Abstract
Sphingomonas paucimobilis is a low-virulence gram-negative bacillus known to cause various ocular infections such as endophthalmitis, panophthalmitis and keratitis that are usually associated with an underlying risk factor such as peri-partum or postpartum phase, cataract surgery, contact lens use, neurotrophic keratopathy or ocular trauma. We report a case of spontaneously occurring perforated corneal ulcer caused by the organism in a young man managed by penetrating keratoplasty. The course was followed by endophthalmitis with graft infection culminating in phthisis bulbi despite aggressive medical and surgical management. Along with reporting this case, we also present a review of literature on ocular infections caused by the same organism.
Keywords: ophthalmology, anterior chamber, Iris
Background
Microbial keratitis is a potentially vision threatening condition that occurs due to invasion of cornea by infective micro-organisms such as bacteria, fungi and viruses. Bacterial corneal ulcers can be caused by both gram-positive and gram-negative organisms. Gram-negative organisms are usually associated with more severe and rapidly progressive corneal ulcers compared with gram-positive organisms due to their ability to produce lytic enzymes.1
Sphingomonas paucimobilis, previously known as Pseudomonas paucimoblis, is a type of gram-negative aerobic bacillus known to cause various systemic and ocular infections.2–17 It is usually associated with endophthalmitis and panophthalmitis and can also cause microbial keratitis. S. paucimobilis is considered a low-virulence organism due to the lack of lipopolysaccharide A in its cell wall. Only one case of microbial keratitis caused by this organism has been reported so far from India.17 Here, we report a case of fulminant keratitis in a young healthy male caused by S. paucimobilis that culminated into phthisis bulbi despite aggressive medical and surgical management. As there is lack of comprehensive literature available on ocular infections caused by the same organism, we also review the current literature on this topic.
Case presentation
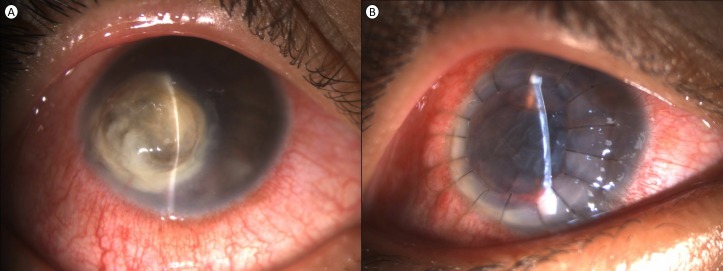
A 17-year-old man presented to our centre with complaints of spontaneous onset pain, redness, watering, photophobia and progressive diminution of vision oculus dextrus (OD) for 14 days. He was diagnosed as microbial keratitis elsewhere, was started on fortified antibiotics (cefazolin 5% and tobramycin 1.3%) and was referred to us for further management in view of lack of response to medical management. The patient was a systemically healthy male with no history of ocular trauma, hospital admissions, contact lens use, tap water exposure, previous ocular surgery, use of steroids (topical or systemic) or similar episodes in the past. He belonged to lower socioeconomic status and was studying in first year of graduation. Visual acuity was reduced to perception of light and inaccurate projection of rays OD and 20/20 oculus sinister (OS). Ocular examination revealed an inferonasal perforated corneal ulcer with yellowish white 7×7 mm well-defined full-thickness infiltrate, flat anterior chamber, barely visible anterior segment and posterior segment details and a grossly anechoic B-scan ultrasonography OD and normal examination OS (figure 1A).
Figure 1.
Slit lamp examination of a patient at presentation. (A) Perforated corneal ulcer with yellow infiltrate. (B) Well-buried sutures and edematous graft as seen on day 1 post-therapeutic keratoplasty.
Investigations
A clinical diagnosis of perforated corneal ulcer was made, and corneal scraping samples were sent for microbiological examination as different types of microbes such as fungi and gram-positive organisms can present in a similar way. Gram’s stain showed polymorphonuclear cells and gram-negative bacilli. KOH (potassium hydroxide) mount did not reveal any organism. Cultures were sent in blood agar, chocolate agar, Robertson-cooked meat medium, Saboraud’s dextrose agar, Lowenstein Jensen medium and non-nutrient agar with Escherichia coli overlay for aerobic and anaerobic bacteria, fungi, Mycobacterium tuberculosis, Nocardia and acanthamoeba. Herpes simplex virus—polymerase chain reaction was also performed on the sample.
Treatment
Therapeutic penetrating keratoplasty was performed immediately without waiting for results of culture sensitivity in lieu of perforated corneal ulcer and distorted AC anatomy (figure 1B). During the surgery, dense iridoocorneal adhesions were dissected gently to reveal an underlying total lens abscess without associated vitreous exudates. Corneal infiltrate was debulked, lens tissue was removed and limited anterior vitrectomy was performed. The patient was left aphakic and the donor cornea was sutured to the host corneal tissue with 16 ‘10–0’ monofilament nylon sutures. Trephined host corneal tissue and samples obtained from lens abscess were sent for microbiological examination mentioned previously. At the end of the surgery, cefazolin and voriconazole were injected in the unicameral eye.
Outcome and follow-up
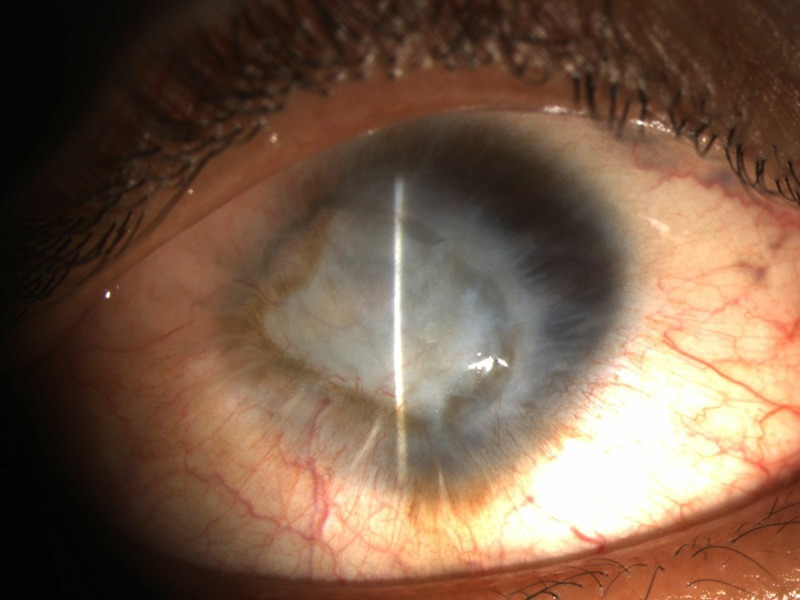
Patient was continued on topical fortified antibiotics (cefazolin 5% and tobramycin 1.3%), and 2 days after surgery, endophthalmitis was noted. The patient was not given any steroids after surgery. However, after overnight incubation in blood agar, an oxidase-positive gram-negative aerobic bacillus with yellow-pigmented non-hemolytic colonies was isolated from host corneal and lens samples sent during surgery. The organism was found negative for citrate production. Pseudomonas and S. parapaucimobilis were differentiated from the grown organism, S. paucimobilis in our case, by its non-hemolytic and non-citrate producing nature, respectively. The organism was found sensitive to ceftazidime, cefoperazone/sulbactam, amikacin, gentamicin, tigecycline and trimethoprim/sulfamethoxazole and resistant to tobramycin, cefazolin, piperacilin/tazobactam, ceftriaxone, meropenem and ciprofloxacin. The donor corneoscleral rim culture was found sterile. Immediate pars plana vitrectomy and injection of intravitreal 2.25 mg/0.1 mL ceftazidime and 200 μg/0.1 mL gentamycin sulfate were performed, and the patient was started on hourly fortified gentamycin 14 mg/mL and oral trimethoprim/sulfamethoxazole 80 mg/400 mg two times per day based on culture sensitivity results. Undiluted vitreous samples sent for microbiological culture grew the same organism and were negative for any other organisms. Due to non-response to the above regime, repeat pars plana vitrectomy along with injection of intravitreal ceftazidime and gentamycin was undertaken after 48 hours. The patient developed graft infection after 48 hours for which graft exchange was done along with repeat pars plana vitrectomy and intravitreal injection of ceftazidime and gentamycin. The endophthalmitis and the graft infection did not resolve despite aggressive management and the visual acuity deteriorated to no perception of light. The visually evoked responses from the affected eye became extinguished and the patient refused any further surgical intervention in view of nil visual prognosis. The eyeball started shrinking in size at 1 week follow-up (figure 2). The culture media were reviewed till 6 weeks after inoculation to rule out the presence of other causative organisms.
Figure 2.
Shrunken eyeball and opacified cornea secondary to endophthalmitis and graft infection.
Discussion
S. paucimobilis (formerly known as P. paucimobilis), a gram-negative bacillus of low virulence, is an opportunistic pathogen known to cause infections in both healthy and immunocompromised individuals.2 It is also found as a commensal in the conjunctival sac and rarely isolated from clinical specimens.3 The organism has been grown from mobile phones of hospital inpatients and hospital water distribution systems.4 5 Various risk factors for acquiring systemic infection by the organism include community-acquired infection, diabetes mellitus and alcoholism. However, the source of organism for microbial keratitis in our case remains uncertain. Until, reported ocular infections caused by the organism including panophthalmitis, endophthalmitis and keratitis were seen either in peripartum phase or occurred after cataract surgery, penetrating ocular trauma or contact lens use (table 1).6–15 However, in our patient, microbial keratitis was spontaneous in onset and no such risk factor could be identified. Adams reported a case of recurrent acute endophthalmitis postcataract surgery caused by the same organism that responded well to vitrectomy.6 While Acharya et al reported a postcataract surgery indolent microbial keratitis caused by S. paucimobilis responsive to patch graft and fortified antibiotics, Roca reported a neurotrophic keratopathy-associated perforated corneal ulcer caused by the same organism that required penetrating keratoplasty. Ratnalingam reported a case of contact lens associated S. paucimobilis keratitis with perforation warranting tectonic keratoplasty. In their case, graft reinfection was noted 1 week later which resolved with medical management.10 Alharbi described endophthalmitis post keratoplasty caused by the same organism responsive to gentamycin and ceftazidime.18 However, S. paucimobilis associated spontaneously occurring perforated corneal ulcer associated with lens abscess followed by endophthalmitis and graft infection postkeratoplasty non-responsive to aggressive management, to the best of our knowledge, is being reported for the first time.
Table 1.
List of ocular infections caused bySphingomonas paucimobilis
| Author, year | Age, sex | Risk factor | Presentation | Time of presentation | Outcome | Antibiotics used |
| Adams, 2006 | 73 years, female | Post cataract surgery | Recurrent Acute Endophthalmitis | Less than 24hours after clear corneal phacoemulsification | Complete resolution after vitrectomy | Susceptible to gentamycin and ciprofloxacin and resistant to vancomycin and ceftazidime, coinfection withRothia dentocariosa |
| Seo, 2008 | 62 years, male | Post cataract extraction | Acute onset delayed endophthalmitis | 3-months post-operative | Visual acuity 20/300 | Susceptible to ampicillin, aztreonam, cefoperazone, ceftazidime, gentamycin, imipenem and piperacillin, resistant to tobramycin |
| Rahman, 2011 | 26 years, female | Peri-partum | Endogenous endophthalmitis | -- | Visual acuity 6/9 | Responsive to Intravitreal amikacin and vancomycin |
| Kriet, 2011 | 39 years, female | Post-partum | Endogenous Panophthalmitis | -- | Purulent corneal melt, Evisceration | Intravitreal ceftazidime and topical gentamycin, ciprofloxacin and rifamycin |
| Ratnalingam, 2013 | 41 years, female | Contact lens related | Infectious keratitis | -- | Re-infection after tectonic keratoplasty, counting fingers at 2 months, failed graft | Sensitive to ciprofloxacin, gentamycin and augmentin |
| Droutsas, 2015 | 30 years, male | Post retained metallic foreign body in vitreous cavity following penetrating trauma | Endophthalmitis | -- | 6/9 at 6 months | Sensitive to aminoglycosides, tetracycline, chloramphenicol and ceftazidime |
| Esen/ Ozcan, 2015 | 31 years, female | Post-partum | Endogenous panophthalmitis | -- | Evisceration | Sensitive to ceftazidime and gentamycin, moxifloxacin and amoxicillin-clavulanic acid |
| Huang, 2015 | -- | Post cataract surgery | Acute onset endophthalmitis | -- | No evisceration | -- |
| Kelkar, 2016 | -- | Post clear corneal phacoemulsification | Endophthalmitis | -- | Visual acuity 20/200 at final follow up visit | Response to vancomycin, ceftazidime and prednisolone f/b vitrectomy |
| Roca, 2018 | 59 years, male | Neurotrophic keratopathy | Perforated corneal ulcer | -- | Corneal graft no complications at 1 year follow up | -- |
| Acharya, 2019 | 46 years, female | Post cataract surgery | Microbial keratitis | 6 weeks | Patch graft, visual acuity 20/40 at final follow up | Sensitive to amikacin, cefazoline, ceftazidime, cephalexin, ciprofloxacin, gatifloxacin, moxifloxacin and tobramycin |
| Our case | 17 years, male | Spontaneous onset | Microbial keratitis | -- | Phthisis bulbi | Sensitive to ceftazidime, cefoperazone/sulbactam, amikacin, gentamicin, tigecycline and trimethoprim/ sulfamethoxazole; Resistant to tobramycin, cefazolin, piperacilin/tazobactam, ceftriaxone, meropenem and ciprofloxacin |
Endophthalmitis caused by S. paucimobilis may be either early or delayed onset after surgery. In our case, early onset endophthalmitis without its presence in the preoperative period could be explained by intraoperative lens rupture. It is possible that the organism gained entry into crystalline lens after corneal perforation and acted as a reservoir of organisms released into vitreous cavity during surgical manipulations. The same mechanism may also explain graft infection in our case. However, what remains unexplained is the constant unresponsiveness of the organism to aggressive medical and surgical management as in all the previously reported cases of ocular infections caused by S. paucimobilis, the infection responded well to commonly used antibacterial agents.4 6–17 Nevertheless, antibiotics resistant cases and cases requiring evisceration have also been reported by others.6 7 9 12 In our case, although in vitro susceptibilities showed sensitivity of organism to various antibiotics, this did not translate in vivo and this could be because the organism behaved differently in vivo than in vitro.
Although the organism is also known to contaminate corneal grafts transport medium, sterile donor rim disproved this mode of endophthalmitis and graft infection in our case.19 It is also suggested that the organism may act as a substrate for other organisms and devastating ocular infections could be due to a coexistent causative organism.6 20 21 However, presence of any such causative coexistent organism was ruled out in our case due to negative microbiological smear and culture specimens for other organisms. We believe that devastatingly severe infection in our case may be due to multiple factors such as delayed presentation, lack of high index of suspicion for this rare organism, inability to isolate it from scraping specimens in early stages due to its low virulence and involvement of lens by the organism.
To conclude, our case report highlights that severe microbial keratitis culminating into phthisis bulbi can be rarely caused by low-virulence organism such as S. paucimobilis and emphasises on maintaining high index of suspicion early in the course of the disease to preserve ocular anatomy and function.
Learning points.
Sphingomonas paucimobilis is a gram-negative bacillus of low virulence and can act as opportunistic pathogen to cause infections in both healthy and immunocompromised individuals.
The organism can cause ocular infections such as panophthalmitis, endophthalmitis and microbial keratitis. Spontaneously occurring perforated corneal ulcer associated with lens abscess followed by endophthalmitis and graft infection postkeratoplasty can also be caused by the organism.
It is possible that the organism can behave differently in vivo and may not adhere to in vitro susceptibility of antibiotics.
Although the organism has low virulence, non-responsiveness to aggressive surgical and medical management finally culminating in phthisis bulbi can also be caused by the organism.
Footnotes
Contributors: RA: manuscript writing; MG: data collection; AM: patient care; NS: manuscript editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Al-Mujaini A, Al-Kharusi N, Thakral A, et al. Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J 2009;9:184–95. [PMC free article] [PubMed] [Google Scholar]
- 2. Toh H-S, Tay H-T, Kuar W-K, et al. Risk factors associated with Sphingomonas paucimobilis infection. J Microbiol Immunol Infect 2011;44:289–95. 10.1016/j.jmii.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Liu Z-R, Chen H, et al. Comparative study of bacterial status from conjunctival sac of the elder Qiang minority and Han people with dry eye in Sichuan, China. Int J Ophthalmol 2012;5:343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinod Kumar B, Hobani YH, Abdulhaq A, et al. Prevalence of antibacterial resistant bacterial contaminants from mobile phones of hospital inpatients. Libyan J Med 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muchesa P, Leifels M, Jurzik L, et al. Coexistence of free-living amoebae and bacteria in selected South African hospital water distribution systems. Parasitol Res 2017;116:155–65. 10.1007/s00436-016-5271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adams WE, Habib M, Berrington A, et al. Postoperative endophthalmitis caused by Sphingomonas paucimobilis. J Cataract Refract Surg 2006;32:1238–40. 10.1016/j.jcrs.2006.01.094 [DOI] [PubMed] [Google Scholar]
- 7. Seo SW, Chung IY, Kim E, et al. A case of postoperative Sphingomonas paucimobilis endophthalmitis after cataract extraction. Korean J Ophthalmol 2008;22:63–5. 10.3341/kjo.2008.22.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahman W, Hanson R, Westcott M. A rare case of Peripartum endogenous bacterial endophthalmitis. Int Ophthalmol 2011;31:113–5. 10.1007/s10792-010-9399-3 [DOI] [PubMed] [Google Scholar]
- 9. Kriet MM, Bouya Y, Louaya S. [Endogenous postpartum panophthalmitis induced by sphingomonas paucimobili]. Bull Soc Belge Ophtalmol 2011:37–40. [PubMed] [Google Scholar]
- 10. Ratnalingam V, Thean PY, Retnasabapathy S, et al. Contact-lens related keratitis caused by an atypical organism. Asian J Ophthalmol 2013;13:73–5. [Google Scholar]
- 11. Posttraumatic Sphingomonas paucimobilis endophthalmitis, 2019. Available: https://www.hindawi.com12/journals/criopm/2015/192864/ [DOI] [PMC free article] [PubMed]
- 12. Esen E, Özcan A, Şimşek F, et al. Postpartum endogenous panophthalmitis caused by Sphingomonas paucimobilis. Cukurova Medical Journal 2015;40:29–32. [Google Scholar]
- 13. Huang Y, Zhan Y, Xie L. [Clinical observations of acute-onset endophthalmitis after clear corneal phacoemulsification]. Zhonghua Yan Ke Za Zhi 2015;51:918–23. [PubMed] [Google Scholar]
- 14. Kelkar AS, Kelkar JA, Barve PM, et al. Post-clear corneal phacoemulsification endophthalmitis: profile and management outcomes at a tertiary eye care center in Western India. J Ophthalmic Inflamm Infect 2016;6:48 10.1186/s12348-016-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duan F, Wu K, Liao J, et al. Causative microorganisms of infectious endophthalmitis: a 5-year retrospective study. J Ophthalmol 2016;2016:1–7. 10.1155/2016/6764192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roca M, García A, Penas.Pardo L, et al. Sphingomonas paucimobilis keratitis in a patient with neurotrophic keratopathy and severe neurosensory hypoacusis: treatment with penetrating keratoplasty and amniotic membrane grafting. Oman J Ophthalmol 2018;11:291–3. 10.4103/ojo.OJO_98_2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acharya M, Farooqui JH, Spencer H, et al. Sphingomonas paucimobilis keratitis post cataract surgery: first case report from India. Nep J Oph 2019;11:82–5. 10.3126/nepjoph.v11i1.25426 [DOI] [PubMed] [Google Scholar]
- 18. Alharbi SS, Alrajhi A, Alkahtani E. Endophthalmitis following keratoplasty: incidence, microbial profile, visual and structural outcomes. Ocul Immunol Inflamm 2014;22:218–23. 10.3109/09273948.2013.841956 [DOI] [PubMed] [Google Scholar]
- 19. Bourigault C, Daniel L, Jourdain S, et al. Contamination à Sphingomonas paucimobilis : à propos de sept cas isolés sur des milieux de conservation et de transport de greffons cornéens. Pathol Biol 2007;55:127–30. 10.1016/j.patbio.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 20. Bottone EJ, Madayag RM, Qureshi MN. Acanthamoeba keratitis: synergy between amebic and bacterial cocontaminants in contact lens care systems as a prelude to infection. J Clin Microbiol 1992;30:2447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Semantic Scholar Incidence and microbiological profile of mycotic keratitis in a tertiary care eye Hospital: a retrospective analysis. Available: https://www.semanticscholar.org/paper/Incidence-and-microbiological-profile-of-mycotic-in-Alkatan-Athmanathan/7b592da076af4dc2546ca51bc6b6fb54e1141935 [Accessed 24 Jun 2019]. [DOI] [PMC free article] [PubMed]