Abstract
Extragastrointestinal stromal tumour (EGIST) occurs outside the gastrointestinal tract and has histopathological and molecular characteristics similar to gastrointestinal stromal tumour (GIST). This tumour is rare and aggressive. A male patient was admitted with anaemia and lower limb oedema. CT scan showed a tumour in the mesentery and retroperitoneum, suspected to be a small bowel GIST. During laparotomy an unresectable mass was found compressing the retroperitoneal structures. Pathology and immunohistochemistry (CD117) confirmed an EGIST. EGIST arises from Cajal-like cells or from pluripotent stem cells outside the gastrointestinal tract. It is aggressive and has a worse prognosis than GIST. Immunohistochemistry is crucial for diagnosis. Surgery aimed at debulking as much of a tumour mass as possible is the cornerstone of treatment. The role of imatinib is not clear. EGIST is rare and has a bad prognosis, and there is no consensus on grading and management. A low threshold of suspicion is crucial for early diagnosis.
Keywords: general surgery, surgical oncology
Background
Extragastrointestinal stromal tumour (EGIST) occurs outside the gastrointestinal tract and has histopathological and molecular characteristics similar to gastrointestinal stromal tumour (GIST), hence its name.1–7 Reith et al 4 from Florida, USA, were the first to use the name EGIST in 2000.3 It is a very rare entity, representing less than 1% of all gastrointestinal malignancies.1 2 5 6 Because it has non-specific clinical presentation and is difficult to differentiate from other pathologies on imaging findings, its preoperative diagnosis is not common.1 2 EGIST is rare and the retroperitoneal location is not frequent, with only 60 cases published in English literature,1 which is why its imaging characteristics, management and follow-up are not fully understood. Hence all case reports are crucial to help doctors deal with this aggressive disease. In this setting, the authors present a case of a male patient diagnosed with a mesenteric and retroperitoneal EGIST during laparotomy who died 2 months after surgery.
Case presentation
A 77-year-old male patient resorted to the emergency department complaining of asthenia, lower limb oedema and melaena during the previous month. He had no abdominal pain, anorexia or weight loss. Vital signs were within normal range. Abdominal examination was normal, without tenderness or masses, and with normal bowel sounds. Rectal digital examination revealed dark stools, without intraluminal masses. Blood tests revealed severe anaemia (53 g/L). A transfusion was initiated with 3 units of red blood cells (RBC). Endoscopy was performed diagnosing gastritis with mucosal erosions as the possible cause of anaemia. The patient was discharged home with oral iron supplement and proton pump inhibitor. One week later the patient returned to the emergency department with aggravated lower limb oedema and asthenia. Physical examination was the same, but blood tests revealed a 43 g/L anaemia. Another RBC transfusion was initiated. Because the patient was getting worse, he was admitted to the internal medicine ward for study completion.
Investigations
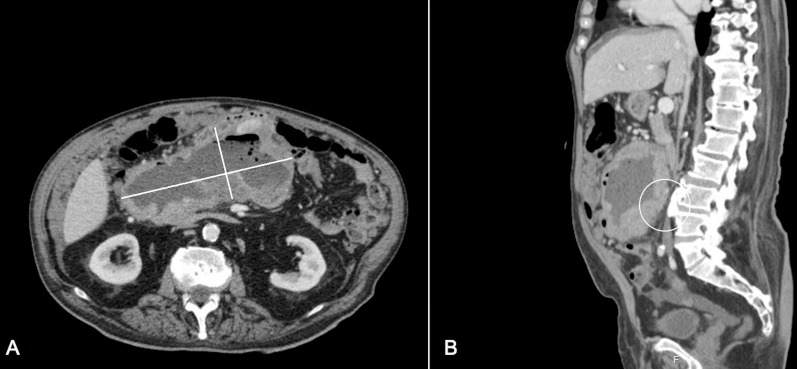
A colonoscopy was performed showing a normal colonic mucosa. Abdominal CT scan revealed a huge tumour mass 21×16.4 cm in size in the mid-abdomen, with lobulated edges, and with a hypodense core with necrosis and air bubbles inside, suggestive of a mesenteric tumour or exophytic tumour from the small bowel (figure 1). Additionally, diffuse mesenteric nodules were found, suggestive of peritoneal carcinomatosis. Adrenal and liver metastases could not be excluded. A percutaneous core biopsy was conducted, and pathology concluded a mesenchymal-like neoplasm without expression of CD117, desmin, S100 or beta-catenin. Surgery was called.
Figure 1.
Abdominal CT scan shows a mesenteric tumour mass with central necrosis and air bubbles, extending into the retroperitoneum and pushing the posterior structures. The tumour mass is compressing/invading the inferior vena cava (white circle in B). (A) Axial plane. (B) Sagittal plane.
Differential diagnosis
A small bowel GIST was the most probable diagnosis, and it could be the cause of the constant symptomatic anaemia.
Treatment
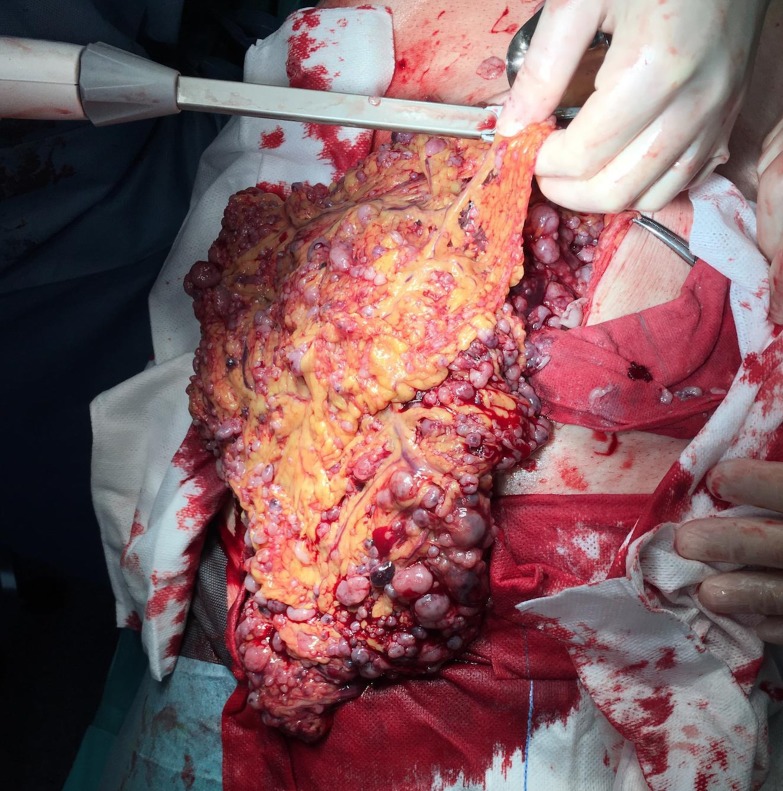
Two months after the first medical evaluation, a midline laparotomy was performed. A huge solid tumour mass in the mid-abdomen was found, which was multicentric, occupying the entire small bowel mesentery extending into the retroperitoneum, with encasement of the superior mesenteric vessels and compression/invasion of the inferior vena cava, and with moderate volume of bloody peritoneal fluid. Additionally, an ‘omental-cake’ with small whitish haemorrhagic lesions and diffuse peritoneal nodules was found (figure 2). The tumour was unresectable. With the intention to achieve tumour debulk to control symptoms and increase imatinib response, the surgical team decided to perform an omental resection. Pathology revealed an EGIST with high mitotic index (>5 mitoses/50 High Power Fields (HPF)), disseminated, and positive for c-KIT (CD117), DOG1 and CD34.
Figure 2.
Omental-cake with countless tumour nodules.
Outcome and follow-up
In the postoperative period there was a fast decline in the patient’s clinical status. Severe renal function impairment arose due to retroperitoneal extension, along with a progressive increase in lower limb oedema due to inferior vena cava compression. No oral intake was possible due to the extrinsic bowel obstruction by the huge mass. The patient died 2 weeks after surgery.
Discussion
EGIST has its origin outside the gastrointestinal tract and has histopathological characteristics similar to GIST, hence its name. It is a very rare entity, representing 1% of all gastrointestinal malignancies and about 5%–10% of all GISTs.1 2 5–7 A mean age of 45.8–59 years has been reported, and women are more affected than men.4 5 7
GIST arises from the interstitial cells of Cajal present in the intestinal wall and responsible for its motility. Because of this, GIST usually presents as an exophytic lesion projecting into the lumen or to the peritoneal cavity. According to some authors, EGIST arises from Cajal-like cells present outside the intestinal wall.1 3 5 Another hypothesis is that it originates from the pluripotent stem cells outside the gastrointestinal tract.1 5 There have only been 60 and 114 reported cases of EGIST in the retroperitoneum and the mesentery, respectively.1 2 The case presented represents a mix of these two locations, with a mesentery tumour extending into the retroperitoneum. Our patient had a mesenterico-retroperitoneal EGIST.
Although histology is similar to GIST, EGIST has several characteristics different from GIST. While GIST usually presents with complications such as fistula or gastrointestinal bleeding due to mucosa ulcerations, EGIST is usually asymptomatic, unless adjacent structures are compressed.1 In fact, EGIST rarely presents with haemorrhage.2 According to a review conducted by Iqbal et al,3 the most frequent presentation of EGIST is abdominal pain, followed by palpable abdominal mass. A growing abdominal mass is a common finding, with tumours reaching up to 32 cm in size (mean of 10 cm).3 Cystic degeneration of an EGIST has also been reported and must be considered in the differential diagnosis of abdominal cystic lesions.6 The mesentery, retroperitoneum, omentum, pancreas, liver, gall bladder, urinary bladder, pleura, prostate, seminal vesicles, pelvis and vagina are possible locations of EGIST, while the entire gastrointestinal tract can be affected by GIST.1–3 Metastasis can be present on diagnosis, with the liver being the most affected organ. Enlargement of abdominal lymph nodes is also a possibility in the case of an EGIST, contrasting the typical absence of lymph nodes in GIST.3
In the case presented, the clinical presentation included anaemia and lower limb oedema. Although the latter can be easily justified by compression of the retroperitoneal structures, anaemia is not fully understood since the tumour was independent from the bowel, although the large number of peritoneal haemorrhagic nodules with moderate amount of bloody peritoneal fluid could be the reason for it. Additionally, could the extrinsic compression of the small bowel by the mesenteric/retroperitoneal mass promote mucosal ulcerations and gastrointestinal bleeding? This is an eventual possibility since the patient had dark stools. Interesting is the fact that the large abdominal tumour, 21 cm in size, was not noted on serial physical examinations. This can be a reason for the late diagnosis and bad prognosis of this disease. A huge mass like the one reported is in accordance with other case reports.
According to European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN), CT scan and positron emission tomography should be used for early-stage lesions.1 However, features on imaging findings that could differentiate EGIST from other tumours (lymphoma, liposarcoma, histiocytoma, leiomyosarcoma, fibrosarcoma) are not totally known due to the small amount of case reports. They have been described as soft tissue masses with necrotic areas, haemorrhagic cystic areas and calcifications.1 CT-guided biopsy can be of good help in achieving a preoperative diagnosis. Although the imaging findings of our case described a soft tissue mass with necrotic areas and air bubbles, it was not possible to diagnose it as an EGIST. In fact, this diagnosis was only suspected after surgery. A suspicion of EGIST did not arise even after a biopsy showing a mesenchymal-like tumour.
Histopathology and immunohistochemistry are gold standard and are crucial for the diagnosis of EGIST.1 As stated above, the characteristics are similar to those present in GIST, with spindle cell type and/or epithelioid type. Positive staining for CD117 (c-KIT) is necessary for confirmation (100%). Additionally, other immunological markers can be found in EGIST, namely BCL-2 (80%), CD34 (70%), smooth muscle actin (30%), desmin (5%) and DOG1.1 3 5 The case presented is not different, and immunohistochemistry was positive for CD117 (c-KIT), CD34 and DOG1, confirming the diagnosis of EGIST.
One major problem when dealing with EGIST is that there is no consensus on grading, management and prognosis. While tumour size and mitotic count dictate the grading and prognosis of GIST, these are not yet valid in EGIST, although there are some authors who use in EGIST the same approach used for GIST.6 7 The only data available is that EGIST has an early age of onset, has usually a larger tumour, is more aggressive and has a worse prognosis than GIST. This is probably due to higher mitotic indices, large tumour sizes, lymph node involvement and metastasis.1 2 5 7 EGIST has a 1-year and 5-year overall survival rate of 91.7% and 48.9%, respectively, while GIST has a 1-year overall survival rate of 94% and a 5-year overall survival rate of 82.4%.2 In 2015, a retrospective analysis of 51 patients conducted by Yi et al 5 from South Korea concluded that a mitotic rate >5/50 HPF had a trend towards higher recurrence, although without statistical significance. The same authors concluded that a tumour size >5 cm was not associated with higher recurrence.5 Additionally, an analysis of 48 patients found that a tumour size >10 cm had no impact on outcome, and that 34% of patients develop metastasis or die within 24 months of follow-up.4 5 Iqbal et al 3 reported a recurrence rate of 43% and an overall survival of 34 months (13 patients studied), while Yi et al 5 reported an overall survival of 36.7 months. It seems clear that, although cellularity, mitotic activity (>2/50 HPF) and necrosis have been presented as potential prognostic factors (size appears not to be a prognostic factor), more studies with higher number of patients are needed to obtain valid conclusions.3–5
Although there are guidelines for GIST management, this is not true for EGIST. The most usual approach to treat an EGIST is surgery and debulking the tumour whenever possible, along with resectable infiltrated structures.1 2 4 5 The utility and efficacy of imatinib mesylate remain uncertain due to the different behaviour of EGIST compared with GIST, although its use is advised for unresectable tumours.1 2 5 Iqbal et al 3 reported the use of imatinib as adjuvant therapy in five patients with unresectable disease and three patients with R2 resection. A partial response was seen in three patients, stable disease was achieved in two patients, and progressive disease was noted in three patients. In cases of recurrence, the same authors achieved a stable disease with imatinib dose escalation in two of five patients.3 Genetic analysis can help to understand the role of imatinib in EGIST treatment. KIT mutation at exon 11 is associated with a good response to imatinib treatment (opposite to wild-type), and it was found in only 37.5% of EGIST cases, contrasting the majority of patients with GIST.5 Following these data, a question arises: is poor response to imatinib to be expected? More studies are necessary.
In our case, due to the recurrent and symptomatic anaemia presented by the patient and the preoperative suspicion of a small bowel GIST, we believe it was a good option to operate before initiating imatinib. This decision may be in contrast to CT findings; however, neither the CT nor the biopsy raised the suspicion of an EGIST. This diagnosis was only possible after surgery and pathology of the removed specimen. Additionally, the patient could not wait for an eventual response to imatinib and surgery could help control symptoms. Since the tumour was extragastrointestinal and unresectable, our aim was to resect and debulk as much of the tumour mass as possible, which is why an omentectomy was performed. Unfortunately, the postoperative diagnosis of EGIST worsened the prognosis. In the postoperative period a fast decline in the general clinical status of the patient was seen, and we could not initiate imatinib mesylate, as this was our objective.
In conclusion, EGIST is a rare mesenchymal tumour similar to GIST, but with a worse prognosis. A low threshold of suspicion is necessary in order to diagnose this aggressive entity. Surgery is the main treatment and the role of imatinib mesylate is still unclear. Due to the rarity of EGIST, more studies are necessary to fully understand its behaviour and promote better treatment programmes.
Learning points.
Extragastrointestinal stromal tumour is a rare mesenchymal tumour located outside the gastrointestinal tract.
CD117 positivity is crucial for diagnosis.
Surgery is the main treatment aimed at debulking as much of the tumour mass as possible.
The utility and efficacy of imatinib mesylate remain uncertain.
More studies are necessary to fully understand its behaviour.
Footnotes
Contributors: CCA: data collection, writing, review. TVC: writing, review. MA, LC: review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Laroia ST, Yadav T, Rastogi A, et al. Malignant retroperitoneal stromal tumor: a unique entity. World J Oncol 2016;7:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pandit N, Das GP, Dahal M, et al. An unexpected extra-gastrointestinal stromal tumor (E-GIST) on the jejunal mesentery. J Surg Case Rep 2018;2018 10.1093/jscr/rjy339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iqbal N, Sharma A, Iqbal N. Clinicopathological and treatment analysis of 13 extragastrointestinal stromal tumors of mesentery and retroperitoneum. Ann Gastroenterol 2015;28:105–8. [PMC free article] [PubMed] [Google Scholar]
- 4. Reith JD, Goldblum JR, Lyles RH, et al. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol 2000;13:577–85. 10.1038/modpathol.3880099 [DOI] [PubMed] [Google Scholar]
- 5. Yi JH, Park B-B, Kang JH, et al. Retrospective analysis of extra-gastrointestinal stromal tumors. World J Gastroenterol 2015;21:1845–50. 10.3748/wjg.v21.i6.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamura D, Adachi Y, Kinjo Y, et al. Extra-gastrointestinal stromal tumor with a large cyst. J Surg Case Rep 2019;2019 10.1093/jscr/rjy354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatipoğlu E. Extragastrointestinal stromal tumor (EGIST): a 16-year experience of 13 cases diagnosed at a single center. Med Sci Monit 2018;24:3301–6. 10.12659/MSM.907654 [DOI] [PMC free article] [PubMed] [Google Scholar]