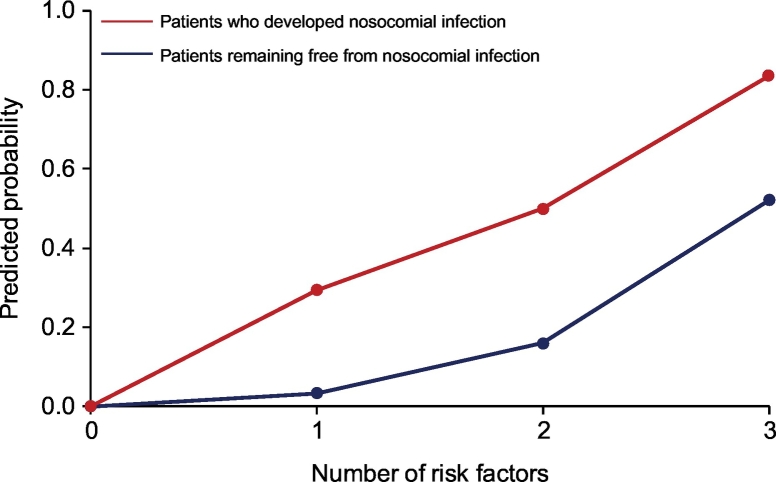
The frequency of nosocomial infections, a well-known trigger of ACLF, was significantly higher in patients who developed nACLF than in those who remained free from this complication (20/59 [34%] vs. 23/351 [7%], p ≪0.001). In 11 (55%) cases, nACLF and nosocomial infection were diagnosed concomitantly, while in the remaining 9 (45%), nACLF developed after a median of 8 (4-16) days from the diagnosis of nosocomial infection. When included in the multivariable CRR model, the onset of nosocomial infection was an independent predictor of nACLF, being associated with a 6.8-fold increased risk of developing the syndrome (Table 4). Interestingly, in each group of patients stratified according to the number of risk factors, the probability of developing nACLF was higher in patients developing nosocomial infection, rising from 3% to 29%, 16% to 50% and 52% to 83% in patients with 1, 2 or 3 risk factors, respectively (Fig. 4). Notably, the predicted probability of developing nACLF in patients not presenting any risk factor remained 0 even when they developed nosocomial infection (Fig. 4).
Table 4.
Multivariable CRR according to the Fine and Gray method with backward selection of factors associated with the development of ACLF during hospitalization.
| Parameter | sHR (95% CI) | p value |
|---|---|---|
| Nosocomial infection (yes vs no) | 6.80 (3.06–15.13) | ≪0.001 |
| MELD score (1-point increase) | 1.13 (1.08–1.18) | ≪0.001 |
| Hemoglobin (1 g/dl increase) | 0.82 (0.69–0.99) | 0.040 |
| Leukocyte (109/L increase) | 1.12 (1.07–1.17) | ≪0.001 |
In-hospital mortality, liver transplantation and hospital discharge were considered as competing events. Data are presented as sub-distribution hazard ratio and 95% confidence interval. Variable entered in the first step included also ascites, serum albumin, C-reactive protein and heart rate at hospital admission. The disease severity score to be included in the analysis was selected by the receiver-operating characteristic analysis.
ACLF, acute-on-chronic liver failure; CRR, competing risk regression; MELD, model for end-stage liver disease.
Fig. 4.
Probability of the development of ACLF during hospitalization in patients presenting 0, 1, 2 or 3 risk factors according to the development of nosocomial infection during hospitalization. ACLF, acute-on-chronic liver failure.
Outcome of nACLF
Among patients developing nACLF, 3 (5%) patients received LT and 16 (27%) died during hospital stay, a rate comparable to that observed in patients with ACLF at admission (18 [17%], p =0.123), but significantly higher than that observed in patients who remained free from ACLF (2 [1%], p ≪0.001). Moreover, no significant differences were found between patients with ACLF at admission and those developing nACLF when the 28- (25% vs. 26%, p = 0.529) and 90-day (42% vs. 45%, p = 0.491) mortality rates from the diagnosis of the syndrome were analyzed.
Among patients with nACLF who died during hospital stay, the most frequent causes of death were sepsis and liver failure (both in 31% of cases), followed by hepatorenal syndrome in 25% of cases and multi-organ failure in 13% of cases. Predictors of in-hospital mortality among parameters recorded at the time of nACLF diagnosis were reported in Table S1. Leukocyte and CRP level, which are closely related to systemic inflammation, and INR were associated with a significantly higher hazard for in-hospital mortality. No association was found with the type of organ failure except for respiratory failure, while, as expected, a significant association was observed with the grade of nACLF and CLIF-OF score (Table S1).
Discussion
The present study confirmed that ACLF is highly prevalent in patients with cirrhosis admitted to hospital because of AD, as the syndrome was diagnosed in 21% of cases at admission and subsequently occurred in 14% of cases during hospitalization. As expected, those who developed nACLF had high short-term mortality, with up to 27% dying during their hospital stay.3 Thus, the ability to identify patients at high risk of nACLF is of great importance, considering that they are hospitalized and, therefore, can be easily placed under close medical surveillance, while preventive measures can also be implemented.
From the present study, it clearly emerged that patients who developed nACLF were carrying a more severe disease at the time of hospital admission than those who remained free from this complication. Indeed, the values of all prognostic scores were significantly higher in patients who ultimately developed nACLF. These patients more often presented with ascites. Notably, although bacterial infections were frequently diagnosed at admission, their prevalence did not differ between the 2 groups of patients. This suggests that this complication, a main precipitating event of ACLF,[3], [17] does not herald the occurrence of nACLF if it has not precipitated the syndrome at the time of admission. Rather, nACLF was independently predicted by other specific parameters such as MELD score, leukocyte count and hemoglobin level.
While it may appear intuitive that nACLF is heralded by a greater severity of cirrhosis, the finding that this complication is independently predicted by leukocyte count in the absence of bacterial infection and hemoglobin level deserves discussion.
Sustained systemic “sterile” inflammation, deriving from the abnormal translocation of bacterial products from the gut and the spread of molecules deriving from cell apoptosis and necrosis in the liver, is a well-recognized pathophysiological factor underlying multi-organ dysfunction/failure in decompensated cirrhosis and ACLF.[18], [19] In the latter context, a relatively increased leukocyte count, which represents a rough but sensitive marker of inflammation, is associated with a greater severity and a higher mortality.3 The present study showed that this parameter also predicts the development of nACLF, a finding already reported in the CANONIC study.3 In addition, we identified the cut-off value of 5.6x109/L leukocytes able to discriminate patients at higher risk of nACLF even in the absence of any other predicting factor. Moreover, any further 109/L increase above this value enhanced the OR for nACLF by 1.14.
Interestingly, the leukocyte count was not among the independent predictors of ACLF at 1-year in outpatients with stable cirrhosis.4 The reasons for this discrepancy are not immediately identifiable. However, it sounds reasonable to consider the leukocyte count as a predictor of ACLF in the short term in a context like AD, where a burst of inflammation has already been primed, while other factors, pre-eminently related to the severity of the underlying cirrhosis, prevail in the long-term prediction in patients with a stable disease.
Another most interesting finding of the present study was that anemia also independently predicts the development of nACLF. The prognostic role of a reduced hemoglobin concentration in patients with cirrhosis has emerged sparsely in different settings. Indeed, hemoglobin concentration was inversely correlated with the extent of portal hypertension as assessed by hepatic venous pressure gradient,20 and the severity of cirrhosis as assessed by MELD score.21 A low level of hemoglobin was a predictor of health-related quality of life22 and a decreased survival in patients with cirrhosis.[21], [23] Moreover, a starting Hb ≤10 g/dl was an independent risk factor for early postoperative pulmonary complications after LT24 and a single measurement of hemoglobin predicted overall survival in patients with hepatocellular carcinoma.25 The prognostic importance of a reduced hemoglobin concentration has also been reported in patients with ACLF, in whom it predicted delayed mortality.[26], [27]
More pertinent to the findings of the present study is the recent report that a median level of 10.8 g/dl of blood hemoglobin concentration was among the independent predictors of ACLF development in stable outpatients with cirrhosis.4 Our results showed that this is also the case in the setting of nACLF, with a cut-off value of 9.8 g/dl. There are a number of factors contributing to anemia in patients with cirrhosis, including chronic occult blood loss leading to iron depletion, malnutrition, reduced hepatic synthesis of tetrahydrofolate, hypersplenism and hemolysis.28 The chronic inflammatory milieu that characterizes advanced cirrhosis might also play a role, as inflammation is known to favor macrophage iron sequestration, reduced erythropoietin synthesis and reduced responsiveness of erythropoietin receptors.29 In the setting of cirrhosis with AD, acute gastrointestinal bleeding should also be considered. Notably, in the present study, the prevalence of this complication at the time of admission to hospital was low (about 5% of cases) and did not differ between patients who then developed nACLF and those who did not. Thus, the predicting power of anemia in our patients cannot be seen as a consequence of hypovolemia induced by an acute bleeding. Rather, it is conceivable than anemia may predispose to inflammation-induced peripheral organ dysfunction and, ultimately, failure by reducing tissue oxygen availability.
The results of the present investigation also allowed us to stratify patients with cirrhosis and AD into different, clear-cut levels of risk for the development of nACLF in a very simple way, assessable shortly after admission to hospital. In fact, the categorization of the 3 predicting factors according to their optimal cut-offs (9.8 g/dl for hemoglobin, 5.6x109/L for leukocyte count and 13 points for MELD score) can be used to estimate different incidences of nACLF. A most interesting finding was that nACLF never occurred in patients who did not present any risk factor. Moreover, the incidence of the syndrome paralleled the number of the risk factors, from 6% in patients presenting 1 factor to 59% in those presenting all 3 factors, irrespective of their combination.
A last issue assessed by the present study was the impact of nosocomial bacterial infections on the occurrence of nACLF. As possibly expected, since bacterial infections are a well-recognized precipitating event,30 their frequency was far higher in patients who then developed nACLF. Their predicting importance was further highlighted by the finding that they were independently associated with the development of nACLF at the multivariable LR analysis, which also included MELD score, leukocyte count and blood hemoglobin concentration. The occurrence of nosocomial bacterial infections was obviously not included in the first multivariable LR analysis, which was based on factors available at admission. When the risk for nACLF evaluated by the combination of MELD score, leukocyte count and hemoglobin concentration was re-assessed grouping patients according to the occurrence of nosocomial bacterial infections, it clearly emerged that this event substantially amplified the risk. Indeed, while the risk for nACLF ranged from 3% to 52% in patients who remained free from bacterial infections, it was enhanced from 29% to 83% in those who suffered this complication. It should be noted that, once more, all patients who did not present any of the 3 risk factors remained free from nACLF even if they developed a nosocomial bacterial infection. Trying to transfer our findings into clinical practice, the risk for nACLF in patients with cirrhosis hospitalized because of AD warrants a first evaluation at admission, which should be repeated if they develop a bacterial infection.
In conclusion, the present prospective investigation identified 3 factors predicting the occurrence of nACLF in patients with cirrhosis hospitalized because of AD and provided a means to stratify this risk at admission. This evaluation is based on very simple and quickly achievable data, such as MELD score calculation and a whole blood cell count; it can also be profitably employed in patients subsequently developing nosocomial bacterial infections. We acknowledge that our results would need validation in a different patient cohort, but we think that they provide a solid foundation on which to build further insight.
Financial support
The study was supported by a grant from Italian Ministry of Health (rf-2010-2310623), a grant from the Emilia-Romagna Region (PRUa1GR-2012-002) and by Fondazione del Monte di Bologna e Ravenna. The funders did not have any involvement in study design, in the collection, analysis and interpretation of data, in the drafting of the manuscript, and in the decision to submit the article for publication.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
PC, MaBe, MD, GZ, MaBa: study concept and design, interpretation of data, drafting of the manuscript; GZ, MaBa, MT, MiBa, SB, MaTa, LN, AF, LM, AA, GI: collection of data; MaBa, GZ: analysis of data; GZ, MaBa, MD, AS, FT, PV, MaBe, PC: critical revision for important intellectual content.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.07.005.
Supplementary data
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Prim. 2016;2 doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 3.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1-9. [DOI] [PubMed] [Google Scholar]
- 4.Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017;67:1177–1184. doi: 10.1016/j.jhep.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N Engl J Med. 1996;334:693–700. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Thermeau TM, Ksberg CL. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 8.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T. Evidence-Based Incorporation of Serum Sodium Concentration Into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 10.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Jalan R, Pavesi M, Saliba F, Amoros A, Fernandez J, Holland-Fischner P. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Moore K, Wong F, Gines P, Bernardi M, Ochs A, Salerno F. The management of ascites in cirrhosis: Report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 13.Blei AT, Córdoba J. Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 14.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. http://www.ncbi.nlm.nih.gov/pubmed/10673079. Accessed March 20, 2019. [DOI] [PubMed] [Google Scholar]
- 16.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P. Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. December 2018 doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64 doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 20.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker R, Armstrong MJ, Bruns T, Hodson J, Rowe IA, Corbett CD. Reticulocyte count and hemoglobin concentration predict survival in candidates for liver transplantation. Transplantation. 2014;97:463–469. doi: 10.1097/01.TP.0000437429.12356.03. [DOI] [PubMed] [Google Scholar]
- 22.Les I, Doval E, Flavià M, Jacas C, Cardenas G, Esteban R. Quality of life in cirrhosis is related to potentially treatable factors. Eur J Gastroenterol Hepatol. 2010;22:221–227. doi: 10.1097/MEG.0b013e3283319975. [DOI] [PubMed] [Google Scholar]
- 23.Moller S, Hobolth L, Winkler C, Bendtsen F, Christensen E. Determinants of the hyperdynamic circulation and central hypovolaemia in cirrhosis. Gut. 2011;60:1254–1259. doi: 10.1136/gut.2010.235473. [DOI] [PubMed] [Google Scholar]
- 24.Jiang G-Q, Bai D-S, Chen P, Fan J, Tan J-W, Peng M-H. Starting hemoglobin value predicts early phase prognosis after liver transplantation. Transplant Proc. 2011;43:1669–1673. doi: 10.1016/j.transproceed.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 25.Finkelmeier F, Bettinger D, Köberle V, Schultheiß M, Zeuzem S, Kronenberger B. Single measurement of hemoglobin predicts outcome of HCC patients. Med Oncol. 2014;31:806. doi: 10.1007/s12032-013-0806-2. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Zheng MH, Yang Y, Wei W, Yang Q, Hu A. Increased delayed mortality in patients with acute-on-chronic liver failure who have prior decompensation. J Gastroenterol Hepatol. 2015;30:712–718. doi: 10.1111/jgh.12787. [DOI] [PubMed] [Google Scholar]
- 27.Zheng MH, Shi KQ, Lin XF, Xiao DD, Chen LL, Liu WY. A model to predict 3-month mortality risk of acute-on-chronic hepatitis B liver failure using artificial neural network. J Viral Hepat. 2013;20:248–255. doi: 10.1111/j.1365-2893.2012.01647.x. [DOI] [PubMed] [Google Scholar]
- 28.Mehta AB, McIntyre N. Haematological disorders in liver disease. Forum (Genova) 1998;8:8–25. http://www.ncbi.nlm.nih.gov/pubmed/9514991. Accessed March 18, 2019. [PubMed] [Google Scholar]
- 29.Cullis JO. Diagnosis and management of anaemia of chronic disease: current status. Br J Haematol. 2011;154:289–300. doi: 10.1111/j.1365-2141.2011.08741.x. [DOI] [PubMed] [Google Scholar]
- 30.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material