Abstract
Purpose:
Adolescents with anorexia nervosa (AN) have decreased dehydroepiandrosterone (DHEA) and estrogen concentrations that may contribute to skeletal deficits. We sought to determine whether DHEA + estrogen replacement (ERT) prevented bone loss in young adolescents with AN.
Methods:
We recruited females with AN (n=70, ages 11–18 y) into a 12-month, randomized, double-blind placebo-controlled trial. Participants were randomized to oral micronized DHEA 50 mg + 20 mcg ethinyl estradiol/0.1 mg levonorgestrel daily (n=35) or placebo (n=35). Outcomes included serial measures of bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA; total body, hip, spine) and peripheral quantitative computed tomography (pQCT; tibia). Magnetic resonance imaging of T1-weighted images of the left knee determined physeal status (open/closed).
Results:
Sixty-two subjects completed the trial. Physeal closure status was the strongest predictor of aBMD changes. Among girls with open physes, those who received DHEA+ERT showed a decline in BMD-Z-scores compared to those receiving placebo, while there was no effect in those with at least one closed physis. Treatment did not affect any pQCT measures, regardless of physeal closure status.
Conclusions:
Combined DHEA + ERT did not significantly improve DXA or pQCT BMD measurements in young adolescent girls with AN, in contrast to an earlier trial showing benefit in older adolescents and young women. In girls with open physes, the mean change in the placebo arm was greater than that of the DHEA+ERT group. We conclude that DHEA+ERT is ineffective for preserving bone health in growing young adolescents with AN at the dose and route of administration described in this report.
Keywords: Anorexia nervosa, malnutrition, adrenal hormone, dehydroepiandrosterone, peripheral quantitative computed tomography, DXA
Introduction
Adolescents with anorexia nervosa (AN) are known to have a striking suppression of bone formation, loss of bone mineral density (BMD) and skeletal strength, and are at increased risk for fracture [1–5]. The consequences of these threats to bone health are especially devastating for this young population. Adolescents are still attaining their peak bone mass [6,7], and the loss of BMD and skeletal strength in these patients is thought to be irreversible. The mechanisms of bone loss in AN include impaired bone formation and accelerated bone resorption [8–10] resulting from a combination of hypogonadism, hypercortisolism, low body mass, and poor nutrition, and are possibly related to alterations in bone marrow composition [11].
The implications for lifelong skeletal health are substantial; patients with AN have a seven-fold increased incidence of fractures, which may occur at multiple sites [3]. Effective therapies for preventing bone loss in patients with AN have remained elusive [4,12–14]. We previously conducted a randomized trial to investigate the effects of combination dehydroepiandrosterone (DHEA) + estrogen replacement therapy (ERT) in older adolescents and young women with AN (mean age 18 years) [15]. Whole body and lumbar spine BMD Z-scores were preserved in the DHEA+ERT group even after controlling for weight gain, but decreased over 18 months in the placebo group. Bone resorption markers (N-telopeptides) were suppressed in the treatment group alone. However, the impact of DHEA + ERT on bone density, geometry and strength in growing adolescents is not yet known. The underlying mechanism of action of this therapy remains unclear, as does the effect on the appendicular skeleton and potential impact of physeal closure.
While dual-energy X-ray absorptiometry (DXA) has been the most widely used clinical tool to assess bone mass, bone strength depends on skeletal geometry, bone material properties, and internal architecture which are not reflected directly in DXA measurements. Additionally, cortical and trabecular bone mineral content (BMC) are superimposed within a DXA measure, which may conceal distinct disease and treatment effects on the separate components of BMC. Peripheral QCT (pQCT) allows for direct selective measurement of trabecular and cortical volumetric BMD (vBMD), and additional cross-sectional geometry measures that are highly correlated with fracture load [16].
Building upon our previous work we sought to determine the impact of 12 months of DHEA + ERT on both the axial and appendicular skeleton, as reflected by measures of bone density, skeletal strength, and geometry in a cohort of young adolescent girls with AN. We hypothesized that aBMD of the total body and lumbar spine and vBMD of the radius and tibia would be maintained in adolescent girls with AN receiving DHEA+ERT, while losses would be observed in participants randomized to placebo.
Materials and Methods:
From 2012–2015, we screened 191 adolescents (Supplemental Figure) for participation in a multiple-site, randomized, double blind, placebo-controlled trial (Clinicaltrials.gov, ). Effects on bone marrow composition in the appendicular skeleton by magnetic resonance imaging (MRI) have previously been published [17]. Eligible patients were age 11–17 years; were post-menarchal or had a bone age ≥13 years; and met DSM-5 criteria for AN or atypical AN. Subjects were excluded if they had other medical conditions known to affect bone health or if they were receiving medications known to affect the skeleton. The study was approved by the Boston Children’s Hospital Institutional Review Board; written informed consent was obtained from the parent/guardian, and assent from the adolescent.
Of those screened, 155 met eligibility criteria and 76 female adolescents were enrolled and assigned sequential randomization codes, interpretable as drug or placebo only by the pharmacy staff according to a key prepared by the statistician. The sequence of assignments was randomly permuted in blocks of 2, 4, and 6 to ensure unpredictability as well as temporal balance. The pharmacy dispensed drug or placebo in identical gelatin capsules indistinguishable by patients or staff. Assignments were not revealed until the entire trial was complete [17].
The treatment arm (n=35) received 12 months of oral micronized DHEA (50 mg daily; Belmar Pharmacy; IND 52192) [4,15,18]. DHEA was given with conjugated equine estrogens (0.3mg daily; Premarin®, Wyeth) for the first 3 months to minimize estrogen-related side effects, a regimen that has been well tolerated in our clinical practice and previous trials [15]. Subsequently, subjects in the treatment arm received 9 months of DHEA+ERT (20µg ethinyl estradiol/ 0.1mg levonorgestrel; Alesse®, Wyeth). The other group (n=35) received placebo for the entire 12 months. All participants received routine care including medical, nutritional, and psychological monitoring. Participants returned for assessments at 3, 6, 9, and 12 months. Medication compliance was measured at each visit with pill counts. All subjects were advised to consume the recommended daily intake of calcium (1300 mg) and vitamin D (400 IU). If participants were found to be vitamin D deficient at baseline (25OHD <20 ng/mL), they received a treatment dose of vitamin D3 2000 IU daily for 3 months. After follow up 25OHD measurements at the 3 month study visit, participants resumed a supplementation dose of vitamin D daily.
Skeletal Assessments
The primary outcomes were measurements through serial cross-sections of the left tibia obtained at baseline, 6 months, and 12 months by pQCT using a Stratec XCT 3000 pQCT device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4mm, slice thickness of 2.3mm, and scan speed of 20 mm/s. Scans were obtained using established measures [19]. A scout view was first obtained to place a reference line at the proximal border of the fused distal tibia growth plate. The location of measurements was determined using percentages of the tibia length proximal to this reference line to avoid issues related to height. At the 3% metaphyseal site, the site of maximal cortical thickness, scans were analyzed for trabecular vBMD (mg/cm3). At the 38% meta-diaphyseal site, scans were analyzed for cortical vBMD, cortical BMC (mg), cortical cross-sectional area (CSA, mm2), periosteal circumference (mm), endosteal circumference (mm), and polar section modulus (Zp, mm3). Calf muscle and fat CSA (mm2) were assessed at the 66% diaphyseal site. As previously described [20,21], pQCT measure of muscle density (mg/cm3) was also obtained. Scans were analyzed using Stratec software, Version 6.20. The manufacturer’s phantom was scanned daily for quality assurance. The average coefficient of variation (CV) ranged from 0.2 to 0.6%.
DXA (Discovery A, Hologic, Inc., Bedford, MA) measurements were obtained for the total body less head (TBLH; i.e., with skull excluded), posteroanterior lumbar spine (L1-L4), and total hip on the same model scanner in Boston and Providence. Skeletal outcomes included BMC (g) and areal BMD (g/cm2). Measurements were compared with age- and sex-matched controls and aBMD Z-scores (number of standard deviations away from the average value of the reference group) were calculated using pediatric software [22]. Scans of the phantom were performed daily. The CV was <1.0%. Image analysis was performed using QDR for Windows XP 12.4 software (Hologic, Inc).
All subjects underwent magnetic resonance imaging (MRI; Trio systems, Siemens Medical Inc., Erlangen, Germany) of the right knee at baseline, using previously published methods.[17] Coronal T1 weighted images (TR 600 msec, TE 9.7 msec, FOV 140mm, 3mm slice thickness, 0.3mm gap) were obtained through the knee. Distal femoral and proximal tibial physes were assessed as open or closed as a marker of skeletal maturation. Closed physes by MR were defined as the absence of cartilage signal intensity between the epiphysis and metaphysis with marrow signal intensity extending between those two structures. We defined “open physes” as the presence of open physes at both the tibia and the femur.
Biochemical Assessments
Biomarkers of bone formation [serum levels of osteocalcin (OC) and bone-specific alkaline phosphatase (BSAP)] and bone resorption [serum C-telopeptides (CTx)] were measured every 3 months. OC was measured by ELISA (ALPCO Diagnostics, Salem, NH; %CV 5.0–6.5%); CTx was measured by immunoradiometric assay (Immunodiagnostic Systems, Tyne and Wear, United Kingdom; %CV 5.2–6.8%); and BSAP was measured by electrochemiluminescence immunoassay (ECLIA; Beckman Coulter, Brea, CA; %CV 1.5–2.6%). Serum 25-hydroxyvitamin D [25OHD; RIA (Diasorin, Inc., Stillwater, MN); %CV ≦8.3%] was measured at baseline.
Hormonal measures included serum estradiol and testosterone concentrations [CIA (Beckman Coulter, Fullerton, CA); %CV ≤ 8.0% for estradiol and ≤ 7.0% for testosterone], leptin [RIA (Millipore, Billerica, MA); human (total) ghrelin [RIA (Millipore), %CV 5.2–7.2%]; adiponectin [RIA (Millipore); %CV 6.9–9.3%]; cortisol [CT RIA [Diagnostics Systems Laboratories (DSL), Webster, TX]; %CV 6.1–12.0%], and DHEAS [double-antibody ELISA (DSL), %CV 4.8–5.3%]. Insulin-like growth factor I (IGF-I) concentrations were measured in the Maine Medical Center Laboratory in Scarborough, ME using ELISA (Immunodiagnostic Systems, Inc, Fountain Hills, AZ; %CV 4.6–7.2%). Safety studies included liver function tests (AST, ALT) every six months and a lipid panel at the beginning and end of the study.
Additional Study Measurements
Height (cm) was measured using standardized procedure with a wall-mounted stadiometer. Weight (kg) was measured in the morning, post-voiding, with subjects wearing a hospital gown. BMI was calculated as Weight (kg) / [Height (m)]2. CDC growth data were used to calculated percentage median body mass index and BMI Z-score for each participant.[23]. Body composition measures [total body lean mass (kg), total body fat mass (kg), percentage body fat] were obtained by DXA. All participants responded to a structured interview to obtain demographic information and health history, which included information about menstrual history, smoking status, personal history of fracture, and family history of osteoporosis.
Statistical Analysis
To estimate power, we used data from our prior trial on the variability and pre-post correlation of aBMD Z-score (standard deviation0.89, Pearson r=0.93). A target of 60 completers provided 80% power to detect a difference between treatment arms of 0.25 for change in total body aBMD Z-score over 12 months, an effect smaller by 23% than that observed in the prior trial. All analyses followed the intention-to-treat principle, ascribing the randomly assigned treatment to each participant regardless of compliance. We analyzed each outcome using repeated-measures analysis of variance, with an unstructured covariance matrix and with interative reweighting to minimize the influence of outliers. Outcomes with severely skewed distribution were log-transformed for analysis. Models were adjusted for concurrent weight (time-varying) and baseline anthropometrics (age, height), menarchal status, duration of amenorrhea, and physeal status (open at both tibia and femur or closed at one site or both) by adding appropriate covariate terms to the repeated-measures model. Arm × visit interaction was tested to address the primary hypothesis of treatment effect. Arm × visit × physeal status interaction was additionally tested to determine whether physeal closure modified the treatment effect. Adjusted closure-specific means and differences were constructed from parameters of the fitted model. For each stratum of treatment and physeal status we constructed a contrast testing H0: Baseline = 6 mo = 12mo, yielding an F-statistic with 2,61 df and a level of statistical significance that we denote Pchange. For each stratum of physeal status we constructed a contrast testing H0: parallel time course in Active and Placebo, yielding an F-statistic with 2,61 df and a level of statistical significance that we denote Pdiff. We took P<0.05 to indicate a statistically significant effect. We used SAS software (Cary, NC, version 9.4) for all computations.
Results:
Among the randomized subjects, 6 became ineligible or withdrew before completion of the baseline visit, resulting in a final sample of 70 (35 DHEA+ERT, 35 placebo), aged 15.5 ± 1.9 yr (mean ± SD) [17]. The treatment groups did not differ with respect to baseline characteristics (Table 1). Subjects had a low BMI (18.7 ± 1.7 kg/m2; range 12.8–22.4) and percentage median body weight (%MBW), consistent with their AN. Most were Caucasian and non-Hispanic. The median duration of amenorrhea was 4 months (range 1–18 months). Girls who had not yet had their first menses (n=11) were not included in the calculation of duration of amenorrhea. No subjects were spontaneously menstruating; those designated as amenorrheic for 1 month were 13 participants receiving oral contraceptive pills at recruitment who discontinued them ≥1 month before study participation. Changes in bone mineral content Z-score over 12 months were different in subjects who had used oral contraceptives compared to subjects who did not; we controlled for the duration of amenorrhea in our analyses to account for these differences. Three subjects receiving DHEA+ERT and 5 receiving placebo dropped out for reasons unrelated to the study; 62 adolescents completed the trial.
Table 1:
Baseline characteristics of a sample of 70 adolescents with AN receiving 12 months of either DHEA+ERT or placebo
| DHEA + ERT (n=35) |
Placebo (n=35) |
P* | |
|---|---|---|---|
| Mean ± SD | |||
| Age, y | 15.5 ± 1.9 | 15.5 ± 2.0 | 0.97 |
| Height, cm | 159.5 ± 7.4 | 161.5 ± 8.5 | 0.30 |
| Weight, kg | 48.0 ± 6.2 | 48.8 ± 6.9 | 0.60 |
| BMI, kg/m2 | 18.8 ± 1.6 | 18.7 ± 1.8 | 0.69 |
| BMI Z-score | −0.6 ± 0.7 | −0.7 ± 0.9 | 0.58 |
| Percentage of mBBMI, % | 94 ± 8 | 93±10 | 0.76 |
| Median (Min-Max) | |||
| Time since AN diagnosis, mo | 4 (1–36) | 6 (1–60) | 0.17 |
| Duration of amenorrhea, moǂ | 4 (1–17) | 4 (1–18) | 0.67 |
| DHEAS, mcg/dL | 185 (46–445) | 125 (39–431) | 0.01 |
| Estradiol, pg/mL | 29 (1–208) | 26 (2–274) | 0.52 |
| N (%) | |||
| Race: Caucasian Other |
30 (86) 5(14) |
31 (89) 4(11) |
1 |
| Ethnicity: Hispanic Non-Hispanic |
3 (9) 32 (91) |
1 (3) 34 (97) |
0.61 |
| Previous fracture: Yes No |
10 (29) 25 (71) |
12 (34) 23 (66) |
0.80 |
| Family history of osteoporosis Yes No/Unknown |
9 (26) 26 (74) |
8 (23) 27 (77) |
1 |
| Vitamin D status (25(OH)D, ng/mL) Deficient (<20) Insufficient (20–30) Sufficient (>30) |
4 (11) 12 (34) 19 (54) |
3 (9) 16 (46) 16 (46) |
1 |
| Physeal closure status: Both open Femur open, tibia closed Both closed |
5 (14) 9 (26) 21 (60) |
12 (34) 6 (17) 17 (49) |
0.17 |
AN, anorexia nervosa; DHEAS, dehydroepiandrosterone sulfate; ERT, estrogen replacement therapy; 25(OH)D, 25-hydroxy-vitamin D; BMI, body mass index; mBMI, median body mass index;
From Student t, Wilcoxon rank-sum, or Fisher Exact test, testing for equal distribution in DHEA+ERT and placebo arms.
Excluding 11 subjects with primary amenorrhea (4 DHEA+ERT, 7 placebo)
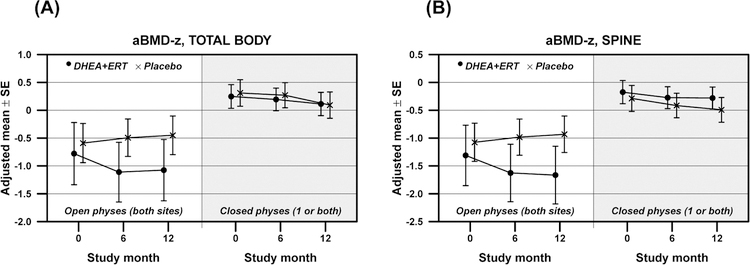
Physeal closure status was the strongest predictor of changes in aBMD (Figure 1). In participants with open physes at baseline, changes in total body BMD-Z score differed significantly between participants receiving DHEA+ERT vs. placebo subjects (Table 2; Pdiff =0.02). Similar results were seen at the lumbar spine and total hip, where subjects with baseline open physes receiving active drug showed a decline in BMD-Z score compared with those receiving placebo. In contrast, in participants with closed physes, no treatment differences in BMD Z-scores were detected at any measured site (Figure 1; Pdiff >0.05 for all). The same pattern was demonstrated in measures of BMC Z-scores at the total body, total hip, and lumbar spine (Table 2).
Figure 1:
Changes in measurements of areal bone mineral density Z-score (aBMD-Z) in adolescents randomized to receive either DHEA+ERT (n=35; represented by •) or placebo (n=35; represented by ×) over 12 months, by baseline physeal closure status. Figure 1A shows values obtained for the total body, while Figure 1B demonstrates those at the lumbar spine.
Table 2.
Changes in bone measures over 12 months in 70 girls with AN in response to treatment with active drug (DHEA+ERT) or placebo.
| Measure | Treatment | Change from Baseline* | |||||
|---|---|---|---|---|---|---|---|
| Open physes (both sites) | Closed physes (1 site or both) | ||||||
| 12-mo change ± SE |
Pchange | Pdiff | 12-mo change ± SE |
Pchange | Pdiff | ||
| DXA Measurements | |||||||
| aBMD-Z, total body | Active Placebo |
−0.30 ± 0.15 0.14 ± 0.10 |
0.06 0.35 |
0.02 | −0.13 ± 0.07 −0.22 ± 0.08 |
0.10 0.006 |
0.43 |
| aBMD-Z, total body less head | Active Placebo |
0.01 ± 0.15 0.14 ± 0.10 |
>0.99 0.30 |
0.73 | −0.02 ± 0.07 −0.13 ± 0.08 |
0.79 0.019 |
0.04 |
| aBMD-Z, spine | Active Placebo |
−0.35 ± 0.17 0.14 ± 0.11 |
0.020 0.40 |
0.009 | −0.11 ± 0.07 −0.20 ± 0.09 |
0.10 0.08 |
0.62 |
| aBMD-Z, hip | Active Placebo |
−0.22 ± 0.16 0.13 ± 0.10 |
0.005 0.42 |
0.004 | −0.15 ± 0.07 −0.25 ± 0.08 |
0.12 0.013 |
0.45 |
| BMC-Z, total body less head | Active Placebo |
−0.21 ± 0.17 0.14 ± 0.10 |
0.26 0.16 |
0.06 | −0.08 ± 0.07 −0.16 ± 0.09 |
0.17 0.14 |
0.18 |
| BMC-Z, spine | Active Placebo |
−0.75 ± 0.30 −0.20 ± 0.19 |
0.008 0.56 |
0.05 | −0.21 ± 0.13 −0.35 ± 0.16 |
0.17 0.04 |
0.76 |
| BMC-Z, hip | Active Placebo |
−0.43 ± 0.20 −0.09 ± 0.13 |
0.002 0.40 |
0.003 | −0.12 ± 0.09 −0.15 ± 0.11 |
0.16 0.32 |
0.41 |
| pQCT Measurements | |||||||
| Trabecular vBMD-Z, 3% | Active Placebo |
0.32 ± 0.19 0.13 ± 0.12 |
0.23 0.04 |
0.47 | −0.11 ± 0.08 −0.03 ± 0.11 |
0.35 0.85 |
0.67 |
| Cortical vBMD-Z, 38% | Active Placebo |
0.05 ± 0.22 0.25 ± 0.14 |
0.24 0.10 |
0.60 | −0.17 ± 0.09 0.12 ± 0.12 |
0.21 0.53 |
0.16 |
| Section modulus, 38% | Active Placebo |
−0.27 ± 0.13 −0.06 ± 0.09 |
0.13 0.47 |
0.32 | −0.07 ± 0.06 −0.13 ± 0.07 |
0.24 0.22 |
0.62 |
AN, anorexia nervosa; DHEA, dehydroepiandrosterone; ERT, estrogen replacement therapy; DXA, dual energy X-ray absorptiometry; aBMD-Z, areal bone mineral density Z-score; BMC-Z, bone mineral content Z-score; pQCT, peripheral quantitative computed tomography; vBMD-Z, volumetric bone mineral density Z-score
From repeated-measures analysis over the time course of the study (baseline, 6 mo, 12 mo), adjusted for concurrent weight and baseline anthropometrics (age, height), menarchal status, and duration of AN. Estimate ± standard error is shown for 12-mo. change from baseline. Pchange tests for change within treatment group over the three measurement times. Pdiff tests for treatment effect, i.e., difference in time course between Active and Placebo.
Treatment did not affect any pQCT measures over the 12-month trial, regardless of physeal closure status (Table 2). No significant changes in trabecular vBMD, cortical vBMD, section modulus, or muscle area occurred between girls receiving DHEA + ERT and those receiving placebo (Pdiff >0.05 for all).
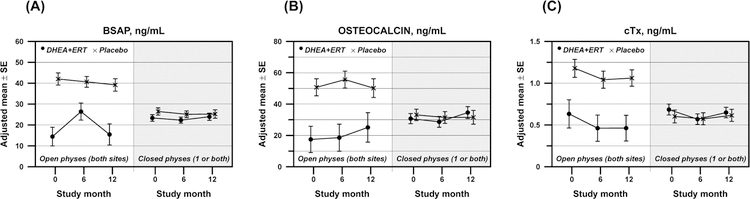
Bone turnover markers again responded differently based upon baseline physeal closure. In girls with open physes, BSAP increased transiently in girls receiving DHEA + ERT, while subjects taking placebo showed no change (Figure 2A; Pdiff =0.02). No treatment differences were seen in girls with closed physes (Pdiff =0.72). Girls with open physes receiving DHEA + ERT showed small, non-significant increases in osteocalcin over 12 months; however, the trajectory of change was the same in girls receiving placebo (Figure 2B; Pdiff =0.40). Again, no differences occurred in girls with closed physes. Changes in CTx were also not different between the two treatment groups, regardless of physeal closure status (Figure 2C).
Figure 2:
Changes in serum measures of bone turnover over 12 months in adolescents randomized to receive either DHEA+ERT (n=35; represented by •) or placebo (n=35; represented by ×). (A) bone specific alkaline phosphatase (BSAP), (B) osteocalcin, and (C) C-telopeptides (CTx)
Safety data were also reviewed (Supplemental Table). Both treatment groups gained weight over the course of the study (median change in BMI Z-score 0.20 SD over 12 mos, p=0.03). Change in BMI did not differ on the basis of physeal closure status, nor on the basis of treatment assignment. The observed changes in BMI Z-score over the course of the study were not correlated with any bone measurement change over time. Liver function tests remained within the normal range for all participants in the trial over the 12 months. Both self-reported compliance and compliance measured by pill count were excellent. Median self-reported compliance was 97.8% at 3 months (range 67–100%) and 99.4% at 12 months (range 50–100%); by pill counts, compliance ranged from 98.3% at 3 months (range 96.7–100%) to 100% at 12 months (range 50–100%). Despite these measures, serum measurements of DHEAS concentration suggest that subjects were compliant for the first 6 months of participation, but may have waned in their medication adherence over the last 6 months (Figure 3; Pdiff <0.001 for both open and closed physes). No treatment group differences in serum measurements of estradiol, total testosterone, leptin, or ghrelin were observed (Supplemental Table; Pdiff >0.05 for all).
Figure 3:
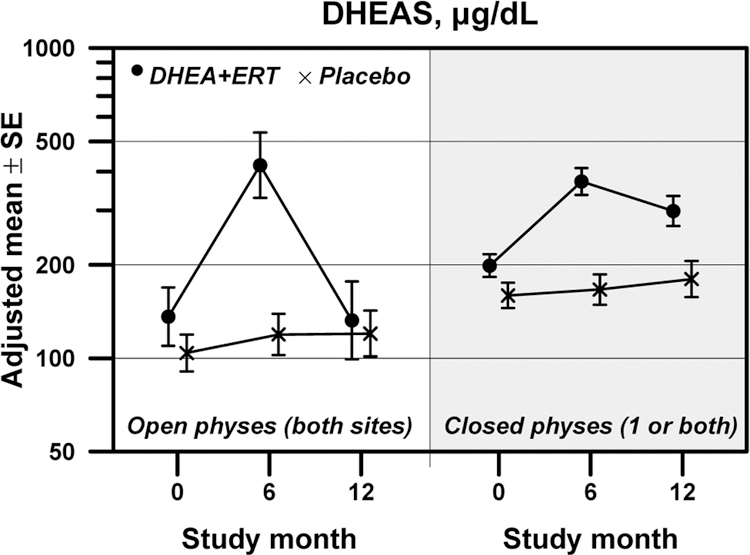
Changes in serum measures of dehydroepiandrosterone sulfate (DHEAS) over 12 months in adolescents randomized to receive either DHEA+ERT (represented by •) or placebo (represented by ×). The left side of Figure 3 represents girls with both physes open, while the right side represents girls with one or both physes closed.
Discussion
In young adolescents with AN and open physes, replacement of adrenal and gonadal hormones led to significant decreases in markers of bone health over 12 months of treatment. Physeal closure status was an important effect modifier of the impact of hormonal therapy on these bone outcomes. Subjects with open physes at baseline showed declines in areal BMD and BMD Z-scores in response to this therapy. In subjects with closed physes, no change, either positive or negative, occurred over 12 months of treatment. Skeletal outcomes measured by pQCT did not change over time in response to DHEA+ERT in either group. The data suggest that the potential skeletal benefits derived from this therapy apply only to older adolescents and young women who have completed their growth and have closed epiphyses.
For this study, we recruited only girls who were post-menarchal or who had attained an expected bone age of 13 years or more. We anticipated that these girls would have already gone through their growth spurt, and would be in the growth deceleration phase with 96% of final height attained [24]. The current findings are in contrast to our previous research which was conducted in older, more substantially malnourished adolescents and young women with closed physes [15]. Older adolescents and young women in our previous studies have shown an arrest of bone loss, as determined by DXA outcomes of BMD, after combined therapy with adrenal and gonadal hormone replacement. We also showed hip strength variables, which were derived from DXA measurements, to be improved after 18 months of the same ERT regimen [25]. Interestingly, in this same cohort, ERT led to arrest of bone marrow changes associated with mild to moderately severe AN [17]. Despite our recruitment strategy for the current study, almost half of participants had at least one open physis on MRI. In these girls, DHEA+ERT was not beneficial. The onset of AN prior to puberty leads to more profound skeletal deficits and in particular, compromised osteoblast activity, with lower BMD seen at both axial and appendicular sites[26]. In contrast, patients with onset of disease during adulthood are more likely exhibit defects solely in the axial skeleton.
Additionally, a young age of onset of the disease has the potential to more negatively impact peak bone mass, since the disease may persist for a greater duration of time. We previously studied a small cohort of adolescents admitted for hemodynamic stabilization and tracked bone biomarkers while on bed rest [27]. Similarly, we found the most compromised bone formation and suppressed bone formation markers in the youngest adolescents (i.e., age 11–14 vs. 15–17 yr), potentially due to the status of the physis [27].
Increases in bone specific-alkaline phosphatase (BSAP) were observed in those subjects who were growing (i.e., in those subjects who had open physes) and receiving DHEA+ERT. Markers in subjects receiving placebo and those with closed physes remained unchanged. The increases in BSAP suggest that the participants with open physes were still very receptive to bone growth. Osteocalcin, a measure of mature osteoblast function, also increased over time but was not different between girls receiving DHEA+ERT or placebo. Osteoclast activity (measured via serum C-telopeptides) did not change; this result is not surprising given that the primary bone insult in AN is a lack of osteoblastic activity. It is unclear why the increases in BSAP did not lead to other signs of bone formation, such as improvement in measures of bone density or bone geometry over time. Our findings are in contrast to our previous study evaluating marrow changes as a result of DHEA + ERT[17]. We hypothesize that the observed marrow changes may take longer to be reflected in clinical measures of skeletal health such as DXA. The differences between our DXA and pQCT outcomes in patients with open physes, with data diverging in treatment vs. placebo by DXA yet showing minimal change by pQCT, may highlight the limitations of DXA. In the pQCT measurements (which would not be confounded by bone size), no harmful changes were seen over time in the group with open physes receiving DHEA + ERT. However, we would not recommend further study or clinical use of DHEA + ERT in young adolescents, given the potential adverse effects in this young group. These questions will need to be addressed in future studies, as well as the effect of oral estrogen compared to transdermal regimens, examining both the effects on circulating bone biomarkers and bone density, geometry, and structure in the axial and peripheral skeleton.
Study limitations should be acknowledged. Our cohort includes subjects with AN diagnosed via DSM-5 criteria and patients with mild severity of malnutrition, in contrast to our earlier trials that met the more stringent weight criteria of the DSM-IV [4,15]. However, the goal of including these participants was to make our study findings more generalizable to the typical patients seen by clinicians in practice. It should also be acknowledged that the interpretation of bone biomarker data in growing adolescents is complex compared to adults or older adolescents with closed epiphyses.
Conclusion:
In conclusion, a combination regimen of oral DHEA + ERT for 12 months did not improve bone density, geometry, or strength compared to placebo in young adolescents with AN with open epiphyses. The benefits with respect to attenuation of bone loss and enhancements in bone strength appear to be limited to older adolescents and young women. Therefore, other therapeutic strategies such as transdermal estrogen [14] should be consideredin young patients affected with this disease, in addition to standard multidisciplinary care including medical, nutritional, and psychological support.
Supplementary Material
Supplemental Figure: CONSORT diagram of subject recruitment
Implications and Contribution:
In a 12-month randomized clinical trial, dehydroepiandrosterone and estrogen replacement therapy supplementation did not improve bone health in young adolescents with open physes who have anorexia nervosa.
Acknowledgements:
The authors thank Drs. Kirsten Ecklund, Robert Mulkern, and Sridhar Vajapeyam, Department of Radiology at Boston Children’s Hospital, for their assistance with the physeal measurements and MR results. Our study was supported by NIH R01 AR060829, NICHD K23 HD060066, NIH UL1 RR-025758 (Harvard Clinical and Translational Science Center), the Boston Children’s Hospital Department of Medicine and CTSU, and the Brown Alpert Medical School Department of Orthopaedics.
Funding Sources: National Institutes of Health, R01 AR060829, NICHD K23 HD060066, and NIH UL1 RR-025758 (Harvard Clinical and Translational Science Center); the Boston Children’s Hospital Department of Medicine and Clinical and Translational Study Unit, and the Brown Alpert Medical School Department of Orthopaedics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All of the authors declare that they have no conflicts of interest in this work.
Trial Registration: clinicaltrials.gov,
References:
- [1].Smink FRE, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep 2012;14:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet 2010;375:583–93. [DOI] [PubMed] [Google Scholar]
- [3].Rigotti NA, Neer RM, Skates SJ, et al. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 1991;265:1133–8. [PubMed] [Google Scholar]
- [4].Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab 2002;87:4935–41. [DOI] [PubMed] [Google Scholar]
- [5].Misra M Long-Term Skeletal Effects of Eating Disorders with Onset in Adolescence. Ann N Y Acad Sci 2008;1135:212–8. [DOI] [PubMed] [Google Scholar]
- [6].Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab 1992;75:1060–5. [DOI] [PubMed] [Google Scholar]
- [7].Bonjour J-P, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev 2014;35:820–47. [DOI] [PubMed] [Google Scholar]
- [8].Gordon CM, Goodman E, Emans SJ, et al. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr 2002;141:64–70. [DOI] [PubMed] [Google Scholar]
- [9].Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002;87:4177–85. [DOI] [PubMed] [Google Scholar]
- [10].Klibanski A, Biller BM, Schoenfeld DA, et al. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 1995;80:898–904. [DOI] [PubMed] [Google Scholar]
- [11].Ecklund K, Vajapeyam S, Feldman HA, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res 2010;25:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002;15:135–43. [DOI] [PubMed] [Google Scholar]
- [13].Liu SL, Lebrun CM. Effect of oral contraceptives and hormone replacement therapy on bone mineral density in premenopausal and perimenopausal women: a systematic review. Br J Sports Med 2006;40:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 2011;26:2430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Divasta AD, Feldman HA, Giancaterino C, et al. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012;61:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu D, Manske SL, Kontulainen SA, et al. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int 2007;18:991–7. [DOI] [PubMed] [Google Scholar]
- [17].Vajapeyam S, Ecklund K, Mulkern RV, et al. Magnetic resonance imaging and spectroscopy evidence of efficacy for adrenal and gonadal hormone replacement therapy in anorexia nervosa. Bone 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gordon CM, Grace E, Emans SJ, et al. Changes in bone turnover markers and menstrual function after short-term oral DHEA in young women with anorexia nervosa. J Bone Miner Res 1999;14:136–45. [DOI] [PubMed] [Google Scholar]
- [19].DiVasta AD, Feldman HA, O’Donnell JM, et al. Skeletal outcomes by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in adolescent girls with anorexia nervosa. Osteoporos Int 2016;27:3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mostoufi-Moab S, Magland J, Isaacoff EJ, et al. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. J Bone Miner Res 2015;30:1657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baker JF, Von Feldt J, Mostoufi-Moab S, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:1612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 2007;92:2087–99. [DOI] [PubMed] [Google Scholar]
- [23].Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000:1–27. [PubMed]
- [24].Carswell J, Stafford DEJ. Amenorrhea and the polycystic ovary syndrome. In: Gordon CM, Katzman D, Rosen D, et al. , editors. Adolesc. Heal. Care A Pract. Guid, Philadelphia, PA: Lippincott Williams & Wilkins; 2007, p. 3–26. [Google Scholar]
- [25].DiVasta ADD, Beck TJJ, Petit MAA, et al. Bone cross-sectional geometry in adolescents and young women with anorexia nervosa: a hip structural analysis study. Osteoporos Int 2007;18:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seeman E, Karlsson MK, Duan Y. On exposure to anorexia nervosa, the temporal variation in axial and appendicular skeletal development predisposes to site-specific deficits in bone size and density: a cross-sectional study. J Bone Miner Res 2000;15:2259–65. [DOI] [PubMed] [Google Scholar]
- [27].DiVasta ADD, Feldman HAA, Quach AEE, et al. The effect of bed rest on bone turnover in young women hospitalized for anorexia nervosa: a pilot study. J Clin Endocrinol Metab 2009;94:1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: CONSORT diagram of subject recruitment