Summary
In the past, patients with liver cirrhosis were thought to be prone to increased bleeding risk. However, those with compensated liver cirrhosis actually have normal coagulative balance, which can become altered when liver function worsens, or infection, bleeding, or acute kidney insufficiency occur. When this happens, it is now recognized that patients with liver cirrhosis are at higher risk of thrombotic rather than haemorrhagic complications. Anticoagulation plays a favourable role both when used therapeutically or prophylactically. Successful anticoagulation is associated with a lower rate of decompensation and with improved survival. To date, treatment has involved the use of low molecular weight heparins and vitamin K antagonists. Preliminary data suggest that novel non-vitamin K antagonist oral anticoagulants can be used safely in patients with liver cirrhosis.
Keywords: Coagulopathy, Thrombophilia, Chronic liver disease, Portal Hypertension, Decompensation
Key points
Patients with compensated liver cirrhosis have a nearly normal coagulative balance
In patients with compensated liver cirrhosis, bleeding (mostly variceal) is usually the consequence of worsening portal hypertension
Onset and progression of chronic liver failure are associated with derangement of coagulative balance
Modification in the equilibrium between thrombotic and antithrombotic proteins can result in a thrombotic, rather than haemorrhagic, condition
Anticoagulants obtain recanalization of portal vein thrombosis in about half of treated cases but the impact on natural history should be obtained by randomized clinical trials, which are not available.
There is evidence that activation of the coagulative cascade has a role in sustaining chronic liver injury
Prophylactic anticoagulation might be useful not only in preventing PVT but also decompensation, but controlled studies are awaited.
Alt-text: Unlabelled Box
Coagulation in cirrhosis
Anomalies/disorders
The liver is responsible for the production of the majority of factors involved in the coagulative process. In healthy individuals, the haemostatic balance is maintained by the equilibrium between pro- and anticoagulant proteins, whose levels are normally well above the quantity required for normal function as only a small percentage of them (less than 50%) are necessary for haemostasis.1 This abundance of coagulative factors makes the system very stable and scarcely influenced by external perturbation factors. In chronic liver disease, especially in patients with cirrhosis, there is a reduced synthesis of both procoagulant and anticoagulant proteins, characterized by the reduction of liver-dependent pro-haemostatic clotting factors (FII, V, VII, IX, X and XI), reduced levels of fibrinolytic proteins but increased tPA, and decreased endogenous anticoagulants such as proteins C, protein S, and antithrombin.[2], [3] Factor VIII and von Willebrand factor (vWF) are instead increased.[4], [5] Although Factor VIII is produced in the liver (in sinusoidal cells), it is also physiologically synthesized in endothelial cells outside the liver, which explains why its levels can be high despite the presence of chronic liver disease. Increased vWF levels reflect enhanced production by the endothelium or reduced clearance by the liver.
Thrombocytopenia, secondary to the splenomegaly induced by portal hypertension and platelet function defects, only slightly affects coagulative performance due to high levels of platelet activators like FVIIIa and Vwf, and reduced VWF-cleaving ADAMTS13.6 The quantitative increase of vWF in cirrhosis can compensate for the qualitative and quantitative defects of platelets. The plasma of patients with cirrhosis was shown to be able to induce increased adhesion both in platelets from individuals and patients with low or normal platelet counts.7
On the whole, these data have led several authors to debate the existence of coagulopathy in cirrhosis. Patients with cirrhosis are no longer considered prone to bleeding because of their coagulative parameters but are rather considered as being in a less stable but still normal coagulative state, which can be defined as “rebalanced haemostasis”.[1], [8]
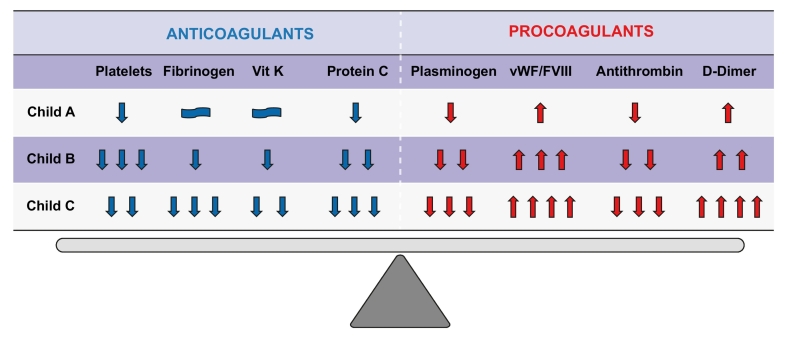
It should be underlined, however, that patients with cirrhosis are not a homogeneous entity and that the essentially normal coagulative status described above can be found in a subgroup of patients with cirrhosis, i.e. those with compensated disease. Being in a compensated or decompensated state has a number of implications. These 2 entities have an entirely distinctive pathophysiology, decidedly different prognosis and respond differently to acute stress.[9], [10], [11], [12] In compensated patients with preserved liver function, spontaneous bleeding is extremely rare and bleeding episodes (variceal rupture or portal hypertensive gastropathy/enteropathy) are the consequence of portal hypertension and not of the coagulative imbalance.[12], [13] In these patients, mechanical complications of invasive procedures are the most common cause of bleeding episodes, which usually resolve without the need for treatment.[14], [15], [16] Patients with decompensated cirrhosis, especially those with ascites, and patients with acute-on-chronic liver failure (ACLF) have a severe systemic inflammation driven by an abnormal bacterial translocation, which causes organ dysfunction, renal failure and more unstable coagulation.[9], [17] In decompensated patients, coagulative performance becomes impaired and bleeding risk, both spontaneous and related to invasive procedures, increases consistently, regardless of the alteration in traditional coagulation tests.[12], [18] Infections and acute kidney insufficiency (AKI)16 have recently been recognized as risk factors for the disruption of the unstable haemostatic balance of cirrhotic patients. Possible mechanisms include increased fibrinolytic activity and alterations in platelet function in the presence of renal impairment and release of heparin-like factors during infection. However, thorough investigations into the mechanisms involved are still lacking18(Fig. 1).
Fig. 1.
Haemostatic balance in cirrhotic patients in different Child-Pugh classes. Modifications of pro- and anti-coagulants factors during progression of chronic liver disease.
On the whole, it appears that coagulative status is subordinate to portal hypertension in determining bleeding risk. The results of the randomized controlled trials of recombinant factor VIIa (rFVIIa) vs. placebo in patients with advanced cirrhosis and active variceal bleeding support this hypothesis.[19], [20] The individual studies were unable to identify differences between patients treated with rFVIIa or placebo in the ability to control 24-hour bleeding, or to prevent rebleeding or death at day 5. Only a meta-analysis of the individual data from the 2 studies was able to identify a beneficial effect of rFVIIa in the selected subgroup of patients with advanced cirrhosis and active bleeding from oesophageal varices at the time of endoscopy.21 However, the authors underlined the risk of increased arterial thrombo-embolic events as a consequence of treatment, thus suggesting that rFVIIa treatment should be reserved for patients with a lack of bleeding control after standard treatment. No evidence-based data are available regarding the usefulness of fresh frozen plasma to correct coagulative abnormalities in acute or chronic liver disease or to improve outcomes in variceal bleeding.22
A final warning should be given regarding novel thrombopoietin agonist drugs, whose use for bleeding prophylaxis in invasive procedures has been associated with higher portal vein thrombosis (PVT) occurrence. Early studies with eltrombopag were associated with high PVT occurrence, likely because of administration of an ill-chosen therapeutic dosage.23 Later studies, with novel molecules like lusutrombopag, demonstrated a lower rate of thrombotic complications.24 However, the most relevant and clear-cut finding of all these studies was the recognition of the safety of invasive procedures both in treated and in control groups, despite abnormalities in routine coagulative tests (like prothrombin time [PT] or partial thromboplastin time [PTT]). This further reinforces the concept that complications are associated much more with mechanical injuries than with coagulative abnormalities.
Evaluation of coagulative abnormalities
The standard tests of haemostasis (PT/international normalized ratio [INR] and platelet count), widely used to estimate coagulopathy and bleeding risk in patients undergoing invasive procedures, do not reflect the coagulative complexity of patients with cirrhosis. The traditional level of INR below 1.5 as the cut-off for safe invasive procedures25 has recently been questioned.13 Fibrinogen levels seem better predictors of bleeding in patients with high INR.[26], [27], [28]
Similarly, while the guidelines usually suggest using a platelet count of 50x103/mm3 to determine whether it is safe to perform invasive procedures, there is no sound data on this threshold. A low platelet count reflects a larger spleen size and is influenced by the degree of portal hypertension. However, a low platelet count is not associated with higher bleeding risk in patients with compensated cirrhosis, even in those on anticoagulants.[29], [30]
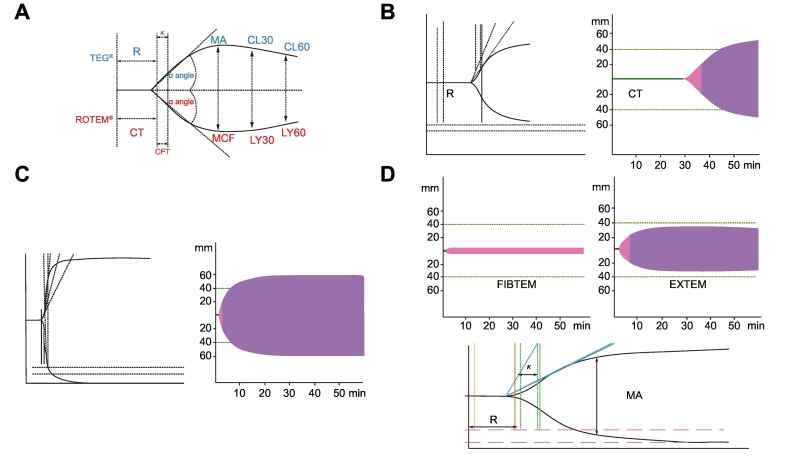
Global tests such as viscoelastic tests like thromboelastography (TEG) and thromboelastometry (ROTEM) are able to explore initial clot formation in non-centrifuged blood and final clot strength in whole blood, as well as the presence of hyper fibrinolysis or premature clot dissolution, although they are not able to assess platelet dysfunction and the effects of vWF.31
TEG, as well as ROTEM, gives a graphic picture of clot formation (Fig. 2). The blood, incubated at 37°C is suspended in a heated cup, connected to a detector system that uses a torsion wire in TEG and an optical detector in ROTEM. TEG can reveal changes in clot formation due to altered coagulation factors, fibrinogen, platelets and fibrinolysis. TEG-guided haemostatic therapy has been shown to be safe in trauma, surgery and in cirrhotic patients undergoing liver transplantation.32 Recently, TEG has shown its utility as a key tool to determine the need for blood transfusions in cirrhotic patients with severe coagulopathy (based on traditional indices: INR ≫1.8 and platelet count ≪50x103/mm3) undergoing invasive procedures.14 A recent meta-analysis in a variety of elective and emergency surgical settings confirmed that TEG-guided transfusion protocols reduced the requirement for blood product transfusion and improved patient outcomes (including survival in an emergency setting).33
Fig. 2.
Changes in TEG® and ROTEM® tests in normal conditions, cirrhosis and anticoagulation. A: Whole blood viscoelastic tests can be detected using ROTEM (optical method, bottom line) or TEG (electromechanical method; upper line). ROTEM measures similar parameters on the graphic compared to TEG: R time is clotting time (CT) represent the period of initial fibrin formation. K time is clot formation time (CFT), which measures the rate of clot formation and reflects fibrin rate build up and cross linking, MA is maximum clot firmness (MCF) represents the ultimate strength of the clot (platelet and fibrin) and LY30 is (CL30) measures the rate of amplitude reduction from MA at 30 minutes detects fibrinolysis.
B: TEG (left) ROTEM (right) showing prolonged clotting time and R in a patient treated with Heparin.
C: TEG (left) ROTEM (right). Hypercoagulable state with short CT, CFT, R and K. increased MCF and MA.
D: ROTEM (upper) and TEG (bottom) in a patient with decompensated OH- cirrhosis showing hyocoagulable state with prolonged CT, R and K and reduced MCF and MA and decreased A10, MCF on FIBTEM showing hypofibrinogenemia.
Thrombin generation tests (TGTs) evaluate thrombin generation and decay, enabling assessment of the balance between the two. Coagulation of the test plasma (platelet poor or platelet rich) is activated by small amounts of tissue factor and phospholipids; the reaction of thrombin generation is continuously monitored by means of a thrombin-specific fluorogenic substrate. Endogenous thrombin potential, defined as the net amount of thrombin that test plasmas can generate on the basis of the relative strength of the pro- and anticoagulant drivers, is the most important parameter derived from the thrombin-generation curve. TGT assays are promising for the investigation of hypo- or hypercoagulability.[34], [35], [36] TGT assays have been used extensively to show the rebalanced/hypercoagulable state in patients with liver disease. However, because of their technical complexity and a lack of studies assessing their utility, the use of TGT assays in the clinical setting of cirrhosis needs to be validated.
On the whole, whenever possible, point-of-care coagulation tests like TEG or ROTEM should be used, as they are able to give an accurate picture of the coagulative performance of the cirrhotic patient, when it is needed, helping to determine whether transfusion of blood components is required.
Thrombosis in cirrhosis
Deep vein thrombosis
Observational studies in the general population have found that patients with liver disease have a higher risk of ischaemic stroke and venous thromboembolism (VTE) compared with patients without liver disease.[37], [38], [39] Several surveys have shown an increased risk of venous thromboembolism of approximately 1.7- to 2-fold in patients with liver disease compared to controls.[40], [41], [42], [43] In addition, patients with non-alcoholic steatohepatitis (NASH)-related cirrhosis have a higher risk of VTE events compared to patients with other causes of liver disease.44 Similarly, patients with hepatitis C virus (HCV) infection (with or without cirrhosis) have an increased risk of VTE compared to patients without HCV infection according to a meta-analysis of 3 large epidemiological studies (risk ratio 1.38, 95% CI 1.08–1.77).45 Only 1 study46 found that VTE occurred in patients with moderate/severe chronic liver disease at a rate lower than in general medical patients. In all cases, the presence of cirrhosis in patients with VTE has been associated with increased mortality.[40], [46] In summary, although heterogeneity in the design and results of available studies preclude a definite conclusion on the level of risk for VTE in patients with cirrhosis, it is clear that patients with cirrhosis are not protected from VTE and therefore should be considered for specific prophylaxis. Data are lacking for the stratification of VTE risk, including laboratory features of coagulation.
Splanchnic vein thrombosis
In a seminal study, Wanless and co-workers showed that explanted livers from patients with advanced liver disease harbour widespread thrombotic occlusion of the intrahepatic hepatic venous and portal venous systems.47 The parenchymal areas corresponding to the obstructed territories undergo atrophy and replacement by fibrous tissue, a lesion denominated parenchymal extinction.[47], [48] Narrowing and distortion of the large hepatic veins appear to be common in patients with cirrhosis. However, thrombosis of these large hepatic veins is difficult to characterize in patients with cirrhosis because the predominantly intrahepatic course of the veins makes it difficult to distinguish primary from secondary obstruction. By contrast, thrombosis of the main portal vein, or its right or left branches, being extrahepatic, has proved easy to evaluate with Doppler ultrasound or CT scan in patients with cirrhosis, either at the time of decompensation, or in the work-up or surveillance before transplantation, or during screening for hepatocellular carcinoma.
The prevalence and the incidence of extrahepatic PVT vary mostly according to the severity of associated liver disease. In patients with predominantly Child-Pugh class A cirrhosis, typically investigated during screening for hepatocellular carcinoma, incidence is about 5% and 11% at 1 and 5 years, respectively.49 In patients with advanced Child-Pugh class C cirrhosis, typically considered or listed for liver transplantation, the reported prevalence was up to 15–23% and incidence 7–18%50 to 16.6%51 in the first year.
In about 75% of patients, portal vein thrombi occupy only a portion of the portal venous lumen. Furthermore, in about half of patients, thrombi are only transiently detected, as they spontaneously disappear in the period of 3 to 6 months between successive imaging procedures.[49], [52], [53]
When the risk factors for development of PVT were investigated in prospective cohort studies or cross-sectional surveys, the independent variables were all related to the severity of liver disease: size of oesophageal varices, gastrointestinal bleeding, portal vein blood flow velocity, size of the liver, ascites, encephalopathy, serum albumin, serum bilirubin, or prothrombin levels.[49], [54], [55], [56], [57], [58] PVT appears to be more common in patients with NASH or NASH-related cirrhosis than in cirrhosis related to other causal factors.59 There are heterogeneous data on a relationship with those primary prothrombotic conditions, such as Factor V Leiden or the prothrombin G20210A gene mutation whose diagnosis is not challenged by hepatic dysfunction.[49], [60] Still, even if real, their role is clearly marginal. It remains unclear whether there is an independent relationship between acquired coagulation inhibitors and PVT in patients with cirrhosis.61 Prospective studies on the role hypercoagulability – related with resistance to thrombomodulin – may play in precipitating PVT are limited.[62], [63], [64] Actually, it is difficult to ascertain which specific consequence of chronic liver disease is mostly responsible for PVT development: coagulation anomalies, slowing of portal blood flow, endothelial injury or any other known or still unknown factor. The role played by endothelial dysfunction is complex, as several elements (like bacterial translocation, stage of disease, severity of portal hypertension) contribute to its development.65
The consequences of PVT on the course and outcome of underlying liver disease are difficult to assess because of the link between liver disease severity and the development of PVT. However, several longitudinal studies including a prospective one, failed to document an aggravation of liver disease severity following development of PVT.[49], [52], [53] In non-liver transplant patients with cirrhosis, PVT is not clearly associated with increased mortality.66 Furthermore, in patients listed for liver transplantation, there is no independent negative impact of PVT on pre-transplant mortality after adjustment for the severity of liver disease.67 By contrast, pre-transplant PVT does affect post-transplant mortality, probably because a proper reconstitution of the portal venous inflow is critical for liver graft survival.67 In this regard, there is convincing evidence that if reconstitution of a physiological portal venous inflow to the graft can be achieved, the outcome is very similar to that of patients without PVT, whereas if this is not the case the outcome is worse.68 Of note, the negative impact of PVT on mortality is limited to the first trimester after transplantation, while late survival curves remain parallel in patients with and without pre-transplant PVT.69
In summary, whereas the parenchymal damage associated with intrahepatic PVT is well known, the effect of extrahepatic PVT on the natural history of liver disease is unclear if not doubtful. Hence, PVT appears to be an excellent marker of poor outcomes in cirrhosis, while not being an independent factor for these poor outcomes. However, PVT is probably both a marker and a factor for a suboptimal outcome after liver transplantation.
Anticoagulation in cirrhosis: Potential indications
Thrombosis
Most studies in cirrhotic patients with anticoagulants have been conducted in PVT with low molecular weight heparins (LMWHs), vitamin K antagonists (VKAs) or a combination. Delgado et al.70 found that among the 55 patients with cirrhosis and acute PVT, 60% of patients treated with LMWHs or VKAs, achieved partial or complete recanalization after 6.8 months of treatment. Platelet count ≪50x103/mm3 and use of VKAs were associated with an increased risk of bleeding. The efficacy of warfarin for PVT in patients with cirrhosis was confirmed in patients awaiting liver transplantation as complete or partial resolution of PVT was reported in 39% and 43% of patients, respectively.71 In addition, Francoz et al.50 reported that when using VKA treatment in patients with cirrhosis and splanchnic vein thrombosis awaiting transplantation, the proportion of partial and/or complete recanalization was significantly higher in those who received anticoagulation therapy (42% vs. 0%) after an average 8.1 months of follow up. No difference in bleeding rates was observed between the 2 groups. In a prospective study in 28 cirrhotic patients with PVT treated with LMWH, complete recanalization of the portal vein occurred in 33% and partial recanalization in 50% after a 6-month course of enoxaparin, with no bleeding.72 Senzolo et al.73 also documented the efficacy of LMWHs in 33 cirrhotic patients with PVT treated with nadroparin for 6 months. The efficacy and safety of LMWH therapy was confirmed with different doses of enoxaparin for acute PVT in cirrhotic patients.74 A recent meta-analysis on the effects of anticoagulant therapy (LMWHs or VKAs) in patients with cirrhosis and PVT showed, among 353 included patients, a significantly higher proportion of PVT recanalization (71%) in patients treated with anticoagulants than in patients without (42%).75 In the 6 studies (257 patients) reporting on the rates of bleeding, no difference of major or minor bleeding between groups (with or without anticoagulation therapy) was found (11% for both groups). In addition, data extracted from 6 studies (including 225 patients) showed that PVT progressed in 9% of patients with anticoagulation therapy vs. 33% of patients without.75 There are limited data on direct-acting oral anticoagulants (DOACs) for treatment of PVT in patients with cirrhosis. Case reports and small retrospective studies suggest that DOACs are safe in patients with mild or moderate cirrhosis.[76], [77], [78] Recently Hanafy et al.79 reported a clinical trial comparing low doses of rivaroxaban (10 mg twice daily) vs. warfarin in 80 patients with cirrhosis (Child-Pugh class A or B) who had developed acute PVT. Recanalization of the portal vein was obtained in 85% of patients treated with rivaroxaban, and only 45% of patients treated with warfarin. No bleeding complications were observed in the rivaroxaban group. Larger studies are needed to confirm the safety and efficacy of DOACs to treat PVT in cirrhosis, especially in patients with severe cirrhosis. However, in the future, DOACs will likely replace heparins and VKAs for treatment of PVT in cirrhotic patients, the question still to be addressed is which DOAC and at which dosage for the safest profile.
In clinical practice, the European Association for the Study of the Liver (EASL) guidelines80 recommend LMWH treatment at weight-adjusted dose for the treatment of acute PVT in patients with cirrhosis. LMWH is preferred over unfractionated heparin (UFH), except in patients with severe renal impairment (≪30 ml/min). Anticoagulation therapy should be prolonged for 6 months, using either LMWH or VKAs. Long-term anticoagulation therapy with VKAs should be proposed only after a careful evaluation of risks and benefits, and should be re-evaluated at regular time intervals (e.g. 6 monthly).
In every patient with cirrhosis given any form of anticoagulation therapy, prophylaxis for gastrointestinal bleeding related to portal hypertension must be carefully checked.
However, it has been shown that anticoagulation does not worsen bleeding outcomes in acute conditions,30 nor increase bleeding risk during variceal band ligation.29
No thrombosis
Anticoagulation for PVT prevention
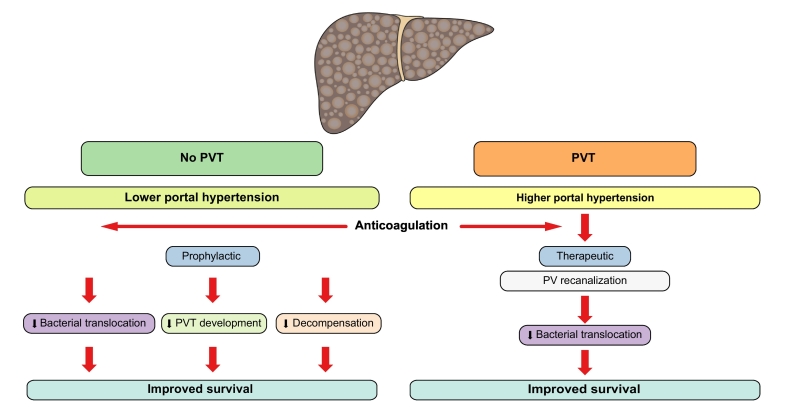
PVT is a common complication in cirrhosis, arising in 10–27% of patients during progression of disease.[49], [51] Its occurrence was previously indicated as a possible cause for disease worsening, especially in the transplant setting. However, it appears that PVT is one of several indicators of progressive liver disease, and the occurrence of decompensation at the same time as PVT is the result of rapidly worsening portal hypertension, as a consequence of upregulation of systemic inflammation and inappropriate activation of the coagulation system, linked with increased bacterial translocation through the damaged intestinal mucosal barrier in patients with portal hypertensive colopathy.51 The prospective study of enoxaparin treatment for prevention of PVT51 showed that anticoagulation not only prevented PVT but also improved bacterial translocation, decreased systemic inflammation, and also prevented hepatic decompensation and improved survival (Fig. 3). A confirmatory study, which instead of enoxaparin uses rivaroxaban, is ongoing; its results will be of paramount importance to confirm not only PVT prevention, which is likely, but also, and most importantly, the positive effect on progression of disease (ClinicalTrials.gov Identifier: NCT02643212). The confirmation of this result will be highly relevant for the treatment of advanced chronic liver disease.
Fig. 3.
Role of prophylactic and therapeutic anticoagulation in the natural history of cirrhosis.
Anticoagulation as an antifibrotic therapy
Portal hypertension is the end result of the architectural distortion occurring during protracted liver damage. Dysregulated activation of fibrogenic cells (hepatic stellate cells [HSCs] and portal myofibroblasts)81 leads to distortion of hepatic architecture. A relevant role in this process is played by the alteration of hepatic microcirculation, with thrombotic occlusions of small intrahepatic veins and sinusoids frequently occurring.47 This is followed by progressive venous obstruction and the occurrence of “parenchymal extinction”, i.e. the irreversible loss of contiguous hepatocytes and their replacement by fibrous tissue.
These considerations strongly suggest that anticoagulation could be a rational approach to prevent and to treat liver fibrosis. A relevant number of experimental studies in different animal models of chronic liver disease (thioacetamide, CCl4, and high-fat diet) and with different types of anticoagulation (LMWH, VKA, new oral anticoagulants) have challenged this hypothesis (reviewed in Turco et al., Curr Hep Rep, 2018).82 On the whole, all studies but one showed a reduction in the development of hepatic fibrosis. Two studies evaluated portal pressure, showing significant reductions, at the same time as decreased HSC activation.[83], [84]
Clinical studies are fewer and results understandably less clear-cut (reviewed in reference 82). Three studies[51], [85], [86] mostly showed improved liver function and reduced rates of decompensation. However, patients undergoing treatment had established cirrhosis and therefore the effect on fibrosis was hardly ascertainable. In the study by Dhar et al.,87 HCV-positive patients with recurrent infection after liver transplant underwent treatment with warfarin. The availability of direct-acting antivirals for HCV soon after the start of the study led to its discontinuation. However, preliminary data, presented as an abstract, showed a significant reduction of fibrosis 1 year after LT in anticoagulated patients in comparison with controls.
On the whole, anticoagulation deserves to be further evaluated as an antifibrogenic treatment. The new oral anticoagulant drugs will offer a better tool to evaluate the effect of long-term anticoagulation on fibrogenesis, once their safety in chronic liver disease has been confirmed.
Practical management
Several warnings have been raised regarding anticoagulation in cirrhosis, relating to safety concerns and the uncertain possibility of achieving effective anticoagulation due to variability in pharmacokinetics and potency of anticoagulants in cirrhosis. Nevertheless, most studies were able to achieve their therapeutic aim without relevant side effects. In the next paragraphs, we will address advantages and disadvantages of available anticoagulant drugs for prophylaxis of thrombotic conditions (VTE and PVT) or for PVT treatment.
Anticoagulant therapeutics
Parenteral anticoagulant
The most widely used agents are UFH, LMWHs, fondaparinux and danaparoids. These drugs act by binding antithrombin (AT) to inhibit thrombin and/or Factor Xa. In cirrhotic patients decreased AT may be associated with reduced efficacy of these treatments, especially in patients with severe disease. However, several in vitro studies showed an increased effect of LMWH in cirrhotic plasma despite low AT or anti Xa levels.[88], [89] No relevant clinical studies on VTE treatment in cirrhotic patients with UFH or LMWHs are available. VTE treatment with UFH needs laboratory monitoring with PTT and/or anti Xa or anti IIa activity. The therapeutic range of PTT is usually between 1.5–2.5 times more than the upper limit of the institutional PTT reference range, but these values are variable depending on the analyzer and reagents. A therapeutic range of 1.5–2.5 corresponds to an anti Xa or anti IIa activity of 0.3–0.7 UI/ml, as AT is often decreased, these tests may underestimate levels of UFH. UFH has been gradually replaced by LMWH. No laboratory monitoring (anti Xa) is required except in patients who are underweight, obese, or have renal insufficiency. Severe renal insufficiency, quite common in cirrhosis, is a contraindication to LMWH and should be tested before starting treatment. The last VTE guidelines from the American College of Chest Physicians recommend LMWH as the preferred anticoagulant in patients with liver disease and coagulopathy.90
No specific guidelines exist regarding the management of different anticoagulation drugs in patients with cirrhosis in case of worsening hepatic and/or kidney function. However, some recommendations can be derived from other guidelines: LMWH, i.e. the treatment of choice in patients with liver disease requiring anticoagulation, should be stopped in case of acute kidney injury and replaced with other heparins not affecting kidney function until normalization of renal function.
Oral anticoagulants
Vitamin K antagonists (VKA) inhibit vitamin K-dependent synthesis of clotting factors II, VII, IX and X. It also decreases the production of the inhibitors protein C and S. Warfarin has traditionally been the oral anticoagulant of choice for the treatment of VTE and the prevention of ischaemic stroke in atrial fibrillation with a recommended target INR of 2–3 in the general population. Due to the narrow therapeutic range and significant drug-drug interactions, biological and clinical surveillance should be performed in patients receiving these treatments in order to maintain an optimal time in therapeutic range (TTR).91 In liver disease, VKA treatment is particularly challenging as warfarin has significant plasma protein binding (~99%), it is predominantly eliminated by the liver through a cytochrome P450-dependent metabolism, and INR at baseline is often elevated. Thus, the dose of warfarin and the target INR are not well defined in this population. Suboptimal dosing in response to an elevated INR at baseline may result in an increased risk of thrombotic events, while titrating to a supratherapeutic INR could result in bleeding complications.92 Patients with liver disease have a lower mean time in TTR, which has been associated with a 2-fold increase in bleeding risk compared with patients without liver disease.93 To date no prospective clinical trial has examined safety and efficacy of warfarin in patients with liver disease. The balance of benefit/risk should be carefully evaluated for each patient, the target INR is usually maintained at 2-3; these patients should be carefully surveyed and monitored and if possible included in a therapeutic educational programme. Furthermore, for prolonged treatment, the benefit/risk ratio should be regularly re-evaluated.
In the last decade DOACs have emerged. DOACs inhibit FXa (apixaban, rivaroxaban, edoxaban) or FIIa (dabigatran), with several randomized trials demonstrating comparable or superior efficacy and safety profile compared with warfarin in patients with atrial fibrillation or VTE. DOACs are given once or twice daily, at fixed doses, and need no laboratory monitoring. DOACs are currently recommended as a first-line treatment in the management of atrial fibrillation or VTE in the guidelines.[90], [91] Unfortunately, patients with liver disease have been systematically excluded from clinical trials. Liver disease could influence several aspects of DOAC pharmacokinetics, including systemic elimination, plasma protein binding, cytochrome P450 mediated metabolism, biliary excretion, and also comorbidities such as renal insufficiency94 (Table 1). However, several observational studies showed the efficacy and safety of DOACs for the treatment of VTE and/or prevention of ischaemic stroke in patients with cirrhosis.[76], [95], [96] These observational results should be interpreted with caution, the sample sizes are small, the analyses were not adjusted for difference in characteristic among the treatment groups, and very few or no patients with severe cirrhosis were included. The Food and Drug Administration (FDA) and European Medicines Agency (EMA) recommend using the Child-Pugh score to guide dosing and use of DOAC: FDA97 and EMA98 do not recommend using DOACs in patients with Child-Pugh C liver disease, or in patients with any liver disease associated with coagulopathy. The DOACs apixaban, dabigatran and edoxaban can be used with caution and/or adjusted doses in patients with Child-Pugh A and B, but the use of rivaroxaban in patients with Child-Pugh B is not recommended. In practice DOACs can be used in patients with atrial fibrillation or VTE in Child-Pugh A cirrhosis, if indicated, and with adjusted doses in Child-Pugh B cirrhosis, but should not be used in Child-Pugh C patients. Renal insufficiency, drug-drug interactions, weight, previous bleeding events and thrombocytopenia should be evaluated before introducing these treatments. The ratio of benefits to risks of these treatments should be carefully assessed for each patient, alongside close biological and clinical surveillance. A periodical re-evaluation of the balance of benefits/risks should be planned for long-term anticoagulation.
Table 1.
Pharmacological characteristics of oral anticoagulation agents.
| Warfarin |
Apixaban |
Dabigatran |
Edoxaban |
Rivaroxaban |
|
|---|---|---|---|---|---|
| Target | VKORC1 | Factor Xa | Factor IIa | Factor Xa | Factor Xa |
| Half-life (h) | 20- 60 | ~12 | 12-17 | 10-14 | 7-13 |
| Prodrug | no | no | yes | no | no |
| Renal clearance (%) | no | 25 | 80 | 50 | 35 |
| Hepatic clearance | 100 | 75 | 20 | 50 | 65 |
| Requires CYP450 | yes | yes | no | minimal | yes |
| Plasma protein binding (%) | 99 | 87 | 35 | 55 | 95 |
| Substrate for P-gP | no | yes | yes | yes | yes |
| Coagulation monitoring | Yes INR | no | no | no | no |
| Coagulation assay | INR | Anti Xa activity | Thrombin Time | Anti Xa activity | Anti Xa activity |
| Reversal agent | 4F-PCC, Vit K | Andexanet alfa | Idarucizumab | Andexanet alfa (Not yet FDA approved) | Andexanet alfa |
Modified from Qamar et al. 2018, with permission.
Venous thromboembolism
Venous thromboembolism (VTE) in cirrhosis is an increasingly recognized clinical problem, proving once more that patients with liver cirrhosis are not spontaneously protected from thrombotic episodes. The low platelet count and the increased INR levels of patients with cirrhosis do not reduce the risk of developing deep vein thrombosis or pulmonary embolism.42 Prophylactic anticoagulation benefit is well established and recommended in guidelines for the general population of inpatients,99 while major concerns still exist regarding the prophylactic use of anticoagulation in patients with cirrhosis for fear of facilitating bleeding events. Several studies have shown that the majority of patients with cirrhosis do not receive prophylaxis against VTE when admitted into hospital wards[100], [101] or prophylaxis when given is suboptimal.102 Interestingly, the reason behind the decision of avoiding VTE prophylaxis was not related to a high INR levels or low platelet count, but it was mostly the consequence of lack of knowledge regarding the high risk of cirrhotic patients of developing VTE.102 Safety and efficacy of prophylactic anticoagulation for VTE in hospitalized cirrhotic patients have been demonstrated beyond doubt.[95], [103] Prophylaxis should therefore be started in patients with cirrhosis in case of surgery, hospitalization or immobilization regardless of INR levels. Special attention should be paid to cirrhotic patients with NASH, who have a higher risk of VTE events, sometimes despite already being on anticoagulation.44
PVT prophylaxis
As outlined in the previous paragraphs, experience with prophylactic treatment for PVT in patients with liver cirrhosis is very limited, as only 1 prospective study has been published so far.51 This prospective randomized trial showed that LMWH not only prevented PVT, but also was also able to prevent decompensation and improve survival. LMWH use even in patients with advanced liver disease was totally safe. Safety has been confirmed in other small uncontrolled studies using LMWH in absence of PVT.[27], [104]
A prospective trial for PVT prophylaxis with the new oral anticoagulant Rivaroxaban is ongoing but no data are so far available (ClinicalTrials.gov Identifier: NCT02643212).
PVT treatment
Several studies, using both heparins (both unfractioned and LMWH) and VKAs have been published. They have been recently reviewed in a comprehensive meta-analysis by Loffredo et al.,75 which shows that patients with liver cirrhosis and PVT responded to anticoagulant therapy with an increased rate of recanalization and with reduced progression of thrombosis and did not experience significant side effects from therapy. Less clear is whether there was a positive effect on outcome, both in term of bleeding episodes and occurrence of decompensation. This is not surprising as all studies but 5 were retrospective, all were small-sized, the treatment protocols were heterogeneous as was the Child-Pugh class or model for end-stage liver disease score at enrolment (Table 2).
Table 2.
Clinical studies assessing the efficacy of anticoagulation in patients with cirrhosis/advanced fibrosis.
| Reference | Type of study | N of patients | Stage of fibrosis/cirrhosis | Type of Anticoagulant | Duration of anticoagulation | Outcome(s) |
|---|---|---|---|---|---|---|
| PATIENTS WITHOUT PVT | ||||||
| Shi, 200385 | Prospective | 52 | HBV related liver fibrosis | Heparin/LMWH | 3 weeks | Improved liver function, reduced collagen proliferation |
| Huang, 200786 | Prospective | 75 | HBV related cirrhosis | LMWH | 3 weeks | Improved liver function, improved portal vein blood flow velocity, improved collagen levels |
| Villa, 201251 | Prospective | 34 | Advanced cirrhosis (CTP B7-C10) | LMWH | 12 months | Reduced decompensation rate, reduced bacterial translocation and inflammation, improved survival |
| Dhar, 201587 | Prospective | n.a. | Post liver transplant HCV-related liver fibrosis | VKAs | 12 months | Decreased fibrosis |
| PATIENTS WITH PVT | ||||||
| Francoz, 200550 | Prospective | 19 | Cirrhosis, awaiting LT | LMWH followed by VKAs | 8 months | 42% of complete PV recanalization |
| Amitrano, 201072 | Retrospective | 28 | Cirrhosis | LMWH | 6 months | 33% of complete and 50 % of partial PV recanalization |
| Garcovich, 2011105 | Retrospective | 15 | Cirrhosis | LMWH | 6 months | 47% of PV recanalization |
| Delgado, 201270 | Retrospective | 55 | Cirrhosis | LMWHs or VKAs | 7 months | 60% of partial or complete PV recanalization |
| Senzolo, 201273 | Prospective | 35 | Cirrhosis | LMWH | 6 months | 36% of complete and 27% of partial PV recanalization |
| Werner, 201371 | Retrospective | 28 | Cirrhosis, awaiting LT | VKAs | 10 months | 39% of complete and 43% of partial PV recanalization |
| Chung, 2014106 | Prospective | 14 | Cirrhosis | VKAs | 4 months | 43% of complete and 27% of partial PV recanalization |
| Risso, 2014107 | n.a. | 50 | Cirrhosis, awaiting LT | n.a. | n.a. | 70% of PV recanalization |
| Cui, 201574 | Prospective | 65 | HBV cirrhosis | LMWH | 6 months | 78% of partial or complete PV recanalization |
| Chen, 201667 | Retrospective | 30 | Cirrhosis | VKAs | 8 months | 68% of PV recanalization |
| Wang, 2016108 | Prospective | 31 | Cirrhosis | VKAs | 12 months | 100% of PV recanalization |
| Zhang, 2017109 | Prospective | 7 | Decompensated cirrhosis | Fondaparinux | 1-3 weeks | 100% of PV recanalization |
| Hanafy, 201879 | Prospective | 80 | HCV cirrhosis | Rivaroxaban vs. VKAs | 6 months | 85% of PV recanalization with rivaroxaban; 45% with VKAs |
| Pettinari-Vukotic, 2018110 | Retrospective | 81 | Cirrhosis | LMWH (69%), Fondaparinux (19%) or VKA (12%) | 13 months | 57% of PV recanalization |
HBV, hepatitis B virus; HCV, hepatitis C virus; LT, liver transplantation; PV, portal vein; PVT, portal vein thrombosis; VKAs, vitamin K antagonists.
Very similar drawbacks can be found in the studies with the recently introduced DOACs. Because no data on their safety in patients with chronic liver disease were available from registration studies, several groups started small pilot studies to ascertain their safety (reviewed in111). All studies confirmed that DOACs could be safely used in chronic liver disease but their efficacy in PVT treatment is still to be ascertained.
Recently, a non-pharmacological approach to PVT treatment with transjugular intrahepatic portosystemic shunt was also developed. Results are reviewed in Valentin et al.112 but will not be discussed in this review, as these studies are not anticoagulation-based.
On the whole, convincing data about a real advantage from treatment of PVT are lacking, as obtaining recanalization of portal vein does not guarantee per se an improvement of the course of chronic liver disease. A prospective study with well-defined endpoints in a well-characterized cohort of patients would probably be the only way of clarifying these issues. An ongoing prospective study, presented at The Liver Meeting™ 2018, in patients with Child-Pugh class A cirrhosis shows that the occurrence of PVT in these patients is low (3% at 1 year and 7% at 3 years) and does not negatively influence the course of disease. However, the effect of anticoagulation for PVT treatment should be prospectively evaluated in more advanced patients with cirrhosis, i.e. Child-Pugh class B/C, as in these patients the possible favourable effect of anticoagulation could be greater.
Financial support
The authors received no financial support to produce this manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhepr.2019.02.006.
Contributor Information
Dominique-Charles Valla, Email: dominique.valla@aphp.fr.
Erica Villa, Email: erica.villa@unimore.it.
Supplementary data
Supplementary material
References
- 1.Monroe DM, Hoffman M. The coagulation cascade in cirrhosis. Clin Liver Dis. 2009;13:1–9. doi: 10.1016/j.cld.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Dahlbäck B. Progress in the understanding of the protein C anticoagulant pathway. Int J Hematol. 2004;79:109–116. doi: 10.1532/ijh97.03149. [DOI] [PubMed] [Google Scholar]
- 3.Castelino DJ, Salem HH. Natural anticoagulants and the liver. J Gastroenterol Hepatol. 1997;12:77–83. doi: 10.1111/j.1440-1746.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 4.Jennings I, Calne RY, Baglin TP. Predictive value of von Willebrand factor to ristocetin cofactor ratio and thrombin-antithrombin complex levels for hepatic vessel thrombosis and graft rejection after liver transplantation. Transplantation. 1994;57:1046–1051. [PubMed] [Google Scholar]
- 5.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 6.Hugenholtz GC, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT, Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology. 2013;58:752–761. doi: 10.1002/hep.26372. [DOI] [PubMed] [Google Scholar]
- 7.Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44:53–61. doi: 10.1002/hep.21231. [DOI] [PubMed] [Google Scholar]
- 8.Weeder PD, Porte RJ, Lisman T. Hemostasis in liver disease: implications of new concepts for perioperative management. Transfus Med Rev. 2014;28:107–113. doi: 10.1016/j.tmrv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Turco L, Garcia-Tsao G, Magnani I, Bianchini M, Costetti M, Caporali C. Cardiopulmonary hemodynamics and C-reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol. 2018;68:949–958. doi: 10.1016/j.jhep.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–576. doi: 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 11.de Franchis R, Baveno VI Faculty Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Schepis F, Turco L, Bianchini M, Villa E. Prevention and Management of Bleeding Risk Related to Invasive Procedures in Cirrhosis. Semin Liver Dis. 2018;38:215–229. doi: 10.1055/s-0038-1660523. [DOI] [PubMed] [Google Scholar]
- 13.Under the Auspices of the Italian Association for the Study of Liver Diseases (AISF) and the Italian Society of Internal Medicine (SIMI). Hemostatic balance in patients with liver cirrhosis: report of a consensus conference. Dig Liver Dis. 2016;48:455–467. doi: 10.1016/j.dld.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 14.De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B. Thromboelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63:566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 15.Zakeri N, Tsochatzis EA. Bleeding Risk with Invasive Procedures in Patients with Cirrhosis and Coagulopathy. Curr Gastroenterol Rep. 2017;19:45. doi: 10.1007/s11894-017-0585-6. [DOI] [PubMed] [Google Scholar]
- 16.Hung A, Garcia-Tsao G. Acute kidney injury, but not sepsis, is associated with higher procedure-related bleeding in patients with decompensated cirrhosis. Liver Int. 2018;38:1437–1441. doi: 10.1111/liv.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8:899–902. doi: 10.1016/j.cgh.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Bosch J, Thabut D, Albillos A, Carbonell N, Spicak J, Massard J. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008;47:1604–1614. doi: 10.1002/hep.22216. PubMed PMID: 18393319. [DOI] [PubMed] [Google Scholar]
- 20.Bosch J, Thabut D, Bendtsen F, D'Amico G, Albillos A, González Abraldes J. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123–1130. doi: 10.1053/j.gastro.2004.07.015. PubMed PMID: 15480990. [DOI] [PubMed] [Google Scholar]
- 21.Bendtsen F, D'Amico G, Rusch E, de Franchis R, Andersen PK, Lebrec D. Effect of recombinant Factor VIIa on outcome of acute variceal bleeding: an individual patient based meta-analysis of two controlled trials. J Hepatol. 2014;61:252–259. doi: 10.1016/j.jhep.2014.03.035. Epub 2014 Apr 5. PubMed PMID: 24713188. [DOI] [PubMed] [Google Scholar]
- 22.Tripodi A. Hemostasis in Acute and Chronic Liver Disease. Semin Liver Dis. 2017;37:28–32. doi: 10.1055/s-0036-1597770. [DOI] [PubMed] [Google Scholar]
- 23.Afdhal NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442-52.e1. doi: 10.1053/j.gastro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, Okoshi M, Tomiyoshi K, Kojima Y, Horigome R, Imai M. Efficacy and safety of repeated use of lusutrombopag prior to radiofrequency ablation in patients with recurrent hepatocellular carcinoma and thrombocytopenia. Hepatol Res. 2019 Jan 2 doi: 10.1111/hepr.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG. Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion. 2014;54:1389–1405. doi: 10.1111/trf.12431. [DOI] [PubMed] [Google Scholar]
- 27.Nadim MK, Durand F, Kellum JA, Levitsky J, O'Leary JG, Karvellas CJ. Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol. 2016;64:717–735. doi: 10.1016/j.jhep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Giannini EG, Giambruno E, Brunacci M, Plaz Torres MC, Furnari M, Bodini G. Low Fibrinogen Levels Are Associated with Bleeding After Varices Ligation in Thrombocytopenic Cirrhotic Patients. Ann Hepatol. 2018;17:830–835. doi: 10.5604/01.3001.0012.0775. [DOI] [PubMed] [Google Scholar]
- 29.Bianchini M, Cavani G, Bonaccorso A, Turco L, Vizzutti F, Sartini A. Low molecular weight heparin does not increase bleeding and mortality post-endoscopic variceal band ligation in cirrhotic patients. Liver Int. 2018;38:1253–1262. doi: 10.1111/liv.13728. [DOI] [PubMed] [Google Scholar]
- 30.Cerini F, Gonzalez JM, Torres F, Puente A, Casas M, Vinaixa C. Impact of anticoagulation on upper-gastrointestinal bleeding in cirrhosis. A retrospective multicenter study. Hepatology. 2015;62:575–583. doi: 10.1002/hep.27783. [DOI] [PubMed] [Google Scholar]
- 31.Thomas W, Samama CM, Greinacher A, Hunt BJ, Subcommittee on Perioperative and Critical Care The utility of viscoelastic methods in the prevention and treatment of bleeding and hospital-associated venous thromboembolism in perioperative care: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:2336–2340. doi: 10.1111/jth.14265. [DOI] [PubMed] [Google Scholar]
- 32.De Pietri L, Bianchini M, Rompianesi G, Bertellini E, Begliomini B. Thromboelastographic reference ranges for a cirrhotic patient population undergoing liver transplantation. World J Transplant. 2016;6:583–593. doi: 10.5500/wjt.v6.i3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dias J.D., Sauaia A., Achneck H.E., Hartmann J., Moore E.E. Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost. 2019;17:984–994. doi: 10.1111/jth.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lancé MD. A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb J. 2015;13:1. doi: 10.1186/1477-9560-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castoldi E, Rosing J. Thrombin generation tests. Thromb Res. 2011;127:S21–S25. doi: 10.1016/S0049-3848(11)70007-X. [DOI] [PubMed] [Google Scholar]
- 36.Tripodi A. Thrombin Generation Assay and Its Application in the Clinical Laboratory. Clin Chem. 2016;62:699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 37.Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: a systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11:S209–S216. doi: 10.1016/j.dsx.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Kuo L, Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW. Liver cirrhosis in patients with atrial fibrillation: would oral anticoagulation have a net clinical benefit for stroke prevention? J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Xu Y, He Z, Zhang H, Lian X, Zhu T. Increased risk of cerebrovascular accident related to non-alcoholic fatty liver disease: aa meta-analysis. Oncotarget. 2018;9:2752–2760. doi: 10.18632/oncotarget.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sogaard KK, Horvath-Puho E, Gronbaek H, Jepsen P, Vilstrup H, Sorensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96–101. doi: 10.1038/ajg.2008.34. [DOI] [PubMed] [Google Scholar]
- 41.Ali M, Ananthakrishnan AN, McGinley EL, Saeian K. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig Dis Sci. 2011;56:2152–2159. doi: 10.1007/s10620-011-1582-5. [DOI] [PubMed] [Google Scholar]
- 42.Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137:1145–1149. doi: 10.1378/chest.09-2177. [DOI] [PubMed] [Google Scholar]
- 43.Ambrosino P, Tarantino L, Di Minno G, Paternoster M, Graziano V, Petitto M. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta-analysis. Thromb Haemost. 2017;117:139–148. doi: 10.1160/TH16-06-0450. [DOI] [PubMed] [Google Scholar]
- 44.Stine JG, Niccum BA, Zimmet AN, Intagliata N, Caldwell SH, Argo CK. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin Transl Gastroenterol. 2018;9:140. doi: 10.1038/s41424-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Hepatitis C Virus Infection and Risk of Venous Thromboembolism: A Systematic Review and Meta-Analysis. Ann Hepatol. 2017;16:514–520. doi: 10.5604/01.3001.0010.0279. [DOI] [PubMed] [Google Scholar]
- 46.Barba R, Gonzalvez-Gasch A, Joya Seijo D, Marco J, Canora J, Plaza S. Venous thromboembolism in patients with liver diseases. J Thromb Haemost. 2018;16:2003–2200. doi: 10.1111/jth.14255. [DOI] [PubMed] [Google Scholar]
- 47.Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247. [PubMed] [Google Scholar]
- 48.Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488–496. doi: 10.1002/hep.510270224. [DOI] [PubMed] [Google Scholar]
- 49.Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou PE, Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660–667. doi: 10.1002/hep.27546. [DOI] [PubMed] [Google Scholar]
- 50.Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691–697. doi: 10.1136/gut.2004.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260.e4. doi: 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Maruyama H, Okugawa H, Takahashi M, Yokosuka O. De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol. 2013;108:568–574. doi: 10.1038/ajg.2012.452. [DOI] [PubMed] [Google Scholar]
- 53.Luca A, Caruso S, Milazzo M, Marrone G, Mamone G, Crinò F. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology. 2012;265:124–132. doi: 10.1148/radiol.12112236. [DOI] [PubMed] [Google Scholar]
- 54.Nonami T, Yokoyama I, Iwatsuki S, Starzl TE. The incidence of portal vein thrombosis at liver transplantation. Hepatology. 1992;16:1195–1198. [PMC free article] [PubMed] [Google Scholar]
- 55.Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736–741. doi: 10.1016/j.jhep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–689. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Abdel-Razik A, Mousa N, Elhelaly R, Tawfik A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the Model for End-stage Liver Disease scoring system. Eur J Gastroenterol Hepatol. 2015 May;27:585–592. doi: 10.1097/MEG.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 58.Stine JG, Wang J, Shah PM, Argo CK, Intagliata N, Uflacker A. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: A matched case-control study. Liver Int. 2018;38:94–101. doi: 10.1111/liv.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21:1016–1021. doi: 10.1002/lt.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saugel B, Lee M, Feichtinger S, Hapfelmeier A, Schmid RM, Siveke JT. Thrombophilic factor analysis in cirrhotic patients with portal vein thrombosis. J Thromb Thrombolysis. 2015;40:54–60. doi: 10.1007/s11239-014-1124-z. [DOI] [PubMed] [Google Scholar]
- 61.Qi X, Chen H, Han G. Effect of antithrombin, protein C and protein S on portal vein thrombosis in liver cirrhosis: a meta-analysis. Am J Med Sci. 2013;346:38–44. doi: 10.1097/MAJ.0b013e31826485fc. [DOI] [PubMed] [Google Scholar]
- 62.Chaireti R, Rajani R, Bergquist A, Melin T, Friis-Liby IL, Kapraali M. Increased thrombin generation in splanchnic vein thrombosis is related to the presence of liver cirrhosis and not to the thrombotic event. Thromb Res. 2014;134:455–461. doi: 10.1016/j.thromres.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Tang W, Wang Y, Zhao X, Wang X, Zhang T, Ou X. Procoagulant imbalance aggravated with falling liver function reserve, but not associated with the presence of portal vein thrombosis in cirrhosis. Eur J Gastroenterol Hepatol. 2015;27:672–678. doi: 10.1097/MEG.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 64.Kalambokis GN, Oikonomou A, Baltayiannis G, Christou L, Kolaitis NI, Tsianos EV. Thrombin generation measured as thrombin-antithrombin complexes predicts clinical outcomes in patients with cirrhosis. Hepatol Res. 2016;46:E36–E44. doi: 10.1111/hepr.12520. [DOI] [PubMed] [Google Scholar]
- 65.Hugenholtz GC, Northup PG, Porte RJ, Lisman T. Is there a rationale for treatment of chronic liver disease with antithrombotic therapy? Blood Rev. 2015;29:127–136. doi: 10.1016/j.blre.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Qi X, De Stefano V, Senzolo M, Xu H, Mancuso A. Splanchnic Vein Thrombosis: Etiology, Diagnosis, and Treatment. Gastroenterol Res Pract. 2015;2015:506136. doi: 10.1155/2015/506136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, Turon F, Hernández-Gea V, Fuster J, Garcia-Criado A, Barrufet M. Nontumoral portal vein thrombosis in patients awaiting liver transplantation. Liver Transpl. 2016;22:352–365. doi: 10.1002/lt.24387. [DOI] [PubMed] [Google Scholar]
- 68.Hibi T, Nishida S, Levi DM, Selvaggi G, Tekin A, Fan J. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg. 2014;259:760–766. doi: 10.1097/SLA.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 69.Ghabril M, Charlton M. Lack of Survival Benefit Following Liver Transplantation With MELD Exception Points for Hepatocellular Carcinoma: Beyond the Unblinding of Lady Justice. Gastroenterology. 2015;149:531–534. doi: 10.1053/j.gastro.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 70.Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MW, García-Criado A. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–783. doi: 10.1016/j.cgh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Werner KT, Sando S, Carey EJ, Vargas HE, Byrne TJ, Douglas DD. Portal vein thrombosis in patients with end stage liver disease awaiting liver transplantation: outcome of anticoagulation. Dig Dis Sci. 2013;58:1776–1780. doi: 10.1007/s10620-012-2548-y. [DOI] [PubMed] [Google Scholar]
- 72.Amitrano L, Guardascione MA, Menchise A, Martino R, Scaglione M, Giovine S. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol. 2010;44:448–451. doi: 10.1097/MCG.0b013e3181b3ab44. [DOI] [PubMed] [Google Scholar]
- 73.Senzolo M, M Sartori T, Rossetto V, Burra P, Cillo U, Boccagni P. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919–927. doi: 10.1111/j.1478-3231.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 74.Cui SB, Shu RH, Yan SP, Yan SP, Wu H, Chen Y. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol. 2015;27:914–919. doi: 10.1097/MEG.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 75.Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of Anticoagulants in Patients With Cirrhosis and Portal Vein Thrombosis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;153:480–487.e1. doi: 10.1053/j.gastro.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 76.Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs. traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98:393–397. doi: 10.1111/ejh.12844. [DOI] [PubMed] [Google Scholar]
- 77.Intagliata NM, Henry ZH, Maitland H, Shah NL, Argo CK, Northup PG. Direct Oral Anticoagulants in Cirrhosis Patients Pose Similar Risks of Bleeding When Compared to Traditional Anticoagulation. Dig Dis Sci. 2016;61:1721–1727. doi: 10.1007/s10620-015-4012-2. [DOI] [PubMed] [Google Scholar]
- 78.De Gottardi A, Trebicka J, Klinger C, Plessier A, Seijo S, Terziroli B. VALDIG Investigators. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2017;37:694–699. doi: 10.1111/liv.13285. [DOI] [PubMed] [Google Scholar]
- 79.Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vasc Pharmacol. 2019;113:86–91. doi: 10.1016/j.vph.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 80.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016 Jan;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 81.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 82.Turco L, Schepis F, Villa E. The Role of Anticoagulation in Treating Portal Hypertension. Curr Hepatology Rep. 2018;17:200. doi: 10.1007/s11901-018-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerini F, Vilaseca M, Lafoz EE, García-Irigoyen O, García-Calderó H, Tripathi DM. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016;64:834–842. doi: 10.1016/j.jhep.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Vilaseca M, García-Calderó H, Lafoz E, García-Irigoyen O, Avila MA, Reverter JC. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65:2031–2044. doi: 10.1002/hep.29084. [DOI] [PubMed] [Google Scholar]
- 85.Shi J, Hao JH, Ren WH. Effects of heparin on liver fibrosis in patients with chronic hepatitis B. World J Gastroenterol. 2003;9:1611–1614. doi: 10.3748/wjg.v9.i7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang JS, Luo X, Yu JX, Liu W, Chen XW, Xie L. Indigenous and imported low molecular weight heparin in the treatment of chronic hepatitis B and cirrhosis with hepatitis B virus: a prospective randomized controlled clinical study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:408–411. [PubMed] [Google Scholar]
- 87.Dhar A, Tschotazis E, Brown R, Manousou P, Millson C, Aldersley M. Warfarin anticoagulation for liver fibrosis in patients transplanted for Hepatitis C (WAFT-C): Results at one year. J Hep. 2015;62:S263–S864. [Google Scholar]
- 88.Senzolo M, Rodriguez-Castro KI, Rossetto V, Radu C, Gavasso S, Carraro P. Increased anticoagulant response to low-molecular-weight heparin in plasma from patients with advanced cirrhosis. J Thromb Haemost. 2012;10:1823–1829. doi: 10.1111/j.1538-7836.2012.04824.x. [DOI] [PubMed] [Google Scholar]
- 89.Potze W, Arshad F, Adelmeijer J, Blokzijl H, van den Berg AP, Porte RJ. Routine coagulation assays underestimate levels of antithrombin-dependent drugs but not of direct anticoagulant drugs in plasma from patients with cirrhosis. Br J Haematol. 2013;163:666–673. doi: 10.1111/bjh.12593. [DOI] [PubMed] [Google Scholar]
- 90.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 92.Levi M, Hovingh GK, Cannegieter SC, Vermeulen M, Buller HR, Rosendaal FR. Bleeding in patients receiving vitamin K antagonists who would have been excluded from trials on which the indication for anticoagulation was based. Blood. 2008;111:4471–4476. doi: 10.1182/blood-2007-11-123711. [DOI] [PubMed] [Google Scholar]
- 93.Efird LM, Mishkin DS, Berlowitz DR, Ash AS, Hylek EM, Ozonoff A. Stratifying the risks of oral anticoagulation in patients with liver disease. Circ Cardiovasc Qual Outcomes. 2014;7:461–467. doi: 10.1161/CIRCOUTCOMES.113.000817. [DOI] [PubMed] [Google Scholar]
- 94.Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral Anticoagulation in Patients With Liver Disease. J Am Coll Cardiol. 2018;71:2162–2175. doi: 10.1016/j.jacc.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 95.Intagliata NM, Henry ZH, Shah N, Lisman T, Caldwell SH, Northup PG. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhotic patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34:26–32. doi: 10.1111/liv.12211. [DOI] [PubMed] [Google Scholar]
- 96.Goriacko P, Veltri KT. Safety of direct oral anticoagulants vs. warfarin in patients with chronic liver disease and atrial fibrillation. Eur J Haematol. 2018;100:488–493. doi: 10.1111/ejh.13045. [DOI] [PubMed] [Google Scholar]
- 97.U.S. Food and Drug Administration Apixaban. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf Available at:
- 98.European Medicines Agency Apixaban. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_ Information/human/ 002148/WC500107728.pdf Available at:
- 99.Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155:625–632. doi: 10.7326/0003-4819-155-9-201111010-00011. [DOI] [PubMed] [Google Scholar]
- 100.Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–394. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 101.Aldawood A, Arabi Y, Aljumah A, Alsaadi A, Rishu A, Aldorzi H. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9:1. doi: 10.1186/1477-9560-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang LS, Alukaidey S, Croucher K, Dowling D. Suboptimal use of pharmacological venous thromboembolism prophylaxis in cirrhotic patients. Intern Med J. 2018;48:1056–1063. doi: 10.1111/imj.13766. [DOI] [PubMed] [Google Scholar]
- 103.Barclay SM, Jeffres MN, Nguyen K, Nguyen T. Evaluation of pharmacologic prophylaxis for venous thromboembolism in patients with chronic liver disease. Pharmacotherapy. 2013;33:375–382. doi: 10.1002/phar.1218. [DOI] [PubMed] [Google Scholar]
- 104.Bechmann LP, Sichau M, Wichert M, Gerken G, Kröger K, Hilgard P. Low-molecular-weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31:75–82. doi: 10.1111/j.1478-3231.2010.02358.x. [DOI] [PubMed] [Google Scholar]
- 105.Caracciolo G, Garcovich M, Zocco MA, Ainora ME, Roccarina D, Annicchiarico BE. Clinical outcome of portal vein thrombosis (PVT) in cirrhotic patients: observe or treat? Hepatology (Baltimore, Md) 2011;54:1261A–1262A. [Google Scholar]
- 106.Chung JW, Kim GH, Lee JH, Ok KS, Jang ES, Jeong SH. Safety, efficacy, and response predictors of anticoagulation for the treatment of nonmalignant portal-vein thrombosis in patients with cirrhosis: a propensity score matching analysis. Clin Mole Hepatol. 2014;20:384–391. doi: 10.3350/cmh.2014.20.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Risso A, Stradella D, Martini S, Rizzetto M, Salizzoni M. Liver transplantation in cirrhotic patients with portal vein thrombosis: a single centre experience. Dig Liver Dis. 2014;46 [Google Scholar]
- 108.Wang Z, Jiang MS, Zhang HL, Weng NN, Luo XF, Li X. Is post-TIPS anticoagulation therapy necessary in patients with cirrhosis and portal vein thrombosis? A randomized controlled trial. Radiology. 2016;279:943–951. doi: 10.1148/radiol.2015150369. [DOI] [PubMed] [Google Scholar]
- 109.Zhang ZH, Zhang JW, He P, Zhou Y, Sun CY. Fondaparinux is effective for acute portal vein thrombosis in decompensated cirrhotic patients. Medicine. 2017;96 doi: 10.1097/MD.0000000000008256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pettinari I, Vukotic R, Stefanescu H, Pecorelli A, Morelli M, Grigoras C. Clinical Impact and Safety of Anticoagulants for Portal Vein Thrombosis in Cirrhosis. Am J Gastroenterol. 2018 Dec 11 doi: 10.1038/s41395-018-0421-0. [DOI] [PubMed] [Google Scholar]
- 111.Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018;170:102–108. doi: 10.1016/j.thromres.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 112.Valentin N, Korrapati P, Constantino J, Young A, Weisberg I. The role of transjugular intrahepatic portosystemic shunt in the management of portal vein thrombosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:1187–1193. doi: 10.1097/MEG.0000000000001219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material