Abstract
Background and Purpose:
The risk of arterial ischemic events after intracerebral hemorrhage (ICH) is poorly understood given the lack of a control group in prior studies. This study aimed to evaluate the risk of acute ischemic stroke and myocardial infarction (MI) among patients with and without ICH.
Methods:
We performed a retrospective cohort study using claims data from Medicare beneficiaries from 2008–2014. Our exposure was acute ICH, identified using validated ICD-9-CM diagnosis codes. Our primary outcome was a composite of acute ischemic stroke and MI, while secondary outcomes were ischemic stroke alone and MI alone. We used Cox regression analysis to compute hazard ratios (HR) during 1-month intervals after ICH. Sensitivity analyses entailed exclusion of patients with atrial fibrillation and valvular heart disease.
Results:
Among 1,760,439 Medicare beneficiaries, 5,924 had ICH. The 1-year cumulative incidence of an arterial ischemic event was 5.7% (95% confidence interval [CI], 4.8–6.8) in patients with ICH and 1.8% (95% CI, 1.7–1.9) in patients without ICH. After adjusting for potential confounders, the risk of an arterial ischemic event remained significantly increased for the first 6 months after ICH and was especially high in the first month (HR, 6.7; 95% CI, 5.0–8.6). In secondary analysis, the risk of ischemic stroke was increased in the first 6 months after ICH (HR, 6.1; 95% CI, 3.5–9.3), but the risk of MI was not (HR, 1.6; 95% CI, 0.3–2.9). In sensitivity analyses excluding patients with atrial fibrillation and valvular heart disease, the association between ICH and arterial ischemic events was similar to that of the primary analysis.
Conclusions:
In a large population based cohort, we found that elderly patients with ICH had a substantially increased risk of ischemic stroke in the first 6 months after diagnosis. Further exploration of this risk is needed to determine optimal secondary prevention strategies for these patients.
Keywords: Intracerebral hemorrhage, Ischemic Stroke, Myocardial Infarction
Intracerebral hemorrhage (ICH) has high morbidity and mortality.1 Patients who survive the acute phase of ICH often make substantial recovery during the months and years after the event.2 Such recovery may be interrupted by a recurrent vascular event such as an acute ischemic stroke or myocardial infarction (MI). For example, covert brain infarcts after ICH are independently associated with a 5-fold higher odds of major disability or death.3
Large case series have reported rates of acute ischemic stroke after ICH to vary from 3–7%4, 5, while rates of MI after ICH may be as high as 6%.6, 7 In a recent prospective observational study, about 20% of ICH patients had incident ischemic events, with a cumulative incidence of 5.9% at the end of 1 year.8 Additionally, after infections, acute vascular events are the second most common reason for readmissions after ICH.9, 10 Prior studies however have not included a control group; consequently, the exact nature of the risk of an arterial ischemic event after ICH remains uncertain. Furthermore, among ICH survivors with atrial fibrillation, rates of strokes may be as high as 10.5% even with a low median CHA2DS2-Vasc score of 211, which normally corresponds to an annual stroke rate of 2–3%.12 This suggests that patients with ICH may have a predisposition for thromboembolic sequelae. We therefore sought to explore the magnitude and timing of the risk of arterial ischemic events after ICH in a large, heterogeneous, nationally representative cohort of Medicare beneficiaries.
Methods
Study Design
We performed a retrospective cohort study using both inpatient and outpatient claims data on a 5% sample of Medicare beneficiaries. The U.S. Centers for Medicare and Medicaid Services (CMS) provides health insurance to a large majority of U.S. citizens once they reach 65 years of age. CMS makes available datasets with de-identified patient information based on data on claims submitted by hospitals and providers. The data provided includes up to 25 International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes.13 A unique and anonymous identifier code links multiple claims for a given patient, thus allowing for a comprehensive longitudinal analysis of each beneficiary’s care over time. This study was approved by the Weill Cornell Medicine institutional review board. Since a de-identified dataset was provided by CMS, individual patient consent did not apply to this study. The claims data used in this analysis are restricted per the terms of Medicare’s data use agreement and therefore cannot be shared directly with other investigators. However, investigators can obtain access to these data by application to the CMS.
Patient Population
We obtained data from inpatient and outpatient claims of Medicare beneficiaries between January 2008 and December 2014. In keeping with the standard practice in analyzing Medicare data,13 we limited our cohort to beneficiaries with continuous coverage in traditional fee-for-service Medicare (both Parts A and B) for at least 1 year or until death, if applicable. Although Medicare eligibility generally begins at 65 years of age, we included only patients 66 years or older in order to allow time for beneficiaries to enter medical care and for their providers to document any pre-existing medical comorbidities.
Measurements
Our exposure variable was ICH, identified by the ICD-9-CM code 431.x in any diagnostic position, which has a sensitivity of 82% and a specificity of 93% as compared to medical record review.14, 15 Patients with any diagnosis codes for trauma were not included. We also excluded patients with any diagnosis of cerebrovascular disease (using the ICD-9-CM codes 430, 433.x1, 434.x1, and 436) or MI (using the ICD-9-CM code 410.x1) preceding the index hospitalization for ICH to prevent misclassification of chronic events. Additionally, we also excluded patients who had an ischemic stroke or MI concurrently with the ICH hospitalization. This was done to prevent inclusion of patients who had ICH from hemorrhagic transformation of ischemic stroke or from procedural complications following stroke or MI. We collected data on demographic characteristics, such as age, sex, and race/ethnicity. Using previously used ICD-9-CM code algorithms, we also measured the following cardiovascular risk factors and relevant comorbidities: hypertension, diabetes, atrial fibrillation, congestive heart failure, valvular heart disease, peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, alcohol abuse, and tobacco use. The burden of comorbidities was quantified using the Charlson comorbidity index16, 17, which is a weighted score of 17 comorbidities identified through ICD-9-CM codes. This index has been used in previous studies of ICH to adjust for illness burden.18, 19
Our primary outcome was a composite of acute ischemic stroke and MI, while secondary outcomes were ischemic stroke alone and MI alone. The sensitivity and specificity of diagnosis codes for acute ischemic stroke are 86% and 95%, respectively.15, 20 Similarly, the ICD-9-CM code for MI has been shown to have a sensitivity and specificity > 85% with a positive predictive value of over 93%.18
Statistical Analysis
To examine the association between ICH and arterial ischemic events, we fit separate Cox regression models for the groups with and without ICH. These multivariable models adjusted for demographics, vascular risk factors, and Charlson comorbidities. We left these covariates in the models regardless of their significance level in univariate analyses. The proportional hazard assumption was violated; therefore, we used the corresponding survival probabilities of these separate models to calculate hazard ratios (HR) in 4-week intervals after discharge from ICH hospitalization, and confidence intervals (CI) were computed using the nonparametric bootstrap method.
We performed sensitivity analyses, stratified by presence of atrial fibrillation and valvular heart disease because these patients have an inherently high risk for arterial ischemic events and therefore are usually anticoagulated. Statistical analyses were performed using R software v 3.3.1 (R Foundation for Statistical Computing). The threshold for statistical significance was p<0.05.
Results
Study Population
Among 1,760,439 Medicare beneficiaries analyzed in this study, 5,924 (0.3%) were diagnosed with ICH. Patients with ICH were older than patients without ICH (79.4 vs. 73.5 years), and they more often had hypertension, coronary artery disease, atrial fibrillation and valvular heart disease (Table 1).
Table 1.
Characteristics of Patients Stratified by Presence of Intracerebral Hemorrhage
| Characteristica | ICH (N = 5,924) |
No ICH (N = 1,754,515) |
|---|---|---|
| Age, mean (SD), y | 79.4 (7.9) | 73.4 (7.7) |
| Female | 2,604 (43.9) | 752,998 (42.9) |
| Race | ||
| White | 5,094 (85.2) | 1,510,647 (86.1) |
| Black | 501 (8.5) | 138,208 (7.9) |
| Other | 376 (6.3) | 105,660 (6.0) |
| Hypertension | 5,301 (89.5) | 893,421 (50.9) |
| Diabetes | 2,140 (36.1) | 362,894 (20.7) |
| Congestive heart failure | 1,082 (18.3) | 4,119 (0.2) |
| Peripheral vascular disease | 628 (10.6) | 1,518 (0.1) |
| Chronic obstructive pulmonary disease | 1,207 (20.4) | 183,128 (10.4) |
| Chronic kidney disease | 995 (16.8) | 84,138 (4.8) |
| Atrial fibrillation | 1,686 (28.4) | 130,992 (7.5) |
| Valvular disease | 839 (14.2) | 111,247 (6.3) |
| Tobacco use | 743 (12.5) | 21,893 (1.3) |
| Alcohol use | 525 (8.9) | 44,094 (2.5) |
Abbreviations: ICH, intracerebral hemorrhage; SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Primary Outcome
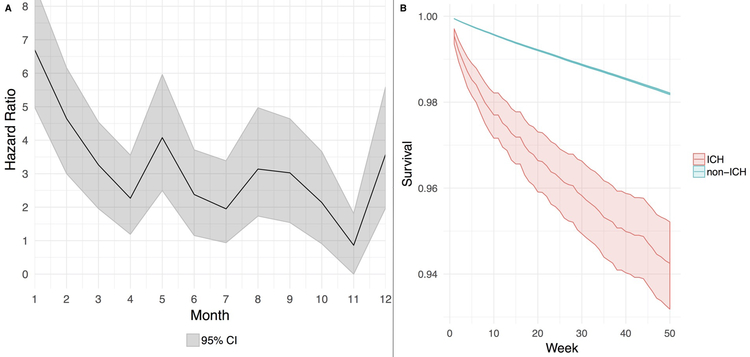
Arterial ischemic events were diagnosed in 137,912 study patients (7.8%). Patients diagnosed with an arterial ischemic event were older and had more vascular risk factors than patients who were not diagnosed with an arterial ischemic event (Table 2). In multivariable Cox models, the risk of an arterial ischemic event was significantly increased during the first 6 months after ICH, peaking in the first (hazard ratio [HR], 6.7; 95% CI, 5.0–8.6) and second months (HR, 4.6; 95% CI, 3.0–6.2) after diagnosis (Figure 1 and Supplemental Figure I). The slope of event-free survival was significantly lower for patients with ICH than for those without; the 1-year cumulative incidence of an arterial ischemic event was 5.7% (95% CI, 4.8–6.8%) in patients with ICH and 1.8% (95% CI, 1.7–1.9%) in patients without ICH.
Table 2.
Characteristics of Patients Stratified by Presence of Ischemic Stroke or Myocardial Infarction
| Characteristica | Stroke/MI (N = 137,912) |
No Stroke/MI (N = 1,622,527) |
|---|---|---|
| Age, mean (SD), y | 77.3 (7.9) | 73.1 (7.6) |
| Female | 61,798 (44.8) | 693,804 (42.6) |
| Race | ||
| White | 116,961 (84.8) | 1,398, 733 (86.2) |
| Black | 13,888 (10.1) | 124,821 (7.7) |
| Other | 7,063 (5.1) | 98,973 (6.1) |
| Hypertension | 91,677 (66.5) | 807,045 (49.7) |
| Diabetes | 43,295 (31.4) | 321,739 (19.8) |
| Congestive heart failure | 864 (0.6) | 4,377 (0.3) |
| Peripheral vascular disease | 238 (0.2) | 1,908 (0.1) |
| Chronic obstructive pulmonary disease | 21,770 (15.8) | 162,565 (10.0) |
| Chronic kidney disease | 13,419 (9.7) | 71,714 (4.4) |
| Atrial fibrillation | 19,182 (13.9) | 113,436 (7.0) |
| Valvular disease | 14,840 (10.8) | 97,928 (1.2) |
| Tobacco use | 2,708 (2.0) | 19,928 (1.2) |
| Alcohol use | 5,058 (3.7) | 39,561 (2.4) |
Abbreviations: MI, myocardial infarction; SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Figure 1:
Cox regression model showing risk of an arterial thrombotic event after intracerebral hemorrhage (Panel A) and corresponding event-free probabilities (Panel B) among patients with and without intracerebral hemorrhage.
Abbreviations: CI, confidence interval; ICH, intracerebral hemorrhage.
Secondary Outcomes
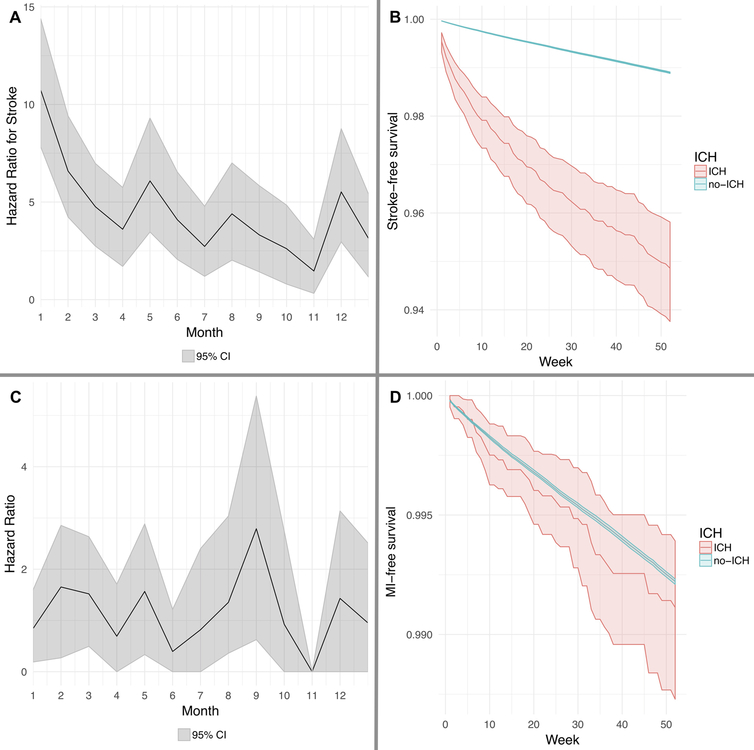
In secondary outcome analysis, the risk of acute ischemic stroke was significantly increased during the first 6 months after ICH (HR, 6.1; 95% CI, 3.5–9.3), but the risk of MI was not (HR, 1.6; 95% CI, 0.3–2.9) (Figure 2, and Supplemental Figure II).
Figure 2:
Cox regression model showing risk of an ischemic stroke (Panel A) and corresponding event-free probabilities (Panel B) among patients with and without intracerebral hemorrhage. Cox regression model showing risk of a myocardial infarction (Panel C) and corresponding event-free probabilities (Panel D) among patients with and without intracerebral hemorrhage. Abbreviations: CI, confidence interval; ICH, intracerebral hemorrhage.
Note: Hazard ratio of 0 indicates very few events in the specified time interval.
Sensitivity Analysis
In a sensitivity analysis excluding patients with atrial fibrillation and valvular heart disease, the 1-year cumulative incidence of an arterial ischemic event was 5.0% (95% CI, 3.8–6.4%) in patients with ICH and 1.5% (95% CI, 1.4–1.6%) in patients without ICH. In multivariable Cox modeling, the risk of an arterial ischemic event was significantly increased during the first few months after ICH, peaking in the first month after diagnosis (HR, 6.2; 95% CI, 4.0–8.9) (Table I in the online only data supplement). Similarly, among patients with atrial fibrillation and valvular heart disease, the risk of an arterial ischemic event was significantly elevated in the first 3 months after ICH.
In post-hoc analyses, we studied the relationship between ICH and arterial ischemic events, stratified by sex, race, and region and observed a short-term heightened risk of arterial ischemic events after ICH compared to patients without ICH (Table I in the online only data supplement).
Discussion
In a large cohort of Medicare beneficiaries, we observed a heightened risk of an arterial thrombotic event in the first 6 months after ICH discharge compared to patients without ICH. While there was an elevated risk of ischemic stroke, the risk of MI was similar among patients with and without ICH. The increased risk of ischemic stroke after ICH was present but attenuated when excluding patients with atrial fibrillation and valvular heart disease—conditions that typically require anticoagulation.
Prior studies evaluating the risk of ischemic stroke after ICH have lacked control groups thereby precluding an estimation of relative risk. Additionally, most previous studies did not evaluate risk during discrete time periods, despite likely violation of the proportional hazard assumption and non-uniform risk over time. In this context, our study provides novel findings that indicate an acutely elevated risk of ischemic stroke in the short term after ICH. Our study captured only clinical events and not radiological infarcts, and may have underestimated the thrombotic risk. This is because incident diffusion-restricted lesions in the acute phase of ICH range from 13–41%3, 21, in contrast to clinical ischemic stroke events which are relatively less common.4, 5 We did not identify any association between ICH and subsequent MI; however, our study was retrospective and relied on claims data so it is possible that subtle or covert coronary ischemic events were missed leading to an underestimation of thromboembolism risk after ICH.
The elevated risk of ischemic stroke may be attributable to antithrombotic drug cessation after the diagnosis of ICH and the unclear optimal time frame for resumption of these medications. For example, studies have reported that fewer than 50% of patients resume antithrombotic agents in the first year after ICH despite strong indications.22 However, prior studies suggest that only about 20% of patients are on antithrombotic medications prior to ICH23, and rates of observed ischemic strokes among ICH survivors with atrial fibrillation exceed those of expected events for a given CHA2DS2-Vasc score.11 Taken together, these factors fail to implicate cessation of antithrombotic medications as the sole mechanism of increased thrombotic risk after ICH. Another potential explanation is poor risk factor control after ICH. Uncontrolled blood pressure has been demonstrated in one third to half of patients after an ischemic stroke24, 25, and these patients have significantly worse blood pressure control compared to patients with other cardiovascular conditions such as acute MI.26 Data on blood pressure control after ICH discharge were not available in the Medicare dataset and hence could not be studied.
Our study has several important limitations. First, we lacked information on antithrombotic medication use and interruption. We tried to mitigate this concern by performing a sensitivity analysis that excluded patients with atrial fibrillation and valvular heart disease, who arguably have the strongest indication to resume antithrombotic medications. Furthermore, it is not clear why antithrombotic cessation would lead only to ischemic stroke and not MI. Second, errors in misclassification resulting from our use of ICD-9-CM codes are also possible. For instance, the ICH primary diagnosis code may have been replaced with a more generic stroke diagnosis code for subsequent readmissions after ICH for non-cardiovascular complications, which may have resulted in the overestimation of the ischemic stroke risk in our study. The ICD-9-CM codes for ICH, stroke and MI however, have been previously validated to have high specificity and positive predictive value.14, 27 Moreover, ICD-9-CM codes for major diagnoses in these types of administrative datasets have generally been shown to be reliable28, and any miscoding is likely to bias the results toward the null, which would support the results of our analyses. Third, the claims based nature of the Medicare dataset prevented us from elucidating the etiology of ischemic strokes. It is also possible that our study was subject to surveillance bias because an ICH patient is probably more likely to have a follow-up brain MRI to evaluate the mechanism of ICH than an ECG/troponin; therefore, some ischemic stroke events may have been incidental findings picked up because of frequent brain imaging, particularly punctate areas of diffusion restriction, which can be clinically silent. This could have resulted in a higher risk of ischemic stroke but not MI after ICH. Fourth, our study is subject to selection bias due to inclusion of patients older than 66 years, which limits the generalizability of our results; however, this age group accurately reflects the median age of ICH patients in the general population.29 Finally, the Medicare data lacked information on ICH etiology and severity factors such as hematoma volume or location, presence of intraventricular hemorrhage, and Glasgow Coma Scale score. Given the large sample size of patients, our results may be biased by residual confounding or factors previously not considered to influence thrombotic outcomes.
Conclusions
There appears to be a heightened short-term risk of ischemic stroke after ICH. Although this risk is the highest in the first month, it remains significantly elevated for approximately 6 months. Further study of the link between ICH and subsequent ischemic stroke may yield new strategies for improving long-term recovery after ICH.
Supplementary Material
Acknowledgments:
Funding/Support: SBM is supported by the National Institutes of Health (NIH) (K23NS105948) and the Leon Levy Foundation. AM is supported by the NIH (grant KL2TR002385), the American Heart Association (18CDA34110419) and the Leon Levy Foundation. KNS is supported by the NIH (U24NS107215, U24NS107136, RO1NR018335, and U01NS106513), and the American Heart Association (17CSA33550004). BBN is supported by the NIH (K23NS091395) and the Florence Gould Endowment for Discovery in Stroke. HK is supported by the NIH (U01NS095869 and R01NS097443) and the Michael Goldberg Research Fund.
CI has served on the scientific advisory board for Broadview Ventures. MMS receives salary support from Amen for investigator initiated research. MMS receives salary support from Amen for investigator-initiated research. KNS serves as the PI for the Novartis-sponsored S1P ICH trial, the co-PI in the Bard-sponsored INTREPID trial, and the co-PI in the Biogen-sponsored CHARM trial, and receives funding from Hyperfine Imaging. BBN serves as a member of the data and safety monitoring board for the PCORI-funded TRAVERSE trial and has received personal fees for medicolegal consulting on stroke. H.K serves as the co-PI for the NIH-funded ARCADIA trial which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition.
Footnotes
Disclosures: All other authors report no conflict of interest for this study.
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176 [DOI] [PubMed] [Google Scholar]
- 2.Hemphill JC 3rd, Farrant M, Neill TA Jr. Prospective validation of the ich score for 12-month functional outcome. Neurology. 2009;73:1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on mri, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123–127 [DOI] [PubMed] [Google Scholar]
- 5.Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2009;40:3567–3573 [DOI] [PubMed] [Google Scholar]
- 6.Butler AC, Tait RC. Restarting anticoagulation in prosthetic heart valve patients after intracranial haemorrhage: A 2-year follow-up. British journal of haematology. 1998;103:1064–1066 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: A nationwide cohort study. Circulation. 2015;132:517–525 [DOI] [PubMed] [Google Scholar]
- 8.Casolla B, Moulin S, Kyheng M, Henon H, Labreuche J, Leys D, et al. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke. 2019;50:1100–1107 [DOI] [PubMed] [Google Scholar]
- 9.Liotta EM, Singh M, Kosteva AR, Beaumont JL, Guth JC, Bauer RM, et al. Predictors of 30-day readmission after intracerebral hemorrhage: A single-center approach for identifying potentially modifiable associations with readmission. Crit Care Med. 2013;41:2762–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen T, Liu B, Wan X, Zhang X, Zhang J, Zhou X, et al. Risk factors associated with 31-day unplanned readmission in 50,912 discharged patients after stroke in china. BMC Neurol. 2018;18:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. Jama. 2015;313:824–836 [DOI] [PubMed] [Google Scholar]
- 12.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The swedish atrial fibrillation cohort study. Eur Heart J. 2012;33:1500–1510 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Medicare Limited Dataset Files. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/. Accessed August 11, 2018.
- 14.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781 [DOI] [PubMed] [Google Scholar]
- 15.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of chronic diseases. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. Journal of clinical epidemiology. 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 18.Bar B, Hemphill JC 3rd. Charlson comorbidity index adjustment in intracerebral hemorrhage. Stroke. 2011;42:2944–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez Caballero PE, Lopez Espuela F, Portilla Cuenca JC, Ramirez Moreno JM, Pedrera Zamorano JD, Casado Naranjo I. Charlson comorbidity index in ischemic stroke and intracerebral hemorrhage as predictor of mortality and functional outcome after 6 months. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22:e214–218 [DOI] [PubMed] [Google Scholar]
- 20.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: A systematic review. PLoS One. 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennlert J, Asplund K, Carlberg B, Wiklund PG, Wisten A, Asberg S, et al. Antithrombotic treatment following intracerebral hemorrhage in patients with and without atrial fibrillation. Stroke. 2015;46:2094–2099 [DOI] [PubMed] [Google Scholar]
- 23.Lerario MP, Gialdini G, Lapidus DM, Shaw MM, Navi BB, Merkler AE, et al. Risk of ischemic stroke after intracranial hemorrhage in patients with atrial fibrillation. PLoS One. 2015;10:e0145579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roumie CL, Zillich AJ, Bravata DM, Jaynes HA, Myers LJ, Yoder J, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46:465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roumie CL, Ofner S, Ross JS, Arling G, Williams LS, Ordin DL, et al. Prevalence of inadequate blood pressure control among veterans after acute ischemic stroke hospitalization: A retrospective cohort. Circ Cardiovasc Qual Outcomes. 2011;4:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravata DM, Daggy J, Brosch J, Sico JJ, Baye F, Myers LJ, et al. Comparison of risk factor control in the year after discharge for ischemic stroke versus acute myocardial infarction. Stroke. 2018;49:296–303 [DOI] [PubMed] [Google Scholar]
- 27.Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke; a journal of cerebral circulation. 1999;30:2523–2528 [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of icd-9-cm and icd-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.