Abstract
Hypocupremia is a rare and under-recognised cause of bone marrow dysplasia and myeloneuropathy. A 47-year-old Caucasian woman had progressive ascending peripheral neuropathy and gait ataxia over 3 months and fatigue, dyspnoea and unintentional weight loss over 8 months. She had profound macrocytic anaemia and neutropenia. Initial workup included normal serum vitamin B12. Bone marrow biopsy was suggestive of copper deficiency. Serum copper levels were later confirmed to be undetectable. The patient received oral copper repletion which resulted in complete normalisation of haematological abnormalities 16 weeks later. However, neurological deficits persisted. This case describes a delayed diagnosis of hypocupremia as initially suggested through invasive testing. Associating myeloneuropathy with cytopenia is imperative for accurate and prompt diagnosis of hypocupremia, which can be confirmed by serum analysis alone. Developing an accurate differential diagnosis can help prevent unnecessary procedures. Furthermore, initiating prompt copper repletion prevents further neurological impairment. Neurological deficits are often irreversible.
Keywords: haematology (incl blood transfusion), neurology, pathology
Background
Copper deficiency is an uncommon nutritional deficiency since copper is needed in small quantities and can be easily obtained from most foods. However, certain conditions that may contribute to this nutritional deficiency have been identified. Malabsorption disorders (celiac disease, Crohn disease or as a result of gastrointestinal or bariatric surgeries), lack of supplementation from prolonged parenteral nutrition, copper chelation and zinc ingestion have all been implicated in causing hypocupremia.1–4 Given its rarity and similar presentation to B12 deficiency, the diagnosis of hypocupremia can often be missed. A thorough history, coupled with appropriate serum analysis, can aid in developing the differential diagnosis of myeloneuropathy with cytopenia. With copper replacement, haematological abnormalities often reverse completely and promptly. However, neurological deficits often persist with residual symptoms. Prompt diagnosis is essential in preserving neurological functioning.
Case presentation
A 47-year-old Caucasian woman presented with numbness and tingling that started in her feet and gradually ascended towards her waist over 3 months. She has had worsening balance and difficulty in walking. She also noted tingling in her fingers for 3 days. She has had palpitations, shortness of breath and reported a 30-pound weight loss over 8 months. She denied any significant medical or surgical history. She works as a waitress, has smoked a 10+ pack for years and denied alcohol or illicit drug use. Family history was not significant for neurological disease. The patient also complained of generalised fatigue, dyspnoea, occasional nausea, vomiting and esophageal reflux. Vital signs were significant for tachycardia. Physical examination was significant for pale conjunctiva and oral mucosa. She had no palpable lymphadenopathy or hepatosplenomegaly.
Initial laboratory studies showed profound neutropenia with a white blood cell count of 1.8×109/L and absolute neutrophil count of 0.2×103/μL. Macrocytic anaemia, with a haemoglobin count of 5.2g/dL, haematocrit count of 17% and mean corpuscular volume of 109 fL was also present. Reticulocyte count was 21.6×109/L (0.9% of erythrocytes). Platelet count was 239×109/L. Haemoglobin A1C was 5.0%. Electrolytes, lactate dehydrogenase (LDH), iron, ferritin and total iron binding capacity were all normal. Vitamin B12 was 238 pg/mL, folate was 7.2 ng/mL and methylmalonic acid was 236 nmol/L, which were all within normal range. HIV and syphilis serologies were negative. Serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) were unremarkable. Antinuclear antibody, SS-A and SS-B antibodies, antigliadin antibody, SCL70, Jo1, dsDNA and antiphospholipid antibodies were negative.
The initial concern was that the patient had a paraneoplastic syndrome and bone marrow involvement. A CT of the chest with intravenous contrast was unremarkable for nodules in the lung or lymphadenopathy. An esophagogastroduodenoscopy was performed showing mild antral gastritis and a moderate-sized hiatal hernia. Testing for Helicobacter pylori was negative. CT of the abdomen and pelvis was later performed on her as an outpatient which did not show any abnormalities to suggest neoplastic disease. At this time, neurology was consulted. A focused neurological examination revealed decreased vibratory sensation in the lower extremities with no deficits to light touch, pinprick or temperature sensation. Tinel’s and Phalen’s signs were negative. She also had a positive Romberg sign, gait ataxia (falling after taking a single step), dysmetria seen with finger-to-nose and heel-to-shin testing, dysdiadochokinesia seen with testing of rapid alternating hand movements, increased patellar reflexes, depressed Achilles reflexes and positive bilateral Babinski sign. Her mental status, cranial nerves and motor strength were otherwise intact.
CT of the head without intravenous contrast revealed no acute intracranial abnormality. MRI of the brain, with and without intavenous contrast, demonstrated no acute intracranial abnormality or abnormal restricted diffusion, abnormal mass or mass effect. MRI of the cervical spine, with and without intravenous contrast, was obtained and initially interpreted as normal with no abnormal enhancements. It was only in retrospect, months after a definitive diagnosis was made, that T2-weighted intensity of the dorsal cervical spinal cord (figure 1) was identified.
Figure 1.
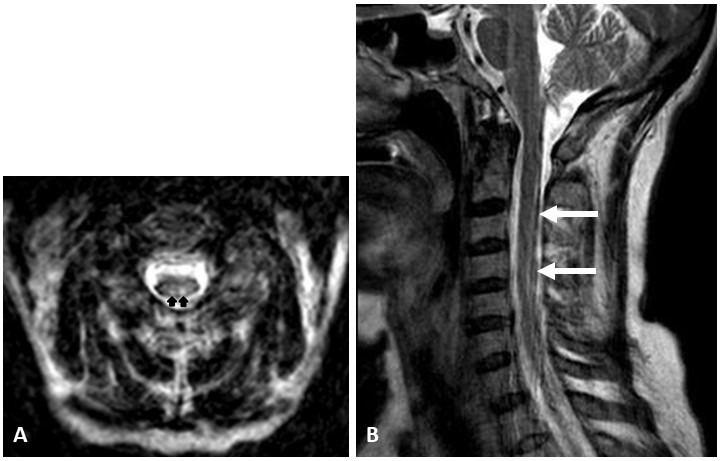
Axial (A) and sagittal (B) T2-weighted MRI of the cervical cord demonstrates increased signal in the dorsal columns (arrows).
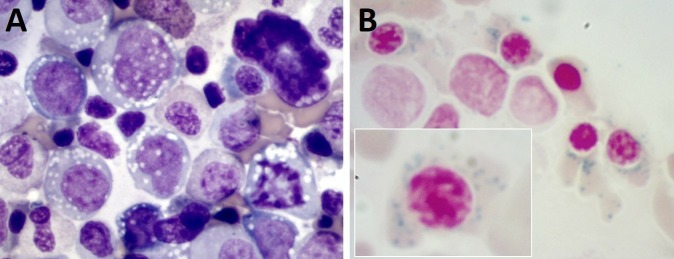
A bone marrow biopsy was performed to assess neutropenia and anaemia, revealing cytoplasmic vacuolisation of erythroid and myeloid precursors and ringed sideroblasts (figure 2). These findings prompted testing serum copper level which was <5 μg/dL (undetectable). Serum zinc level was slightly elevated at 121 μg/dL.
Figure 2.
(A) Bone marrow aspirate smear shows cytoplasmic vacuolisation in all cell lineages (H&E, 40x). (B) Iron staining of bone marrow aspirate shows numerous ringed sideroblasts (40x, inset 100x).
Further history revealed that the patient had been using excessive Fixodent, a denture adhesive cream, daily for over 20 years. Our patient was advised to stop using zinc-containing denture creams. Treatment involved oral copper repletion with elemental copper 8 mg daily for 1 week, 6 mg daily for 1 week, 4 mg daily for 1 week and then 2 mg daily until serum copper levels normalised. After 16 weeks of copper supplementation, serum analysis showed complete resolution of cytopenia with normal serum copper. Her neurological deficits stabilised but persisted.
Differential diagnosis
With a combination of myeloneuropathy and cytopenia, studies should be undertaken to evaluate a differential diagnosis that includes deficiencies in copper, vitamin B12 or folate, certain lymphoproliferative disorders, paraneoplastic syndromes associated with malignancy and HIV infection.
Copper, vitamin B12 and folate deficiencies can be readily assessed via serum analyses. On initial serum testing, vitamin B12 was 238 pg/mL. While this value was near the lower limit, subsequent testing of methylmalonic acid was normal at 236 nmol/L. A normal (and not elevated) level of methylmalonic acid effectively ruled out vitamin B12 deficiency. A normal folate level of 7.2 ng/mL ruled out folate deficiency. Vitamin E level tests were advised as an outpatient, but the patient did not have this serum analysis done. Unfortunately, copper deficiency was not initially considered, so serum copper level was not analysed.
However, copper deficiency was suspected from the bone marrow findings. These included cytoplasmic vacuolisation in multiple cell lines and ringed sideroblasts. Ringed sideroblasts are seen in a variety of conditions including myelodysplastic syndromes (MDS), vitamin B12 or folate deficiencies, copper deficiency, lead toxicity and Wilson disease. Congenital and drug-induced causes exist as well. However, the presence of vacuoles in the cytoplasm of multiple cell lines (both erythroid and myeloid precursor cells) is characteristically seen in copper deficiency, which prompted confirmation with a serum analysis of the copper level. Serum copper was in fact confirmed to be undetectable at a level of <5 μg/dL. Serum zinc levels were subsequently requested and found to be elevated at 121 μg/dL. Zinc overload has been implicated in copper deficiency. On retrospective review, our patient had been excessively using a zinc-containing brand of denture adhesive cream.
Additionally, given our patient’s symptoms of cerebellar ataxia (dysmetria and dysdiadochokinesia), there was a concern for cerebellar lesions. However, MRI of the brain and cervical spine did not demonstrate a cerebellar lesion or abnormal enhancement to suggest a stroke or multiple sclerosis. Vitamin E deficiency was also considered. Testing for vitamin E deficiency was advised during an outpatient neurology follow-up appointment. However, the patient did not go to have this test completed.
Lymphoproliferative disorders such as lymphoma, leukaemia and autoimmune lymphoproliferative syndrome are typically characterised by lymphadenopathy and splenomegaly. While the bone marrow biopsy demonstrated cytoplasmic vacuolisation, suggestive of lymphoma, our patient did not have lymphadenopathy or splenomegaly on physical examination. Serum LDH was also within normal limits. SPEP and UPEP were unremarkable. Given these results, the diagnosis of a lymphoproliferative disorder was less likely.
MDS can have variable presentations but will almost always demonstrate a macrocytic or normocytic anaemia. Leucopenia is seen in about half of MDS cases while varying degrees of thrombocytopenia are seen in a quarter of MDS cases. In our patient, macrocytic anaemia and neutropenia may suggest MDS, and a bone marrow biopsy would be warranted to further evaluate for MDS. However, the additional symptoms of lower extremity neuropathy, gait abnormalities and abnormal neurological examination cannot be ignored in the constellation of symptoms. Given the combination of neurological and haematological findings, nutrient deficiency should be studied. Serum studies to evaluate for these deficiencies should be obtained prior to unnecessary invasive procedures.
Outcome and follow-up
Our patient discarded all Fixodent denture adhesive creams and changed to Polygrip denture adhesive cream which is a zinc-free brand. She completed oral copper repletion and has had normal serial serum copper levels and complete blood count. Follow-up serum studies 7 months later demonstrated normal copper (124 μg/dL), folate (8.0 ng/mL), vitamin B12 (443 pg/mL) and iron (52 μg/dL) levels. At this time, outpatient nerve conduction studies demonstrated low amplitude sural sensory response (distal 3.3 uμV (normal >6)). Nerve conduction of the median motor and median sensory, tibial motor and peroneal motor nerves was normal. Nerve conduction studies were not performed when the patient was first diagnosed with hypocupremia in the hospital.
Despite a normal serum copper level, she continues to have difficulty ambulating and is unable to operate motor vehicles. Her cerebellar ataxia (dysmetria and dysdiadochokinesia) has significantly improved with copper repletion, while gait ataxia has moderately improved. She requires a cane for support while ambulating. She continues to have painful neuropathy and spasticity of the lower extremities, requiring pregabalin 300 mg three times per day, duloxetine 60 mg daily and tizanidine 4 mg two times per day.
Discussion
Copper is an essential micronutrient. It is a cofactor of mitochondrial cytochrome c oxidase which allows for proper metabolic processes such as haemoglobin synthesis, neurotransmission, cellular respiration, formation of connective tissue and antioxidant defence to occur. The normal human enterocyte takes in dietary copper via the copper transporter Ctr1 with the stomach and proximal duodenum as the sites of greatest absorption.5 Once in the enterocyte, copper is transported into the blood via the Cu-ATPase, ATP7A, located on the basolateral membrane.6 7 Majority of copper is stored in hepatocytes and is released into the blood stream as needed to maintain homeostasis in the body. Copper is bound to copper-dependent enzymes such as cytochrome c oxidase, monoamine oxidases, lysyl oxidase, dopamine B-hydroxylase and ceruloplasmin.8 If these copper-dependent enzymes lack copper, as seen in a copper-deficient state, haematological or neurological deficits occur.
The recommended dietary allowance (RDA) of copper for adult men and women is 900 μg/day. The RDA of copper for pregnant and lactating women is 1000 μg/day and 1300 μg/day, respectively. The median intake of dietary copper in the USA is approximately 1.0–1.1 mg/day for women and 1.2–1.6 mg/day for men. Copper is widely available in foods. Common dietary sources of copper in the USA include organ meats, nuts, seeds, seafood, whole grains, wheat bran cereals and cocoa products.9
The effect of zinc overload on copper absorption in adults has been well described. Zinc absorption primarily occurs in the jejunum by metallothionein which is then transported to the liver, much like copper absorption.10 Excessive zinc intake causes an upregulation of metallothionein in enterocytes. Because metallothionein has a higher binding affinity for copper than for zinc, copper remains bound to metallothionein and is retained in the enterocytes, thus reducing its absorption into the plasma.11 While this response is used as therapy in patients with Wilson disease, this competitive inhibition of copper absorption can be seen in excessive zinc supplementation and use of zinc-containing denture adhesive creams.3 4
Adults using dentures in the USA make up 13% of the total population, with an estimate of 8.6 million edentulous adults in 2050.12 Reports of excessive use of zinc-containing denture adhesive cream causing hypocupremia have been documented. Nations et al found that zinc concentrations range from 17 000 to 34 000 μg/g in Fixodent and Polygrip denture creams.4 The RDA of zinc intake is 8 mg/day for women and 11 mg/day for men. The tolerable upper intake level of zinc for adults is 40 mg/day, which is the highest level of daily zinc intake that is likely to pose no risk of adverse health effects.13 The average user of Fixodent denture cream absorbs 2 mg of zinc per day with typical use, which should not pose any health risks. However, excessive and improper use of denture cream can cause zinc toxicity.
Copper deficiency has also been described in patients after Roux-en-Y gastric bypass surgery with incidence ranging from 12%–30% in the USA.14–17 Nutritional deficiencies are common following gastric bypass surgery as a large portion of the stomach and duodenum are bypassed. Copper deficiency has also been described in malabsorption syndromes such as celiac disease, tropical sprue and inflammatory bowel disease.2 18 As in other nutritional deficiencies, copper absorption is impaired due to mucosal tissue damage from autoimmune or inflammatory processes of the intestines.
Hypocupremia causes a macrocytic anaemia as well as neutropenia. The pathogenesis of anaemia in hypocupremia is multifactorial. Copper and iron interact through ceruloplasmin so that ferrous iron is oxidised and can bind to transferrin for transport. Without copper, this process is interrupted. Furthermore, alterations in copper-dependent cytochrome c oxidase impair mitochondrial heme biosynthesis. Neutropenia likely occurs due to reduced survival of peripherally circulating mature granulocytes and impaired granulocyte maturation in the bone marrow. The haematological effects manifest prior to neurological abnormalities and rapidly correct once copper is repleted, as was the case with our patient.19 Normalisation of bone marrow dysplasia has been seen at 8 months following copper repletion.19
Copper deficiency, associated with myelopathy, was first reported in literature in 2001.20 As seen with vitamin B12 deficiency, copper deficiency resembles subacute, combined, degeneration with a spastic ataxic gait and dorsal column deficits. Neuroimaging with MRI of the spinal cord shows increased T2 signal in the paramedian dorsal spine cord. Brain MRI findings tend to be non-specific. Electromyography and nerve conduction studies can demonstrate varying degrees of axonal neuropathy. Reports include myopathic potentials, impairment in central conduction and neuronopathy.21 Nerve biopsy can show evidence of axonal degeneration while muscle biopsy reveals vacuolar changes.21 Despite adequate copper repletion, the degree of neurological improvement is variable and residual neurological deficits persist. Copper repletion does, however, prevent further neurological deterioration.
Cerebellar ataxia has rarely been reported in literature in association with copper deficiency. Inaba et al reported a case of peripheral neuropathy, myelopathy and cerebellar ataxia in a patient with copper deficiency.22 Interestingly, another case report demonstrated cerebellar atrophy in a patient with copper deficiency.23 The patient underwent extensive ataxia workup to rule out other causes; it was concluded that hypocupremia had a possible association with cerebellar atrophy.
Hypocupremia is easily diagnosed with a serum copper level test. However, if the copper deficiency is mild, serum ceruloplasmin level is more reliable than serum copper level. Serum zinc level test is indicated if the copper level is low. Further tests that may be obtained include urine copper level to examine for excess excretion. Flow cytometric analysis may show hematogone hyperplasia in the bone marrow. As seen in our patient, serum copper analysis was sufficient to reach the diagnosis. Evaluating a simple serum level can potentially save a patient from undergoing unnecessary procedures and progression of the disease.
Patient’s perspective.
I have a lot of doctor appointments now. I have to wear these compression stockings to work. My balance still isn’t what it should be and it’s hard to walk. I am on my feet all day at work. I take a lot of medicines and it’s ridiculous.
At first, I was really mad. I wasn’t mad at the doctors. I was mad at the people who created the situation. Then I found out that I wasn’t the only case. I found out that some people were paralysed from this and that some people have died from this. A lawyer wouldn’t touch this case. I was very mad. I had tried to hire a lawyer to file lawsuit, but no one would take this case. I eventually came out on the other side of this and I’m okay with it now… it could be worse. I was one of the lucky ones.
I was mad at Fixodent. Now today whenever I’m at the grocery store and I see somebody pick up a tube of Fixodent, I would tell them my story. There ’s no reason for them to put zinc in their product when Polygrip doesn’t use it in their product, and it works just fine. I’m using Polygrip now. I have not used Fixodent ever since I was told about the zinc.
It’s hard to walk sometimes. When I’m not at work, I’m exhausted. I still have to use the cane when I’m walking longer distances because I wobble. I think my medical care since the diagnosis has been awesome. I feel much better since my diagnosis in the hospital. I had no idea my symptoms were from the denture cream. I remember it started a month after I moved in with my daughter. I could barely walk, I had no energy, I was sleeping most of the day, I had lost a lot of weight.
I am still seeing the neurologist. He has done nerve conduction tests and a biopsy of my nerves in the legs which did confirm neuropathy. Right now I’m managing the neuropathy with lyrica and cymbalta. I used to be on maximum doses of gabapentin but then we switched to lyrica and cymbalta because they work better. I still occasionally see the haematologist. My blood levels are entirely normal but she still likes to see me occasionally about every 6 months just to make sure.
I don’t understand why this happened to me. It’s frustrating. I got dentures in my 20 s and had been using Fixodent for over 20 years. I had to get dentures in my 20s. I was told by the doctor that because I had back-to-back pregnancies, it took all the calcium out of my teeth and several teeth had to come out. I didn’t have any dental care so all my teeth had to come out since they all rotted. I got my dentures at age 27 and this happened to me at 47, so yeah it’s been exactly 20 years. I used about a tube or two of denture cream a week and I think after 20 years of using it, it was finally too much.
Learning points.
When myeloneuropathy and cytopenia present in combination, the differential diagnosis should include nutritional deficiencies, lymphoproliferative disorders, paraneoplastic syndromes associated with malignancy and HIV infection.
Consider deficiencies in copper along with vitamin B12, B6 and folate when presented with both cytopenia and myeloneuropathy.
Include the use of zinc-containing products and zinc overload as a cause of copper deficiency.
Initiate prompt copper repletion to preserve neurological function.
Footnotes
Contributors: CLR, SA and LL contributed substantially to the design of the work, analysis of data, drafting of the work and providing intellectual content. All authors have approved the final manuscript and agree to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kumar N. Copper deficiency myelopathy (human swayback). Mayo Clin Proc 2006;81:1371–84. 10.4065/81.10.1371 [DOI] [PubMed] [Google Scholar]
- 2. Halfdanarson TR, Kumar N, Hogan WJ, et al. . Copper deficiency in celiac disease. J Clin Gastroenterol 2009;43:162–4. [Internet] 10.1097/MCG.0b013e3181354294 [DOI] [PubMed] [Google Scholar]
- 3. Hedera P, Peltier A, Fink JK, et al. . Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin II. The denture cream is a primary source of excessive zinc. Neurotoxicology 2009;30:996–9. 10.1016/j.neuro.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 4. Nations SP, Boyer PJ, Love LA, et al. . Denture cream: an unusual source of excess zinc, leading to hypocupremia and neurologic disease. Neurology 2008;71:639–43. [Internet] 10.1212/01.wnl.0000312375.79881.94 [DOI] [PubMed] [Google Scholar]
- 5. Myint ZW, Oo TH, Thein KZ, et al. . Copper deficiency anemia: review article. Ann Hematol 2018;97:1527–34. 10.1007/s00277-018-3407-5 [DOI] [PubMed] [Google Scholar]
- 6. Monty J-F, Llanos RM, Mercer JFB, et al. . Copper exposure induces trafficking of the Menkes protein in intestinal epithelium of ATP7A transgenic mice. J Nutr 2005;135:2762–6. [Internet] 10.1093/jn/135.12.2762 [DOI] [PubMed] [Google Scholar]
- 7. Ravia JJ, Stephen RM, Ghishan FK, et al. . Menkes copper ATPase (ATP7A) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem 2005;280:36221–7. 10.1074/jbc.M506727200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turnland JR. Modern nutrition in health and disease : Copper. Philadelphia: Lippincott;, 2000.. [Google Scholar]
- 9. National Academy of Sciences Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc : Copper. 1st ed Washington DC: National Academies Press, 2002: 224–57. [PubMed] [Google Scholar]
- 10. Weigand E. Absorption of trace elements: zinc. Int J Vitam Nutr Res Suppl 1983;25:67–81. [PubMed] [Google Scholar]
- 11. Fosmire GJ. Zinc toxicity. Am J Clin Nutr 1990;51:225–7. 10.1093/ajcn/51.2.225 [DOI] [PubMed] [Google Scholar]
- 12. Rozier RG, White A, Slade G. Trends in oral diseases in the U.S. population. J Dent Educ 2017;81:e97–109. 10.21815/JDE.017.016 [DOI] [PubMed] [Google Scholar]
- 13. National Academy of Sciences. Zinc : Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. 1st. Washington DC: National Academies Press, 2002: 442–501. [PubMed] [Google Scholar]
- 14. King D, Siau K, Senthil L, et al. . Copper deficiency myelopathy after upper gastrointestinal surgery. Nutr Clin Pract 2018;33:515–9. 10.1177/0884533617713955 [DOI] [PubMed] [Google Scholar]
- 15. Sonu RJ, Rashidi HH. Concurrent copper and iron deficiency in a gastric bypass patient: a great mimicker of MDS. Blood 2015;125:2582 [Internet] 10.1182/blood-2015-02-627117 [DOI] [PubMed] [Google Scholar]
- 16. Griffith DP, Liff DA, Ziegler TR, et al. . Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obesity 2009;17:827–31. 10.1038/oby.2008.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gletsu-Miller N, Broderius M, Frediani JK, et al. . Incidence and prevalence of copper deficiency following Roux-en-Y gastric bypass surgery. Int J Obes 2012;36:328–35. 10.1038/ijo.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sternlieb I, Janowitz HD. Absorption of copper in malabsorption syndromes. J Clin Invest 1964;43:1049–55. 10.1172/JCI104988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huff JD, Keung Y-K, Thakuri M, et al. . Copper deficiency causes reversible myelodysplasia. Am J Hematol 2007;82:625–30. [Internet] 10.1002/ajh.20864 [DOI] [PubMed] [Google Scholar]
- 20. Schleper B, Stuerenburg HJ. Copper deficiency-associated myelopathy in a 46-year-old woman. J Neurol 2001;248:705–6. [Internet] 10.1007/s004150170118 [DOI] [PubMed] [Google Scholar]
- 21. Kumar N, Gross JB, Ahlskog JE. Copper deficiency myelopathy produces a clinical picture like subacute combined degeneration. Neurology 2004;63:33–9. 10.1212/01.WNL.0000132644.52613.FA [DOI] [PubMed] [Google Scholar]
- 22. Inaba M, Torii T, Shinoda K, et al. . [Peripheral neuropathy, myelopathy, cerebellar ataxia, and subclinical optic neuropathy associated with copper deficiency occurring 23 years after total gastrectomy]. Rinsho Shinkeigaku 2011;51:412–6. Japanese 10.5692/clinicalneurol.51.412 [DOI] [PubMed] [Google Scholar]
- 23. Mittal SO, Machado DG. Hypocupremia: a possible association with late cortical cerebellar atrophy. Tremor Other Hyperkinet Mov 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]