Abstract
Macrophages perform critical functions for homeostasis and immune defense in tissues throughout the body. These innate immune cells are capable of recognizing and clearing dead cells and pathogens, and orchestrating inflammatory and healing processes that occur in response to injury. In addition, macrophages are involved in the progression of many inflammatory diseases including cardiovascular disease, fibrosis, and cancer. Although it has long been known that macrophages respond dynamically to biochemical signals in their microenvironment, the role of biophysical cues has only recently emerged. Furthermore, many diseases that involve macrophages are also characterized by changes to the tissue biophysical environment. This review will discuss current knowledge about the effects of biophysical cues including matrix stiffness, material topography, and applied mechanical forces, on macrophage behavior. We will also describe the role of molecules that are known to be important for mechanotransduction, including adhesion molecules, ion channels, as well as nuclear mediators such as transcription factors, scaffolding proteins, and epigenetic regulators. Together, this review will illustrate a developing role of biophysical cues in macrophage biology, and also speculate upon molecular targets that may potentially be exploited therapeutically to treat disease.
Summary sentence:
Review on the role of biophysical cues in regulating macrophage function, and potential molecular mediators of mechanotransduction.
Graphical Abstract
Introduction
Macrophages are innate immune cells that can adopt a spectrum of phenotypes by responding to signals in their surrounding environment [1, 2]. These cells play a critical role in maintaining tissue homeostasis by clearing cell debris, foreign materials, and orchestrating inflammatory and healing processes [3, 4]. In response to damaged tissue or pathogens, macrophages polarize toward a classically activated phenotype (also known as M1), and secrete pro-inflammatory cytokines to recruit other immune cells. Upon stimulation with wound healing cytokines, they polarize toward an alternatively activated phenotype (often referred to as M2), and help to dampen inflammation and promote healing and tissue regeneration. These M1 and M2 phenotypes were initially thought to exist as binary cell states, but macrophages in vivo are often found to express markers associated with both phenotypes [2]. In addition, macrophages are now known to express diverse transcriptional profiles that are distinct from the traditional M1 and M2 paradigm, particularly when they are exposed to signals present in diseases such as cardiovascular disease, chronic inflammation, and cancer [5]. The complexity and versatility of macrophage activation within the context of disease highlight their remarkable ability to respond, and potentially contribute, to dynamic changes in microenvironmental cues.
Our current understanding of macrophages is largely based on their response to soluble, diffusible signals including chemokines, cytokines, and damage- or pathogen-associated molecular patterns, among many others [6]. However, macrophages exist within complex matrix environments, as they are either recruited to or reside within tissues throughout the body, where they may be exposed to a wide array of biophysical cues. Therefore, it is important to also consider how the biophysical factors present within different cellular environments, including mechanical forces and tissue physical properties, might contribute to macrophage function. Biophysical cues have been shown to modulate the function of many different cell types. In cardiovascular tissues, shear stress regulates healthy function of the endothelium, and mechanical stretch promotes cardiomyocyte contractility and electrical conduction [7, 8]. In bone, tension and compression are required for tissue remodeling and osteogenesis after injury [9]. In addition to externally applied forces, tissue stiffness and topography can also regulate cell function. In seminal work performed just over a decade ago, mesenchymal stem cells were shown to commit to specific lineages depending on the stiffness of the tissue culture substrate - on materials with brain-like stiffness, they adapted a neurogenic phenotype, whereas on substrates of increasing stiffness, they adapted myogenic and osteogenic phenotypes [10]. These studies suggest an essential role for biophysical cues in development and regulation of healthy function of cells within tissues.
Interestingly, many diseases have been associated with changes in biophysical properties of tissues [11, 12]. For example, tumors are characterized by heightened collagen deposition and matrix stiffness, and these factors are known to contribute to disease progression and metastasis [13]. Lipid and calcium-rich atherosclerotic plaques alter the flow profiles within blood vessels and also have higher stiffness, which are associated with increased endothelial inflammation and permeability, and thus enhanced leukocyte recruitment to the vessel wall [14]. During abnormal wound healing and tissue fibrosis, remodeling of the extracellular matrix (ECM) often results in scar tissue that is stiffer than native tissue and exhibits thicker and denser collagen fibrils [15]. Notably, macrophages are known to play an important role in the progression of all of these diseases, and in some cases their presence is correlated with poor prognosis [16, 17]. Thus, a better understanding of how the biophysical environment influences the phenotype and behavior of macrophages will likely unveil new strategies and molecular targets for treatment of disease.
In this review, we will discuss studies investigating the role of biophysical cues in regulating macrophage function, including their migration, phagocytotic behavior, and polarization towards inflammatory and healing phenotypes. Interestingly, the mechanisms underlying their responses appear to be unique from cells that have traditionally been studied in mechanobiology, likely because the molecular machinery involved in mechanotransduction such as adhesive and cytoskeletal structures are quite different. We will first describe studies that show a role for stiffness, topography, and mechanical forces in macrophage behavior, and then provide insight to potential molecular mediators of these responses.
2. Regulation of macrophages by biophysical cues
2.1. Matrix composition
The extracellular matrix (ECM) is composed of proteins, polysaccharides, and glycoproteins, providing a structural scaffold upon which cells adhere and organize to form tissues, and undergoes dynamic changes in both composition and physical properties during tissue healing and in disease [18]. Collagen is the most abundant ECM protein found in the body and forms fibrils to give tissue tensile strength, whereas elastin associates with collagen in many tissues and provides tissue elasticity through its coiled conformation. In addition, fibronectin is a globular protein that facilitates cell attachment, and other specialized ECMs exist within basement membranes, which is found basolateral to cell monolayers throughout the body, and is composed of collagen type IV, laminin, among many other molecules. Upon injury, native ECM structures are damaged and replaced by a provisional matrix composed mainly of fibrin that is eventually remodeled to form collagen-rich scar. Many ECM molecules have domains that interact with adhesion receptors found on the surface of cells. Furthermore, ECM can bind soluble factors including growth factors and alter their activity and/or presentation to cells. Thus, the ECM is not only the adhesive and structural support for cells, but can also provide instructive cues to manipulate their behavior.
To investigate the effects ECM composition on macrophages, cells can be cultured on surfaces coated with various ECM proteins, and their behavior examined. In an early study, bone marrow-derived macrophages (BMDMs) cultured on dishes coated with fibronectin, laminin, type I collagen or type IV collagen were found to have a slower growth rate when compared to cells cultured on tissue culture plastic alone, which naturally adsorbs soluble ECM proteins found in serum [19]. Cells adopted different morphologies on different ECMs, with collagen IV and laminin causing a rounder shape, compared to the other ECMs. Despite clear changes in morphology, secretion of inflammatory cytokines including IL-6 and TNFα was similar across the different culture conditions. These results were corroborated in our recent study, where we also observed similar levels of TNFα secreted by BMDMs cultured on Matrigel, vitronectin, and fibrinogen in addition to the ECM proteins above [20]. We further found that the expression of Arginase-1 (Arg1), a pro-healing marker, was enhanced when macrophages were cultured on laminin, Matrigel and vitronectin compared to the other ECMs [20]. Interestingly, these ECMs have been associated with tumors, which are often laden with protective M2-like macrophages, often referred to as myeloid suppressor cells [21]. Taken together, these results suggest that the ECM itself has minimal impact on inflammatory activation, but may play a more significant role in polarization towards a pro-healing phenotype.
Although ECM coatings on two-dimensional (2D) culture surfaces provide some insight to the effect of composition on macrophage function, it poorly recapitulates the three-dimensional (3D) environment that cells experience in the body. In the same early study described above, BMDMs were cultured in agar suspensions containing ECM proteins, IL-6 secretion was significantly reduced compared to control agar with no ECM protein [19], suggesting that adhesion to the matrix in a 3D environment modulates macrophage cytokine secretion. Our laboratory observed that macrophages cultured on top of fibrin gels generated by polymerization of fibrinogen using thrombin, exhibit reduced secretion of TNFα when compared to cells cultured on polystyrene surfaces [22]. Addition of the adhesive peptide arginine-glycine-aspartic acid (RGD) to non-adhesive hydrogels composed of polyethylene glycol also led to inhibition of inflammatory activation, suggesting that interaction with RGD-binding integrin receptors may be important [23, 24]. In addition to their activation, the effects of the physical structure on migration of macrophages through 3D gels has also been studied [25]. As perhaps expected, a minimum matrix density is required for motility, but enhanced density of fibrin impedes macrophage migration. Gel matrix supplemented with fibronectin or crosslinked with factor XIII also reduced migration [26]. Together, these studies suggest that the 3D ECM environment impacts macrophage activation and motility. Further studies will be needed to characterize the diversity of ECM molecules present during healthy and disease states, and probe their individual and combinatorial effects on macrophage activity.
2.2. Substrate stiffness
ECM-based 3D materials differ significantly from traditional cell culture materials in their stiffness, the ability of a material to resist deformation in response to applied force, which itself has been shown to affect the behavior of cells [10, 27] . Tissue stiffness can be characterized by elastic modulus, which ranges from 1 kPa for brain to 10 kPa for muscle, and S~GPa for bone [28]. Many tumors and fibrous scar tissues have stiffnesses up to 10 fold greater than the native healthy tissue [29, 30]. Atherosclerotic plaques are often even stiffer because of the presence of calcium deposits [31]. Experimentally, crosslinking can be used to control the stiffness of ECM-based materials, but this method also leads to changes in the ligand density, as well as the pore size and fibrillar architecture of the matrix. Engineered synthetic materials such as polyacrylamide or polyethylene glycol offer the ability to tune material stiffness on a nonadhesive background, which is then further modified with adhesion proteins [32–34]. Although these materials generally do not reproduce the fibrillar architecture of ECM materials, they allow for independent manipulation of matrix mechanical properties and ligand density, and have led to tremendous insights about the role of stiffness on cell function.
Using such engineered systems, murine alveolar macrophages (AM) and bone marrow derived macrophages (BMDM), as well as human peripheral blood mononuclear cell-derived macrophages have all generally been observed to have enhanced adhesion and spreading when cultured on stiffer surfaces (hundreds of kPa) compared to soft surfaces (~1–10 kPa) [35–39]. In most of these studies, stiffness-dependent changes in adhesion were also associated with increased actin and cytoskeletal stiffness. In addition, macrophages cultured on substrates of increasing stiffness generally exhibit increase in proliferation and migration, [37], as well as increased phagocytosis [38, 39] (Fig. 1 a and b), With respect to phenotype polarization, several groups have found that culture of macrophages (THP-1, RAW 264.7, or BMDM) on stiffer materials potentiates inflammatory activation [24, 40], although there are some reported discrepancies [41]. Nonetheless, subcutaneous implantation of polyethylene glycol hydrogels with different stiffnesses led to greater macrophage recruitment and fibrous capsule thickness in response to stiffer compared to softer materials, suggesting an important ramification of material stiffness on the immune-mediated foreign body response in vivo [24]. In the context of cancer, a recent study showed that matrix stiffness potentiated the ability for tumor associated macrophages to regulate the invasive potential of tumor cells in co-culture model of lung adenocarcinoma [42] The presence of macrophages and increased matrix stiffness together enhanced the epithelial to mesenchymal transition of human adenocarcinoma cells and increased tumor cell proliferation and invasiveness. Given that matrix stiffness has also been shown to enhance the activation of macrophages themselves, tumor stiffness may contribute to the protective activity that is commonly asociated with tumor-associated macrophages [24, 40]. One possibility is that increased stiffness along with the presence of protective cytokines enhances macrophage alternative activation, although this hypothesis has yet to be tested.
Figure 1: Effect of biophysical cues on macrophage function.
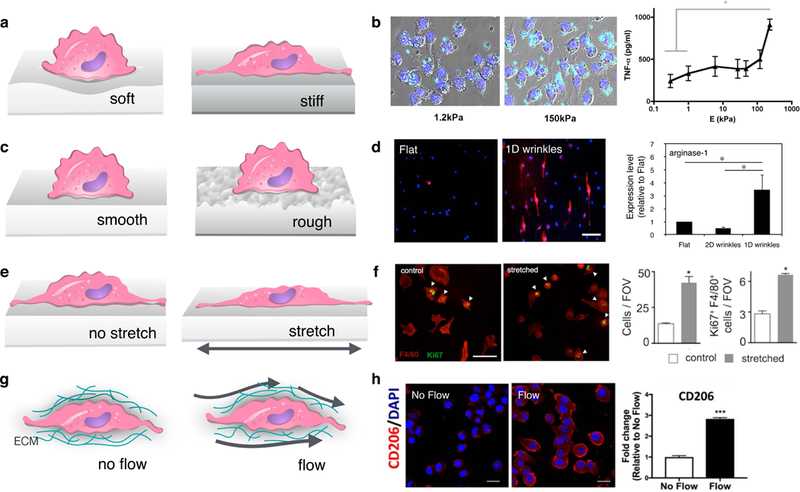
(a, c, e, g) Schematics showing the interaction of macrophages with surfaces of different stiffness (a) or topology (c) and external mechanical forces including stretch (e) and interstitial flow (g). (b) Image of IgG coated latex beads (cyan) uptaken by RAW264.7 macrophages on stiff compared to soft surfaces (left), adapted from [38], and TNFα secretion by primary murine macrophages on surfaces different stiffness (right), adapted from [40]. (d) Immunofluorescence image of Arg1 (M2 marker, red) and nuclei (blue) of macrophages cultured on flat and 1D wrinkled surfaces (left), and quantification of Arg1 expression as measured by Western blot (right), adapted from [59]. (f) Immunofluorescence image of F4/80 (red) and Ki67 (green) cell proliferation marker of murine peritoneal macrophages undergoing cyclic mechanical stretch (4%, biaxial, 0.67 Hz) compared to static controls (left) and quantification of cell number and Ki67 (right), adapted from [71]. (h) Immunofluorescence images of Dapi (blue) and CD206 (M2 marker, red) in BMDM exposed to interstitial flow (3 μm/s) compared to static controls (left), and quantification of CD206 levels (right), adapted from [83].
Differences in observations described above may be caused by varied experimental design parameters associated with in vitro studies, including the choice of macrophage source, time allowed for adhesion, as well as the type of adhesive protein or phagocytic target used. Increases in the stiffness of the target itself is also thought to enhance its phagocytic uptake [43, 44]. In addition, since substrate stiffness generally causes changes in adhesion, it is possible that different numbers of cells are adhering on soft versus stiff surfaces as demonstrated in the study [45]. Given the known effects of macrophage homotypic paracrine interactions on their function [46], it is necessary to consider variations in cell density across the culture conditions in order to eliminate any confounding factors. Furthermore, the effects could be biphasic, as has been observed for cell spreading and inflammatory activation [41, 47]. Thus, it becomes important to study a wide and detailed range of stiffnesses in order to comprehensively characterize their effects.
2.3. Topographical cues and adhesive geometry
Substrate topography, or physical features on the surface of a material, is a significant biophysical cue that can guide macrophage behavior. Much of our understanding of this phenomenon stems from investigations of biomaterials for medical implants. Early studies demonstrated that macrophages preferentially adhere to rough surfaces [48], and exhibit heightened spreading, elongation, and motility when cultured on textured compared to untextured surfaces [49, 50]. Furthermore, macrophages cultured on sandblasted and etched titanium surfaces secreted more proinflammatory cytokines compared to smooth surfaces, both with and without lipopolysaccharide (LPS) stimulation [51]. When implanted femorally in rats, TiO2 surfaces containing rough features promoted pro-inflammatory M1 polarization and impaired osteogenicity compared to smooth controls [52]. Extending into 3D, material porosity also impacts infiltration and activation of macrophages. Porous hydrogel implants placed subcutaneously in mice rats produced significantly thinner and vascularized fibrous capsules than their non-porous controls [53]. Such porous implants also induced spatial differences in macrophage polarization, with cells in the pores shifted towards an M1 phenotype, while macrophages on the surface exhibited less M2 marker expression. Here, improved foreign body response was attributed to increased macrophage infiltration and enrichment of M1 cells, which has been reported by others to be necessary for enhanced wound healing [54, 55].
Effects of surface topography are thought to be independent of the chemistry of the material and several studies have used engineered materials in order to investigate specific defined features that might cause changes in macrophage function. Using photolithographic techniques, micron-scale surface patterns can be generated with a wide range of shapes and sizes. Micropatterned posts appear to induce a greater M2-like polarization in macrophages than line patterns, but interestingly the spacing between the features had a more significant effect on macrophage behavior than the size of the features themselves [56]. Another study also compared macrophages cultured on micropillars, microgrooves and unpatterned surfaces, and found distinct gene expression profiles, particularly programs involved in transcription, translation, protein trafficking, DNA repair, and cell survival [57]. Topographical features that elicit cellular elongation appear to enhance macrophage wound healing responses. In work from our own laboratory, macrophages cultured on an array of microgrooves of varied dimensions tend to express more Arg1, an M2 marker, with increased degree of elongation [58]. In addition, biomimetic wrinkle substrates that cause macrophage elongation also promote Arg1 expression and IL-10 secretion in vitro (Fig. 1 c and d). These findings were further corrobrated with the host response following implantation in vivo. Grooved materials implanted within the subcutaneous space of mice showed heightened Arg1 expression and reduced collagen density in the tissue surrounding the biomaterial when compared to flat materials [59]. In 3D, fibrous geometries generated by electrospinning pores appear to alter macrophages, with more M2 macrophage response to microscale fibers in comparison to nanoscale fibers [60, 61]. A study performed on nanofibrous PLLA substrates showed that fiber diameter and not alignment was the primary feature that caused changes in macrophage activity [62]. Moreover, the impact of the topography on macrophages was dynamic, with pro-inflammatory cytokine secretion reduced in the first 24 h, but no differences are observed after 7 days of culture. This result highlights the temporal nature of the effects of substrate adhesion on macrophage behavior.
The effects of surface topography are often correlated with changes in cell shape, which intriguingly is also associated with macrophage differentiation and polarization. Monocytes treated with granulocyte-macrophage colony stimulating factor (GM-CSF) to induce differentiation to macrophages or macrophages stimulated with LPS yield flat but rounded cells. In contrast, differentiation with macrophage colony stimulating factor (M-CSF) or stimulation with IL-4 and/or IL-13 causes macrophage elongation [63–65]. Using microcontact printing, we showed that cell shape itself can contribute to function of macrophages. In this method, adhesive proteins are transferred to a substrate using a photolithographically generated stamp. Cells seeded on the patterned substrates adhere to the proteins and adopt the adhesive geometry of features determined by the stamp [66]. Using this technique, we found that cellular elongation causes an increase Arg1, and potentiates the effects of IL-4/IL-13. Furthermore, elongation mitigates the effects of inflammatory stimuli LPS and IFNγ. While the mechanisms underlying these effects remain mostly unknown, these shape-induced changes are lost with the addition of inhibitors of cytoskeletal polymerization and contractility [65]. Interestingly, a recent study that also used micropatterning tools showed that restricting cell spreading can suppress late inflammatory gene response to LPS [67]. This effect was associated with the actin-bound transcription factor, myocardin related transcription factor A (MRTF-A), which is released upon cell spreading and potentiates inflammatory gene activation. While this study revealed a novel mechanism underlying control of macrophage behavior, much work is still needed to elucidate the molecules involved in macrophage sensing of their topographical surroundings.
Most studies investigating topography have been performed on engineered materials that are very stiff, and the effects of topographical cues in a soft environment still remain relatively unexplored. Soft polymeric materials, often hydrogels, are more challenging to fabricate with precisely tuned topography or architecture. Furthermore, soft materials can be deformed by contractile traction forces exerted by cells, and therefore the topography changes over time [68]. Engineering the self assembly conditions of soft ECM hydrogels could potentially be used to control fibril length, thickness, density, and mechanical properties, and may provide more physiologic architectures [69]. However, cells will also remodel these natural ECM environments and the dynamics need to be considered. Thus, further work is needed to investigate the combinatorial effects of substrate topography and stiffness.
2.4. External forces
Externally applied mechanical forces such as stretch and shear stresses regulate the function of many cell types, especially those that reside within mechanically active tissues. Macrophages exist within tissues throughout the body including those that are mechanically active and are, therefore, also exposed to dynamic external forces that often change with disease. For example, in cardiovascular tissues a 10% strain amplitude is characteristic of healthy blood vessels, but strain levels increase during hypertension or myocardial infarction [70–72]. In the lungs, inhalation and exhalation results in cyclic mechanical stretch, but ventilator-induced stretch exacerbates strain [73]. Studies investigating the role of mechanical stretch commonly utilize in vitro systems, whereby cells are cultured on flexible silicone membranes that are stretched using a computer-controlled motor. To manipulate the shear environment, cells are cultured within flow chambers or microfluidic systems, and culture media is flowed through using a pump. These engineered systems can provide a wide variety of defined regimens to manipulate the mechanical environment of cells.
Several studies have found that mechanical stretch causes macrophages to elongate along the direction of stretch [74, 75]. Stretch has also been observed to increase macrophage expression of matrix metalloproteinase-9 (MMP-9), an enzyme involved in extracellular matrix remodeling [76, 77]. The role of stretch in influencing macrophage inflammatory activation remains less clear, with higher levels of inflammatory cytokine secretion observed in some studies and others showing minimal effects [77–79]. When pre-polarized with inflammatory stimuli interferon-γ (IFN-γ) and LPS, cyclic stretch had no effect on inflammatory markers [79]. However, simultaneous addition of IFN-γ/LPS with mechanical stretch resulted in increased secretion of the inflammatory markers IL-6 and TNFα, in alveolar macrophages compared to unstretched controls [76]. Finally, cyclic mechanical stretch has been shown to increase the proliferation of peritoneal macrophages (Fig. 1 e and f) [71]. This observation was thought to explain the increased presence of macrophages in the heart following myocardial infarction. Stretch is, therefore, capable of altering macrophage morphology, function, as well as proliferative state.
Shear stress caused by blood flow in vessels has a well-established effect on endothelial function, leading to changes in monocyte/macrophage recruitment. Endothelial cells exposed to healthy shear levels (12 dyn/cm2 ) exhibit significant decreases in bound THP-1 monocytes compared to static controls [80]. However, lower shear stresses and flow reversal conditions, both of which are common in vessel bifurcations and have been shown to play a role in plaque development, result in a higher expression of vascular cell adhesion molecule-1 (VCAM-1) by endothelial cells, and thus greater leukocyte attachment and recruitment [81, 82]. Together, these studies suggest that healthy shear stress maintains endothelial function with minimal leukocyte recruitment, and pathological shear conditions contribute to the adhesion and recruitment of monocyte/macrophages to tissues.
Fluid flow has also recently been shown to directly influence macrophage functional activation and migration. Li. et al. developed a microfluidic system to expose macrophages within collagen gels to interstitial flow, or flow through tissues, which was aimed at recapitulating flow emanating from a tumor to the surrounding stromal tissues [83]. Using this system, the group showed that interstitial fluid flow increased the expression of phosphorylated STAT3/6 and CD206 expression, markers associated with macrophage pro-healing activation (Fig. 1 g and h), which was abolished by antibody-mediated blocking of β1 integrins. In addition, macrophages were observed to migrate against the direction of flow. The results, thus, suggest that interstitial flow may be involved in polarizing and recruiting pro-healing macrophages to tumors, and that targeting β1 integrins could potentially modulate these effects. Further characterization of the effects of shear stresses on altering macrophage function and activity, as well as uncovering the responsible molecular mechanisms in transducing these signals, will help elucidate the role of mechanical forces in both healthy macrophage function and disease development.
As with studies of stiffness described earlier, the lack of a clear consensus in the role of mechanical forces, particularly the effects of stretch, in influencing macrophage function likely stems from differences in experimental parameters including source of cells, mechanical stimulation profiles, doses of cytokines or chemokines, extracellular matrix used to coat culture substrates, and experimental timecourse. In addition, while the effects of stretch on inflammatory activation have been studied, the effects of cyclic stretch on macrophage function in a healing environment remain unknown. While published studies have independently explored different strain profiles, a systematic study of macrophage response to various amplitudes or frequencies of strain would provide more comprehensive knowledge. Understanding the effects of mechanical stimulation combined with other biophysical cues, such as substrate stiffness and topography, also remain unknown. Furthermore, the adhesion molecules and intracellular signaling pathways responsible for stretch-mediated changes have not been explored. Future work elucidating these molecular mechanisms will contribute to our understanding about how mechanical forces regulate macrophage function during disease, and potentially uncover new molecular targets for therapy.
3. Molecular mechanisms of mechanotransduction
3.1. Integrin and cytoskeletal-mediated interactions
Adhesion receptors are thought to play a major role in sensing mechanical cues and transducing them into biological signals that regulate cell function, a process referred to as mechanotransduction. This includes integrins, heterodimeric transmembrane receptors composed of α and β subunits, which bind to the ECM and connect the extracellular environment with the intracellular cytoskeleton [84]. Integrins play a central role in various cellular activities including morphological changes, motility, proliferation and differentiation. Given the changes in adhesion that occur as monocytes transition to macrophages, it is not surprising that differentiation leads to expression of integrins and integrin-associated molecules such as focal adhesion kinase (FAK) [85–87]. Our own analysis of publicly available RNA-seq data [88] shows that differentiation of HL60 myeloid progenitor cells to macrophages with phorbol 12-myristate 13-acetate (PMA) treatment is largely associated with the upregulation of integrin and cadherin adhesome genes [89, 90], which include adhesion receptors as well as adaptor and intracellular signaling molecules (Fig. 2). While adhesome genes are generally upregulated with macrophage differentiation, there does appear to be a smaller portion of genes only transiently upregulated during early exposure to PMA. In addition, exposure of differentiated macrophages to LPS also influences the expression of several adhesome genes. Similarly, when primary human macrophages are activated by different polarizing stimuli, including LPS, IFNγ, IL4, adhesome gene expressions are dynamically controlled [5], exhibiting patterns of both up and down-regulation (Fig. 3). LPS increased overall adhesome gene expression, which was further enhanced upon addition of IFNγ. While stimulation with both LPS and IFNγ appears to have synergistic effects on adhesome gene regulation, the effects do not appear to be additive and IFNγ stimulation alone has only a mild impact on adhesome gene expression. Interestingly, ultrapure LPS treatment, which specifically targets TLR4 , did not elicit a strong effect. These data suggest that as macrophages undergo differentiation and activation, they also display global modulation of adhesion receptor and adhesion-mediated signaling protein expression.
Figure 2: Heat map of adhesome gene expression during macrophage differentiation and LPS stimulation.
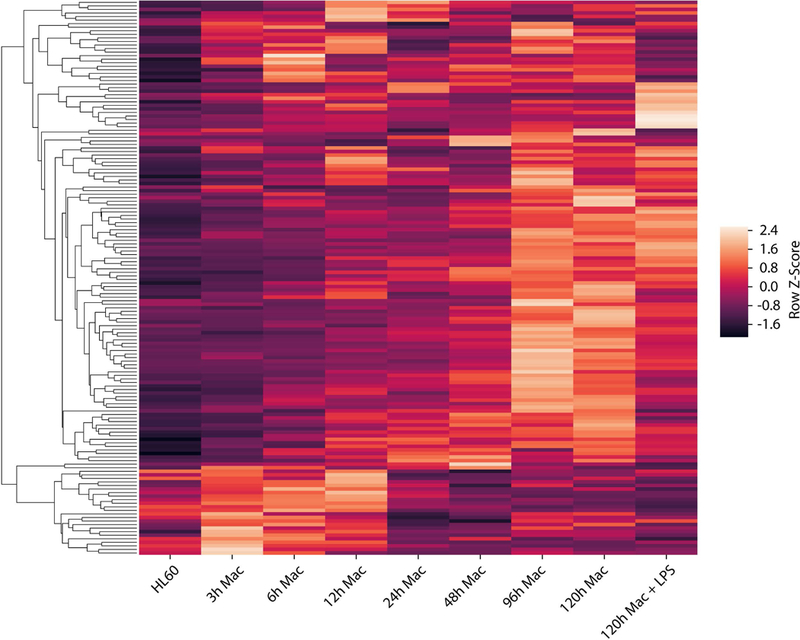
Transcriptional analysis of differentiating HL-60 cells after 0–120 hrs of treatment with phorbol 12-myristate 13-acetate (PMA) [88]. Genes from integrin and cadherin adhesomes, as previously defined [89, 90], are shown. Data was filtered for minimum expression based on FPKM (fragments per kilobase of transcript per million mapped reads). The remaining genes were then normalized across all conditions and hierarchically clustered using the unweighted pair group method with arithmetic mean (Z-Score range: −2.4 to 2.4).
Figure 3: Regulation of macrophage adhesome gene expression by stimulation with polarizing signals.
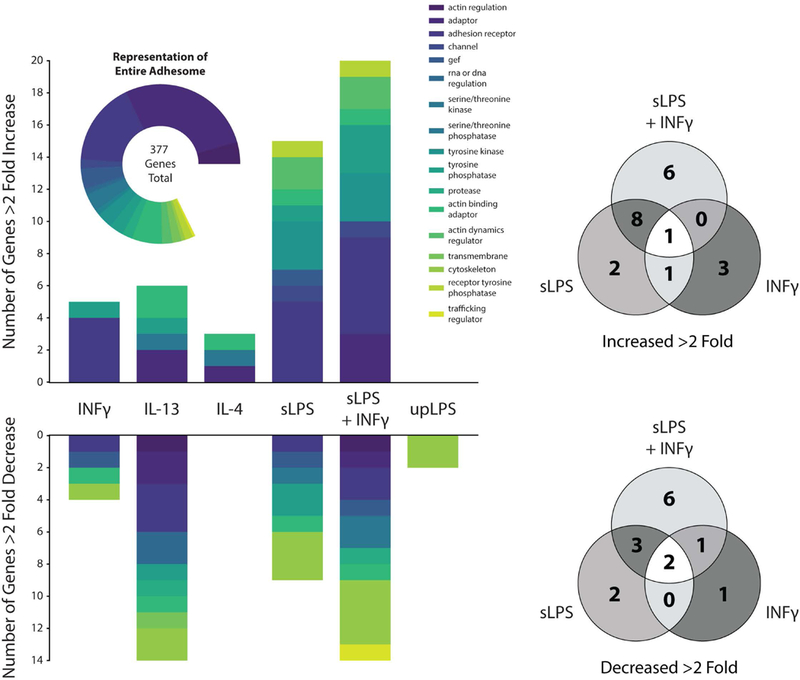
(a) Graph of human macrophage integrin and cadherin adhesome genes that increased or decreased by greater than 2-fold upon stimulation with combinations of interferon-γ (INFγ), standard LPS (sLPS), ultrapure LPS (upLPS), IL-4 and IL-13. Plots were generated using microarray expression data from Xue et al. [5]. Genes expressed below a baseline threshold were filtered out and the remaining genes were compared using a pairwise Log2 fold change analysis relative to an unstimulated control, which was made publicly available by the authors (b) Venn diagrams showing numbers of genes from (a) overlapping across different pro-inflammatory conditions.
Early studies showed a linkage between adhesion receptors and the cytoskeleton using a magnetic twisting device, whereby an ECM-coated magnetic bead was applied to cells, and then mechanically activated with magnetic force [91]. Forces applied to the cell surface were observed to directly cause cytoskeletal rearrangements through integrins, which clustered to form focal adhesions. It has now become clear that focal adhesions respond to diverse mechanical cues including applied forces as well as topographical cues and substrate rigidity [92, 93]. These adhesive structures are thought to act as signaling hubs, activating downstream signaling pathways that not only regulate cytoskeletal dynamics but also control many cellular behaviors including proliferation, migration, and differentiation. In contrast to fibroblasts, endothelial cells, or mesenchymal stem cells, which are used in the many of studies that have established our existing understanding of mechanotransduction, macrophages mostly form podosomes instead of focal adhesions upon integrin engagement (Fig. 4, box 1). Podosomes are adhesive structures defined by an actin-rich core surrounded by a ring structure composed of integrins [94] and integrin-associated proteins such as talin, paxillin and vinculin [95]. Similar to focal adhesions, these structures are critical for adhesion and migration, and thought to act as mechanosensors since they are also connected to the intracellular cytoskeleton. Furthermore, traction forces exerted at podosomes are enhanced with increased substrate rigidity, and podosome structures are displaced in response to applied forces [96]. Despite many similarities between podosome structures and focal adhesions, the precise mechanisms by which podosomes sense the mechanical environment of macrophages still remain relatively unknown.
Figure 4: Schematic of potential macrophage mechanotransduction pathways.
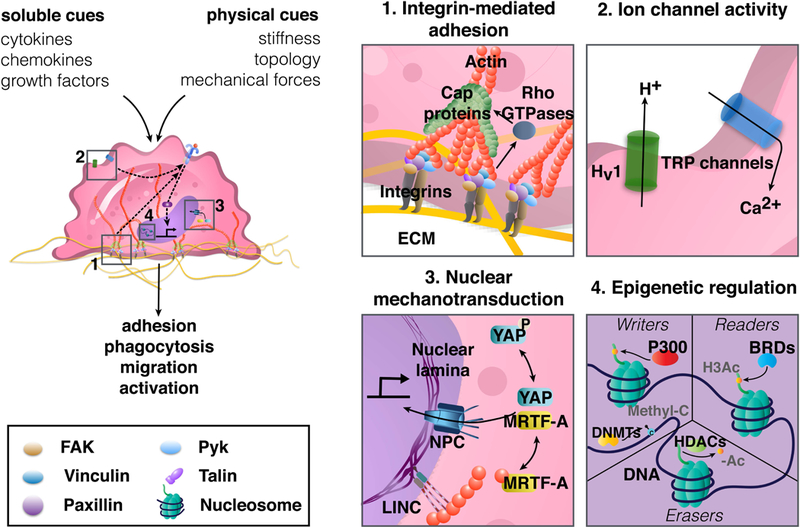
Macrophages integrate soluble and physical cues from their microenvironment to regulate their adhesion, phagocytosis, migration, and activation (left). Podosomal type adhesions contain integrins, which physically anchor cells to the ECM, and bind intracellularly to adhesion-associated scaffolding and signaling proteins and the actin cytoskeleton (box 1). Forces transmitted across integrins and cytoskeleton, as well as plasma membrane tension, regulate stretch-activated ion channels (box 2). Biophysical cues trigger nuclear translocation of transcriptional regulators to direct gene expression (box 3), and may also regulate epigenetic enzymes including writers and erasers, which add and remove chromatin modifications respectively, while also enabling readers that detect modifications (box 4). BRDs: bromodomain containing proteins, DNMTs: DNA methyltransferases, ECM: extracellular matrix, FAK: focal adhesion kinase, H3Ac: histone H3 acetylation, HDACs: histone deacetylases, Methyl-C: cytosine methylation, MRTF-A: myocardin–related transcription factor, NPC: nuclear pore complex, Pyk: protein tyrosine kinase, TLRs: toll-like receptors, TRP: transient receptor potential, YAP: yes associated protein.
Integrin clustering, either in the form of focal adhesions or podosomes, leads to the binding of intracellular signaling and adaptor proteins including paxillin, talin, focal adhesion kinase (FAK), Pyk2, and Src, among numerous others [97]. In macrophages, these interactions are critical for adhesion, migration, and phagocytosis. Expression levels of β2 and β3 integrins have been correlated with adhesiveness and migratory capacity of macrophages, and their inhibition leads to reduced adhesion and migration, respectively [35, 98, 99]. In addition, deletion of FAK and/or paxillin in primary BMDMs impaired recruitment of macrophage to sites of inflammation [100, 101]. Interestingly, surface nanostructures have been shown to activate FAK and Src in macrophages, [102, 103], but their role in mechanotransduction is still mostly undefined. To perform phagocytosis, macrophages employ several receptors including IgG, Fc receptors (FcR), complement receptors, scavenger receptors, integrins, and TLRs [104]. Several integrin subtypes (αMβ2 and αVβ3) and integrin-associated proteins including talin have been shown to be involved in phagocytosis of opsonized beads and red blood cells [105, 106]. Furthermore, coordinated activities among different integrin subtypes or small G- proteins with other receptors including complement receptors are thought to be critical for phagocytic activity [106, 107]. Thus, there is a clear role for integrin-mediated interactions in regulation of macrophage migration and phagocytosis.
Upon stimulation with different polarizing signals, macrophages undergo significant changes in their adhesion, evident through cell morphology [65], as well as expression of adhesome-related genes (Fig. 3). These adhesive changes are not only a consequence of activation, but can also feed back to regulate the polarization response. αM integrin has been shown to inhibit macrophage inflammatory activation since macrophages derived from αM knockout mice exhibited enhanced TNFα secretion in response to LPS when compared to macrophages from wildtype mice [108]. However, this negative regulation has not been observed in all macrophage populations [109], suggesting perhaps that the mechanisms are not conserved across macrophages derived from different sources or that other experimental parameters may be involved in regulation of inflammation by integrins. Methods to engage different integrin subtypes have also achieved varied results. One study showed that engagement of, αvβ3, using an immobilized antibody enhanced TNFα secretion of human primary macrophages in response to LPS [110]. In contrast, a recent study compared THP-1-derived macrophage culture on adhesive gelatin hydrogels to cells on a non-adhesive polyethylene glycol gel, and observed decreased inflammatory activation [23]. This latter study also found higher expression of pro-healing markers in cells on gelatin compared to polyethylene glycol, and implicated a role for α2β1 using a blocking antibody. These results corroborate the idea that integrin-mediated adhesion inhibits macrophage inflammatory activation, and further suggest that integrin adhesions enhance pro-regenerative activities of macrophages, albeit through a different integrin subtype and in the context of a soft environment. Clearly, the role of integrins in macrophage phenotype polarization is complex and will require further investigation.
Adhesion activates several downstream signaling pathways including Rho small GTPases, [111, 112]. Rho GTPases are key regulators of cytoskeletal dynamics and cell contractility, and crosstalk with other signaling pathways such as mitogen activated protein (MAP) kinase signaling to mediate cellular activities such as proliferation and differentiation. Numerous studies have demonstrated that mechanical cues modulate these signaling pathways [92, 113]. In macrophages, Rho GTPases are most well characterized for their roles in motility and transendothelial migration, as well as phagocytic uptake [107, 114, 115], and MAP kinase signaling is involved in cytokine production and inflammatory response [116, 117]. One study demonstrated that mechanical stimulation through administration of external pressure increased p38 MAPK signaling and phagocytosis in THP-1-derived macrophages [118]. Aside from this, however, evidence for Rho GTPase and MAPK signaling in macrophage mechanosensing are lacking. Thus, integrins and proteins associated with podosomes as well as downstream signaling molecules have a significant role in macrophage adhesion, migration and activation, but further studies are needed to better understand the role of biophysical cues in their regulation.
3.2. Ion Channels
Cells can also transmit forces through ion channels, which may be activated by membrane tension to control the passage of ions to regulate membrane potential as well as many cell functions (Fig. 4, box 2). Transmission of divalent cations, such as calcium in particular, have been shown to influence activation, proliferation, and migration of immune cells [119]. The transient receptor potential (TRP) family of channels consist of 30 proteins, which are subdivided into six subgroups: TRPC (canonical),TRPM (melastatin), TRPV (vanilloid), TRPA (ankyrin), TRPML (mucolipin), and TRPP (polycystin) [120, 121]. These channels are nonselective to cations as they are permeable to sodium as well as calcium and are known to respond to diverse cues within the microenvironment, such as pH, temperature, osmolarity, and mechanical stimuli [119]. Macrophages express TRPV4, TRPV2, TRPC6, and TRPM7, which have roles in inflammatory activation and phagocytosis. TRPV4 appears to negatively regulate LPS-induced inflammation, but enhances both LPS-mediated phagocytosis and ox-LDL uptake [122, 123]. In contrast, a recent study found that TRPM7 positively regulates LPS-induced inflammatory activation by enhancing endocytosis of the LPS-TLR4 complex, and its depletion has been shown to protect mice from LPS-induced peritonitis [124]. Finally, manipulation of the expression and activity of TRPV2 and TRPC6 reveals that these channels are required for phagocytosis in macrophages [125, 126]. While all of these channels have been shown to be regulated by biophysical cues in other cell types [127–129], thus far, only TRPV4 activity is known to be influenced by the mechanical environment in macrophages [122]. BMDMs seeded on stiffer substrates exhibited increased LPS-mediated intracellular calcium influx and phagocytic capacity compared to cells on soft surfaces, and pharmacological inhibition or reduced expression of TRPV4 resulted in decreased phagocytosis in BMDMs cultured on stiff substrates. The results obtained, thus, suggest an important role for TRP ion channel activity in macrophage function, and suggests that mechanical forces and membrane tension are involved.
Macrophages also express channels for other ions on their cell surface, including the voltage-gated proton channel Hv1. Hv1 is responsible for the regulation of protons into the cell and is associated with the NAPDH oxidase (NOX) complex in innate immune cells. Phagocytosis of microorganisms by macrophages activate the NOX complex which leads to the transfer of electrons into the forming phagosome and formation of superoxide anions that are involved in killing microorganisms [130, 131]. While the mechanical regulation of this channel specifically in macrophages is relatively unknown, membrane stretch administered through a high speed pressure clamp on excised membrane patches from oocytes has been shown to open this proton channel in the absence of strong depolarization [132]. Further work, however, still needs to be conducted to better characterize the effects of the mechanical microenvironment on the regulation of the Hv1 proton channel in macrophages.
Ion channels are clearly important in modulating numerous macrophage functions; however, the role of the mechanical environment in regulating channel activity is still poorly understood. The majority of channels discussed in this section are dependent on membrane tension for activation, and thus the stiffness of the environment, which enhances tension, is likely to modulate their activity. While the effects of stiffness on TRPV4 have been studied, the role of other physical stimuli including mechanical stretch, shear stress, topography, or adhesive cues on the multitude of ion channels expressed in macrophages are still unknown. As with integrin subtypes, the effects of channel manipulation on the activity or expression of other ion channels or surface receptors are poorly characterized and could likely impact the overall cellular response. The effects of mechanical cues may yet provide novel insight into the mechanisms responsible for changes in macrophage activation in their physiological environment.
3.3. Nuclear Mechanotransduction
Mechanical cues can regulate gene expression through control of transcription factors and coactivators, primarily by galvanizing the nuclear translocation of these factors that may be otherwise marked for degradation or sequestration in the cytoplasm [133, 134]. Mechanistically, this is thought to occur through either (1) the association of transcription factors with cytoskeleton or related signaling components, or (2) direct coupling of the cytoskeleton to nuclear pores that open in response to forces and thus influence the import/export of molecules (Fig. 4, box 3). Both of these mechanisms are applicable to the mechanosensitive transcriptional co-activator Yes-associated protein (YAP), and its role in coordinating the cellular response to mechanical cues [135, 136]. YAP and the related protein transcriptional co-activator with PDZ-binding motif (TAZ) play a vital role in organ size control, by regulating cell proliferation during development [137]. Recent work has shown that YAP/TAZ is regulated by stiffness, cell shape, and mechanical forces [138, 139], and its dysregulation is associated with diseases including atherosclerosis, fibrosis, and cancer [138]. In myeloid cells, YAP/TAZ has been found to be involved in monocyte adhesion to inflamed endothelium [140], TGFβ1-induced fibrotic M2 polarization [141], and tumor associated M2 polarization [142], but a definitive role for YAP in macrophage mechanotransduction remains elusive. The myocardin-related transcription factor A (MRTF-A) is another transcriptional regulator that has been observed to be influenced by mechanical cues [143]. MRTF-A is a cytoplasmic actin-bound protein that when freed upon actin polymerization, relocates to the nucleus [144], where it binds serum response factor (SRF) and activates promoters with serum response elements [133]. MRTF-A is thought to be essential for LPS-induced proinflammatory activation [145], and a recent study revealed that spatial confinement of macrophages reduces MRTF-A nuclear localization by downregulating actin polymerization, and led to reduced expression of LPS-activated inflammatory programs [67]. Interestingly, YAP and MRTF-A share striking similarities in their response to mechanical forces. For example, both YAP and MRTF-A translocate into the nucleus with increased cellular or matrix stiffness, and also appear to require nuclear envelope proteins including lamin A/C and emerin, a component of the inner nuclear membrane responsive to isolated nuclear force [146–149]. Finally, the inflammatory program controlled by NF-κB nuclear translocation has also been observed to be controlled by mechanical inputs [150–152]. There have also been observations of altered levels of nuclear NF-κB in macrophages in response to differences in substrate stiffness [40] and topography [153]. Together, these studies implicate transcriptional activator shuttling in macrophage sensing of mechanical cues in their environment.
In addition to altered nuclear transport of transcriptional regulators, mechanical forces may rely on an alternative path embodied by physical linkages, to regulate chromatin organization and gene expression [154–156]. Such cell-wide mechanical integration allows for the transmission of mechanical signals from extracellular interfaces to the nuclear scaffolding, and are be capable of propagating signals several order of magnitudes faster than biochemical signals that originate upstream at cell membrane [157]. An important component in this is the linker of nucleoskeleton and cytoskeleton (LINC) complex, which connects the cytoskeleton in the cytoplasm with the nuclear lamina on the inside of the nuclear envelope [158, 159]. Nuclear lamins and lamin-associated proteins can interact directly with DNA, histones and other heterochromatin proteins [160]. This may establish control of genes by subnuclear positioning that localizes active genes close to nuclear pore complexes [161], or sequesters inactive heterochromatin to the nuclear periphery [154, 162–164]. Mutants of lamin A/C are known to causes a spectrum of laminopathies, resulting in tissue-specific abnormalities or early aging [165]. Lamins have also been implicated in mechanotransduction in several cell types. In stem cells, lamin A expression increases as cells are cultured on substrates of increasing stiffness, and its knockdown in cells cultured on stiff surfaces reduces osteogenesis and increases adipogenesis [166]. In fibroblasts, lamin A/C deficiency is associated with increased nuclear deformability, defective mechanotransduction, impaired motility, and decreased viability under mechanical strain [167, 168]. As with adhesion and adhesion-mediated signaling molecules, expression of proteins associated with the LINC complex and nuclear lamina are significantly upregulated during differentiation of macrophages from monocytes [169], although a role for lamins in mechanotransduction has not yet been identified in macrophages. Interestingly, lamin A/C upregulation in adipose tissue derived macrophages from obesity-induced type 2 diabetic mice, was associated with increased NF-κB activity, suggesting a potential role in chronic inflammation [170]. These data suggest that the nuclear envelope and lamina may be involved in monocyte differentiation, and possibly in macrophage motility and function, but the definite roles of these nuclear envelope proteins in macrophage mechanotransduction remain to be determined.
3.4. Epigenetic regulation
While the inherently different mechanical cues present in various tissues may induce epigenetic changes that are responsible for tissue-specific variations in macrophage behavior, direct evidence of such mechanisms has yet to be revealed. However, epigenetic mechanisms clearly play a major role in directing macrophage differentiation and activation. For example, both H3K4 methylation and H3K27 acetylation regulate tissue-specific macrophage gene expression in the brain and lungs [171]. Furthermore, transcriptional profiles and chromatin accessibility are divergent in macrophages derived from monocytes compared to those derived directly from myeloid progenitors or tissue-resident macrophages [88]. Interestingly, recent studies have revealed that certain biophysical cues can regulate epigenetic pathways that are known to be important in governing macrophage behavior. Specifically, histone deacetylase (HDAC) activity is reduced in both human mesenchymal stem cells (MSCs) [172] and mouse fibroblasts when cultured on patterned microgroove surfaces. These changes in HDAC activity were also associated with global increases in H3Ac, H3K4 di- and trimethylation, and cellular elongation more broadly [173]. Notably, elongation is a feature of the alternative pro-healing macrophage phenotype [65, 174], and cell-adhesive geometries of varying shape, aspect ratio, and size as well as actomyosin contractility were found to regulate HDAC3 [175], which is required for macrophage inflammatory signaling [176]. Furthermore, confining macrophage spreading on fibronectin coated surfaces was shown to decrease inflammatory gene expression and HDAC3 activity in LPS stimulated macrophages when compared to cells that were allowed to spread freely [67]. Confinement also induced an increase in H3K36me2, a marker of chromatin compaction and gene suppression, near inflammatory gene promoters Il-6 and Nos2 (or iNOS) but not at the promoters of Il-1β and Cxcl9 suggesting their may be more than one avenue for mechanical cues to modulate epigenetic marks at genes important for macrophage activation [67].
Other epigenetic mechanisms are also known to control and regulate macrophage polarization and inflammatory function. For example, histone H3 acetylation (H3Ac) modifications, which are typically found near active promoter or enhancer sequences, increase near inflammatory genes, including Il6, in macrophages stimulated with LPS [177]. Additionally, LPS-induced gene expression patterns are reduced by inhibiting bromodomain (BRD) protein binding to H3Ac using a small molecule inhibitor iBET [177], suggesting that blocking histone acetylation could prevent inflammatory gene activation. However, somewhat counterintuitively, inhibitors of histone deacetylase (HDAC) enzymes such as trichostatin A (TSA), which increase global histone acetylation levels, are also known to suppress inflammation in macrophages [178]. One study in a mouse B cell line (Ba/F3) showed that genome-wide increases in histone acetylation due to TSA treatment actually direct BRD proteins away from target gene promoters (including gene targets of JAK/STAT signaling, which are also involved in macrophage activation) by increasing the total number of binding sites available, causing BRD proteins to bind more diffusely throughout the genome [179]. While this has yet to be shown at the chromatin level in macrophages, this reported mechanism could potentially resolve these apparent conflicting observations. In addition, the colocalization of p300, a histone acetyl transferase, to sites of H3K4me1 was shown to be important in the activation of enhancers near LPS-inducible proinflammatory genes, including Tnfa (tumor necrosis factor alpha) and Sod2 (superoxide dismutase 2), and facilitates an inflammatory response through the myeloid transcription factor PU.1 [180].
While histone modifications clearly play a role in priming, activating, and enhancing pro-inflammatory gene expression, DNA cytosine methylation is also an important regulator of macrophage activation. Hypermethylation of CpGs near Socs1, a gene known to negatively regulate the inflammatory JAK2/STAT3 pathway, occurs following LPS stimulation in RAW264.7 macrophages [181]. Pharmacological inhibition of DNA methyltransferase 1 (DNMT1) or knockdown through siRNA decreases methylation at the Socs1 promoter, increasing its expression and downregulating inflammatory signaling [181]. Additionally, DNMT1 and DNMT3b are responsible for hypermethylation of the peroxisome proliferator-activated receptor γ1 (Pparγ) promoter in response to obesity-associated factors including saturated fatty acids, which leads to pro-inflammatory STAT signaling [182, 183]. Finally, macrophage inflammation during atherosclerosis can also be suppressed by the inhibition of DNMTs with 5-aza-dC treatment [184]. Interestingly, it was shown that characteristic atherosclerotic oscillatory shear flow profiles induce DNMT1-mediated hypermethylation in human umbilical vein endothelial cells and rat carotid arteries [185], although the ability of mechanical forces to regulate methylation enzyme activity or methylation patterns in macrophages remains unknown [181]. Given that epigenetic modifications have also been associated with many diseases that involve dysregulation of mechanical cues and macrophages [186], it is possible that regulation of epigenetic mechanisms through biophysical cues may explain some macrophage behaviors that are relevant to health and disease.
4. Concluding Remarks
In this review, we describe an emerging paradigm that suggests mechanical cues modulate the function of macrophages. These adhesive innate immune cells respond to a variety of mechanical cues such as material stiffness and topography or architecture as well as externally applied mechanical forces. Often, these cues synergize with soluble factors including damage and pathogen associated molecular patterns or cytokines to regulate the function of macrophages. As with most studies in mechanobiology, many of the models used to probe the role of biophysical cues rely on in vitro systems – culture of macrophages on materials with different physical characteristics, or within engineered platforms that subject cells to mechanical stimulation. These systems allow for manipulation of the biophysical microenvironment, but also pose several challenges and considerations. Macrophage cell lines or primary macrophages derived from bone marrow or blood are commonly used since these populations of cells are relatively easy to access and yield numerous cells to use for studies in vitro. However, cell lines and macrophages differentiated from hematopoetic or monocytic precursors on plastic petri dishes could very well behave differently from those found in the body. Furthermore, macrophages in vivo are not only derived from circulating monocytes, but also exist resident within tissue. It is possible that macrophages of different origins respond uniquely to biophysical signals, particularly since cells within the heart and lungs experience significantly more basal mechanical stimulation compared to cells in the brain or skin. Another challenge of in vitro systems is that experimental parameters can vary – cell culture media, seeding densities, stimulation cytokine concentrations, experiment duration, among many others. In our own experience, all of these parameters impact macrophage function, and it is therefore necessary to consider the effects of these variables when analyzing and interpreting results.
Much of our knowledge in this area has stemmed from studies performed in the context of biomaterials engineering, since implanted materials clearly alter the biophysical environment, and are known to interact with abundant macrophages [187]. Several groups have found that stiffer materials enhance inflammatory activation, and grooved or fibrous materials promote alternative activation [40, 58]. In some cases, these findings have been correlated with in vivo studies, where materials are implanted within animals and macrophages in the surrounding tissue are characterized [24, 59]. In this context, although only one cell layer of macrophages is in direct contact with the material, even cells that are further into the tissue are observed to be affected, perhaps because they were previously in contact with the material or because of paracrine effects caused by cells at the surface. Nonetheless, even with these important insights, it is still unclear what material characteristics will elicit the macrophage response needed for optimal wound healing and minimal scarring. Heightened inflammation leads to chronic inflammation, and excessive alternative activation could in fact lead to fibrosis. Moreover, macrophages in vivo are often highly heterogeneous, and thought to exist in numerous activation states as well as transitioning among different states at any given time. Most likely, timely activation and inactivation of macrophages is needed to promote optimal healing. Advanced biomaterials engineering strategies with dynamically changing properties may be needed to achieve such temporal control.
Mechanical forces are known to modulate wound healing and tissue repair. In the skin, mechanical tension from contraction of myofibroblasts promotes wound closure. While these forces are necessary to heal the wound, exacerbated levels are thought to contribute to scar. Nonetheless, clinically used therapies often leverage mechanical stimulation to improve tissue regeneration. For example, negative pressure wound therapy (NPWT) applies a biomaterial foam and negative pressure to a large skin wound, causing both macroscale and microscale deformations that speed wound closure [188]. Similarly in the bone, exercise and compressive forces are needed to maintain bone health and physical exercise is an integral part of treatment to heal bones [189]. It is thought that mechanical stimulation leads to heightened cell proliferation, angiogenesis, and osteogenesis, but how this therapy specifically influences the function of immune cells remains unexplored [190, 191].
While macrophages clearly express molecules with established roles in mechanosensing in many other cell types, the molecular mechanisms underlying mechanotransduction in macrophages remain mostly elusive. Sensing of all biophysical cues including material stiffness, topography and mechanical forces likely involve overlapping mechanisms that include integrin-mediated adhesion and ion channel activity. However, the expression of many subtypes, each with potentially unique roles as well as the ability to compensate for one another, makes their study a challenge. Recent advances in tools to profile entire proteomes or transcriptomes of cells may help to understand the interactions between these molecules and their activation of downstream signaling pathways. The identification of molecules and signaling pathways that transduce mechanical forces remains an important area of investigation, as it may unveil new strategies to target macrophage activity for treatment of disease.
Acknowledgements
The authors would like to thank Esther Yu-tin Chen for assistance with figure artwork. This work was supported by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Disease (NIAID) Grant R21AI128519–01 to W.F.L and by an National Science Foundation (NSF) grant (DMS1763272) and a grant from the Simons Foundation (594598 QN) to T.L.D. H.A. was supported by NIH National Institute T32 Training Grant in Cardiovascular Applied Research and Entrepreneurship 5 T32 HL116270–3.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- AM
Alveolar macrophage
- Arg1
Arginase 1
- BMDM
bone marrow derived macrophage
- CD11b
Cluster of Differentiation 11b
- CD206
Cluster of Differentiation 206
- ECM
Extracellular matrix
- FAK
Focal adhesion kinase
- FcR
Fc Receptor
- GM-CSF
granulocyte- macrophage colony- stimulating factor
- GTPase
enzymes that hydrolyse guanosine triphosphate
- HDAC
Histone deacetylase
- iBET
inhibitor of bromodomain and extra-terminal motif
- IFNγ
interferon gamma
- IgG
Immunoglobulin G
- IL-10
Interleukin 10
- IL-13
Interleukin 13
- IL-1β
Interleukin 1β
- IL-4
Interleukin 4
- IL-6
Interleukin 6
- iNOS
inducible nitric oxide synthase
- JAK
Janus kinase
- LINC
linker of nucleoskeleton and cytoskeleton
- LPS
Lipopolysaccharide
- M-CSF
macrophage colony- stimulating factor
- MAPK
Mitogen- activated protein kinase
- MMP-9
matrix metalloproteinase
- MRTF-A
Myocardin related transcription factor A
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PLLA
polylactic acid
- PMA
Phorbol 12-myristate 13-acetate
- Pparγ
Peroxisome proliferation-activator receptor gamma
- Pyk2
protein tyrosine kinase 2
- RGD
arginine-glycine-aspartate
- RNA
Ribonucleic acid
- STAT
Signal transducer and activator of transcription
- TAZ
transcriptional co-activator with PDZ-binding motif
- TGF β−1
transforming growth factor 1
- THP-1
human monocytic cell line
- Ti02
titanium dioxide
- TLR4
Toll like receptor-4
- TNFα
tumor necrosis factor alpha
- TRP
transient receptor potential
- TRPA
TRP (ankyrin)
- TRPC
TRP (canonical)
- TRPM
TRP (melastatin)
- TRPML
TRP (mucolipin)
- TRPP
TRP (polycystin)
- TRPV
TRP (vanilloid)
- TSA
trichostatinA
- VCAM-1
vascular cell adhesion molecule-1
- YAP
Yes-associated protein
Footnotes
Conflict of Interest Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Gordon S, Pluddemann A, Martinez Estrada F (2014) Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 262, 36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosser DM and Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA and Vannella KM (2016) Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496, 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL (2014) Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray PJ (2017) Macrophage Polarization. Annu Rev Physiol 79, 541–566. [DOI] [PubMed] [Google Scholar]
- 7.Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoppel WL, Kaplan DL, Black LD 3rd (2016) Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliv Rev 96, 135–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich PJ and Lanyon LE (2002) Mechanical strain and bone cell function: a review. Osteoporos Int 13, 688–700. [DOI] [PubMed] [Google Scholar]
- 10.Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–89. [DOI] [PubMed] [Google Scholar]
- 11.Ingber DE (2003) Mechanobiology and diseases of mechanotransduction. Ann Med 35, 564–77. [DOI] [PubMed] [Google Scholar]
- 12.Jaalouk DE and Lammerding J (2009) Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickup MW, Mouw JK, Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15, 1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palombo C and Kozakova M (2016) Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 77, 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Wynn TA and Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18, 1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabas I and Bornfeldt KE (2016) Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res 118, 653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong JW and Chapes SK (1994) Effects of extracellular matrix proteins on macrophage differentiation, growth, and function: comparison of liquid and agar culture systems. J Exp Zool 269, 178–87. [DOI] [PubMed] [Google Scholar]
- 20.Luu TU and Liu WF (2018) Regulation of Macrophages by Extracellular Matrix Composition and Adhesion Geometry. Regenerative Engineering and Translational Medicine, 1–9. [Google Scholar]
- 21.Yin Z, Li C, Wang J, Xue L (2018) Myeloid-derived suppressor cells: Roles in the tumor microenvironment and tumor radiotherapy. Int J Cancer. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF (2017) Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater 47, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha BH, Shin SR, Leijten J, Li YC, Singh S, Liu JC, Annabi N, Abdi R, Dokmeci MR, Vrana NE, Ghaemmaghami AM, Khademhosseini A (2017) Integrin-Mediated Interactions Control Macrophage Polarization in 3D Hydrogels. Adv Healthc Mater 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blakney AK, Swartzlander MD, Bryant SJ (2012) The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A 100, 1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciano PS, Colvin RB, Dvorak AM, McDonagh J, Dvorak HF (1986) Macrophage migration in fibrin gel matrices. Lab Invest 54, 62–70. [PubMed] [Google Scholar]
- 26.Lanir N, Ciano PS, Van de Water L, McDonagh J, Dvorak AM, Dvorak HF (1988) Macrophage migration in fibrin gel matrices. II. Effects of clotting factor XIII, fibronectin, and glycosaminoglycan content on cell migration. Journal of immunology (Baltimore, Md. : 1950) 140, 2340–9. [PubMed] [Google Scholar]
- 27.Wells RG (2008) The role of matrix stiffness in regulating cell behavior. Hepatology 47, 1394–400. [DOI] [PubMed] [Google Scholar]
- 28.Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–43. [DOI] [PubMed] [Google Scholar]
- 29.Janmey PA and Miller RT (2011) Mechanisms of mechanical signaling in development and disease. J Cell Sci 124, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handorf AM, Zhou Y, Halanski MA, Li WJ (2015) Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezvani-Sharif A, Tafazzoli-Shadpour M, Avolio A (2018) Progressive changes of elastic moduli of arterial wall and atherosclerotic plaque components during plaque development in human coronary arteries. Med Biol Eng Comput. [DOI] [PubMed] [Google Scholar]
- 32.Tse JR and Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol Chapter 10, Unit 10 16. [DOI] [PubMed] [Google Scholar]
- 33.Stowers RS, Allen SC, Suggs LJ (2015) Dynamic phototuning of 3D hydrogel stiffness. Proc Natl Acad Sci U S A 112, 1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunyer R, Jin AJ, Nossal R, Sackett DL (2012) Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS One 7, e46107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fereol S, Fodil R, Labat B, Galiacy S, Laurent VM, Louis B, Isabey D, Planus E (2006) Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskeleton 63, 321–40. [DOI] [PubMed] [Google Scholar]
- 36.Fereol S, Fodil R, Laurent VM, Planus E, Louis B, Pelle G, Isabey D (2008) Mechanical and structural assessment of cortical and deep cytoskeleton reveals substrate-dependent alveolar macrophage remodeling. Biomed Mater Eng 18, S105–18. [PubMed] [Google Scholar]
- 37.Adlerz KM, Aranda-Espinoza H, Hayenga HN (2016) Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur Biophys J 45, 301–9. [DOI] [PubMed] [Google Scholar]
- 38.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H (2012) Cell elasticity determines macrophage function. PLoS One 7, e41024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Previtera ML, Peterman K, Shah S, Luzuriaga J (2015) Lipid rafts direct macrophage motility in the tissue microenvironment. Ann Biomed Eng 43, 896–905. [DOI] [PubMed] [Google Scholar]
- 40.Previtera ML and Sengupta A (2015) Substrate Stiffness Regulates Proinflammatory Mediator Production through TLR4 Activity in Macrophages. PLoS One 10, e0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irwin EF, Saha K, Rosenbluth M, Gamble LJ, Castner DG, Healy KE (2008) Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Ed 19, 1363–82. [DOI] [PubMed] [Google Scholar]
- 42.Alonso-Nocelo M, Raimondo TM, Vining KH, Lopez-Lopez R, de la Fuente M, Mooney DJ (2018) Matrix stiffness and tumor-associated macrophages modulate epithelial to mesenchymal transition of human adenocarcinoma cells. Biofabrication 10, 035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beningo KA and Wang YL (2002) Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci 115, 849–56. [DOI] [PubMed] [Google Scholar]
- 44.Beningo KA and Wang YL (2002) Flexible substrata for the detection of cellular traction forces. Trends Cell Biol 12, 79–84. [DOI] [PubMed] [Google Scholar]
- 45.Kothapalli D, Liu SL, Bae YH, Monslow J, Xu T, Hawthorne EA, Byfield FJ, Castagnino P, Rao S, Rader DJ, Pure E, Phillips MC, Lund-Katz S, Janmey PA, Assoian RK (2012) Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep 2, 1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue Q, Lu Y, Eisele MR, Sulistijo ES, Khan N, Fan R, Miller-Jensen K (2015) Analysis of single-cell cytokine secretion reveals a role for paracrine signaling in coordinating macrophage responses to TLR4 stimulation. Sci Signal 8, ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hind LE, Dembo M, Hammer DA (2015) Macrophage motility is driven by frontal-towing with a force magnitude dependent on substrate stiffness. Integr Biol (Camb) 7, 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rich A and Harris AK (1981) Anomalous preferences of cultured macrophages for hydrophobic and roughened substrata. J Cell Sci 50, 1–7. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt JA and von Recum AF (1992) Macrophage response to microtextured silicone. Biomaterials 13, 1059–69. [DOI] [PubMed] [Google Scholar]
- 50.Wojciak-Stothard B, Curtis A, Monaghan W, MacDonald K, Wilkinson C (1996) Guidance and activation of murine macrophages by nanometric scale topography. Exp Cell Res 223, 426–35. [DOI] [PubMed] [Google Scholar]
- 51.Refai AK, Textor M, Brunette DM, Waterfield JD (2004) Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A 70, 194–205. [DOI] [PubMed] [Google Scholar]
- 52.Ma QL, Zhao LZ, Liu RR, Jin BQ, Song W, Wang Y, Zhang YS, Chen LH, Zhang YM (2014) Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 35, 9853–9867. [DOI] [PubMed] [Google Scholar]
- 53.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD (2014) Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng 42, 1508–16. [DOI] [PubMed] [Google Scholar]
- 54.Smith TD, Tse MJ, Read EL, Liu WF (2016) Regulation of macrophage polarization and plasticity by complex activation signals. Integr Biol (Camb) 8, 946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G (2015) Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartneck M, Schulte VA, Paul NE, Diez M, Lensen MC, Zwadlo-Klarwasser G (2010) Induction of specific macrophage subtypes by defined micro-patterned structures. Acta Biomater 6, 3864–72. [DOI] [PubMed] [Google Scholar]
- 57.Singh S, Awuah D, Rostam HM, Emes RD, Kandola NK, Onion D, Htwe SS, Rajchagool B, Cha B-H, Kim D, Tighe PJ, Vrana NE, Khademhosseini A, Ghaemmaghami A (2017) Unbiased Analysis of the Impact of Micropatterned Biomaterials on Macrophage Behavior Provides Insights beyond Predefined Polarization States. ACS Biomaterials Science & Engineering 3, 969–978. [DOI] [PubMed] [Google Scholar]
- 58.Luu TU, Gott SC, Woo BW, Rao MP, Liu WF (2015) Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl Mater Interfaces 7, 28665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, Luu TU, Chen A, Khine M, Liu WF (2016) Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles. Biomater Sci 4, 948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, Gao X, Li D, Li Y, Zheng XL, Zhu Y, Kong D, Zhao Q (2014) The effect of thick fibers and large pores of electrospun poly(epsilon-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 35, 5700–10. [DOI] [PubMed] [Google Scholar]
- 61.Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL (2013) Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 34, 4439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L (2011) Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules 12, 1900–11. [DOI] [PubMed] [Google Scholar]
- 63.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS (2008) Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol 172, 1112–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jay SM, Skokos E, Laiwalla F, Krady MM, Kyriakides TR (2007) Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation. Am J Pathol 171, 632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF (2013) Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A 110, 17253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan JL, Liu W, Nelson CM, Raghavan S, Chen CS (2004) Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng 10, 865–72. [DOI] [PubMed] [Google Scholar]
- 67.Jain N and Vogel V (2018) Spatial confinement downsizes the inflammatory response of macrophages. Nat Mater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, Wang K, Gu X, Leong KW (2017) Biophysical Regulation of Cell Behavior-Cross Talk between Substrate Stiffness and Nanotopography. Engineering (Beijing) 3, 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geckil H, Xu F, Zhang X, Moon S, Demirci U (2010) Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond) 5, 469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X. m., Ensenat D, Wang H, Schafer AI, Durante W (2003) Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett. 541, 52–56. [DOI] [PubMed] [Google Scholar]
- 71.Sager Hendrik B, Hulsmans M, Lavine Kory J, Moreira Marina B, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan Omar F, Dahlman James E, Borodovsky A, Fitzgerald K, Anderson Daniel G, Weissleder R, Libby P, Swirski Filip K, Nahrendorf M (2016) Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circulation Research 119, 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jufri NF, Mohamedali A, Avolio A, Baker MS (2015) Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vascular Cell 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lionetti V, Recchia FA, Marco Ranieri V (2005) Overview of ventilator-induced lung injury mechanisms. Current Opinion in Critical Care 11, 82. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto T, Delafontaine P, Schnetzer KJ, Tong BC, Nerem RM (1996) Effect of Uniaxial, Cyclic Stretch on the Morphology of Monocytes/Macrophages in Culture. J Biomech Eng 118, 420–422. [DOI] [PubMed] [Google Scholar]
- 75.Atcha H, Davis CT, Sullivan NR, Smith TD, Anis S, Dahbour WZ, Robinson ZR, Grosberg A, Liu WF (2018) A Low-Cost Mechanical Stretching Device for Uniaxial Strain of Cells: A Platform for Pedagogy in Mechanobiology. J Biomech Eng 140, 081005–081005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat J-L, Nicod LP, Chevrolet J-C (1998) Activation of human macrophages by mechanical ventilation in vitro. American Journal of Physiology-Lung Cellular and Molecular Physiology 275, L1040–L1050. [DOI] [PubMed] [Google Scholar]
- 77.Oya K, Sakamoto N, Ohashi T, Sato M (2011) Combined stimulation with cyclic stretching and hypoxia increases production of matrix metalloproteinase-9 and cytokines by macrophages. Biochemical and Biophysical Research Communications 412, 678–682. [DOI] [PubMed] [Google Scholar]
- 78.Matheson LA, Jack Fairbank N, Maksym GN, Paul Santerre J, Labow RS (2006) Characterization of the Flexcell™ Uniflex™ cyclic strain culture system with U937 macrophage-like cells. Biomaterials 27, 226–233. [DOI] [PubMed] [Google Scholar]
- 79.Dziki JL, Giglio RM, Sicari BM, Wang DS, Gandhi RM, Londono R, Dearth CL, Badylak SF (2017) The Effect of Mechanical Loading Upon Extracellular Matrix Bioscaffold-Mediated Skeletal Muscle Remodeling. Tissue Engineering Part A 24, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsao PS, Lewis NP, Alpert S, Cooke JP (1995) Exposure to Shear Stress Alters Endothelial Adhesiveness. Circulation. [DOI] [PubMed] [Google Scholar]
- 81.Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL (1995) Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler. Thromb. Vasc. Biol. 15, 2–10. [DOI] [PubMed] [Google Scholar]
- 82.Honda HM, Hsiai T, Wortham CM, Chen M, Lin H, Navab M, Demer LL (2001) A complex flow pattern of low shear stress and flow reversal promotes monocyte binding to endothelial cells. Atherosclerosis 158, 385–390. [DOI] [PubMed] [Google Scholar]
- 83.Li R, Serrano JC, Xing H, Lee TA, Azizgolshani H, Zaman M, Kamm RD, Weaver VM (2018) Interstitial flow promotes macrophage polarization toward an M2 phenotype. MBoC 29, 1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–87. [DOI] [PubMed] [Google Scholar]
- 85.Kume A, Nishiura H, Suda J, Suda T (1997) Focal adhesion kinase upregulated by granulocyte-macrophage colony-stimulating factor but not by interleukin-3 in differentiating myeloid cells. Blood 89, 3434–42. [PubMed] [Google Scholar]
- 86.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M (2000) Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology 100, 364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Nichilo MO and Burns GF (1993) Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate alpha v integrin expression on cultured human macrophages. Proc Natl Acad Sci U S A 90, 2517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramirez RN, El-Ali NC, Mager MA, Wyman D, Conesa A, Mortazavi A (2017) Dynamic Gene Regulatory Networks of Human Myeloid Differentiation. Cell Syst 4, 416–429 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaidel-Bar R (2013) Cadherin adhesome at a glance. J Cell Sci 126, 373–8. [DOI] [PubMed] [Google Scholar]
- 90.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9, 858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–7. [DOI] [PubMed] [Google Scholar]
- 92.Katsumi A, Orr AW, Tzima E, Schwartz MA (2004) Integrins in mechanotransduction. J Biol Chem 279, 12001–4. [DOI] [PubMed] [Google Scholar]
- 93.Sun Z, Guo SS, Fassler R (2016) Integrin-mediated mechanotransduction. J Cell Biol 215, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, Gerber-Scokaert D, Planus E, Albiges-Rizo C (2008) Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol 87, 491–506. [DOI] [PubMed] [Google Scholar]
- 95.Gimona M, Buccione R, Courtneidge SA, Linder S (2008) Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol 20, 235–41. [DOI] [PubMed] [Google Scholar]
- 96.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N (2008) Self-organized podosomes are dynamic mechanosensors. Curr Biol 18, 1288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10, 21–33. [DOI] [PubMed] [Google Scholar]
- 98.Yakubenko VP, Belevych N, Mishchuk D, Schurin A, Lam SC, Ugarova TP (2008) The role of integrin alpha D beta2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res 314, 2569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weerasinghe D, McHugh KP, Ross FP, Brown EJ, Gisler RH, Imhof BA (1998) A role for the alphavbeta3 integrin in the transmigration of monocytes. J Cell Biol 142, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH (2007) Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol 179, 1275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams LM and Ridley AJ (2000) Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. Journal of immunology (Baltimore, Md. : 1950) 164, 2028–36. [DOI] [PubMed] [Google Scholar]
- 102.Ghrebi S, Hamilton DW, Douglas Waterfield J, Brunette DM (2013) The effect of surface topography on cell shape and early ERK½ signaling in macrophages; linkage with FAK and Src. J Biomed Mater Res A 101, 2118–28. [DOI] [PubMed] [Google Scholar]
- 103.Lee S, Choi J, Shin S, Im YM, Song J, Kang SS, Nam TH, Webster TJ, Kim SH, Khang D (2011) Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium. Acta Biomater 7, 2337–44. [DOI] [PubMed] [Google Scholar]
- 104.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23, 901–44. [DOI] [PubMed] [Google Scholar]
- 105.Lim J, Wiedemann A, Tzircotis G, Monkley SJ, Critchley DR, Caron E (2007) An essential role for talin during alpha(M)beta(2)-mediated phagocytosis. Mol Biol Cell 18, 976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blystone SD, Graham IL, Lindberg FP, Brown EJ (1994) Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J Cell Biol 127, 1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoppe AD and Swanson JA (2004) Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell 15, 3509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han C, Jin J, Xu S, Liu H, Li N, Cao X (2010) Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 11, 734–42. [DOI] [PubMed] [Google Scholar]
- 109.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, Scott D, Franzoso G, Cook HT, Botto M (2014) Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun 5, 3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Antonov AS, Antonova GN, Munn DH, Mivechi N, Lucas R, Catravas JD, Verin AD (2011) alphaVbeta3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-kappaB activation. J Cell Physiol 226, 469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohashi K, Fujiwara S, Mizuno K (2017) Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem 161, 245–254. [DOI] [PubMed] [Google Scholar]
- 112.Hall A and Nobes CD (2000) Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci 355, 965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orr AW, Helmke BP, Blackman BR, Schwartz MA (2006) Mechanisms of mechanotransduction. Dev Cell 10, 11–20. [DOI] [PubMed] [Google Scholar]
- 114.Pradip D, Peng X, Durden DL (2003) Rac2 specificity in macrophage integrin signaling: potential role for Syk kinase. J Biol Chem 278, 41661–9. [DOI] [PubMed] [Google Scholar]
- 115.Aepfelbacher M, Essler M, Huber E, Czech A, Weber PC (1996) Rho is a negative regulator of human monocyte spreading. Journal of immunology (Baltimore, Md. : 1950) 157, 5070–5. [PubMed] [Google Scholar]
- 116.Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY (2014) Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm 2014, 352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rao KM (2001) MAP kinase activation in macrophages. J Leukoc Biol 69, 3–10. [PubMed] [Google Scholar]
- 118.Shiratsuchi H and Basson MD (2005) Activation of p38 MAPKalpha by extracellular pressure mediates the stimulation of macrophage phagocytosis by pressure. Am J Physiol Cell Physiol 288, C1083–93. [DOI] [PubMed] [Google Scholar]
- 119.Feske S, Wulff H, Skolnik EY (2015) Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 33, 291–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clapham DE, Runnels LW, Strübing C (2001) The trp ion channel family. Nature Reviews Neuroscience 2, 387–396. [DOI] [PubMed] [Google Scholar]
- 121.Vig M and Kinet J-P (2009) Calcium signaling in immune cells. Nat. Immunol. 10, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scheraga RG, Abraham S, Niese KA, Southern BD, Grove LM, Hite RD, McDonald C, Hamilton TA, Olman MA (2016) TRPV4 Mechanosensitive Ion Channel Regulates LPS-Stimulated Macrophage Phagocytosis. J Immunol 196, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goswami R, Merth M, Sharma S, Alharbi MO, Aranda-Espinoza H, Zhu X, Rahaman SO (2017) TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation. Free Radic. Biol. Med. 110, 142–150. [DOI] [PubMed] [Google Scholar]
- 124.Schappe MS, Szteyn K, Stremska ME, Mendu SK, Downs TK, Seegren PV, Mahoney MA, Dixit S, Krupa JK, Stipes EJ, Rogers JS, Adamson SE, Leitinger N, Desai BN (2018) Chanzyme TRPM7 Mediates the Ca2+ Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation. Immunity 48, 59–74.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ (2010) TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 11, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riazanski V, Gabdoulkhakova AG, Boynton LS, Eguchi RR, Deriy LV, Hogarth DK, Loaëc N, Oumata N, Galons H, Brown ME, Shevchenko P, Gallan AJ, Yoo SG, Naren AP, Villereal ML, Beacham DW, Bindokas VP, Birnbaumer L, Meijer L, Nelson DJ (2015) TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc Natl Acad Sci U S A 112, E6486–E6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL (2006) A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A 103, 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao E, Yang HQ, Gan Y-H, Duan D-H, He L-H, Guo Y, Wang SQ, Zhang Y (2015) Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells 33, 615–621. [DOI] [PubMed] [Google Scholar]
- 129.Pottosin I, Delgado-Enciso I, Bonales-Alatorre E, Nieto-Pescador MG, Moreno-Galindo EG, Dobrovinskaya O (2015) Mechanosensitive Ca2+-permeable channels in human leukemic cells: Pharmacological and molecular evidence for TRPV2. Biochimica et Biophysica Acta (BBA) - Biomembranes 1848, 51–59. [DOI] [PubMed] [Google Scholar]
- 130.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C (1999) Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10, 29–38. [DOI] [PubMed] [Google Scholar]
- 131.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE (2009) Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci U S A 106, 7642–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pathak MM, Tran T, Hong L, Joós B, Morris CE, Tombola F (2016) The Hv1 proton channel responds to mechanical stimuli. The Journal of General Physiology 148, 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Janmey PA, Wells RG, Assoian RK, McCulloch CA (2013) From tissue mechanics to transcription factors. Differentiation 86, 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mammoto A, Mammoto T, Ingber DE (2012) Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 125, 3061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P (2017) Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 171, 1397–1410 e14. [DOI] [PubMed] [Google Scholar]
- 136.Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13, 591–600. [DOI] [PubMed] [Google Scholar]
- 137.Totaro A, Panciera T, Piccolo S (2018) YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20, 888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Panciera T, Azzolin L, Cordenonsi M, Piccolo S (2017) Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18, 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–83. [DOI] [PubMed] [Google Scholar]
- 140.Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan KL, Li YJ, Chien S (2016) Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 113, 11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Feng Y, Liang Y, Zhu X, Wang M, Gui Y, Lu Q, Gu M, Xue X, Sun X, He W, Yang J, Johnson RL, Dai C (2018) The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang YJ, Yang CK, Wei PL, Huynh TT, Whang-Peng J, Meng TC, Hsiao M, Tzeng YM, Wu AT, Yen Y (2017) Ovatodiolide suppresses colon tumorigenesis and prevents polarization of M2 tumor-associated macrophages through YAP oncogenic pathways. J Hematol Oncol 10, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McGee KM, Vartiainen MK, Khaw PT, Treisman R, Bailly M (2011) Nuclear transport of the serum response factor coactivator MRTF-A is downregulated at tensional homeostasis. EMBO Rep 12, 963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Posern G and Treisman R (2006) Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol 16, 588–96. [DOI] [PubMed] [Google Scholar]
- 145.Yu L, Weng X, Liang P, Dai X, Wu X, Xu H, Fang M, Fang F, Xu Y (2014) MRTF-A mediates LPS-induced pro-inflammatory transcription by interacting with the COMPASS complex. J Cell Sci 127, 4645–57. [DOI] [PubMed] [Google Scholar]
- 146.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J (2013) Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K (2014) Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 16, 376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Speight P, Kofler M, Szaszi K, Kapus A (2016) Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFbeta-regulated Smad3. Nat Commun 7, 11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bertrand AT, Ziaei S, Ehret C, Duchemin H, Mamchaoui K, Bigot A, Mayer M, Quijano-Roy S, Desguerre I, Laine J, Ben Yaou R, Bonne G, Coirault C (2014) Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J Cell Sci 127, 2873–84. [DOI] [PubMed] [Google Scholar]
- 150.Hoffmann A, Natoli G, Ghosh G (2006) Transcriptional regulation via the NF-kappaB signaling module. Oncogene 25, 6706–16. [DOI] [PubMed] [Google Scholar]
- 151.Nam J, Aguda BD, Rath B, Agarwal S (2009) Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS One 4, e5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Young SR, Gerard-O’Riley R, Harrington M, Pavalko FM (2010) Activation of NF-kappaB by fluid shear stress, but not TNF-alpha, requires focal adhesion kinase in osteoblasts. Bone 47, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Waterfield JD, Ali TA, Nahid F, Kusano K, Brunette DM (2010) The effect of surface topography on early NFkappaB signaling in macrophages. J Biomed Mater Res A 95, 837–47. [DOI] [PubMed] [Google Scholar]
- 154.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, Singh R, Khanna N, Belmont AS, Wang N (2016) Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater 15, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Graham DM and Burridge K (2016) Mechanotransduction and nuclear function. Curr Opin Cell Biol 40, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kelkhoff D, Downing T, Li S (2016) Mechanotransduction to Epigenetic Remodeling In Molecular and Cellular Mechanobiology (Chien S, Engler AJ, and Wang PY, eds), Springer; New York, New York, NY: 163–173. [Google Scholar]
- 157.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N (2008) Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A 105, 6626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maniotis AJ, Chen CS, Ingber DE (1997) Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences 94, 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marshall WF, Fung JC, Sedat JW (1997) Deconstructing the nucleus: global architecture from local interactions. Curr Opin Genet Dev 7, 259–63. [DOI] [PubMed] [Google Scholar]
- 161.Akhtar A and Gasser SM (2007) The nuclear envelope and transcriptional control. Nat Rev Genet 8, 507–17. [DOI] [PubMed] [Google Scholar]
- 162.Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, van Steensel B (2006) Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet 38, 1005–14. [DOI] [PubMed] [Google Scholar]
- 163.Bank EM and Gruenbaum Y (2011) The nuclear lamina and heterochromatin: a complex relationship. Biochem Soc Trans 39, 1705–9. [DOI] [PubMed] [Google Scholar]
- 164.Van de Vosse DW, Wan Y, Wozniak RW, Aitchison JD (2011) Role of the nuclear envelope in genome organization and gene expression. Wiley Interdiscip Rev Syst Biol Med 3, 147–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Worman HJ and Bonne G (2007) “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 313, 2121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE (2013) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT (2004) Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113, 370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khatau SB, Bloom RJ, Bajpai S, Razafsky D, Zang S, Giri A, Wu PH, Marchand J, Celedon A, Hale CM, Sun SX, Hodzic D, Wirtz D (2012) The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci Rep 2, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olins AL, Hoang TV, Zwerger M, Herrmann H, Zentgraf H, Noegel AA, Karakesisoglou I, Hodzic D, Olins DE (2009) The LINC-less granulocyte nucleus. Eur J Cell Biol 88, 203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim Y, Bayona PW, Kim M, Chang J, Hong S, Park Y, Budiman A, Kim YJ, Choi CY, Kim WS, Lee J, Cho KW (2018) Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front Immunol 9, 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA (2011) Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3, 112ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S (2013) Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater 12, 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McWhorter FY, Davis CT, Liu WF (2015) Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci 72, 1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jain N, Iyer KV, Kumar A, Shivashankar GV (2013) Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci U S A 110, 11349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao Z, He Q, Peng B, Chiao PJ, Ye J (2006) Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function. J Biol Chem 281, 4540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, Tarakhovsky A (2010) Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Han SB and Lee JK (2009) Anti-inflammatory effect of Trichostatin-A on murine bone marrow-derived macrophages. Arch Pharm Res 32, 613–24. [DOI] [PubMed] [Google Scholar]
- 179.Pinz S, Unser S, Buob D, Fischer P, Jobst B, Rascle A (2015) Deacetylase inhibitors repress STAT5-mediated transcription by interfering with bromodomain and extra-terminal (BET) protein function. Nucleic Acids Res 43, 3524–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, Gregory L, Lonie L, Chew A, Wei CL, Ragoussis J, Natoli G (2010) Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–28. [DOI] [PubMed] [Google Scholar]
- 181.Cheng C, Huang C, Ma TT, Bian EB, He Y, Zhang L, Li J (2014) SOCS1 hypermethylation mediated by DNMT1 is associated with lipopolysaccharide-induced inflammatory cytokines in macrophages. Toxicol Lett 225, 488–97. [DOI] [PubMed] [Google Scholar]
- 182.Wang X, Cao Q, Yu L, Shi H, Xue B, Shi H (2016) Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 1, e87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang X, Wang X, Liu D, Yu L, Xue B, Shi H (2014) Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol Endocrinol 28, 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cao Q, Wang X, Jia L, Mondal AK, Diallo A, Hawkins GA, Das SK, Parks JS, Yu L, Shi H, Shi H, Xue B (2014) Inhibiting DNA Methylation by 5-Aza-2’-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology 155, 4925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou J, Li YS, Wang KC, Chien S (2014) Epigenetic Mechanism in Regulation of Endothelial Function by Disturbed Flow: Induction of DNA Hypermethylation by DNMT1. Cell Mol Bioeng 7, 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zoghbi HY and Beaudet AL (2016) Epigenetics and Human Disease. Cold Spring Harb Perspect Biol 8, a019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smith TD, Nagalla RR, Chen EY, Liu WF (2017) Harnessing macrophage plasticity for tissue regeneration. Adv Drug Deliv Rev 114, 193–205. [DOI] [PubMed] [Google Scholar]
- 188.Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP (2008) The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 122, 786–97. [DOI] [PubMed] [Google Scholar]
- 189.Duncan RL and Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57, 344–58. [DOI] [PubMed] [Google Scholar]
- 190.Lu F, Ogawa R, Nguyen DT, Chen B, Guo D, Helm DL, Zhan Q, Murphy GF, Orgill DP (2011) Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg 66, 296–300. [DOI] [PubMed] [Google Scholar]
- 191.Lancerotto L and Orgill DP (2014) Mechanoregulation of Angiogenesis in Wound Healing. Adv Wound Care (New Rochelle) 3, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]


