Abstract
Bartonella species are fastidious, Gram-negative aerobic rods and a well-recognised pathogen responsible for culture-negative endocarditis. The histopathological appearance of glomerulonephritis (GN) caused by Bartonella endocarditis may include a pauci-immune GN similar to that usually seen in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Herein, we present an unusual case report of Bartonella endocarditis masquerading as ANCA-positive vasculitis, with crescentic GN. A 66-year-old woman, who had undergone aortic valve replacement 2 years prior to admission, presented with confusion and loss of vision in her right nasal field. Following an extensive diagnostic evaluation, the main findings were right central retinal artery occlusion, ground-glass appearance on chest CT and ANCA-positive, anti PR-3 negative, rapidly progressive GN. The patient was scheduled to start treatment with rituximab for presumed ANCA-positive GN, when a positive serological test for Bartonella henselae was received. In view of this result, a diagnosis of endocarditis was made, based on fulfilment of five Duke minor criteria, namely fever, predisposition, arterial emboli, immunological phenomena and serological evidence of active infection with an organism consistent with infective endocarditis. Immunosuppressive treatment was withheld and antibiotic treatment initiated. This case report emphasises the need for maintaining a high index of suspicion regarding the diagnosis of Bartonella infection, which might mimic ANCA-associated GN.
Keywords: valvar diseases, cardiovascular system, infections, renal system, Vasculitis
Background
The clinical overlap between chronic infectious disorders such as (culture-negative) endocarditis and immune-mediated systemic disease, for example, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, engenders one of the most perplexing and intriguing dilemmas faced by the clinician. This diagnostic dilemma becomes exquisitely acute in view of the diametrically opposed treatment modalities resulting from either diagnosis—that is, systemic immunosuppression that may exacerbate infections versus long-term antibiotics. We present the clinical course and deliberations associated with such a case.
Bartonella species are short, fastidious, pleomorphic, Gram-negative aerobic rods.1 Transmission of Bartonella species may be via arthropod vectors or direct inoculation, depending on which species is involved.
Humans usually become infected incidentally, either by haematophagous insects (eg, Bartonella quintana), or via bites or scratches by domestic animals (Bartonella henselae). There is a wide spectrum of clinical manifestations, ranging from a benign and self-limited disease to a potentially life-threatening course, which may be determined by the immune status of the infected human.2
The clinical manifestations of Bartonella infection are notoriously protean, ranging from the classical cat-scratch disease in immunocompetent patients, to bacillary angiomatosis in immunosuppressed ones—and everything in between, including a totally asymptomatic course.3 Bartonella is a well-recognised pathogen responsible for culture-negative endocarditis, and thus has been associated with the various systemic and immunological manifestations related to that condition. Herein, we present an unusual, fulminant case of Bartonella endocarditis masquerading as ANCA-positive vasculitis, with crescentic glomerulonephritis (GN). This unusual presentation of a relatively common infection emphasises the critical importance of maintaining a high index of suspicion regarding the diagnosis of Bartonella infection as well as calling attention to the treacherous clinical overlap between chronic systemic infection and immune-mediated connective tissue disorders.
Case presentation
A 66-year-old woman of Ashkenazi–Jewish origin presented with confusion, starting 2 days prior to admission. The patient reported loss of balance and recurrent falls, as well as a loss of vision in her right nasal field. The patient had a prior history of hypothyroidism, diabetes mellitus type 2, hyperlipidemia, hypertension, gout and ischaemic heart disease. She had undergone coronary artery bypass graft and aortic valve replacement to bioprosthesis 2 years prior to admission.
She denied fever, but reported fatigue and weight loss of 14 kg over the prior 2 months. She denied recent travel abroad and had no pets at home. On physical examination, vital signs showed blood pressure of 136/74 mm Hg, pulse of 83 bpm, oxygen saturation 96% on room air and temperature of 37.5°C. She was alert but mildly confused. Her neurological exam showed no meningeal signs, equal pupils’ size, normal eye movement, but right relative afferent pupillary defect. Examination of the skin revealed a purpuric rash on the lower limbs. The rest of the examination was normal. Initial laboratory data showed sodium of 125 mmol/L, creatinine of 2.74 mg/dL, blood urea nitrogen (BUN) of 35 mg/dL, C reactive protein (CRP) of 55 mg/L, lactate dehydrogenase (LDH) 480 U/L and albumin 28 g/L. Liver enzymes were normal except for an elevated level of gamma-glutamyl transferase (GGT) (132 U/L). A complete blood count showed microcytic anaemia, with haemoglobin of 68 g/L while 6 months earlier it was 12.5 g/dL, there were also mild leucopenia and thrombocytopenia.
Urinalysis showed a protein/creatinine ratio of 1862 mg/g and an estimated 24-hour urinary protein excretion of 1.9 g. Microscopic urine analysis demonstrated microhaematuria, and no casts were found. Urinary tract obstruction was ruled out by catheter insertion and ultrasound examination, which was normal. Brain CT showed no acute findings. Ophthalmological examination showed right central retinal artery occlusion.
Shortly after admission the patient deteriorated, with the abrupt onset of fever, worsening anaemia and leucopenia and Coombs positive haemolysis.
A peripheral blood smear showed anisocytosis, microcytosis, hypochromia, ovalocytes, but no schistocyte. A lumbar puncture was normal. The differential diagnosis at this point included both infectious conditions, mainly infective endocarditis, versus immune-mediated connective tissue disease (vasculitis) as well as immune-mediated haemolytic anaemia.
Thus, empirical treatment was commenced with hydration, blood transfusion, high-dose steroids (prednisone 1 mg/kg daily) and empirical antibiotic treatment with gentamicin and doxycycline. Transthoracic echo and transesophageal echo were performed, ruling out the presence of vegetations on the prosthetic valve. Normal function of the prosthetic aortic valve was demonstrated as well as moderate mitral regurgitation. Despite initial clinical improvement, 3 days after admission, the patient developed pulmonary oedema and respiratory failure, necessitating mechanical ventilation, from which she was subsequently successfully weaned. Further investigations included bone marrow biopsy, which was normal; total body CT, which demonstrated bilateral infiltrates and ground-glass appearance, possibly attributable to congestive heart failure as described above. Skin biopsy taken from her left shin showed purpura. Serological tests demonstrated a positive cryoglobulin test (1%).
ANA was positive at a high titer (1:320). Cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) was positive at a titer higher than 1:20 but ELISA testing for proteinase-3 and myeloperoxidase were negative. Perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) was negative. Rheumatoid factor (RF) was positive. β2-glycoprotein-1, anti-cardiolipin, anti–glomerular basement membrane (anti-GBM), anti-Sjögren's-syndrome-related antigen A (anti-Ro), anti-Sjögren's-syndrome-related antigen B (anti-LA), anti-Smith, anti-ribonucleoprotein (anti-RNP), anti-Jo1 and anti-Scl-70 were all negative. Serum electrophoresis and immunofixation showed faint oligoclonal profile, predominant of IgG, lambda and kappa light chains.
Repeated blood cultures returned sterile and serological testing for Q fever was negative, as were antibodies to Rickettsia conorii and Rickettsia typhi, cytomegalovirus (CMV), parvovirus B19, HIV, hepatitis C virus (HCV) and hepatitis B virus (HBV). A low titer venereal disease research laboratory (VDRL) was found together with a negative treponema pallidum hemagglutination assay (TPHA). Due to the ongoing renal failure, the findings of proteinuria and positive c-ANCA, a renal biopsy was performed.
Renal histological examination demonstrated focal proliferative and necrotising GN with crescents (figure 1).
Figure 1.
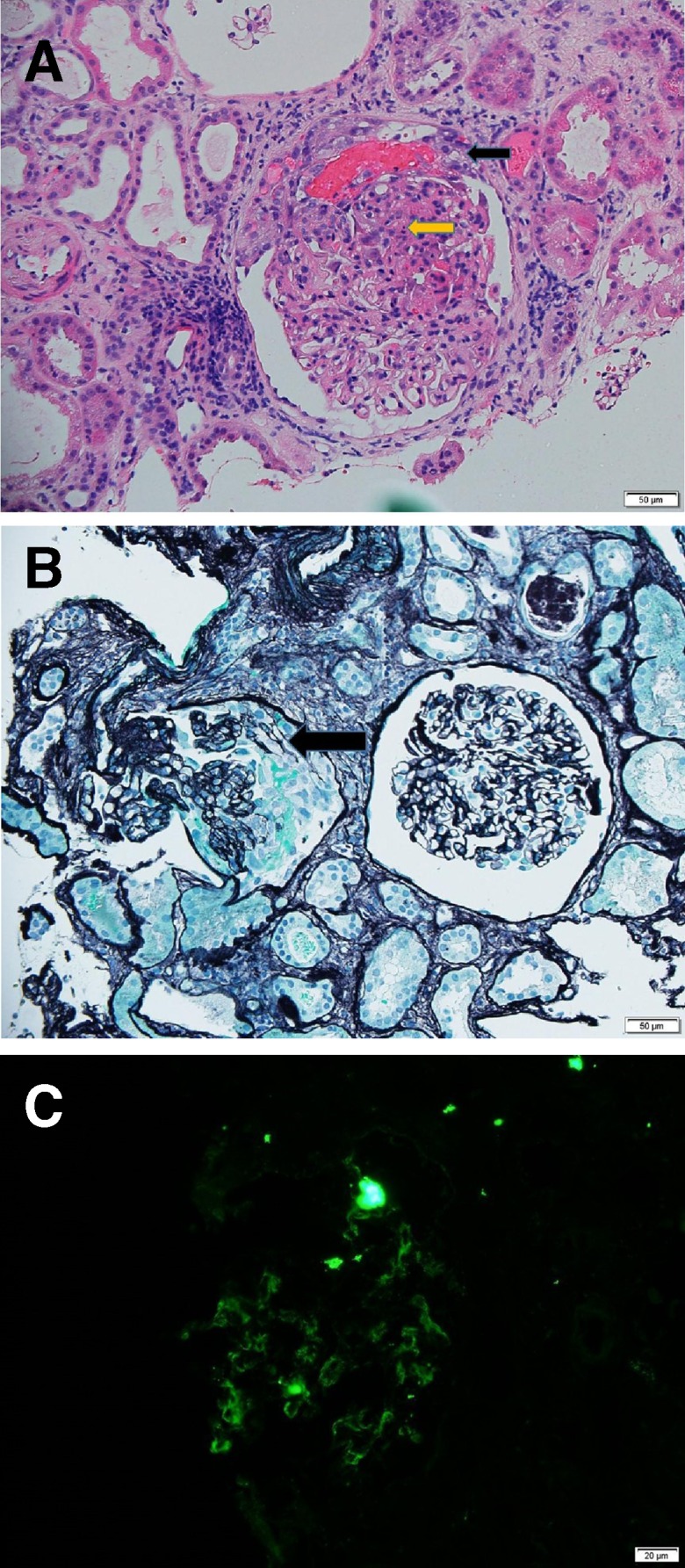
Crescentic GN. (A) Black arrow showing cellular crescent with a fibrin exudate, yellow arrow showing karyorexis (H&E 100×). (B) Black arrow showing cellular crescent (silver stain 100×). (C) direct immunofluorescence of C1q showing mesangial pattern. GN, glomerulonephritis.
Immunofluorescence was positive for C3 and C1q, IgG, IgM, lambda+ located in the mesangial areas.
Due to the accumulation of clues pointing towards the diagnosis of a systemic vasculitis at this juncture (GN, purpura, serological findings including cryoglobulins, neurological manifestations and retinal artery occlusion), together with negative blood and cerebral spinal fluid (CSF) cultures, as well as a TEE without perivalvular leak, abscess or vegetations, the clinical team considered after extensive consultations, to supplement steroid treatment with further immunosuppression. Rituximab treatment was chosen and was scheduled for administration. However, at this point in the course, a positive high-titer (1:1600) anti-B. henselae IgG antibodies, using enzyme immunoassay previously shown to be highly specific for Bartonella infection, was received.4
In view of this finding, indicating the high probability of a Bartonella infection, the diagnosis of culture-negative endocarditis re-emerged as the most likely explanation for the patient’s wide spectrum of symptoms and findings. The probability of Bartonella endocarditis, in particular B. henselae endocarditis, was further increased when the patient admitted taking care of stray cats and having close contact with them, particularly when feeding them on a regular basis. Thus, the combination of a pathogen known to be a leading cause of culture-negative endocarditis, together with a predisposition (prosthetic valve), history of extensive exposure to cats, with ocular embolic events, as well as immunological findings, all could be attributed to endocarditis. Notably, our patient fulfilled all five of the conditions necessary to meet the modified Duke minor criteria (fever, predisposition, arterial emboli, immunological phenomena and serological evidence of active infection with an organism consistent with infective endocarditis) and thus had a definite diagnosis of endocarditis, despite lack of evidence on TEE.
In this context, further immunosuppression appeared to be contraindicated, and a long-term antibiotic regiment was commenced, with gentamicin for 2 weeks and doxycycline for 4 months until anti-B. henselae IgG titer decreased to 1:400.
Further revision of the findings on renal biopsy subsequently were interpreted as being compatible with Löhlein GN—a finding classically associated with infectious endocarditis. PCR for Bartonella from kidney tissue was negative.
Outcome and follow-up
Seven months after her first admission, the patient was feeling well; her visual loss however was unchanged. Her lab tests, including complete blood count (CBC), creatinine and CRP were within normal range and serological tests for RF, VDRL and cryoglobulin were all negative. Proteinuria gradually decreased over follow-up.
Echocardiography showed no change.
Discussion
The reported incidence of culture-negative endocarditis varies, ranging from 2.1% to 35% of all infective endocarditis cases.5 It is currently estimated that culture-negative endocarditis accounts for around 5% of all endocarditis cases.6 Possible explanations to this wide range in incidence may include (1) geographic variation, (2) early use of antibiotics, (3) suboptimal specimen collection, (4) difficulty in growing the pathogen and (5) non-infectious causes, such as marantic endocarditis.
Bartonella endocarditis accounts for 3%–12% of all cases of endocarditis and is the most common cause of culture-negative endocarditis in the USA, with B. henselae and B. quintana being responsible for the majority of cases.5–7 Due to cross-antigenicity between B. henselae and B. quintana serology cannot distinguish between these two closely related pathogens and results are interpreted as Bartonella spp endocarditis unless PCR of infected valves provide a species-specific diagnosis. The common clinical manifestations in Bartonella endocarditis are similar to those seen with other causes of subacute bacterial endocarditis. Non-specific symptoms such as fever, fatigue and weight loss predominate the clinical picture. The most frequently affected valve is the aortic, either in isolation or with another valve. When Bartonella endocarditis involves a prosthetic valve, the pattern may take a more aggressive course, with valve-perforation and rapid development of heart failure. The most commonly observed laboratory abnormalities include elevated inflammatory markers such as erythrocyte sedimentation rate, anaemia, thrombocytopenia, elevated liver enzymes, renal failure, leucocytosis and a positive RF. Although causal association has not been proven, increased rates of seropositivity for B. henselae have been described in patients with leucocytoclastic vasculitis and Henoch-Schönlein purpura, as well as in a case of Coombs-positive autoimmune haemolytic anaemia.6 Kidney disease is a common manifestation of infectious endocarditis, 40%–50% of patients demonstrating parenchymal infarction, haematuria or—most commonly—GN. The histopathological appearance of GN caused by Bartonella endocarditis may include, besides the classical immune-complex-mediated GN, a pauci-immune GN similar to that usually seen in ANCA-associated vasculitis. ANCA positivity spuriously observed in the context of endocarditis, is expected to disappear with resolution of the infection, as was observed in the current case. Notably, the presence of additional autoantibodies such as RF, cryoglobulins and ANA has been reported to be more suggestive of an ANCA-positive bacterial endocarditis rather than a ‘true’ ANCA vasculitis.8 The focal proliferative pattern which we observed in the current case, appears to be characteristic of the GN encountered in cases of Bartonella endocarditis. A recent review of the literature identified 54 case with Bartonella endocarditis associated with both positive and negative ANCA GN.9 This focal pattern is compatible with what was historically described as ‘Löhlein nephritis’ and was originally considered to represent an embolic pathogenesis: ‘In bacterial endocarditis, there occurs the well-recognized focal embolic nephritis (Löhlein), where some of the lesions are due to actual emboli of thrombus lodging in glomerular capillaries’.10 While the finding of focal proliferative GN may well in fact be immune mediated, it nonetheless appears to be relatively typical of endocarditis-associated glomerular inflammation, and thus should alert both the pathologist and the clinician to this possibility.
In conclusion, we have presented here the fulminant case of a patient ultimately diagnosed with Bartonella endocarditis-associated GN. While the correct diagnosis ultimately led to appropriate antibiotic treatment and to a favourable outcome, the extensive clinical and serological overlap with systemic vasculitis engendered significant diagnostic difficulty and could easily have eventuated in the untoward administration of strong immunosuppressive agents. This case thus emphasises the critical importance of reaching the correct diagnosis when faced with this dilemma; the case similarly serves as a reminder of the importance of including Bartonella in the diagnostic workup of culture-negative endocarditis, despite this pathogen not being specifically included in the existing Duke criteria.11 Notably, in the current case, rapid administration of appropriate antibiotics may have obviated the progression to surgery often described in similar cases.12–14 Our case also serves as a reminder of the somewhat unusual histological pattern associated with endocarditis-associated glomerular inflammation as previously discussed.
Learning points.
Infectious endocarditis, and particularly culture-negative endocarditis, must be considered in the differential diagnosis of systemic vasculitis including antineutrophil cytoplasmic antibody-positive vasculitis.
Renal involvement of infectious endocarditis can manifest as rapidly progressive, focal glomerulonephritis, aka ‘Löhlein nephritis’.
Serological testing for Bartonella is of critical importance in the investigation of cases where culture-negative endocarditis is considered part the differential diagnosis, despite not being currently included in the Duke criteria.
Footnotes
Contributors: LB, GC and JA were involved in clinically treating the patient and performing the diagnostic workup during her hospitalisation. MG was involved in the diagnosis and follow-up in the infectious disease clinic. LB was involved in reviewing the pathology, searching the literature and writing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Jacomo V, Kelly PJ, Raoult D. Natural history of Bartonella infections (an exception to Koch’s postulate). Clin. Diagn. Lab. Immunol 2002;9:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents 2014;44:16–25. 10.1016/j.ijantimicag.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 3. Velho PENF, Cintra ML, Uthida-Tanaka AM, et al. . What do we (not) know about the human bartonelloses? Brazilian Journal of Infectious Diseases 2003;7:01–6. 10.1590/S1413-86702003000100001 [DOI] [PubMed] [Google Scholar]
- 4. Michael G, Yehudith K, Avidor B, et al. . Enzyme immunoassay for the diagnosis of cat-scratch disease defined by polymerase chain reaction. Clinical Infectious Diseases 2001;33:1852–8. [DOI] [PubMed] [Google Scholar]
- 5. Spach DH, Callis KP, Paauw DS, et al. . Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. Journal of clinical microbiology 1993;31:692–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subedi S, Jennings Z, Chen SC-A. Laboratory approach to the diagnosis of culture-negative infective endocarditis. Heart, Lung and Circulation 2017;26:763–71. 10.1016/j.hlc.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 7. Okaro U, Addisu A, Casanas B, et al. . Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev 2017;30:709–46. 10.1128/CMR.00013-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukasawa H, Hayashi M, Kinoshita N, et al. . Rapidly progressive glomerulonephritis associated with PR3-ANCA positive subacute bacterial endocarditis. Internal Medicine 2012;51:2587–90. 10.2169/internalmedicine.51.8081 [DOI] [PubMed] [Google Scholar]
- 9. Vercellone J, Cohen L, Mansuri S, et al. . Bartonella Endocarditis Mimicking Crescentic Glomerulonephritis with PR3-ANCA Positivity. Case Reports in Nephrology 2018;2018:1–4. 10.1155/2018/9607582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray J. Classification of nephritis. Postgrad Med J 1937;13:39–44. 10.1136/pgmj.13.136.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durack DT, Lukes AS, Bright DK, et al. . New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 1994;96:200–9. 10.1016/0002-9343(94)90143-0 [DOI] [PubMed] [Google Scholar]
- 12. Fournier PE, Lelievre H, Eykyn SJ, et al. . Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine 2001;80:245–51. 10.1097/00005792-200107000-00003 [DOI] [PubMed] [Google Scholar]
- 13. Raoult D, Fournier P-E, Vandenesch F, et al. . Outcome and treatment of Bartonella endocarditis. Arch Intern Med 2003;163:226–30. 10.1001/archinte.163.2.226 [DOI] [PubMed] [Google Scholar]
- 14. Rolain JM, Brouqui P, Koehler JE, et al. . Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother 2004;48:1921–33. 10.1128/AAC.48.6.1921-1933.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]


