Background & Aims
Autoimmune hepatitis (AIH) is an immune-mediated disease with no curative treatment. Regulatory T cell (Treg) therapy is potentially curative in AIH given the critical role of Tregs in preventing autoimmunity. To work effectively, adoptively transferred Tregs must migrate to and survive within the inflamed liver. We conducted a proof-of-concept study aiming to assess the safety and liver-homing properties of good manufacturing practice (GMP)-grade autologous Tregs in patients with AIH.
Methods
Autologous polyclonal GMP-grade Tregs were isolated using leukapheresis and CliniMACS, labelled with indium tropolonate and re-infused intravenously to 4 patients with AIH. GMP-Treg homing to the liver was investigated with longitudinal gamma camera and SPECT-CT scanning. GMP-Treg immunophenotype, function and immunometabolic state were assessed during the study.
Results
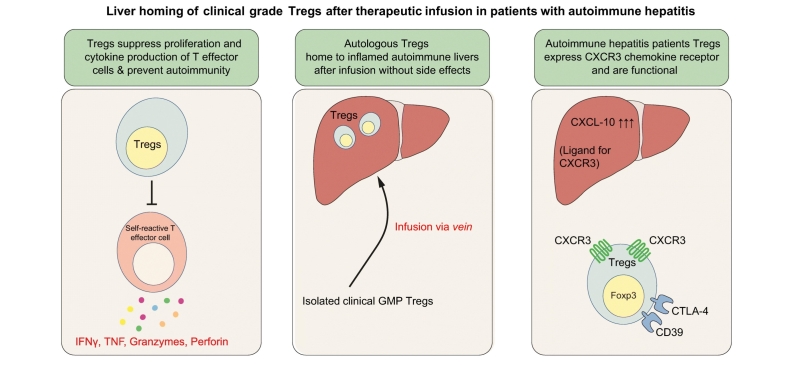
We observed that the isolated Tregs were suppressive and expressed CXCR3, a chemokine receptor involved in recruitment into the inflamed liver, as well as Treg functional markers CD39, CTLA-4 and the transcription factor Foxp3. Serial gamma camera and SPECT-CT imaging demonstrated that 22–44% of infused Tregs homed to and were retained in the livers of patients with autoimmune hepatitis for up to 72 h. The infused cells did not localise to any off-target organs other than the spleen and bone marrow. GMP-Tregs were metabolically competent and there were no infusion reactions or high-grade adverse effects after Treg infusion.
Conclusion
Our novel findings suggest that the liver is a good target organ for Treg cellular therapy, supporting the development of clinical trials to test efficacy in autoimmune hepatitis and other autoimmune liver diseases.
Lay summary
Autoimmune liver diseases occur when the body’s immune cells target their own liver cells. Regulatory T cells (Tregs) prevent autoimmunity, thus they are a potential therapy for autoimmune liver diseases. In patients with autoimmune hepatitis, Treg infusion is safe, with nearly a quarter of infused Tregs homing to the liver and suppressing tissue-damaging effector T cells. Thus, Tregs are a potentially curative immune cell therapy for early autoimmune liver diseases.
Keywords: Autoimmune hepatitis, regulatory T cells, human liver, homing, cell therapy
Graphical abstract
Highlights
-
•
Tregs from patients with autoimmune hepatitis are suppressive, possess functional markers CD39 and CTLA-4, and express CXCR3.
-
•
Treg infusion in autoimmune liver disease is safe without any side effects.
-
•
22-44% of infused Tregs home to and were retained in the livers of patients with autoimmune hepatitis for up to 72 hours.
-
•
GMP-Tregs from patients with autoimmune hepatitis were metabolically competent.
Introduction
Autoimmune hepatitis (AIH) is an immune-mediated liver disease. Its pathogenesis is poorly understood although genetic, dietary and environmental factors have been implicated.1 It is characterised biochemically by the presence of elevated serum aminotransferase levels, histologically by interface hepatitis and immunologically by increased levels of immunoglobulin G (IgG) in the presence of autoantibodies.2 The condition is T cell-mediated and occurs as a result of immune dysregulation, including dysfunctional Tregs,3 which allow auto-reactive T cells to attack hepatocytes resulting in lobular and interface hepatitis.3,4 There is no curative therapy for AIH5 and most patients require life-long immunosuppression, putting them at risk of serious side effects.6,7 If hepatic inflammation is not controlled, persistent hepatitis progresses to cirrhosis and liver failure. Many patients ultimately require liver transplantation6 after which the disease can recur.
Tregs are a subset of CD4 T cells that maintain peripheral immune homeostasis by suppressing a range of effector immune responses to allow immune resolution and establish homeostatic balance between immune activation and tolerance.8,9 In 1995, Sakaguchi and colleagues showed by adoptive transfer that subsets of CD4 T cells expressing the IL-2 receptor α-chain (CD25) prevent autoimmunity by actively suppressing self-reactive lymphocytes.10 CD4+CD25high T cells constitute 5–10% of CD4 T cells in the blood. Their development and function is controlled by Foxp3, defects in which lead to autoimmune and inflammatory syndromes in humans and mice.11 Expression of the IL-7 receptor, CD127 correlates inversely with Foxp3 and Treg suppressive function; hence Tregs are now defined as CD4+CD25highCD127lowFoxp3+ cells.12
Failure of Tregs to suppress effector cells is a typical feature of autoimmunity, including AIH, leading to studies exploring the use of either polyclonal13,14 or antigen-specific Tregs[15], [16], [17] as cellular therapies for autoimmune diseases. To work effectively, adoptively transferred Tregs must home to and mediate suppression at the target tissue. Chemokines direct the trafficking and positioning of leukocytes within tissues18,19 and deficiency in CXCR3 which drives recruitment across hepatic sinusoids3,20 has been associated with the exacerbation of liver disease and abrogation of tolerance in mouse models of immune-mediated hepatitis.21
In this first proof-of-concept study of Treg homing to the human liver, we investigated the functionality and in vivo trafficking of autologous, polyclonal GMP-grade Tregs in patients with AIH. We report the cells are suppressive and metabolically competent and when infused intravenously traffic to the liver and spleen with little uptake in other tissues. Thus, AIH is a good target disease for Treg therapy.
Materials and methods
Patients
Five patients with AIH (median age 39 years, range 22–64 years) were screened. All had good peripheral venous access, met the inclusion and exclusion criteria (Box 1) and were enrolled after obtaining informed consent. The primary outcome was the tissue distribution of the indium-labelled infused Tregs over 72 h following infusion, as assessed by serial gamma camera and SPECT-CT imaging. Secondary outcome measures were the safety and tolerability of Treg infusions and longitudinal changes in immunophenotyping. Primary and secondary objectives and outcome measures of the study were described in Table 1. Ethical approval was obtained for this study (IRAS ID: 177127).
Box 1. Inclusion and exclusion criteria of AUTUMN patients.
Inclusion criteria
-
•
Age ≥18 and ≪70 years at screening
-
•
Diagnostic criteria for autoimmune hepatitis based on recommendations of the International Autoimmune Hepatitis Group
-
•
Non-cirrhotic or compensated cirrhosis
-
•
Leukocyte count ≥4 x 109/L
-
•
World Health Organization (WHO) performance status of 0-1
-
•
Females of childbearing potential must have a negative pregnancy test prior to starting study intervention
-
•
All sexually active women of childbearing potential must agree to use a highly effective method of contraception from the screening visit throughout the study period and for 99 days following Treg infusion
-
•
All sexually active males must agree to use reliable forms of contraception during the study and the 4-month follow-up period
-
•
Patients who can give informed consent
Exclusion criteria
-
•
Post liver transplant or listed for liver transplantation
-
•
Past surgical history of liver resection (including partial/hemi hepatectomy)
-
•
Liver disease other than autoimmune related liver disease
-
•
Positive for blood borne viruses HBV, HCV, HIV, HTLV-1, HTLV-2 or syphilis
-
•
Evidence of other active inflammatory disease or sepsis
-
•
Pregnancy or breastfeeding
-
•
Poor venous access
-
•
Decompensated cirrhosis
-
•
Clinically significant cardiovascular disease (ischaemic heart disease, heart failure)
-
•
A history of any underlying previous malignancy
-
•
Previous allergy to radio-contrast reagents
-
•
Patients with metallic objects fitted to the body
-
•
Any other physical or psychiatric disorder that may interfere with compliance, adequate informed consent, follow up or determine the adverse events
Alt-text: Box 1
Treg, regulatory T cell.
Table 1.
Primary and secondary objectives and outcome measures. CTCAE, Common Terminology Criteria for Adverse Events; SPECT-CT, single photon emission computed tomography; Treg, regulatory T cell.
| Objectives | Outcome measures |
|---|---|
| Primary | Primary |
| To study the trafficking behaviour and tissue localisation of indium-labelled autologous GMP-Tregs for up to 72 h after infusion. | Gamma camera imaging at 4, 24 and 72 h post Treg reinfusion to assess the kinetics of trafficking plus SPECT-CT at 24 h to quantify tissue uptake of Treg. |
| Secondary | Secondary |
| To monitor safety and tolerability of Treg infusions. To measure immunological changes associated with Treg infusions |
Adverse events and signs of toxicity were measured by CTCAE criterion/grading score and any changes in clinical, biochemical, immunological tests Immunological changes were assessed by longitudinal immunophenotyping and measurement of serum cytokines and chemokines. |
Study methodology
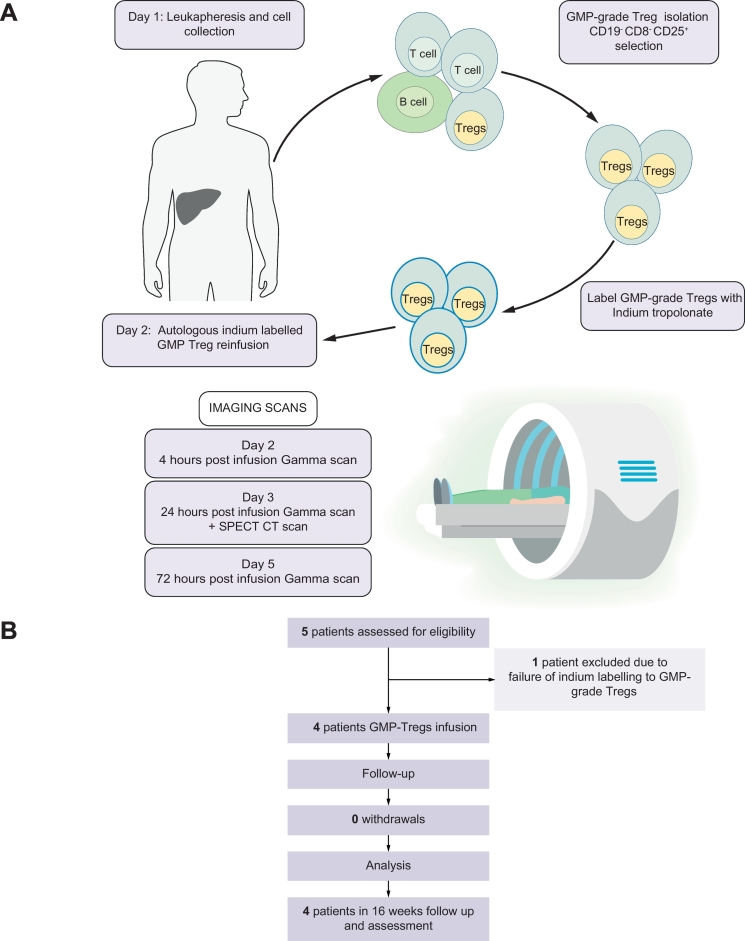
AUtologous T-regulatory cell tracking after InfUsion in AutoiMmuNe Liver Disease (AUTUMN) was a single-centre, single-arm, phase 0, feasibility pilot study, assessing the localisation of autologous GMP-grade Tregs in 4 patients with AIH. Tregs were radiolabelled with indium, infused into patients and tracked over 72 h using gamma and SPECT-CT scanning. Between 16th September 2016 and 11th July 2017, a total of 5 patients were recruited and seen at the Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust (UHB NHSFT), Birmingham, UK. Of these, 4 completed the study. The UHB NHSFT Research & Development (R&D) department and the East of Scotland Research Ethics Service REC in the UK approved the study protocol (IRAS ID: 177127). The University of Birmingham (Birmingham, UK) acted as sponsor and the Cancer Research Clinical Trials Unit (CRCTU), Birmingham, UK monitored the trial and provided annual reports to the Research Ethics Committee. Ethical approval was given by the UK National Research Ethics Service (reference 15//0135) East of Scotland Research Ethics Committee. Fig. 1 outlines the study design (1A) and provides a summary of the study profile (1B).
Fig. 1.
Graphical representation of AUTUMN Study.
(A) Schedule of activities during intervention week between days 1 and 5. (B) Study profile indicating the number of patients to complete each stage of the study from screening, enrollment and leukapheresis to GMP-Treg infusion and 16 weeks follow-up. GMP, Good Manufacturing Practice; Treg, regulatory T cell.
Leukapheresis and GMP-Treg isolation
Leukocytes were obtained by a twice-blood-volume leukapheresis using Spectra Optia Apheresis and the mononuclear cell collection protocol. GMP-grade Tregs were then isolated using CliniMACS technology to deplete CD8 T cells and CD19 B cells and enrich CD4+CD25high cells (see supplementary methods). The purity of GMP Tregs was verified by flow cytometry and cells were transferred to the radiopharmacy department and labelled with clinical grade 111Indium tropolonate (see supplementary methods).
Preparing 111Indium tropolonate
111Indium tropolonate was prepared from 111indium chloride and 0.054% tropolone. The activity of the 111Indium Tropolonate was measured in the vial on a Capintec CRC25r calibrator using factor 216 with a copper filter. Cells for labelling were resuspended in 0.01% plasma in saline. The volume of indium chloride required was calculated to provide 100 MBq and activity measured on the Capintec CRC25r using factor 216 with the copper filter. The measured 111indium tropolonate was added drip-wise to the cells and incubated for 20 min at room temperature, behind lead shielding. Following incubation, cells were made up to 15 ml with 1% plasma in saline and centrifuged at 1,000 rpm for 5 min. Activity in the supernatant was then measured in the Capintec CRC25r calibrator using Factor 216 with the copper filter. This process was repeated. The labelling efficiency of the clinical grade cells was then calculated and the volume of labelled cells required calculated for a patient dose of up to 20 MBq.
Only 4 patients received indium tropolonate labelled Treg infusions because cells from the first patient failed to label sufficiently. The freshly labelled GMP-Tregs were then infused back into patients via a peripheral vein on the same day.
Method for gamma camera scanning and quantitation of uptake at 24 h in SPECT-CT
The activity of the full and used 111Indium tropolonate -In syringe was measured on a CRC25R dose calibrator (Capintec Inc., USA) using a Thomson copper filter (Southern Scientific Ltd., UK). Anterior and posterior partial body images (vertex of head to mid-thigh) were acquired at approximately 4 ± 1 h, 24 ± 4 h and 72 ± 4 h post-injection on a gamma camera (Symbia T, Siemens USA) with medium energy general purpose collimator and scan speed 10 cm/min. In addition, at the 24-h time point, SPECT-CT of the same body region was performed (120 azimuths non-circular acquisition with up to 15 s per view). SPECT images were produced using OSEM iterative with scatter correction and CT attenuation correction (Hybid Reconstruction, Hermes Medical Solutions). Counts in the reconstruction SPECT images were converted to activity concentrations (Bq/ml) using a calibration factor previously obtained by scanning test objects containing known activities of 111 indium. The reconstructed SPECT images were segmented by growing volumes of interest on the reconstructed SPECT slices or delineating organs on the CT image (Hybrid Viewer, Hermes Medical Solutions). The activities were calculated for 7 organs of interest: liver, spleen, lungs, bone marrow (arms, leg, spine, pelvis and head excluding brain), heart, brain and gut (taken to be the volume of the abdomen excluding the liver, spleen and spine). These activities were expressed as a percentage of injected activity after correcting for radioactive decay of 111Indium.
Regions of interests (ROI) were drawn on the right liver lobe and part of the spleen in the anterior and planar whole body scans. Geometric means of background corrected counts in the ROI for each partial organ were calculated at the 3 time points. The geometric mean count for each partial organ in the 24 h whole body scan was “normalised” to the corresponding estimated activity for the whole organ from the quantitative SPECT-CT scan. This enabled the geometric means from the whole body scans at the 3 time points to be converted to activities and, following decay correction, to percentage of injected activity in the liver and spleen.
Clinical laboratory tests
Clinical, biochemical (liver enzymes, bilirubin, renal function), immunological (IgG level, auto-antibodies) and radiology imaging (Ultrasound of liver) data were collected by the NIHR Biomedical Research Centre research nurse and chief investigator.
Immunometabolic analysis and GMP-grade Treg suppression of T responder cell assay
Metabolic analysis was carried out using an Extracellular Flux Analyzer XFe96 (Seahorse Bioscience). Briefly 1.0 x 105 CD4+ Tregs, CD4+ non-Tregs and CD8+ T cells were seeded onto a Cell-Tak (Corning) coated microplate. Mitochondrial and glycolytic parameters were measured via oxygen consumption rate (OCR) (pmoles/min) and ECAR (mpH/min) respectively with use of real-time injections. Cells were resuspended in XF assay media supplemented with 5.5 mM glucose and 1 mM pyruvate and injections of oligomycin (1 μM), carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone) (FCCP; 1 μM) and rotenone and antimycin (both 1 μM) were used. All chemicals were purchased from Sigma unless stated otherwise. Calculations for individual metabolic parameters can be found as per manufacturer’s instructions (Seahorse Bioscience).
Cytokine and chemokine analysis
Levels of cytokines, chemokines and growth factors in the peripheral blood were analysed by luminex on serum samples collected on days 1, 3, 5, week 2, week 4 and week 8. One 4 ml serum-separating tube of peripheral blood was collected at each time point during the intervention. After being left for 1 h to settle at room temperature the sample was centrifuged at 10,000x g for 10 min and the serum aliquoted and frozen at -80°C until the end of the trial when all samples were analysed in parallel. Samples were analysed using the Bio-Plex ProTM Human Cytokine 27-plex Assay, TGF-β 3-plex Assay and Th17 Cytokine IL-22 and IL-23 sets according to manufacturer’s instructions (BioRad).
For further details regarding the materials and methods used, please refer to the CTAT table and supplementary information.
Results
Patient characteristics
Five patients who met the eligibility criteria (Box 1) were enrolled to the study. Four received a single infusion of indium tropolonate labelled autologous, polyclonal GMP-grade Tregs and were followed up for 4 months. Indium labelling of the first patient’s Tregs did not achieve the release criteria, thus no infusion was carried out*. The mean age was 39 years, and mean disease duration was 46 months at the time of screening. Out of 4 patients, 3 had established compensated cirrhosis at the time of Treg infusion. Table 1 describe primary and secondary objectives and outcome measures of the study.
The demographic, biochemical, immunological and liver disease severity profile of the patients are described in Table 2A. All patients were receiving immunosuppressive treatments which are detailed in Table 2B.
Table 2.
Patient characteristics and immunosuppressive medications taken.
| Patient 1* | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| A) Patient characteristics | |||||
| Age, years | 29 | 46 | 26 | 64 | 22 |
| Gender | F | F | M | F | F |
| Ethnicity | Caucasian | Caucasian | African | Caucasian | Caucasian |
| ALT, IU/L | 200 | 24 | 16 | 15 | 19 |
| Creatinine, μM/L | 78 | 63 | 57 | 58 | 59 |
| IgG, g/L | 22 | 21 | 15 | 13.3 | 11.7 |
| ANA | 1:1,600 | Negative | 1:400 | 1:1,600 | 1:400 |
| LKM | Negative | Negative | Negative | Negative | Negative |
| F-Actin | Positive | Positive | Negative | Negative | Negative |
| SLA | Negative | Positive | Negative | Positive | Positive |
| SMA | Positive | 1:160 | 1:320 | 1:40 | 1:80 |
| UKELD | 50 | 46 | 45 | 44 | 43 |
| B) Immunosuppressive medications | |||||
| Budesonide | 6 mg | ||||
| Prednisolone | 15 mg | 7.5 mg | 20 mg | 10 mg | |
| Azathioprine | 50 mg | 100 mg | 75 mg | ||
| Tacrolimus | 4 mg | ||||
| Mycophenolate mofetil | 2 g | ||||
(A) Patients’ characteristics. Patient demographics and blood levels of ALT, creatinine, immunoglobulin G (IgG), auto-antibody profile including ANA, LKM, SLA, SMA and UKELD scores. Patient 1* underwent leukapheresis and GMP-Treg isolation but did not receive cells due to poor labelling. (B) Immunosuppressive medications taken by the patients. Total daily doses are shown. ALT, alanine aminotransferase; ANA, anti-nuclear antibody; LKM, anti-liver, kidney, microsomal antibody; SLA, soluble liver antigen antibody; SMA, anti-smooth muscle antibody; Treg, regulatory T cell; UKELD, United Kingdom end-stage liver disease.
Leukapheresis and GMP Treg isolation
Patients underwent twice-blood-volume leukapheresis at the haematology day unit, Queen Elizabeth Hospital Birmingham and Tregs were isolated from the buffy coat using a GMP-compliant protocol that we developed at the National Health Service Blood and Transplant Unit, Birmingham. CD19+B cells and CD8+T cells were removed then CD25+ cells enriched using a CliniMACS cell-isolator. Between 8.9 × 106 and 86 × 106 purified CD4+CD25high Tregs were obtained (Table 3). The freshly isolated Tregs were 92.2% Foxp3 (range: 76–96.9%) and met the release criteria of high viability (target ≥90%, actual ≫98%), CD4+ percentage (≥95%), and minimal CD8 T cell (target = 1 log reduction, actual ≫2.5 log reduction) or CD19 B cell contamination (target = 1 log reduction, actual ≫2.5 log reduction). GMP-Treg product characteristics are described in Table 3A.
Table 3.
GMP-grade Treg product characteristics and details of indium tropolonate labelling of GMP-Tregs prior to infusion.
| Patient 1* | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| A) GMP-grade Treg product characteristics | |||||
| Total GMP-Tregs | 32.9 x 106 | 8.9 x 106 | 86 x 106 | 26.1 x 106 | 21.5 x 106 |
| CD8 x 106 | 0.01 | 0.019 | 0.28 | 0.017 | 0.015 |
| CD20 x 106 | 0.006 | 0.044 | 0.66 | 0.003 | 0.006 |
| Treg viability, % | 99.53 | 99.7 | 99.02 | 99.56 | 99.43 |
| B) Details of indium tropolonate labelling of GMP-Tregs prior to infusion | |||||
| Initial activity of indium, Mbq | 108 | 88 | 125 | 116 | 116 |
| Post- labelled Treg viability, % | 98 | 95 | 96.5 | 96.2 | 98.5 |
| Mbq labelled GMP-Tregs, Mbq | 0 | 6.8 | 20.6 | 19.8 | 15.4 |
(A) GMP-grade Treg product characteristics. GMP-grade Tregs frequency, purity (log reduction of CD8 and CD19 along with purity of CD4positive CD25high GMP-grade Tregs) and viability following CliniMACS isolation. All products were negative for bacterial, fungal and blood borne viruses (HIV, HTLV, HCV, HBV). *Treg product from patient 1 was not reinfused as a result of poor indium labelling. (B) Details of indium tropolonate labelling of GMP-Tregs prior to infusion. Treg product from patient 1* was not reinfused as a result of poor indium labelling thus, only 4 of the 5 patients were evaluated. Once 111indium tropolonate labelling was completed, the labelling efficiency was calculated and the numbers of labelled GMP-Tregs to be infused were adjusted, aiming for a dose of up to 20 Mbq. Indium-labelled GMP-Tregs were assessed for viability (≫90%) before being infused immediately into the same patient via a peripheral vein injection on the same day of labelling. All infused patients has indium labelling efficiency of more than 90%. Labelling efficiency was calculated by activity of indium-labelled Tregs divided by indium activity in supernatant.
GMP, Good Manufacturing Practice; Treg, regulatory T cell.
111Indium tropolonate labelling of GMP-Tregs
Freshly isolated GMP-Treg were labelled with indium tropolonate. Table S4 reports the total number of CD4+CD25high GMP-Tregs isolated, the amount of indium used, cell viability post indium labelling and final radio-labelling (Mbq). Up to 20 Mbq is the radiological safety approved limit defined by the UK’s Radioactive Substances Advisory Committee for the infusion of indium labelled lymphocytes for imaging. The details of indium tropolonate labelling of GMP-Tregs prior to infusion are described in Table 3B.
Function and phenotype of GMP Tregs prior to infusion
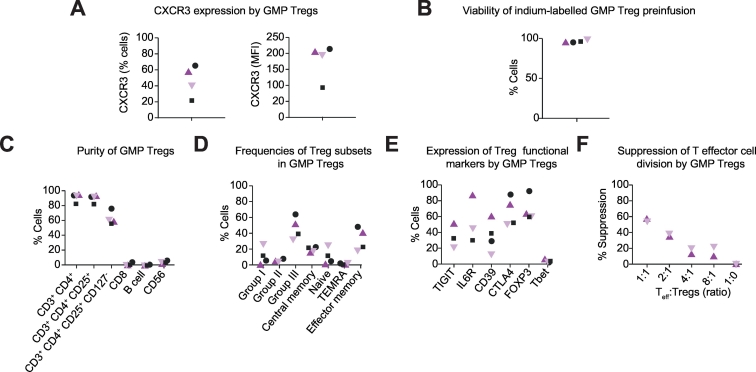
The gating strategy for immune cell subsets was shown in Fig. S1. The majority of the Tregs expressed high levels of the liver-homing chemokine receptor CXCR3 (Fig. 2A)(% cells: median 50%, range 20-65%; IQR 37%) (MFI: median 200, range 95-215; IQR 94). Cells were ≫95% viable (Fig. 2B), pure (Fig. 2C) and sterile as confirmed by bacterial and fungal testing. GMP-grade Tregs had an effector memory phenotype (Fig. 2D) and expressed moderate to high levels of Treg functional markers including CTLA-4 (median 64%, range 55–90%, IQR 35%) and Foxp3 (60–90%;) (Fig. 2E). These phenotypes were not affected by indium labelling (Fig. S2). The Tregs suppressed T responder cells in standard in vitro suppression assays (Fig. 2F).
Fig. 2.
Immunophenotype and viability of the autologous GMP-Tregs.
(A) CXCR3 expression. (B) Viability post indium labelling and immediately before infusion. (C) Purity (CD3+CD4+CD25+CD127neg Tregs expressed as percentage of total). (D) Memory/naïve phenotype showing the proportions of CD45RA+CCR7+ (naïve), CD45RAnegCCR7+ (central memory), CD45RAnegCCR7neg (effector memory) and CD45RA+CCR7neg (tissue-resident effector memory RA-positive, TEMRA). (E) Expressions of Treg functional surface proteins. (F) Suppressive potential. GMP, Good Manufacturing Practice; Treg, regulatory T cell.
Immunometabolic analysis of Tregs
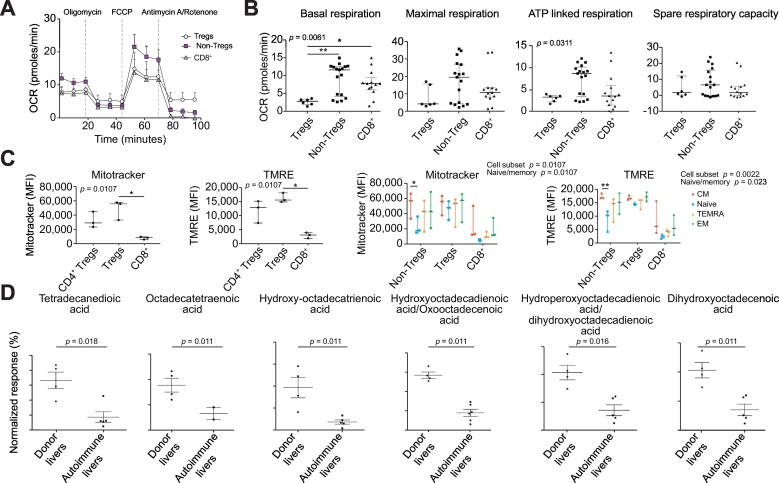
We investigated whether the CD4+CD25highCD127low Tregs from the patients were metabolically competent and thus able to survive and function after infusion. We compared the metabolic profiles of these Tregs with CD4 non-Tregs and CD8 T cell subsets. We measured the mitochondrial OCR (Fig. 3A) and extracellular acidification rate (ECAR, Fig. S3A) in a Seahorse Extracellular Flux Analyzer XFe96. Injections of oligomycin (ATP synthase inhibitor), FCCP (proton ionophore) and antimycin A/rotenone (complex III and I inhibitors) were added to the cells in a timed sequence. The assay revealed differences in the metabolism of the 3 subsets. Tregs had lower basal respiration, ATP-linked respiration and maximal respiration when compared to CD4 non-Tregs and CD8 T cells (Fig. 3B). The spare respiratory capacity was similar for all 3 populations (Fig. 3B) and there were no differences in basal or maximal glycolysis levels between the 3 subsets (Fig. S3B,C). The mitochondrial content and membrane potential of each cell subset was measured using flow cytometry analysis of Mitotracker® Deep Red and tetramethylrhodamine ethyl ester (TMRE) uptake respectively. This revealed that Tregs had an elevated mitochondrial content (Mitotracker; Fig. 3C) and functional capacity (membrane potential) (TMRE; Fig. 3C) when compared with CD4 non-Tregs and CD8 T cells. A subsequent analysis dividing the cells into naïve, central memory (CM), effector memory (EM) and tissue-resident effector memory RA (TEMRA) subsets (Fig. S3D) showed that mitochondrial content and functional capacity were highest in the memory cells (Fig. 3C and Fig. S3E). Since Tregs have been shown to favour mitochondrial fatty acid oxidation as their metabolic pathway for ATP synthesis,22,23
Fig. 3.
Immunometabolic profile of leukapharesis Tregs.
(A) OXPHOS profiles; (B) respiratory parameters including: basal respiration, maximal respiration, ATP-linked respiration and spare respiratory capacity; (C) mitochondrial content (MitoTracker) and membrane potential (TMRE) of CD4 non-Treg, Treg and CD8 T cells are shown. (D) Long chain fatty acid levels in normal donor (n = 4) and autoimmune diseased (n = 7) liver tissue supernatants. Statistical significance was tested by Kruskal-Wallis test with Dunn’s multiple comparisons post-hoc test (A-B); 2-way ANOVA with Tukeys multiple comparisons post hoc test (C) and Mann-Whitney U test with Benjamin-Hochberg correction for multiple testing. Data expressed as mean *p ≤0.05. Treg, regulatory T cell.
Homing of infused indium-labelled polyclonal Tregs
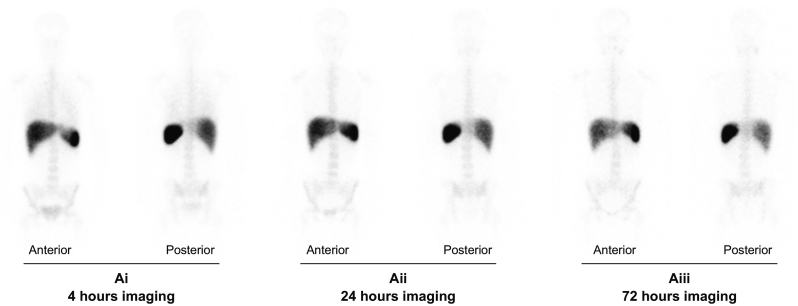
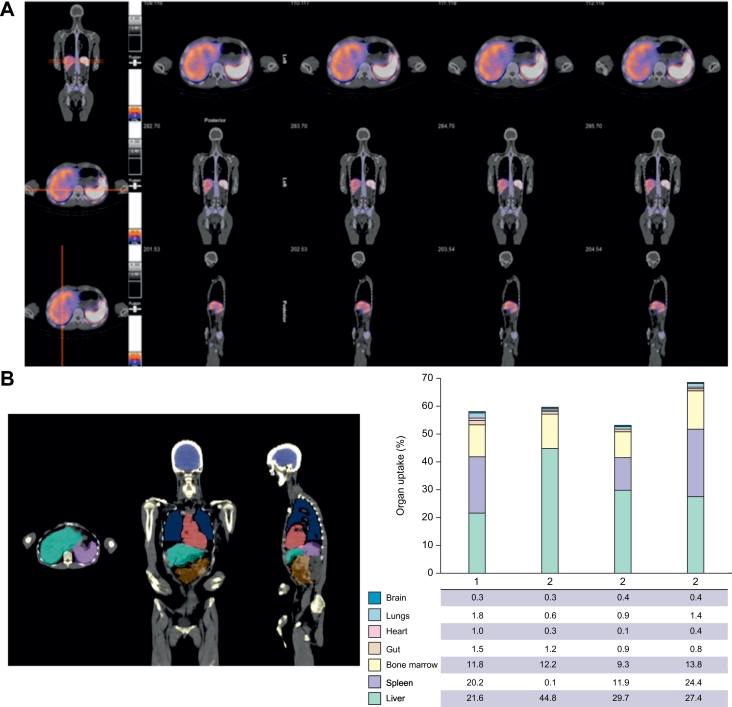
Serial gamma camera imaging was carried out at 4 h (Fig. 4Ai), 24 h (Fig. 4Aii) and 72 h (Fig. 4Aiii) following Treg infusions. Quantification of homing to different organs was investigated by SPECT-CT at 24 h after Treg transfer with axial, coronal and sagittal imaging positions (Fig. 5A). Of infused GMP-Tregs, 22–44% were detected in the liver at 24 h with minimal localisation (1%) in the brain, lungs, heart, gut or pelvic organs (Fig. 5B and 5C; Imaging video in supplementary material). Bone marrow uptake varied between 9–13% and splenic uptake between 11–24% (Fig. 5C). Individual patient Gamma camera image scan following GMP-Treg therapy was shown in Fig. S6 and 7.
Fig. 4.
Serial Gamma camera scanning of indium labelled GMP-Treg after infusion.
Serial Gamma camera imaging performed at 4 h (Ai), 24 h (Aii) and 72 h (Aiii) post GMP-Treg infusion demonstrates the presence of indium tropolonate labelled GMP-Tregs in the liver, spleen and bone marrow. GMP-Tregs were present and remained in the liver for up to 72 h (Aiii). (Anterior = Gamma camera imaging scan from the anterior view; posterior = Gamma camera imaging scan from posterior view). GMP, Good Manufacturing Practice; Treg, regulatory T cell.
Fig. 5.
SPECT-CT axial, coronal and sagittal imaging of GMP-Tregs at 24 h after infusion and cell distribution.
(A) Fused images showing CT (black and white) with functional colours overlay of SPEC-CT data. Top-bottom axial, coronal and sagittal. Moderate to intense uptake of indium labelled GMP-Tregs is shown in the liver and the spleen. (B) Anatomical delineation of brain, heart, lungs, liver, gut and spleen on multi-planar CT. (C) Relative uptake of indium labelled GMP-Tregs in different organs expressed as the percentage of injected activity. GMP, Good Manufacturing Practice; Treg, regulatory T cell.
Safety and clinical parameters
All patients underwent leukapheresis without complication. No significant infusion-related reactions, citrate toxicity or hypotensive events were observed. No grade 3 or 4 adverse events were recorded during the intervention and follow-up period. Biochemical, Immunology, ultrasound and liver fibroscan results before and after GMP-grade Treg infusion was described in Table S1.
Immunophenotyping post-leukapheresis and following GMP-Treg infusion
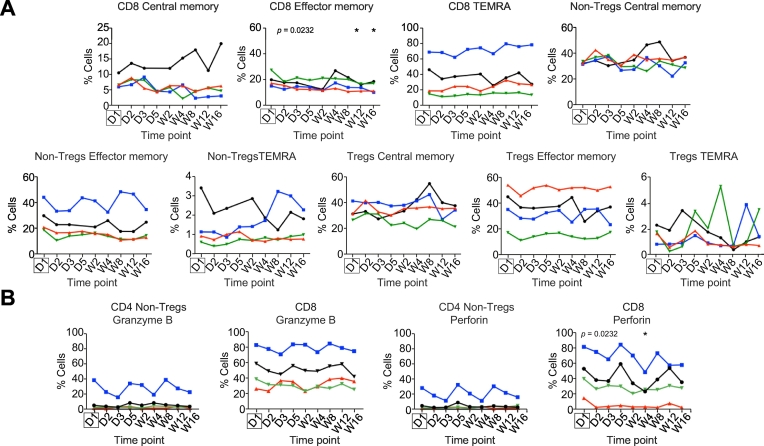
Throughout the study and 16-week follow-up period we monitored the peripheral blood immune cell profiles by multi-colour flow cytometry. Time points of blood sampling during the study are shown in Table S2. There were no statistically significant changes in the frequencies of the main immune cell populations including T cells, natural killer cells and B cells in the peripheral blood immediately after leukaphereis and Treg infusion or during the follow-up period. Modest changes in natural killer T cell frequencies were observed (p = 0.047) (Figs. S4A and S6B). A decrease in CD8 effector memory T cells was detected at weeks 12 and 16 (p = 0.023) (Fig. 6A), whereas the other T cell subsets were unchanged (Fig. 6A). Furthermore, we saw a significant reduction in perforin-expressing CD8 T cells (p = 0.023) (Fig. 6B) during follow-up.
Fig. 6.
Longitudinal changes in circulating immune cell subsets before and after leukapheresis and Treg infusion.
Central memory, effector memory and tissue-resident effector memory RA-positive (TEMRA) subsets of CD8 T cells, CD4 non-Treg and Treg. (B) Granzyme B and perforin-expressing CD4 non-Treg and CD8 T cells. Each patient’s profile between day 1 (D1) and week 16 (W16) is illustrated by a different line: Black: Patient 2; red: Patient 3; blue: Patient 4; green: Patient 5. Significance was assessed using Friedman’s test with Dunn’s Multiple Comparisons post hoc analysis comparing to baseline (D1) (indicated by the box), *p ≤0.05. Treg, regulatory T cell.
Cytokine and chemokine changes in the circulation pre and post Treg infusion
We analysed the patients’ sera for changes in cytokines and chemokines before and up to 8 weeks after Treg infusion. IL-2, an important Treg survival cytokine,24 was detected at only very low levels. CXCL10, the ligand for CXCR3 was detectable in blood throughout the study with no changes seen after Treg infusions. No significant changes were observed in any of the inflammatory and regulatory cytokines measured (Fig. S5).
Discussion
Deficiency or defects in Tregs have been associated with autoimmune liver diseases.3,7,10,20 In the Concanavalin-A murine model of immune-mediated liver injury, adoptive transfer of ex vivo expanded Tregs alleviates injury and restores hepatic tolerance25,26 and clinical trials in autoimmune type-1 diabetes suggest the adoptive transfer of Tregs may be beneficial.13,14,[27], [28], [29] This led us to develop GMP-grade Tregs as a therapy for patients with AIH. However, if Treg cell therapy is to be effective in AIH, infused cells must migrate to and be retained within the liver. Currently, little is known about the homing of adoptively transferred Tregs in mice or humans. Thus, before embarking on clinical trials in liver disease we wanted to determine where infused Treg migrate and study factors that may determine whether they survive and function within inflamed hepatic tissue. In total, 4 patients (3 are cirrhotic) who were in remission underwent GMP-Treg infusion. We demonstrate that infused Tregs home to the inflamed human liver and the spleen with high levels of efficiency in patients with AIH and with minimal uptake into other tissues. Our data support the development of Treg cell therapy for treatment of AIH.
Homing of lymphocytes to inflamed tissue is guided by chemokines.18,30,31 We have reported that CXCL10 is upregulated on inflamed human sinusoids where it can act to recruit Tregs expressing its receptor CXCR3.3 Consistent with this, mice lacking CXCR3 show a loss of immune tolerance and exacerbated hepatitis.21 We infused the autologous Tregs intravenously into 4 patients with AIH, 3 of whom were cirrhotic. We detected Tregs in liver tissue at 4 h after infusion and verified their persistence in the liver for 72 h, after which the indium was no longer detectable due to decay. More than 20% of the infused Tregs localised to the liver and there was minimal uptake in other organs apart from the spleen and bone marrow. This lack of homing to other tissues is encouraging. We expected to see uptake in the lungs as the microvascular pulmonary bed of the lung can trap leukocytes in some circumstances. Significant homing to the spleen was observed and interestingly uptake by the bone marrow, which has been reported as a reservoir for Tregs in mice.32 One patient (patient 3, Fig. S6) had only a rudimentary splenic remnant and in this case more than 40% of the Tregs migrated to the liver. The infusions were well tolerated in all patients with no significant side effects. We detected high levels of CXCR3 on the isolated Tregs, along with high levels of CD11a and CD49d, which may explain why these cells homed efficiently to the inflamed human liver where the CXCR3 ligand, CXCL10 and the integrin ligands ICAM-1 and VCAM-1 are detected on vessels.3,33 The cells also expressed markers critical for Treg function such as CD39, CTLA-4 and Foxp3.
Our study was neither designed nor powered to demonstrate efficacy, so it was not surprising that we did not see any changes in liver enzymes, bilirubin, immunoglobulin G levels, blood cytokines or fibroscan scores after the infusions (Table S5). The maximal dose we infused was 86 million cells; previous studies in diabetes have infused up to 256 million Tregs without reported toxicity.13 These data suggest that we can increase the number of infused cells in future clinical trials to optimise efficacy.
We took the opportunity to look for changes in the frequencies of circulating immune subsets after the infusions. A small but selective and statistically significant reduction in perforin-expressing CD8 T cells is unlikely to be a consequence of simple dilution, as other subsets were unaffected, suggesting a possible effect on effector responses. However, this will need to be confirmed in larger studies.
An important factor in adoptive cell therapy is the ability of the transferred cells to survive and thrive after reinfusion. In this context, our findings that the Tregs have a high mitochondrial mass and intact membrane potential is reassuring, as it suggests that they are metabolically competent.
Tregs are dependent on IL-2 to survive and function in vivo and we detected only very low levels of IL-2 in the blood of the patients. Studies in autoimmune Type 1 diabetes mellitus and systemic lupus erythematosus also show low circulating IL-2 levels and treatment with low dose IL-2 enhances CTLA-4 dependent function of Tregs isolated from patients with AIH via a STAT-5 dependent mechanism.34,35 There is now increasing evidence that supplementing with clinical grade low dose IL-2 enhances Treg frequency and function.34,35 This may be particularly important in the context of autoimmune liver disease, which is characterised by very low levels of hepatic IL-2.20 Tregs have been shown to be functional in vitro in the peripheral circulation,36 however they may not exert their full functionality in the inflamed liver, as has been shown in mice.37 These observations require further investigation in future clinical trials where the infused Tregs are retrieved from the target tissue.
Our study has several limitations. We used CD4+CD25high cells rather than a more highly purified Treg population, which may be obtained by, for instance, additionally selecting cells with low levels of the IL-7 receptor, CD127. We took this approach because we were concerned that further purification steps would reduce the numbers of cells, making radiolabelling difficult. Radiolabelling meant that we could not track infused cells after 72 h. This period was long enough to exclude a transient trapping in the liver sinusoids but future studies, using for example deuterium labelled cells, will allow us to study longer term tissue residency and stability.13 Ideally we would have carried out post-infusion liver biopsies to study the infused Tregs in the liver microenvironment. However, we did not think it was ethically justified to include this in our proof-of-concept study given the risks of liver biopsy. The purity of Tregs is defined by dementylation status, thus epigenetic analysis with Treg-specific demethylated region (TSDR) is important for testing the purity of Treg populations. However, in this proof-of-concept study, we did not manipulate or expand the Tregs. We isolated fresh Tregs and then labelled them with indium tropholonate on the same day, thus we did not assess the Treg purity with TSDR. However, we will apply the TSDR assay to test the purity in the next phase of the dose escalation trial.
In summary, this study demonstrates that after intravenous infusion, GMP-grade Tregs show strong preferential homing to the liver and spleen with minimal localisation to other organs. These Tregs also have the metabolic capacity to survive within inflamed tissues. Thus patients with autoimmune liver diseases may be excellent candidates for Treg therapy.
Financial support
This Research was funded by Medical Research Council Clinician Scientist Grant (G1002552); Sir Jules Thourn Trust, Queen Elizabeth Hospital Birmingham Charity and National Institute of Health Research Birmingham Biomedical Research Centre and the study was carried out at the National Institute for Health Research (NIHR) Wellcome Trust Birmingham Clinical Research Facility.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Y.H.O designed, managed and supervised the study, oversaw, the GMP-grade Treg isolation, laboratory experiments and wrote the manuscript; D.H.A oversaw the study and edited and wrote the manuscript; Y.H.O, D.H.A and G.M.H managed the AIH patients; S.A and R.C radiolabelled the GMP-grade Tregs; P.A and L.J performed the nuclear medicine imaging and analysis; H.C.J, L.E.J, R.E.W and P.L performed the longitudinal immune phenotyping experiments and analysed the data; N.J and Y.H.O designed the immunometabolic studies; N.J, R.E.W and H.C.J performed and analysed the immunometabolic studies; J.T isolated and released the Tregs at the NHS Blood and Transplant Service; H.C.J, R.E.W and P.L prepared the samples for metabolite assay; H.C.J, and L.E.J performed the suppression assay and the luminex; A.A and D.B managed the AUTUMN study; K.G screened, monitored and followed up the AIH patients; J.G supervised and monitored the AIH patients at the NIHR Wellcome Trust clinical research facility; G.H helped identify patients and obtained informed consent. T.W supervised the leukapheresis procedures; P.G oversaw, interpreted and reported both gamma camera nuclear medicine imaging and SPECT-CT images and is the responsible clinician holding the local Radioactive Substances Advisory Committee Licence. All authors reviewed and contributed to the final version of the manuscript.
Ackowledgement
We thank all our patients with autoimmune hepatitis who participated in the study. This Research was funded by 1) Medical Research Council Clinician Scientist Grant (G1002552); 2) Sir Jules Thorn Trust Biomedical Research Award; 3) National Institute of Health Research Liver Biomedical Research Unit, and 4) Queen Elizabeth Hospital Birmingham Charity.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https: https://doi.org/10.1016/j.jhepr.2019.08.001.
Contributor Information
Ye Htun Oo, Email: y.h.oo@bham.ac.uk.
David H. Adams, Email: D.H.Adams@bham.ac.uk.
Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
Representative horizontal SPEC CT real time video demonstrating the homing of GMP Treg to inflamed autoimmune liver.
Representative sagittal SPEC CT real time video demonstrating the homing of GMP Treg to inflamed autoimmune liver.
References
- 1.de Boer YS, van Gerven NM, Zwiers A, Verwer BJ, van Hoek B, van Erpecum KJ. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology. 2014;147:443–452. doi: 10.1053/j.gastro.2014.04.022. e445. [DOI] [PubMed] [Google Scholar]
- 2.Lohse AW, Lohr H, Bilo K, Zumbuschenfelde KHM. Recognition and regulation of LKM-specific T-cell clones in LKM-positive autoimmune hepatitis. Hepatology. 1994;20:A 144. [Google Scholar]
- 3.Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 4.Oo YH, Hubscher SG, Adams DH. Autoimmune hepatitis: new paradigms in the pathogenesis, diagnosis, and management. Hepatol Int. 2010;4:475–493. doi: 10.1007/s12072-010-9183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Than NN, Jeffery HC, Oo YH. Autoimmune Hepatitis: Progress from Global Immunosuppression to Personalised Regulatory T Cell Therapy. Can J Gastroenterol Hepatol. 2016;2016 doi: 10.1155/2016/7181685. 7181685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 7.Taubert R, Hardtke-Wolenski M, Noyan F, Wilms A, Baumann AK, Schlue J. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery HC, Braitch MK, Brown S, Oo YH. Clinical Potential of Regulatory T Cell Therapy in Liver Diseases: An Overview and Current Perspectives. Front Immunol. 2016;7:334. doi: 10.3389/fimmu.2016.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 12.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J. Administration of CD4+CD25highCD127- regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MA, Hannen RF, Cooper D. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant. 2017;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 17.Noyan F, Zimmermann K, Hardtke-Wolenski M, Knoefel A, Schulde E, Geffers R. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am J Transplant. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 18.Oo YH, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. J Autoimmun. 2010;34:45–54. doi: 10.1016/j.jaut.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349:490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen YY, Jeffery HC, Hunter S, Bhogal R, Birtwistle J, Kaur Braitch M. Human intrahepatic tregs are functional, require IL-2 from effector cells for survival and are susceptible to fas ligand mediated apoptosis. Hepatology. 2016;64:138–150. doi: 10.1002/hep.28517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erhardt A, Wegscheid C, Claass B, Carambia A, Herkel J, Mittrucker HW. CXCR3 deficiency exacerbates liver disease and abrogates tolerance in a mouse model of immune-mediated hepatitis. J Immunol. 2011;186:5284–5293. doi: 10.4049/jimmunol.1003750. [DOI] [PubMed] [Google Scholar]
- 22.Howie D, Cobbold SP, Adams E, Ten Bokum A, Necula AS, Zhang W. Foxp3 drives oxidative phosphorylation and protection from lipotoxicity. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Procaccini C, Carbone F, Di Silvestre D, Brambilla F, De Rosa V, Galgani M. The Proteomic Landscape of Human Ex Vivo Regulatory and Conventional T Cells Reveals Specific Metabolic Requirements. Immunity. 2016;44:712. doi: 10.1016/j.immuni.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffery HC, Jeffery LE, Lutz P, Corrigan M, Webb GJ, Hirschfield GM. Low dose interleukin-2 promotes STAT5 phosphorylation, Treg survival and CTLA-4 dependent function in autoimmune liver diseases. Clin Exp Immunol. 2017;188:394–411. doi: 10.1111/cei.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 26.Lapierre P, Beland K, Yang R, Alvarez F. Adoptive transfer of ex vivo expanded regulatory T cells in an autoimmune hepatitis murine model restores peripheral tolerance. Hepatology. 2013;57:217–227. doi: 10.1002/hep.26023. [DOI] [PubMed] [Google Scholar]
- 27.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365:2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 29.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T. A Pilot Study of Operational Tolerance with a Regulatory T Cell-Based Cell Therapy in Living Donor Liver Transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 30.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 32.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 33.Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol. 2012;57:1044–1051. doi: 10.1016/j.jhep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todd JA, Evangelou M, Cutler AJ, Pekalski ML, Walker NM, Stevens HE. Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 36.Peiseler M, Sebode M, Franke B, Wortmann F, Schwinge D. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol. 2012 Jul;57:125–132. doi: 10.1016/j.jhep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 37.Schwinge D, von Haxthausen F, Quaas A, Carambia A. Dysfunction of hepatic regulatory T cells in experimental sclerosing cholangitis is related to IL-12 signaling. J Hepatol. 2017 Apr;66:798–805. doi: 10.1016/j.jhep.2016.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Representative horizontal SPEC CT real time video demonstrating the homing of GMP Treg to inflamed autoimmune liver.
Representative sagittal SPEC CT real time video demonstrating the homing of GMP Treg to inflamed autoimmune liver.