Abstract
Colibactins are genotoxic secondary metabolites produced in select Enterobacteriaceae, which induce downstream DNA double-strand breaks (DSBs) in human cell lines and are thought to promote the formation of colorectal tumors. Although key structural and functional features of colibactins have been elucidated, the full molecular mechanisms regulating these phenotypes remain unknown. Here, we demonstrate that free model colibactins induce DSBs in human cell cultures and do not require delivery by host bacteria. Through domain-targeted editing, we demonstrate that a subset of native colibactins generated from observed module skipping in the nonribosomal peptide synthetase–polyketide synthase (NRPS–PKS) biosynthetic assembly line share DNA alkylation phenotypes with the model colibactins in vitro. However, module skipping eliminates the strong DNA interstrand cross-links formed by the wild-type pathway in cell culture. This product diversification during the modular NRPS–PKS biosynthesis produces a family of metabolites with varying observed mechanisms of action (DNA alkylation versus cross-linking) in cell culture. The presence of membranes separating human cells from model colibactins attenuated genotoxicity, suggesting that membrane diffusion limits colibactin activity and could account for the reported bacterium–human cell-to-cell contact phenotype. Additionally, extracellular supplementation of the colibactin resistance protein ClbS was able to intercept colibactins in an Escherichia coli–human cell transient infection model. Our studies demonstrate that free model colibactins recapitulate cellular phenotypes associated with module-skipped products in the native colibactin pathway and define specific protein domains that are required for efficient DNA interstrand cross-linking in the native pathway.
Graphical Abstract
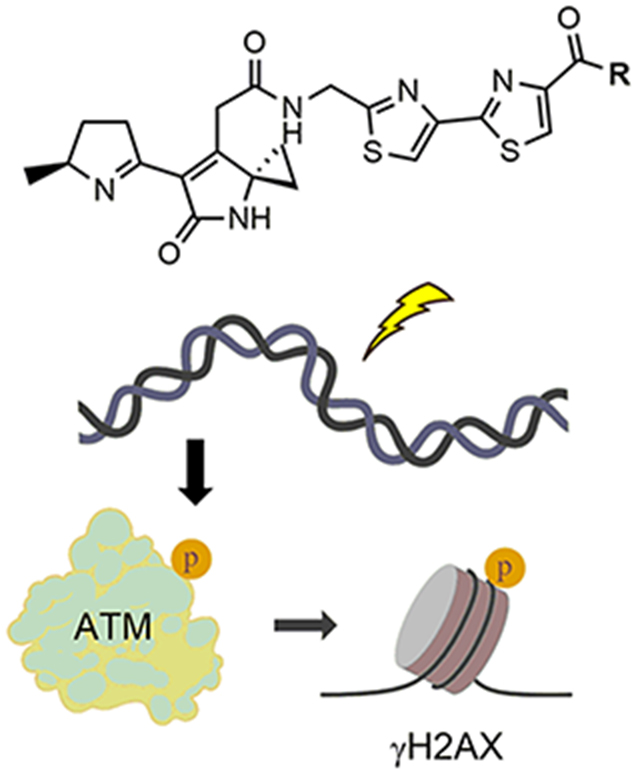
Perturbations in the community structure of the gut microbiota coupled with shifts in host–microbe metabolism can lead to a variety of altered host physiologies associated with diseases, such as inflammatory bowel disease and cancer.1–3 A prominent, yet rare, example of a one-pathway, one-phenotype correlation in the microbiome is that of the colibactin pathway.4–8 The colibactin gene cluster (clb) contains 19 genes (clbA–clbS) that encode the biosynthesis of hybrid polyketide synthase–nonribosomal peptide synthetase (PKS–NRPS) secondary metabolites known as colibactins. The clb locus is found in many strains of Enterobacteriaceae, including select strains of gut-commensal and extraintestinal pathogenic Escherichia coli (ExPEC) and Klebsiella pneumoniae, among others.9,10 The pathway is associated with virulence11 and is significantly more prevalent in patients with inflammatory bowel disease (IBD), colorectal cancer (CRC), and familial adenomatous polyposis (FAB).12–14
A growing number of studies support a causative role for the metabolites in colorectal cancer formation. For example, when mammalian cells are co-cultured with colibactin-expressing strains, DNA double-strand breaks (DSBs) accumulate and the mammalian cells activate ataxia-telangiectasia mutated kinase (ATM) signaling and undergo cell cycle arrest and senescence.9,15,16 clb+ adherent invasive E. coli (AIEC) cells induce tumor formation in three in vivo models of CRC.12,17,18 Synthetic colibactin derivatives of metabolites from clb+ bacteria are genotoxic in vitro.19 Finally, it was recently shown that incubation of exogenous DNA with colibactin-expressing strains induces DNA interstrand cross-links in a pathway-dependent manner.20
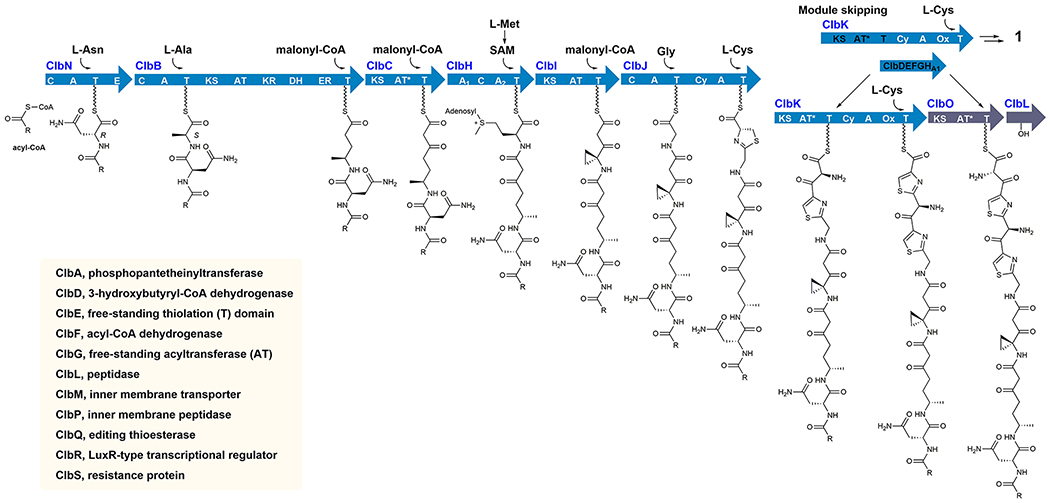
Characterization of colibactins has been difficult; to date, no colibactin has been directly isolated from any producing strain. This has been attributed to their low titers and/or putative instability.21 Progress has been made using a combination of biochemical characterization of pathway enzymes,22–27 comparative metabolomics,21,28,29 isolation from large-scale fermentation of genetically modified mutants,21,30–33 and chemical synthesis.7,19,29,34 This body of research has revealed important aspects of colibactin biosynthesis, structure, and function. First, colibactins are assembled by the NRPS–PKS biosynthetic machinery as prodrugs with an appended N-acyl-d-asparagine side chain.22,28,35 These “precolibactins” are transported into the periplasm by the 12-transmembrane transporter ClbM.36 The N-acyl-d-Asn residue is then removed by the pathway-dedicated membrane-bound peptidase (ClbP).37,38 Upon deacylation, spontaneous cyclization reactions occur to generate genotoxic colibactin structures.7,19,29
Deletion of any one of the biosynthetic genes within the colibactin pathway results in abrogation of DSBs in cell culture.9 Access to fully functionalized colibactins is desirable for understanding their trafficking from the periplasmic space and mode of action in eukaryotic cells. However, to the best of our knowledge, a metabolite that accounts for all of the genes in the clb gene cluster has not been isolated or predicted with experimental support.
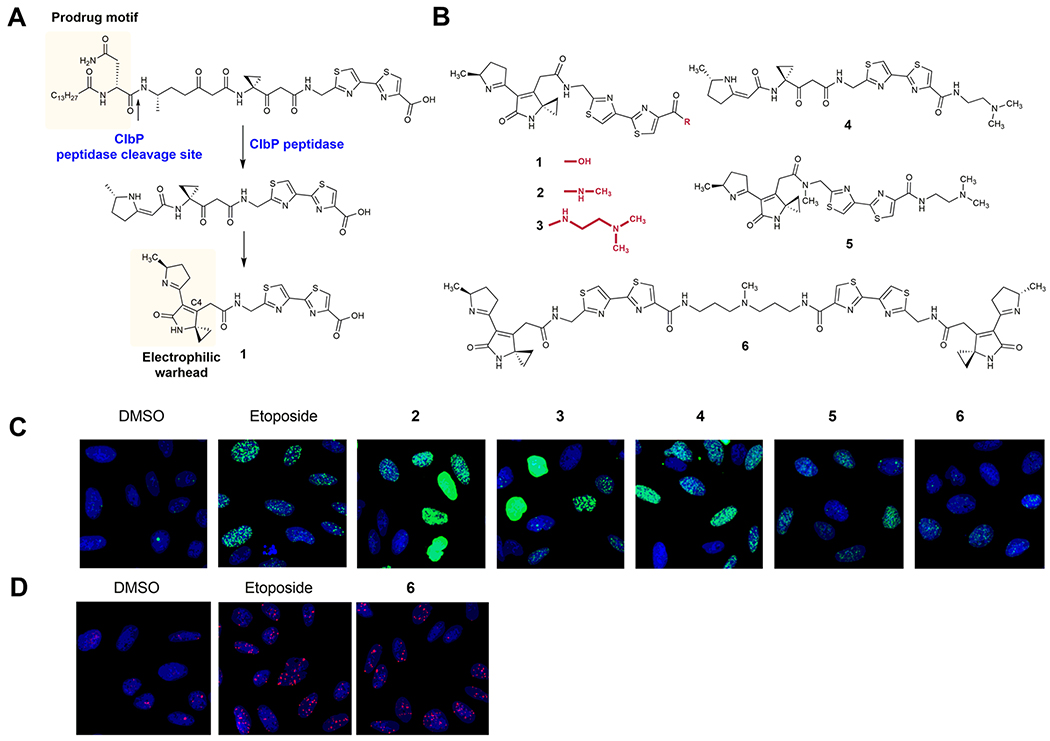
Model (pre)colibactins alkylate and cross-link DNA in vitro.19,21 Previous studies identified colibactin 1, which was detected in ClbP-proficient strains (Figure 1A).29 Derivatives of 1, such as N-methylamide 2 and dimethylethylene diamine derivative 3, alkylated DNA in vitro by addition of nucleotides to the electron deficient cyclopropyl residue.19
Figure 1.
Synthetic colibactins exhibit cellular genotoxicity. (A) Linear precolibactin metabolites undergo a spontaneous cyclization cascade to genotoxic colibactin metabolites such as 1 after ClbP-mediated prodrug cleavage. (B) Structures of synthetic analogues of 1 that previously demonstrated in vitro DNA alkylation activity. (C) HeLa cells were treated with 11 μm of compounds 2–6 for 4 h and subsequently fixed and stained for a marker of cellular DNA damage, γH2AX. As a positive control, 1 μM etoposide is shown for comparison. Quantification of γH2AX induction relative to that of etoposide is shown in the Supporting Information. (D) Of the model colibactins, only dimer 6 shows significant activation of 53BP1, a downstream effector of ATR kinase signaling, a phenotype induced by the native colibactin pathway.
We have shown that linear biosynthetic products are off-loaded from the pathway and that they undergo spontaneous cyclization to unsaturated imines, such as 1,29 via intermediates resembling 4.19 We previously synthesized 4 and found that it is genotoxic in vitro, indicating that cyclization to an unsaturated imine is facile. The N-methylamide analogue 5, which was designed to block formation of an inactive pyridone isomer, was also genotoxic. Finally, dimer 6 was synthesized and examined for functional comparison. With the exception of 6, all compounds incubated with a linearized plasmid demonstrated extensive alkylation and degradation of duplex DNA. The cyclic imine was shown to be much more electrophilic than the β-ketoamide found in precolibactins. Additionally, introduction of a basic amine or cationic sulfonium residue at the terminal thiazole ring increased levels of DNA damage, presumably by enhancing the affinity of the molecule for DNA. Compound 6 cross-linked DNA, as expected.19
Herein, we evaluated the abilities of model colibactins 2–6 to recapitulate the genotoxic phenotypes associated with colibactins produced in transiently infected human tissue cultures. Human cell (HeLa and U2OS) DNA damage was quantified by immunofluorescence imaging of the DNA damage marker phospho-SER139-histone H2AX (γH2AX).39 The effects of the compounds incubated for 4 h at 16 doses ranging from 230 nM to 100 μM were quantified relative to 1 μM etoposide as a positive control (set at 100% effect) and dimethyl sulfoxide (DMSO) vehicle as a negative control (0% effect). All of the compounds induced γH2AX activation and were cytotoxic (Figure 1C). Aside from a few exceptions, we could establish reliable half-maximal inhibitory concentration values (IC50) in the low micromolar range (Table 1 and Figures S1–S5). To further characterize the DNA damage response induced by the model colibactins, we evaluated recruitment of p53-binding protein 1 (53BP1), a downstream effector of ATR kinase signaling.40 Of the suite of compounds evaluated, only 6 demonstrated significant levels of colocalized 53BP1-γH2AX foci, which was comparable to those induced by 1 μM etoposide or infection with clb-expressing strains at a multiplicity of infection (MOI) of 10 (Figure 1D and Figure S6).20 Compounds 2 and 4 generated very weak 53BP1 signaling, with high micromolar concentrations demonstrating approximately 40% activity of the etoposide control (Figures S1 and S4). The coincidence of γH2AX and 53BP1 seen in select compounds supports activation of the eukaryotic DNA DSB repair pathways, without targeted cellular delivery or bacterium–human cell-to-cell contact.
Table 1.
Half-Maximal Effective Concentrations for γH2AX Activationa
| compound | cell line | IC50 |
|---|---|---|
| 2 | HeLa | not determined |
| U2OS | not determined | |
| 3 | HeLa | 2.11 μM |
| U2OS | not determined | |
| 4 | HeLa | 3.15 μM |
| U2OS | 2.87 μM | |
| 5 | HeLa | 10.7 μM |
| U2OS | 35.53 μM | |
| 6 | HeLa | 65.68 μM |
| U2OS | 1.28 μM |
The effect of compounds on γH2AX foci was quantified relative to that of 1 μM etoposide and graphed on an X–Y plot with a nonlinear regression curve fit. IC50 values were calculated unless the fit was ambiguous.
Previously, 2 was shown to be a substrate for the resistance protein ClbS,27,41 a cyclopropane hydrolase that catalyzes hydrolytic ring opening of the cyclopropane.27 We showed that several products were spontaneously generated from the hydrolysis product, including a proposed alkyl hydroperoxide. Both N-methylamide 2 and peroxide-containing hydrolytic intermediates possess a second electrophilic site (C4) in the lactam (Figure 1A), which is proposed to account for the very weak DNA interstrand cross-linking activity previously observed for a truncated precolibactin in vitro.21,27 The potential role for this electrophilic reactivity in vivo is unknown.
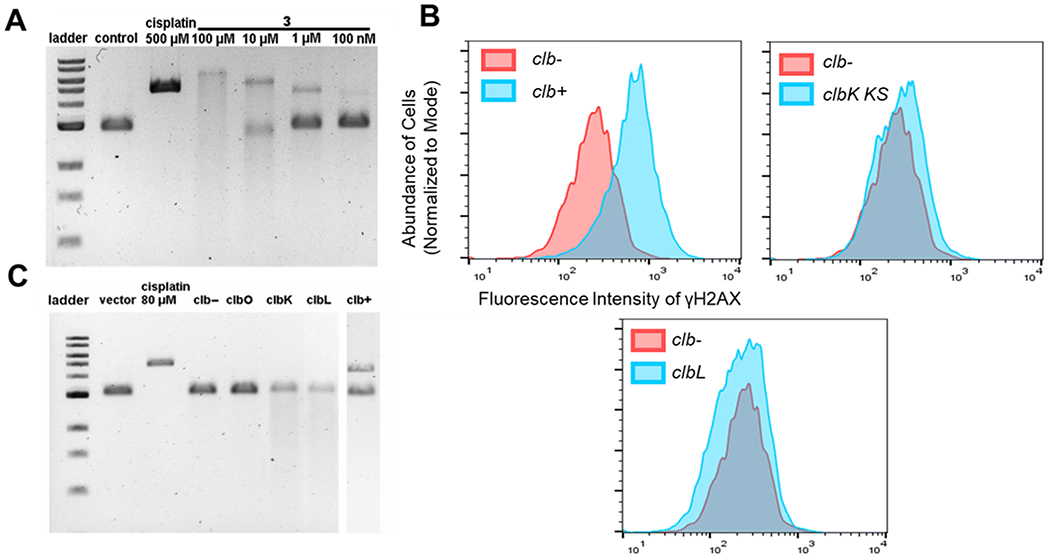
Given the weak 53BP1 activation for 2, we suspected that 2 and 3 might produce unstable cross-links via the electrophilic lactam C4 that were not detected in our earlier study.19 Because the bacteria acidify the medium in the stationary phase, we hypothesized that decreasing the pH of the alkylation assay could increase cross-linking activity. Indeed, reproducible formation of DNA interstrand cross-links was observed when linearized pBR322 DNA was incubated with 10 or 1 μM 2 or 3 at pH 5, as compared to assays conducted at pH 8 (Figure 2A and Figures S7 and S8). We propose that a decreased pH may enhance compound reactivity or the stability of the model colibactin–DNA complexes. The degree of cross-linking induced by compound 3 was time-dependent, while the stabilities of DNA cross-links induced by both 2 and 3 were decreased as the concentration of the base increased during denaturing gel assays (Figure 2A and Figures S7–S9). However, cross-links resulting from natively expressing clb+ strains remain stable after denaturing with higher NaOH percentages (Figure S10). The data indicate that colibactin analogues, which closely resemble known metabolites detected in cell culture, can alkylate and cross-link DNA in vitro, although the cross-links showed different stability compared to that of DNA cross-linked by native clb+ strains, suggesting different cross-link reactivities.
Figure 2.
Model colibactins can strongly alkylate and weakly cross-link DNA in vitro, whereas native colibactins both strongly alkylate and cross-link DNA. (A) Incubation of compound 3 with linearized pBR322 at pH 5 reveals clear cross-links under mild denaturing conditions [50 mM (0.2%) NaOH, on ice]. The cisplatin (500 μM) concentration is used as a positive control for DNA cross-linking. Extensive colibactin-alkylated and cross-linked DNA undergoes further degradation in these assays, which is observed as DNA smears. (B) Active-site point mutations in ClbL and in the PKS module of ClbK establish these biosynthetic proteins/domains as being essential for the function of colibactin in a bacterium–human cell transient infection model. U2OS cells (6.0 × 105) were transiently infected with clb-expressing bacteria or isogenic mutants at an MOI of 10 for 4 h. Cell fixation and γH2AX antibody staining occurred immediately after infection. (C) Incubation of linearized pBR322 with isogenic mutants of the clb pathway for 4 h demonstrates varying levels of DNA alkylation and degradation activity. Denaturing gels were run at 50 mM (0.2%) NaOH.
Compounds 2–6 are approximately 10-fold less potent than etoposide in tissue culture, yet they induce comparable levels of DNA damage at nanomolar concentrations in vitro. We speculated that this discrepancy is due to issues of transport and membrane permeability and might be related to biosynthetic events not represented in the current model colibactins. The model colibactins employed in this study do not contain an α-aminomalonate-derived extender unit that is known to be incorporated by the PKS module of NRPS–PKS hybrid protein ClbK.23,24,30 While the genes dedicated to the synthesis and incorporation of this residue are essential for genotoxicity in the transient infection model, the only colibactin metabolite known to date that contains this motif has not been reported to harbor genotoxicity or DNA cross-link activity similar to that of fully functionalized native colibactins. This is in part due to the retention of the N-acyl-d-asparagine side chain as well as possibly the missing biosynthetic additions provided by the largely uncharacterized enzymes ClbO and ClbL in the pathway. Though the exact functional requirement of the α-aminomalonate unit is unclear, there has been speculation that the basic amine in this residue may increase DNA affinity19,24 and enhance cellular permeability. Indeed, a genetic screen of bleomycin-resistant Saccharomyces cerevisiae revealed mutations in polyamine transporters as a key bleomycin-resistant determinant, implying that amino-containing moieties could enhance nuclear delivery.42,43
To further explore these functional consequences of the clb enzymes for which we have not accounted, we evaluated the genotoxicity of pathway mutants both in vitro and in the transient infection model in comparison to our model colibactins. Active-site point mutations were constructed in the PKS module of ClbK and in the peptidase ClbL based on protein sequence alignments to known ketosynthase and homologous amidase domains, as previously described.29 U2OS cells were transiently infected with DH10B E. coli carrying the full clb pathway, the clbK point mutant (C167A), the clbL point mutant (S179A), or the empty BAC vector control, and γH2AX activation was analyzed by flow cytometry (Figure 2B). Both point mutants ablated the genotoxic effects of the pathway, demonstrating that these specific catalytic activities are essential for clb+ E. coli in inducing DSBs in cell culture.
To remove uncharacterized human cell trafficking aspects of the transient infection model, we next analyzed these strains for their ability to damage exogenously supplied DNA using denaturing gel assays (Figure 2C). E. coli negative controls lacking the pathway (clb−) or fully deleted for the PKS clbO were inactive as expected. However, E. coli with the inactive PKS module in clbK or inactive peptidase clbL exhibited DNA damage but not cross-linking. Because the complete pathway leads to stable DNA interstrand cross-links, as described above and elsewhere,20 relative to the model colibactins (Figure S10), additional structural modifications afforded by the PKS module of ClbK, PKS ClbO, and amidase ClbL transform the potent DNA alkylators with weak to moderate cross-linking activities into a highly efficient DNA interstrand cross-linker. The activity of the clbK and clbL mutants in vitro is consistent with the extensive DNA alkylation and degradation that we have observed for model colibactins, which are analogues of metabolites produced by biosynthetic PKS module skipping of ClbK. Thus, while model colibactins do not fully reproduce the cellular phenotypes induced by fully functionalized colibactins, they do phenotypically recapitulate a subset of characterized on-pathway intermediates. This illustrates how product diversification evolutionarily encoded by the modular NRPS–PKS biosynthetic enzymes can generate functionally distinct products with varying modes of action. In addition, the alkylation activity for clbK and clbL mutants in vitro, but not in the transient infection model where DNA repair is possible, again suggests that these uncharacterized enzymatic modifications may enhance transport, cellular permeability, and potency.
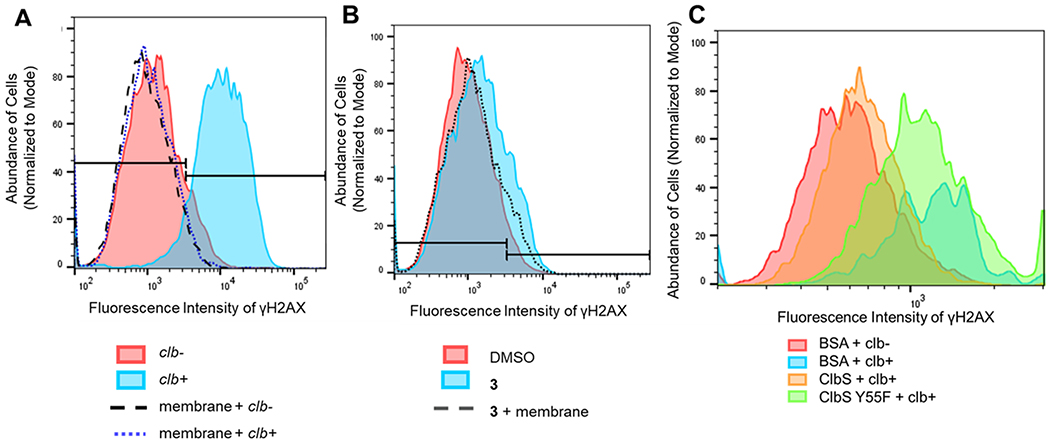
The nature of colibactin transport is largely unknown; however, bacterium–human cell-to-cell contact was reported to be necessary for genotoxicity.9 This has largely puzzled researchers, given that expression of the clb locus does not depend on the presence of mammalian cells44 and outer membrane vesicles isolated from clb-expressing strains in some studies confer genotoxicity when applied,45 yet membranes separating clb-expressing bacteria from mammalian cells or thick adherent intestinal mucus layers abolish genotoxicity.9,46 To test whether the genotoxic effects of the synthetic colibactins were similarly eliminated by membrane separation as with the native colibactins, we treated U2OS cells with 50 μM 3 for 4 h in the presence or absence of separating 0.45 μm membranes. DNA damage via γH2AX activation was then analyzed by flow cytometry (Figure 3). The presence of the membrane completely ablated genotoxicity from clb+ E. coli as expected, and the membrane similarly attenuated the modest γH2AX response of 3 (Figure 3 and Figure S11). To test whether actively dividing bacteria would have affected the activity of 3, we pretreated the membranes with clb− or clb+ bacteria for 30 min before adding 3 (Figure S11). The modest γH2AX response of 3 was also diminished in this experiment. This suggests that diffusion of colibactin across the membrane is slow or potentially deleterious interactions with the membrane itself impede transport into cellular targets, attenuating genotoxic activity.
Figure 3.
(A) Native colibactins from live expressing clb+ or control (clb−) cells were applied to U2OS cells with or without separating membranes. U2OS cells were subsequently evaluated for DNA DSB activity via γH2AX staining. Membranes abrogated native colibactin activity. (B) U2OS cells (6.0 × 105) were treated with 50 μM compound 3 in the presence or absence of a 0.45 μm membrane separating the bacteria from the U2OS cells for 4 h. Cells were immediately fixed and stained for γH2AX and quantified by flow cytometry. Membranes attenuated the modest cellular activity of free 3. (C) Protection from genotoxicity by supplementation of extracellular ClbS. U2OS cells (6.0 × 105) were transiently infected with clb-expressing bacteria at an MOI of 10 for 4 h in the presence of different additives, including ClbS. The medium was replaced, and 50 μg/mL gentamycin was added. Cell fixation and γH2AX antibody staining occurred 16 h after infection to monitor downstream DSB activity, and γH2AX positive cells were quantified using flow cytometry. ClbS protected human cells from genotoxic action, whereas its active-site mutant did not relative to controls.
Given that free model colibactins induce DNA double-strand breaks in human cells, we evaluated whether ClbS could protect human cells when delivered extracellularly. We found that when purified ClbS was exogenously supplemented (at 1 μM in our studies), U2OS cells were protected from clb+ E. coli, as determined by quantification of γH2AX staining (Figure 3C; 10 μM ClbS studies are shown in Figure S12, supporting complete protection of human cells). While this work was in progress, a similar observation was reported.20 Additionally, we had previously identified the active-site Tyr residue Y55 in ClbS as being critical for cyclopropane hydrolase activity.27 When the ClbS Y55F mutant (1 μM) was supplemented to U2OS cells transiently infected with clb+ E. coli, protective effects were not observed (Figure 3C). These data indicate that the previous protein biochemical studies using purified ClbS and model colibactins are consistent with the activities observed in cell cultures. These data also suggest that native colibactins can be intercepted by extracellularly supplemented ClbS. The results of these experiments also suggest that direct cell-to-cell contact might not be required for native colibactin activity. We propose that poor diffusion and chemical instability contribute to the observed bacterium–human cell-to-cell contact phenotype.
In conclusion, we have demonstrated that model colibactin analogues can recapitulate cellular genotoxic phenotypes consistent with native clb+ E. coli infection by formation of γH2AX and 53BP1 foci. In addition, we have shown that these compounds also reconstitute unstable DNA interstrand cross-links in vitro. DNA interstrand cross-linking has recently been supported as the primary mode of action of the mature, final pathway product.20 However, several modifications relative to the model colibactins, which are encoded in the PKS module of ClbK, PKS ClbO, and peptidase ClbL, are needed to recapitulate efficient stable cross-links of the native colibactins produced by the full pathway. (A summary of the biosynthesis of (pre)colibactins is shown in Figure 4.) Our membrane studies, in combination with the ability of exogenous ClbS to intercept and neutralize native colibactins, call into question the absolute necessity of cell-to-cell contact for colibactin’s genotoxic action. Our work also provides important new insights into resolving the remaining questions surrounding colibactin’s mature structure and mode of action.
Figure 4.
Proposed biosynthesis of precolibactins. The precolibactin leading to colibactin metabolite 1 is derived from PKS module skipping in ClbK (see also Figure 1A). PKS ClbO and peptidase ClbL (gray) represent steps in mature (pre)colibactin biosynthesis for which experiments could not account, although PKS ClbO is known to biochemically accept an l-Ser-derived α-aminomalonyl extender unit from ClbDEFG and the first adenylation domain of ClbH (ClbHA1).23,24 Other proteins are listed in the inset. Modular PKS/NRPS enzymes are colored blue. Abbreviations: C, condensation; A, adenylation; T, thiolation; E, epimerization; KS, ketosynthase; AT, acyl-transferase; KR, ketoreductase; DH, dehydratase; ER, enoyl-reductase; AT*, inactive AT; Cy, dual condensation/cyclase; Ox, oxidase.
METHODS
Mammalian Cell Lines and Reagents.
U2OS and HeLa cell lines were obtained from American Type Culture Collection (ATCC). For microscopy, U2OS cells were cultured in McCoy’s 5A medium (Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (Life Technologies). For flow cytometry studies, U2OS cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies)/F12 with 25 mM HEPES and 5% FBS. HeLa cells were cultured in DMEM with 10% FBS. All cells were maintained at 37 °C with 5% CO2. Antibodies for immunofluorescence were purchased from Upstate (phospho-specific H2AX, 05-636), Novus Biologicals (53BP1, NB100–904SS), or Molecular Probes [Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin G (IgG) and Alexa Fluor 647-conjugated goat anti-rabbit IgG]. Antibodies for flow cytometry quantification were purchased from Cell Signaling (P-histone H2AX S139 rabbit) and Life Technologies (Alexa Fluor 647, REF A21245).
Bacterial Strains and Growth Conditions.
The E. coli strains used in this study were DH10B containing the colibactin gene cluster on pBeloBAC11 (clb+) or empty vector alone (clb−).9 The clbL, clbK, and clbO mutants were constructed as previously described.29 Bacteria were grown overnight in LB and diluted into DMEM F12 and 15 mM HEPES with chloramphenicol (25 μg/mL) for assays. Standard growth conditions were 37 °C with 250 rpm agitation.
Synthesis of Colibactin Analogues.
Compounds were prepared as previously described.19
Immunofluorescence Assay.
Cells were seeded at a density of 2500 (HeLa) or 5000 (U2OS) cells per well to achieve total well volumes of 20 μL in 384-well plates (black with an optically clear bottom, Greiner Bio One 781091) using a Thermo Combidrop liquid dispenser. Cells were grown for 24 h, followed by the addition of test compounds using an Echo acoustic liquid handler (Labcyte). For each tested drug concentration, a 20 nL aliquot of the 1000× stock was added to 20 μL of cells to provide a final DMSO concentration of 0.1%. Each plate contained 16 negative vehicle control wells (0.1% DMSO) and 16 positive control wells (1 μM etoposide). The cells were incubated with the compounds for 4 h, fixed, and subjected to immunofluorescence.
Immunofluorescence.
Cells were fixed with 4% paraformaldehyde (PFA) (Electron Microscopy Sciences) in the presence of 0.02% Triton X-100 at room temperature (RT) for 20 min and then incubated in permeabilization/blocking solution [10% FBS and 0.5% Triton X-100 in phosphate-buffered saline (PBS)] at RT for 1 h. Primary antibodies were diluted 1:500 in permeabilization/blocking solution and used to stain cells at 4 °C overnight. Cells were imaged using the InCell 2200 Imaging System (GE Corp.) and analyzed using InCell Analyzer software (GE Corp.) to quantify the number of γH2AX and 53BP1 foci. The effect of the test compounds was normalized to the mean of positive control wells (1 μM etoposide, set as the 100% effect) and the mean of the negative control well (0.1% DMSO, set as the 0% effect).
DNA Cross-Linking Assay.
The 4163 bp plasmid pBR322 was purchased from NEB and linearized with 5 units/μg EcoRI (NEB). The cut plasmid was purified using a polymerase chain reaction (PCR) clean kit (NEB) and eluted into 10 mM Tris (pH 8.0). For each reaction with synthetic colibactins 2–6, 200 ng of linearized DNA (31 μM base pairs) was incubated with the compound in a total volume of 20 μL. Compounds were diluted in DMSO such that each reaction included a fixed 5% DMSO concentration. Reactions were conducted in 10 mM Tris EDTA buffer (pH 8.0) or in 10 mM sodium citrate (pH 5.0) as labeled in the figure. Reactions proceeded for 3 h at 37 °C, unless otherwise noted. For each reaction with bacteria, 600 ng of linearized plasmid DNA was added to 150 μL of DMEM/F12 and 15 mM Hepes (Invitrogen) inoculated with 6 × 106 bacteria pregrown to exponential phase in the DMEM/F12 15 mM Hepes (Invitrogen) medium. The mixture of DNA and bacteria was incubated at 37 °C for 4 h, and the bacteria were then pelleted. The DNA was isolated from the supernatant using the PCR clean kit (NEB) and quantified using nanodrop. The DNA concentration was adjusted to 10 ng/μL using water and stored in −20 °C until it was ready for gel analysis. Pure methylmethanesulfonate (MMS) (Alfa Aesar) and cisplatin (Biovision) stock solutions were diluted into DMSO immediately prior to being used. As controls, 200 ng of DNA was treated with 80 μM cisplatin (Biovision) in 10 mM sodium citrate (pH 5) buffer with a final DMSO concentration of 5%. The DNA was immediately tested via gel electrophoresis after incubation.
DNA Gel Electrophoresis.
For each DNA sample, the concentration was preadjusted to 10 ng/μL. Four microliters (40 ng) of DNA was removed and mixed with 1.5 μL of 6× purple gel loading dye; no SDS (NEB) was present in the nondenatured gel. For denatured gels, 5 μL (50 ng) of DNA was removed each time and separately mixed with 15 μL of 0.2% denaturing buffer (0.27% sodium hydroxide, 10% glycerol, and 0.013% bromophenol blue), 0.4% denaturing buffer (0.53% sodium hydroxide, 10% glycerol, and 0.013% bromophenol blue), or 1% denaturing buffer (1.33% sodium hydroxide, 10% glycerol, and 0.013% bromophenol blue) on an ice bath. The mixed DNA samples were denatured in 4 °C for 10 min and immediately loaded onto 1% agarose Tris Borate EDTA (TBE) gels for 1.5 h at 90 V. The gel was poststained with SybrGold (Thermo Fisher) for 2 h.
Membrane Transport Assay.
U2OS cells (6.0 × 105) were seeded in six-well plates and treated with 50 μM compound 3 diluted in 300 μL suspended on the surface of a 0.45 μm, 30 mm diameter membrane (Milipore) for 4 h. DH10B clb− or clb+ cells at an OD600 of 1.0 were diluted into 300 μL of DMEM at an MOI of 10 and suspended on the surface of the 0.45 μm membrane to separate the bacteria from the U2OS cells.
ClbS Protection Assay.
Expression and purification of ClbS were performed as previously described.27 Briefly, 1 L of BL21 cells carrying either pET28-ClbS-His or pET28-ClbSY55C was induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) and grown overnight at 25 °C. Bacteria were lysed in lysis buffer [50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole (pH 8), with 1 mg mL−1 lysozyme freshly added] and purified on a 2 mL bed volume of HisPur Ni-NTA resin (Thermo Scientific). Proteins were eluted into 250 mM imidazole, 100 mM Tris, 300 mM NaCl, and 10% glyercol (pH 8). Purified proteins were separated over a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel to verify the size and relative purity and then buffer exchanged into 50 mM potassium phosphate buffer (pH 8) using PD-10 desalting columns (GE). U2OS cells (6.0 × 105) were infected with either clb− or clb+ bacteria at an OD600 of 1.0 and an MOI of 10 with either 1 or 10 μM ClbS/ClbSY55C or BSA and incubated at 37 °C for 4 h.
Quantification of H2AX.
Cells were immediately fixed and stained with the γH2AX antibody and quantified by flow cytometry. Cells were fixed with 4% paraformaldehyde in PBS for 10 min, permeabilized with 90% ice-cold methanol for 30 min on ice, and blocked in PBS with 1 mM CaCl2, 1 mM MgCl2, and 10% FBS for 10 min. Primary antibodies were diluted 1:100 in blocking solution and incubated overnight at 4 °C. Secondary antibodies were diluted 1:1000 in blocking solution and incubated at RT for 2 h. Cells were washed and resuspended in PBS before being analyzed on a FACSAria II instrument (BD).
Supplementary Material
ACKNOWLEDGMENTS
Financial support from the National Institutes of Health (1DP2-CA186575 to J.M.C., R01GM110506 to S.B.H., and R01CA215553 to S.B.H. and J.M.C.), the Burroughs Wellcome Fund (1016720 to J.M.C.), the Camille & Henry Dreyfus Foundation (TC-17-011 to J.M.C.), the Yale Cancer Center, and Yale University is gratefully acknowledged. E.E.S. was supported by the National Science Foundation Graduate Research Fellowships Program. A.R.H. was supported by a Charles H. Revson Foundation Senior Fellowship. The abstract graphic was created with BioRender Software.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.8b00714.
H2AX dose–response analysis, DNA interstrand cross-link gels, membrane separation studies, and ClbS protection assays (Figures S1–S12) (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Winter SE, Lopez CA, and Baumler AJ (2013) The dynamic of gut-associated microbial comunities during inflammation. EMBO Rep. 14, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Garrett WS (2015) Cancer and the microbiota. Science 348, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, and Knight R (2016) Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 535, 94–103. [DOI] [PubMed] [Google Scholar]
- (4).Balskus EP (2015) Colibactin: understanding an elusive gut bacterial genotoxin. Nat. Prod. Rep 32, 1534–1540. [DOI] [PubMed] [Google Scholar]
- (5).Trautman EP, and Crawford JM (2016) Linking Biosynthetic Gene Clusters to their metabolites via Pathway Targeted Molecular Networking. Curr. Top. Med. Chem 16, 1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Taieb F, Petit C, Nougayrède JP, and Oswald E (2016) The Enterobacterial Genotoxins: Cytolethal Distending Toxin and Colibactin. EcoSal Plus, DOI: 10.1128/ecosalplus.ESP-0008-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Healy AR, and Herzon SB (2017) Molecular Basis of Gut Microbiome-Associated Colorectal Cancer: A Synthetic Perspective. J. Am. Chem. Soc 139, 14817–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fais T, Delmas J, Barnich N, Bonnet R, and Dalmasso G (2018) Colibactin: More Than a New Bacterial Toxin. Toxins 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nougayrède J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, and Oswald E (2006) Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851. [DOI] [PubMed] [Google Scholar]
- (10).Johnson JR, Johnston B, Kuskowski MA, Nougayrède JP, and Oswald E (2008) Molecular Epidemiology and Phylogenetic Distribution of the Escherichia coli pks Genomic Island. J. Clin. Microbiol 46, 3906–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Putze J, Hennequin C, Nougayrède JP, Zhang W, Homburg S, Karch H, Bringer MA, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, and Dobrindt U (2009) Genetic Structure and Distribution of the Colibactin Genomic Island among Members of the Family Enterobacteriaceae. Infect. Immun 77, 4696–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Arthur J, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis J, Fan T, Campbell B, Abujamel T, Dogan B, Rogers A, Rhodes J, Stintzi A, Simpson K, Hansen J, Keku T, Fodor A, and Jobin C (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, and Bonnet R (2013) High prevalence of mucosa-assoicated E.coli producing cyclomodulin and genotoxin in colon cancer. PLoS One 8, No. e56964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll D.Rm., Housseau F, and Sears CL (2018) Patients with Familian Adenomatous Polyposis Harbor Colonic biofilms containing tumorigenic bacteria. Science 359, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, and Nougayrède J-P (2010) Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci U. S. A 107, 11537–11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Secher T, Samba-Louaka A, Oswald E, and Nougayrède JP (2013) Escherichia coli Producing Colibactin Triggers Premature and Transmissible Senescence in Mammalian Cells. PLoS One 8, No. e77157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bonnet M, Buc E, Sauvanet P, Darcha Cl., Dubois D, Pereira B, Dechelotte P, Bonnet R, Pezet D, and Darfeuille-Michaud A (2014) Colonization of the Human Gut by E. coli and Colorectal Cancer Risk. Clin. Cancer Res 20, 859–867. [DOI] [PubMed] [Google Scholar]
- (18).Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, Sun X, Mohamadzadeh M, Liu X, Martin P, Wang GP, Oswald E, Fodor AA, and Jobin C (2017) Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res. 77, 2620–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Healy AR, Nikolayevskiy H, Patel JR, Crawford JM, and Herzon SB (2016) A mechanistic model for colibactin-induced genotoxicity. J. Am. Chem. Soc 138, 15563–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bossuet-Greif N, Vignard J, Taieb F, Mirey G, Dubois D, Petit C, Oswald E, and Nougayrède JP (2018) The Colibactin Genotoxin Generates DNA Interstrand Cross Links in Infected Cells. mBio 9, e02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Vizcaino MI, and Crawford JM (2015) The colibactin warhead crosslinks DNA. Nat. Chem 7, 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Brotherton CA, and Balskus EP (2013) A prodrug resistance mechanism is involved in colibactin biosynthesis and cytotoxicity. J. Am. Chem. Soc 135, 3359–3362. [DOI] [PubMed] [Google Scholar]
- (23).Brachmann AO, Garcie C, Wu V, Martin P, Ueoka R, Oswald E, and Piel J (2015) Colibactin biosynthesis and biological activity depend on the rare aminomalonyl polyketide precursor. Chem. Commun 51, 13138–13141. [DOI] [PubMed] [Google Scholar]
- (24).Zha L, Wilson MR, Brotherton CA, and Balskus EP (2016) Characterization of polyketide synthase machinery from the pks island facilitates isolation of a candidate precolibactin. ACS Chem. Biol 11, 1287–1295. [DOI] [PubMed] [Google Scholar]
- (25).Guntaka NS, Healy AR, Crawford JM, Herzon SB, and Bruner SD (2017) Structure and functional analysis of ClbQ, an unusual intermediate-releasing thioesterase from the colibactin biosynthetic pathway. ACS Chem. Biol 12, 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zha L, Jiang Y, Henke MT, Wilson MR, Wang JX, Kelleher NL, and Balskus EP (2017) Colibactin assembly line enzymes use S-adenosylmethionine to build a cyclopropane ring. Nat. Chem. Biol 13, 1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Tripathi P, Shine EE, Healy AR, Kim CS, Herzon SB, Bruner SD, and Crawford JM (2017) ClbS is a cyclopropane hydrolase that confers colibactin resistance. J. Am. Chem. Soc 139, 17719–17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Vizcaino MI, Engel P, Trautman E, and Crawford JM (2014) Comparative metabolomics and structural characterizations illuminate colibactin pathway-dependent small molecules. J. Am. Chem. Soc 136, 9244–9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Trautman EP, Healy AR, Shine EE, Herzon SB, and Crawford JM (2017) Domain-targeted metabolomics delineates the heterocycle assembly steps of colibactin biosynthesis. J. Am. Chem. Soc 139, 4195–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Li ZR, Li J, Gu JP, Lai JYH, Duggan BM, Zhang WP, Li ZL, Li YX, Tong RB, Xu Y, Lin DH, Moore BS, and Qian PY (2016) Divergent biosynthesis yields a cytotoxic aminomalonate-containing precolibactin. Nat. Chem. Biol 12, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li Z-R, Li Y, Lai JYH, Tang J, Wang B, Lu L, Zhu G, Wu X, Xu Y, and Qian P-Y (2015) Critical intermediates reveal new biosynthetic events in the enigmatic colibactin pathway. ChemBioChem 16, 1715–1719. [DOI] [PubMed] [Google Scholar]
- (32).Brotherton CA, Wilson M, Byrd G, and Balskus EP (2015) Isolation of a metabolite from the pks island provides insights into colibactin biosynthesis and activity. Org. Lett 17, 1545–1548. [DOI] [PubMed] [Google Scholar]
- (33).Bian X, Plaza A, Zhang Y, and Müller R (2015) Two more pieces of the colibactin genotoxin puzzle from Escherichia coli show incorporation of an unusual 1-aminocyclopropanecarboxylic acid moiety. Chem. Sci 6, 3154–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Healy AR, Vizcaino MI, Crawford JM, and Herzon SB (2016) Convergent and modular synthesis of candidate precolibactins. Structural revision of precolibactin A. J. Am. Chem. Soc 138, 5426–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bian X, Fu J, Plaza A, Herrmann J, Pistorius D, Stewart AF, Zhang Y, and Muller R (2013) In vivo evidence for a prodrug activation mechanism during colibactin maturation. ChemBioChem 14, 1194–1197. [DOI] [PubMed] [Google Scholar]
- (36).Mousa JJ, Newsome RC, Yang Y, Jobin C, and Bruner SD (2017) ClbM is a versatile, cation-promiscous MATE transporter found in the colibactin bioysnthetic gene cluster. Biochem. Biophys. Res. Commun 482, 1233–1239. [DOI] [PubMed] [Google Scholar]
- (37).Cougnoux A, Gibold L, Robin F, Dubois D, Pradel N, Darfeuille-Michaud A, Dalmasso G, Delmas J, and Bonnet R (2012) Analysis of structure-fuction relationships in the colibactin-maturaing enzyme clbP. J. Mol. Biol 424, 203–214. [DOI] [PubMed] [Google Scholar]
- (38).Dubois D, Baron O, Cougnoux A, Delmas J, Pradel N, Boury M, Bouchon B, Bringer M-A, Nougayrède J-P, Oswald E, and Bonnet R (2011) ClbP is a prototype of a peptidase subgroup invovled in biosynthesis of nonribosomal peptide. J. Biol. Chem 286, 35562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Rogakou EP, Pilch DR, Orr AH, Ivanova VS, and Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem 273, 5858–5868. [DOI] [PubMed] [Google Scholar]
- (40).Ciccia A, and Elledge SJ (2010) The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Bossuet-Greif N, Dubois D, Petit C, Tronnet S, Martin P, Bonnet R, Oswald E, and Nougayrède J-P (2016) Escherichia coli ClbS is a colibactin resistance protein. Mol. Microbiol 99, 897–908. [DOI] [PubMed] [Google Scholar]
- (42).Chen J, and Stubbe J (2005) Bleomycins: Towards Better Therapeutics. Nat. Rev. Cancer 5, 102–112. [DOI] [PubMed] [Google Scholar]
- (43).Aouida M, Page N, Leduc A, Peter M, and Ramotar D (2004) A genome-wide screen in Saccharomyces cerevisiae reveals altered transport as a mechanism of resistance to the anticancer drug bleomycin. Cancer Res. 64, 1102–1109. [DOI] [PubMed] [Google Scholar]
- (44).Homburg S, Oswald E, Hacker J, and Dobrindt U (2007) Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol. Lett 275, 255–262. [DOI] [PubMed] [Google Scholar]
- (45).Canas M-A, Gimenez R, Fabrega M-J, Toloza L, Baldoma L, and Badia J (2016) Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the Commensal ECOR12 enter intestinal epthileal cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS One 11 , No. e0160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Reuter C, Alzheimer M, Walles H, and Oelschlaeger TA (2018) An adherent mucus layer attenuates the genotoxic effects of colibactin. Cell. Microbiol 20, e12812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.