Abstract
Essentially all biological processes fluctuate over the course of the day, observed at cellular (e.g., transcription, translation, signaling), organ (e.g., contractility, metabolism), and whole body (e.g., physical activity, appetite) levels. It is therefore not surprising that both cardiovascular physiology (e.g., heart rate, blood pressure) and pathophysiology (e.g., onset of adverse cardiovascular events) oscillate during the 24-hr day. Chronobiological influence over biological processes involves a complex interaction of factors that are extrinsic (e.g., neurohumoral factors) and intrinsic (e.g., circadian clocks) to cells. Here we focus on circadian governance of six fundamentally important processes: metabolism, signaling, electrophysiology, extracellular matrix, clotting, and inflammation. In each case, we discuss: 1) the physiologic significance for circadian regulation of these processes (i.e., ‘The Good’); 2) the pathologic consequence of circadian governance impairment (i.e., ‘The Bad’); and 3) whether persistence/augmentation of circadian influences contribute to pathogenesis during distinct disease states (i.e., ‘The Ugly’). Finally, the translational impact of chronobiology on cardiovascular disease is highlighted.
Keywords: Chronobiology, electrophysiology, extracellular matrix, inflammation, metabolism
PRELUDE
The purpose of this review article is to highlight recent advances made with regards to our understanding of how temporal organization of biological processes across the 24-hr hour day impact cardiovascular physiology and pathology. The majority of these studies fall into two major categories: 1) observational and interventional studies in humans; and 2) experimental studies in animal models (most commonly rats and mice). A great deal of heterogeneity exists between these studies, including the resolution of the time intervals reported (ranging from minutes to hours), the environmental conditions (e.g., light/dark cycles versus constant lighting), the natural sleep-wake patterns of the organism (e.g., diurnal humans versus nocturnal rodents), and whether sleep/wake status or neurohumoral markers of ‘circadian phase’ were assessed. In an attempt to reach a wider audience, this review article often discusses findings in terms of sleep and awake periods, making the assumption that non-shift work humans typically wake up in the morning (around 6am), while rodents wake at the beginning of the dark phase. ‘Time-of-day fluctuation’ is utilized to describe whether a parameter changes as a function of the time of the day, while ‘circadian rhythm’ is reserved to describe 24-hr oscillations in parameters that occur independently of environmental influences (i.e., intrinsically driven). Along those lines, intrinsic and extrinsic will be utilize to describe effects that originate from within or outside (respectively) a cell, organ, or organism (to be specified when the term is utilized).
Introduction
Organisms have evolved numerous strategies allowing them to accommodate daily fluctuations in stimuli/stresses on Earth. In the case of mammals, dramatic changes in both environmental factors (e.g., light intensity, temperature, humidity, predator/prey presence, etc.) and behaviors (e.g., sleep/wake and fasting/feeding cycles) must be dealt with on a daily basis. For example, upon awakening, physical activity increases as the organism forages for food, avoids predation, engages in reproductive and husbandry activities, etc.; these activities must occur, regardless of whether the forage for food is successful. Biological processes influencing cognitive function (including alertness), neuroendocrine activity, metabolic homeostasis, repair mechanisms, and cardiovascular function must occur in a cohesive and coordinated manner, thus ensuring the organism maintains a selective advantage.1, 2
It has become increasingly apparent that temporal control of many of these processes does not operate solely at the level of stimulus-response coupling, but also involves an element of anticipation (i.e., daily rhythms in many biological processes occur prior to, and therefore independent of, alterations in behavioral/environmental factors). Anticipatory mechanisms allow cells/organs/systems to prepare for stimuli/stresses prior to their onset, thus ensuring optimal physiologic responses in a temporally appropriate manner. The purpose of this article is to provide an overview of chronobiological influence over cardiovascular function, the mechanisms responsible for daily temporal partitioning of biological processes, and why this form of regulation may be advantageous (‘The Good’). Moreover, evidence will be discussed suggesting not only that circadian disruption precipitates cardiovascular dysfunction (‘The Bad’), but will also provide specific examples in which persistence/augmentation of circadian governance may play a causal role in cardiovascular disease (‘The Ugly’).
Circadian Rhythms in Cardiovascular Physiology and Pathophysiology
In healthy subjects, blood pressure, heart rate, and cardiac output decrease while sleeping.3, 4 Upon awakening, these parameters increase rapidly in association with neurohumoral fluctuations (e.g., sympathetic and autonomic tone, cortisol, epinephrine, etc.).5–7 Such observations led to early suggestions that time-of-day fluctuations in cardiovascular function occurred in response to changes in mental and physical stresses (secondary to sleep/wake, and possibly fasting/feeding, cycles). However, early studies by Kleitman (in the 1930’s) and Aschoff (in the 1960’s) revealed that approximate 24-hr fluctuations persist for distinct physiologic parameters (e.g., body temperature) when environmental conditions (e.g., lighting) are kept constant.8, 9 Regarding cardiovascular parameters, Reinberg and colleagues similarly reported persistence of approximate 24-hr fluctuations in heart rate and blood pressure when healthy volunteers were housed under constant environmental conditions.10 Recently, studies by Scheer and Shea have utilized a protocol designed to dissociate both environmental and behavioral influences on 24-hr fluctuations in cardiovascular parameters.11–13 More specifically, when either 20-hr or 28-hr environmental/behavioral cycles (combined light/dark, sleep/wake and fasting/feeding cycles; termed dyssynchrony protocol) were enforced in healthy volunteers, 24-hr fluctuations in both heart rate and blood pressure persisted. However, although the endogenous 24-hr rhythm in heart rate was similar to that observed under native conditions (rising in the morning), the endogenous 24-hr rhythm in blood pressure peaked in the early evening (this less prominent evening peak is also observed in free living healthy individuals).11, 12, 14 These studies suggest that time-of-day fluctuations in cardiovascular parameters are driven by a combination of mediators that are both extrinsic (e.g., environment- and/or behavior- related) and intrinsic to the body. One intrinsic timekeeping mechanism is the circadian clock.
Circadian clocks are cell autonomous molecular mechanisms comprised of a series of transcriptional/translational feedback loops with a cycle duration of approximately 24-hrs.1, 2 Although initially considered to be restricted to specialized neurons within the hypothalamus (more specifically the suprachiasmatic nucleus [SCN]; also known as the central clock), it is now clear that circadian clocks are found in all mammalian cells.15, 16 At the core of the mammalian clock are two transcription factors, CLOCK and BMAL1, which upon heterodimerization, bind to E-boxes within the promoter of various target genes (typically leading to induction).17, 18 These target genes include negative feedback loop components, such as Period (PER), Cryptochrome (CRY), and REV-ERB isoforms, which repress the CLOCK/BMAL1 heterodimer (in transcriptional and post-translational manners).19–21 Notably, circadian clock components (both those detailed here, as well as others; see review by Takahashi et al for a more comprehensive description of the circadian clock mechanism2) not only influence expression of one another, but also modulate expression of genes that do not directly feedback onto the CLOCK/BMAL1 heterodimer (so called clock controlled genes; CCGs). CCGs play an important role in modulating cellular function over the course of the day. It has been estimated that 3% to 10% of an organ’s transcriptome is circadian regulated, either directly (i.e., a CCG) or indirectly.22 Both unbiased (e.g., transcriptomic) and candidate (e.g., RT-PCR) approaches implicate clock control of critical cellular processes, including transcription, translation, posttranslational modifications, protein turnover, signaling, electrophysiology, and metabolism (as discussed in greater detail below).
The use of genetically modified mouse models has provided important insights regarding circadian governance of cardiovascular function. A striking phenotype is observed following germline deletion of BMAL1; 24-hr fluctuations in both heart rate and blood pressure are abolished.23 However, these mice exhibit disruption of normal sleep/wake and fasting/feeding cycles, thus hindering dissociation of contributions from cell autonomous clocks versus behaviors.23, 24 Cell type specific modulation of circadian clock components has proven to be very insightful. For example, cardiomyocyte-specific CLOCK mutant (CCM) mice exhibit attenuated time-of-day fluctuations in both heart rate and contractility.25 With regard to blood pressure, both endothelial and smooth muscle cell specific BMAL1 knockout mice have revealed that approximately one third of the daily oscillation in blood pressure is dependent on the smooth muscle cell clock.26, 27 Collectively, studies in both humans (e.g., dyssynchrony protocol) and rodents (e.g., genetically modified mice) suggest that extrinsic (e.g., neurohumoral changes associated with behaviors) and intrinsic (e.g., circadian clocks) influences act in synchrony to mediate 24-hr fluctuations in multiple aspects of cardiovascular physiology (i.e., ‘The Good’).
Circadian clocks are exquisitely sensitivity to a plethora of stimuli/stresses, allowing them to adjust to environmental perturbations (associated with, for example, seasonal fluctuations, geographical location, food sources, etc.).16 However, as a consequence, circadian clocks can become dysregulated during disease states (e.g., obesity, diabetes mellitus, sleep apnea, etc.) and/or secondary to ‘abnormal’ behaviors (e.g., eating during the usual sleep period, etc.).28–31 When considering a hypothetical example (such as mRNA levels of a circadian clock component, whose 24-hr fluctuation fits a simple cosine curve; Figure 1A), circadian dysregulation can manifest at the level of altered mesor (i.e., daily average level), amplitude (i.e., mesor-to-peak difference; Figure 1B), and/or phase (i.e., timing of the trough/peak). Interestingly, shift workers (estimated at over 25% of the workforce) have increased risk of obesity, diabetes mellitus, gastrointestinal disorders, cognitive decline, cancer, and cardiovascular disease (although it should be noted that circadian dysregulation is likely just one of several contributing factors to increased disease risk in this population).32, 33 Similarly, genetic polymorphisms in circadian clock components have also been described in humans, which are associated with increased obesity, diabetes mellitus, and hypertension incidence.34, 35 Genetic manipulation of circadian clock components in rodents also leads to numerous cardiometabolic disease states.36, 37 Moreover, germline deletion of BMAL1 results in age-onset cardiomyopathy, a phenotype that is recapitulated in cardiomyocyte-specific BMAL1 knockout (CBK) mice, revealing a critical role of the cardiomyocyte circadian clock in maintenance of normal cardiac structure and function.38, 39 Collectively, the aforementioned observations suggest that circadian disruption is frequently associated with pathology (i.e., ‘The Bad’).
Figure 1.
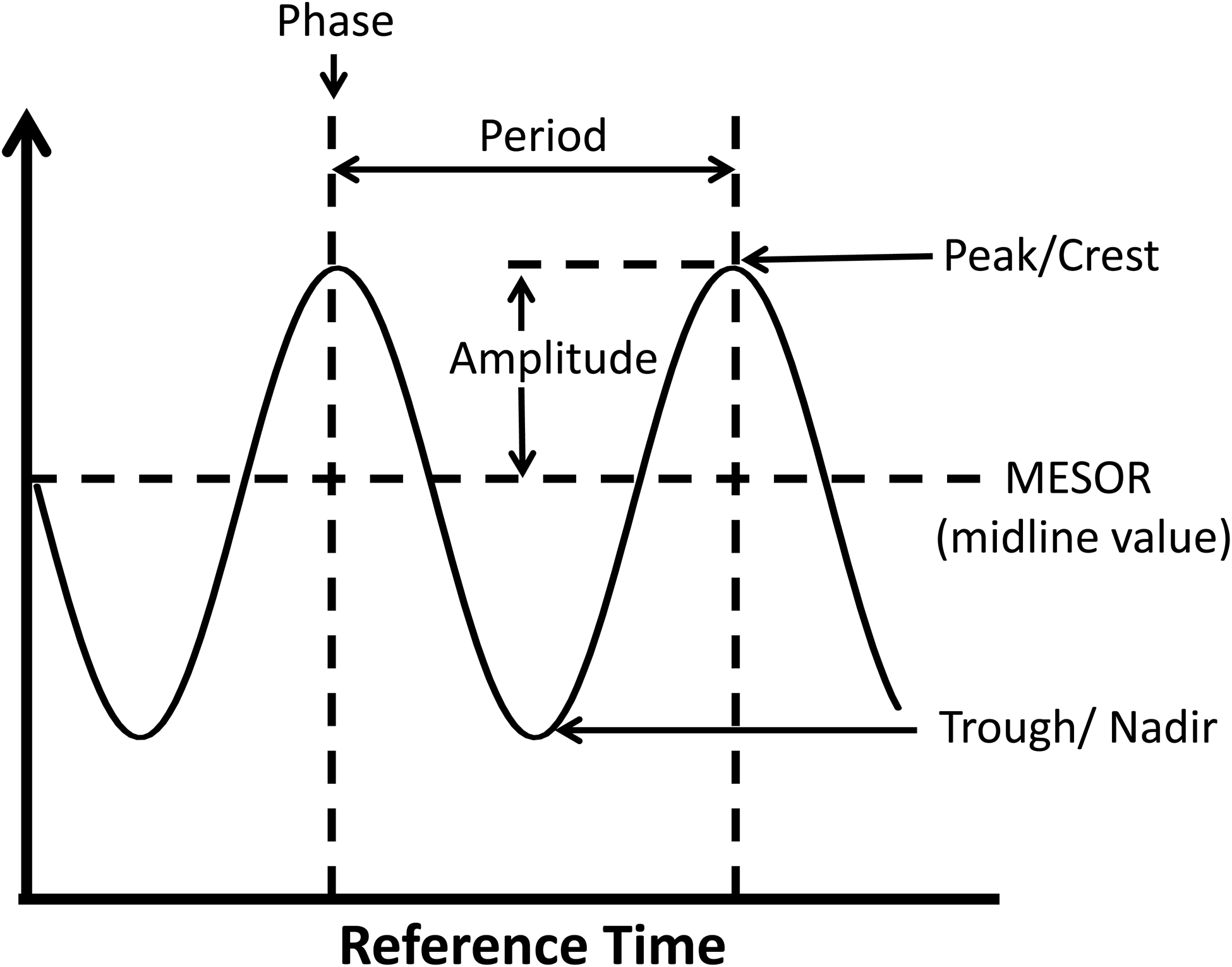
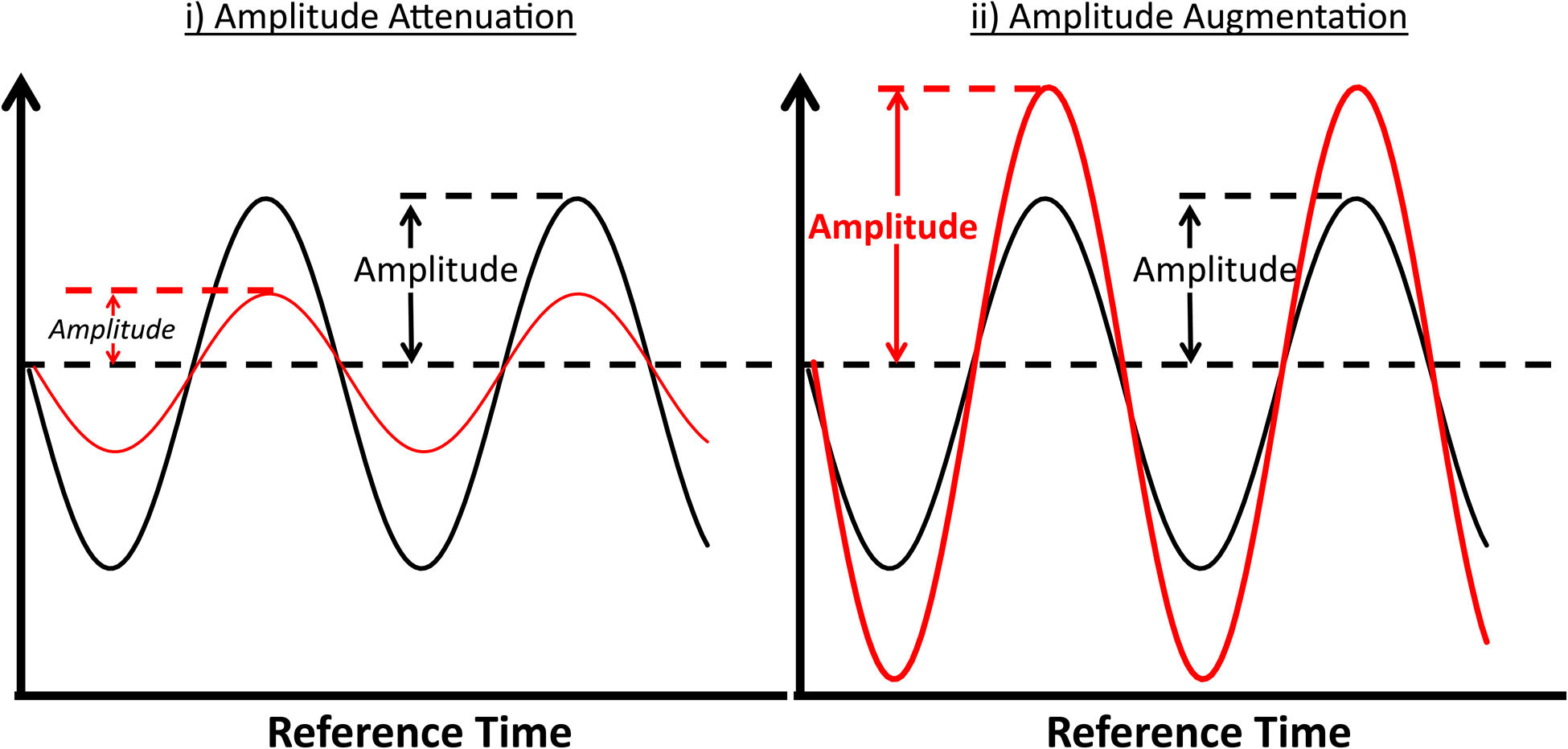
Factors/processes which fluctuate rhythmically with respect to time that fit to a cosine curve can be defined by the peak (maximal value), trough (minimal value), mesor (midline value), amplitude (peak value minus mesor value), phase (timing of the peak or trough), and periodicity (24-hrs, in the case of circadian rhythms) (A). Alterations in 24-hr fluctuations can manifest at the levels of phase (termed a phase shift), periodicity, mesor, and/or amplitude; the figure illustrates attenuation (i) and augmentation (ii) of amplitude, both of which could hypothetically lead to pathology (B).
Not all circadian clocks are regulated in an identical fashion. A striking example of differential regulation involves consideration of the central (i.e., SCN) clock versus peripheral clocks. The SCN is entrained on a daily basis by light (via the retinal-hypothalamic tract), while peripheral clocks are entrained primarily via neurohumoral signals (some of which originate from the SCN).16 Central and peripheral clocks can therefore become misaligned when physical activity or food intake occurs at an inappropriate time-of-day. For example, forcing rodents to consume food only during the sleep period phase shifts peripheral clocks, without effecting the central clock.31 An additional layer of complexity exists: not all peripheral clocks respond equally to a distinct entrainment signal. In the case of altering food availability, the liver clock responds quickly, whereas the heart clock responds slowly.40 As such, internal dyssynchrony can occur, observed at the levels of both central-to-peripheral and peripheral-to-peripheral clock misalignment. This form of circadian misalignment might occur readily in humans, and has the potential not only to attenuate a physiologic process, but also to augment pathology. With respect to the latter, the onset of adverse CV events (e.g., myocardial infarction, stroke, arrhythmia, and abdominal aorta rupture) exhibit time-of-day fluctuations in humans, rising steeply during early morning hours in predisposed individuals (e.g., presence of vulnerable plaque, ischemic heart disease, vessel wall thinning).41–44 This leads to speculation that circadian control of cardiovascular processes may contribute towards disease onset in the presence of risk factors (i.e., ‘The Ugly’). Subsequent sections in this review will elaborate further on this concept, providing specific examples.
Circadian Control of Biological Processes Influencing Cardiovascular (Dys)function
As highlighted above, it is becoming increasingly clear that biological processes known to be essential for cardiovascular function exhibit time-of-day fluctuations. Clearly, it is not feasible to provide a detailed review of all circadian regulated processes. Accordingly, here we have elected to highlight circadian regulation of six biological processes known to play critical roles in cardiovascular function and dysfunction: metabolism, cellular signaling, electrophysiology, extracellular matrix, clotting, and inflammation. In each case, we will discuss: 1) the physiologic significance for circadian regulation of these processes (i.e., ‘The Good’); 2) the pathologic consequence of circadian governance impairment (i.e., ‘The Bad’); and 3) whether persistence/augmentation of circadian influences contributes to pathogenesis during distinct disease states (i.e., ‘The Ugly’).
Metabolism
The Good.
Consistent with fluctuations in energetic demand and nutrient availability during normal sleep/wake and fasting/feeding cycles, metabolic fluxes change dramatically over the course of the day, at both whole body and tissue-specific levels.45, 46 This form of metabolic plasticity serves multiple purposes. In addition to homeostatic roles (e.g., maintenance of blood nutrients within a physiologic range), temporal partitioning of metabolism ensures that a cell’s needs are met at all times of the day. Examples include providing ATP for augmented myosin cross-bridge cycling and ion channel activity during periods of increased physical activity, providing building blocks for replacement of cellular constituents during reparative periods, as well as providing metabolism-derived signaling molecules (e.g., AMP) and posttranslational modification precursors (e.g., UDP-glucosamine for protein O-GlcNAcylation) at an appropriate time-of-day.47
With regard to the cardiovascular system, circadian governance of cardiac metabolism has been investigated to the greatest extent (as reviewed previously47–49). Rodent studies reveal that upon awakening, myocardial oxidative metabolism increases (particularly glucose oxidation, secondary to increased glucose uptake and mitochondrial oxidative capacity), enabling the heart to meet increased energetic demand at this time.25, 50–52 During the latter half of the awake period, anabolic processes become more active, including replenishment of myocardial glycogen and triglyceride (which are subsequently utilized during the sleep period).52, 53 The beginning of the sleep period is a time of increased cellular constituent turnover, when processes such as protein synthesis and degradation (e.g., autophagy) are activated simultaneously (theoretically enhancing cellular function integrity prior to awakening).54, 55 Daily fluctuations in cardiac metabolism appear to be mediated in large part by the cardiomyocyte circadian clock (as these rhythms are severely attenuated following genetic clock ablation in murine models).25, 52, 54 Moreover, many time-of-day fluctuations in cardiac metabolism have been described in ex vivo perfused rodent hearts, during which acute neurohumoral influences are excluded.
Multiple mechanistic links between the cardiomyocyte circadian clock and cardiac metabolism have been proposed, including direct clock control of metabolic enzymes (e.g., diacylglycerol acyltransferase 2 [DGAT2; involved in triglyceride synthesis], nicotinamide phosphoribosyltransferase [NAMPT; involved in NAD metabolism]) and signaling components (e.g., regulatory subunit of phosphoinositide-3-kinase [p85α; involved in insulin signaling]).39 In vivo, factors intrinsic (i.e., cardiomyocyte circadian clock) and extrinsic (i.e., neurohumoral factors) to the heart likely act cooperatively, thus facilitating synchrony of metabolic rhythms. For example, increased contractility and β-adrenergic stimulation upon awakening will both augment glucose uptake and glycolytic flux, while post-prandial secretion of insulin during the awake period will accentuate nutrient uptake and storage.56, 57 Although little is known regarding circadian control of cardiac metabolism in humans, numerous studies have detailed daily fluctuations in whole body metabolism; dyssynchrony and misalignment protocols (see above) suggest that multiple aspects of metabolic homeostasis (e.g., glucose tolerance) are regulated in part by endogenous circadian clocks.13, 58
The Bad.
As temporal partitioning of cardiac metabolism plays important roles in ATP provision, cellular constituent integrity, and signaling, it is not surprising that disruption of circadian governance over cardiac metabolism yields detrimental effects. Genetic manipulation of either CLOCK or BMAL1 in mice results in a model of temporal suspension, such that the clock is essentially ‘paused’ at the beginning of the sleep phase (a time-of-day at which glucose utilization is low, while protein turnover is elevated).25, 39 As such, CCM and CBK mouse hearts exhibit attenuated rates of glucose utilization and augmented rates of protein synthesis independent of the time-of-day.25, 39, 52, 54 These metabolic perturbations would be predicted to limit ATP generation during periods of high energetic demand, and promote cardiac remodeling; this phenotype is observed in both CCM and CBK mice.
Workload challenge studies serve as excellent examples. Exercise simulation ex vivo (i.e., increased afterload plus epinephrine) unveils greatest contractile reserve and glucose utilization in wild-type hearts during the active period.25 In marked contrast, CCM hearts do not show time-of-day fluctuations in either contractile reserve or glucose utilization during this acute workload challenge; these parameters are chronically blunted, at levels similar to those observed in wild-type hearts at the beginning of the sleep phase.25 Similarly, when CCM mice are provided running wheels, these mice exhibit an approximate 2-fold lower voluntary running capacity, compared to wild-type littermates.59 Moreover, when challenged with increased workload chronically (daily isoproterenol injection, for one week), wild-type hearts exhibit greatest hypertrophic (remodeling) responses at the beginning of the sleep phase (consistent with increased protein synthesis at this time); this time-of-day specific remodeling was absent in CCM hearts.60 Interestingly, both CCM and CBK hearts exhibit a pro-hypertrophic phenotype even in the absence of an interventional stimulus/stress.39, 60, 61 In the case of CBK hearts, hypertrophic growth worsens with age, ultimately precipitating dilated cardiomyopathy (a phenotype also observed in germline BMAL1 null mice).39 This pro-hypertrophic phenotype is in part secondary to chronic mTOR-mediated protein synthesis, as the mTOR inhibitor rapamycin normalized protein synthesis and cardiac mass in CBK mice.54
In contrast to rodent studies, little is known regarding the impact of circadian disruption on cardiac metabolism in humans. However, both behavioral (e.g., shift work, sleep disorders, etc) and genetic (e.g., polymorphisms in clock genes) influences on circadian rhythms negatively impact whole body metabolism, predisposing individuals to common cardiometabolic diseases (e.g., obesity, diabetes mellitus).32–35, 62
The Ugly.
While disruption of circadian control over cardiac metabolism is detrimental, we further postulate that persistence of daily oscillations in metabolic processes may also accelerate cardiac disease (particularly in the presence of common cardiovascular risk factors). One example might be the increased risk of left ventricular (LV) hypertrophy reported in non-dipping hypertensives.63 In healthy individuals, blood pressure decreases more than 10% during the sleep period.4 We have hypothesized that circadian governance of metabolism ensures that pro-hypertrophic stimuli (e.g., shear stress associated with increased blood pressure) is not normally present when myocardial cellular constituent turnover is active. During non-dipping hypertension, normal lowering of blood pressure during sleeping is attenuated, such that blood pressure remains elevated at a time when processes involved in cardiac remodeling are augmented (which could hypothetically promote LV hypertrophy).64 Evidence in support of this concept was highlighted in the previous sub-section (i.e., isoproterenol promotes adverse remodeling only during the sleep phase).60
A second hypothetical example is lipotoxicity during dyslipidemic conditions, such as obesity (Figure 2). A mismatch between fatty acid (FA) availability and utilization (oxidation plus storage as triglyceride) can lead to accumulation of lipid-based signaling molecules (e.g., ceramide, phospholipids, acyl-carnitines) within cardiomyocytes, resulting in lipotoxic cardiac dysfunction.65–67 Prior studies report circadian regulation of triglyceride turnover in the heart, with high rates of triglyceride synthesis during the awake period, and high rates of lipolysis during the sleep period.53 Persistence of this circadian governance during dyslipidemic states (e.g., obesity) augments expansion of the myocardial triglyceride pool specifically during the awake period (i.e., a time at which both triglyceride synthesis capacity and substrate are elevated).53 This expanded triglyceride pool can subsequently undergo lipolysis during the sleep period (a time at which oxidative metabolism is low), thus facilitating lipotoxic species generation. Given that multiple lipid-based species are known to influence cellular signaling, inflammation, and electrophysiology, we postulate that augmentation of triglyceride turnover rhythms contributes toward the increased incidence of myocardial infarction and sudden cardiac death risk in the early hours of the morning, particularly in obese individuals.
Figure 2.
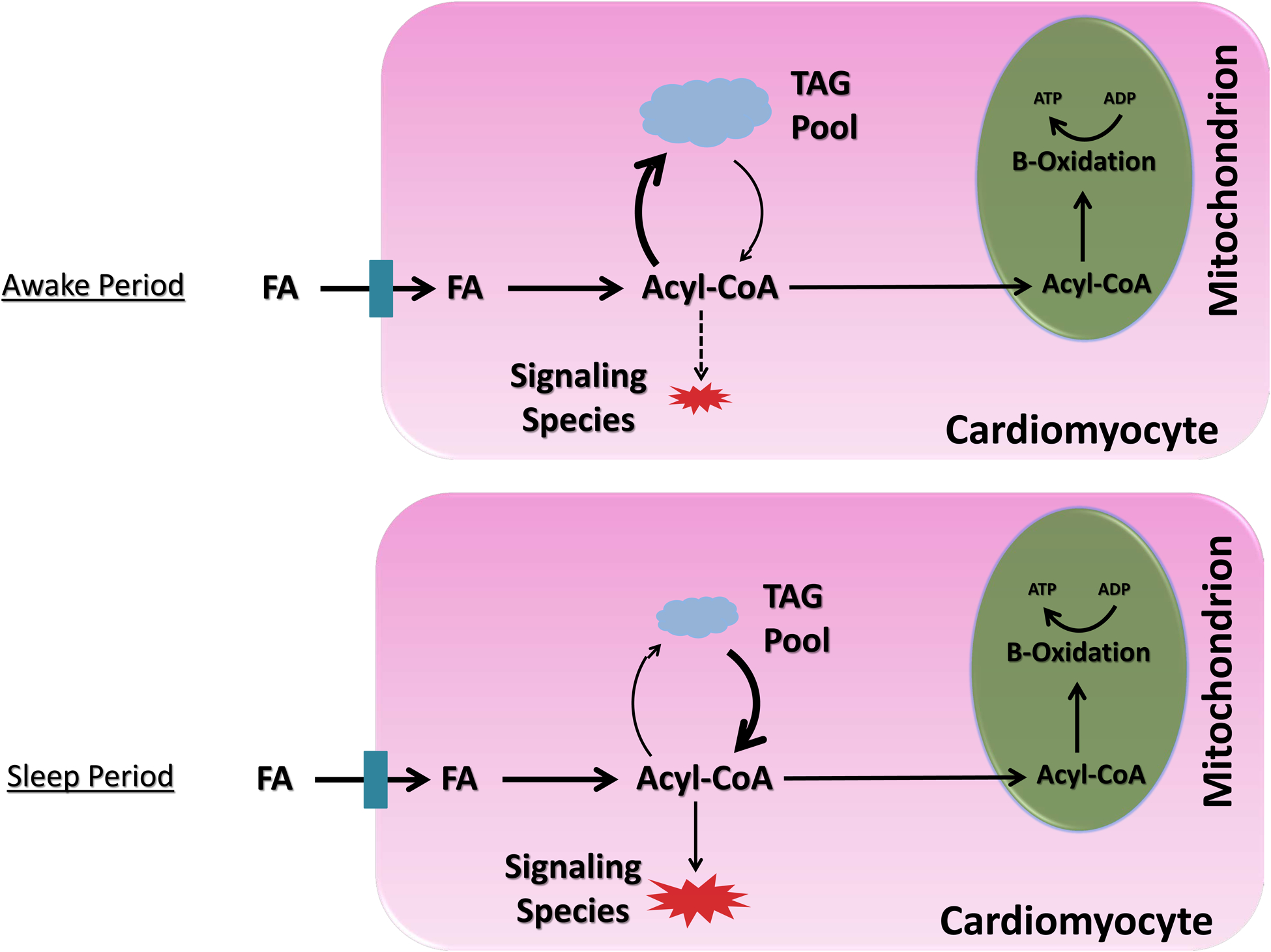
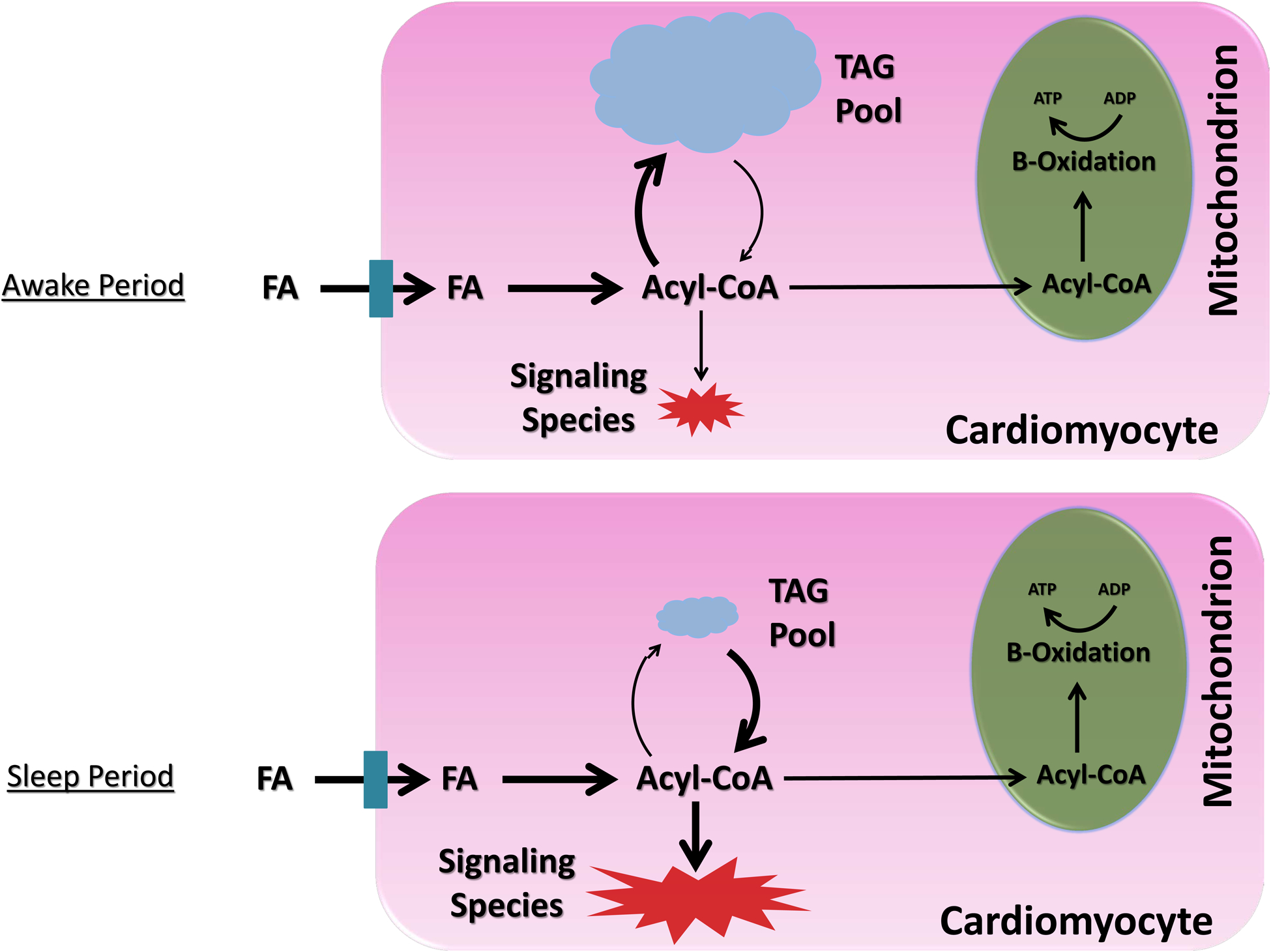
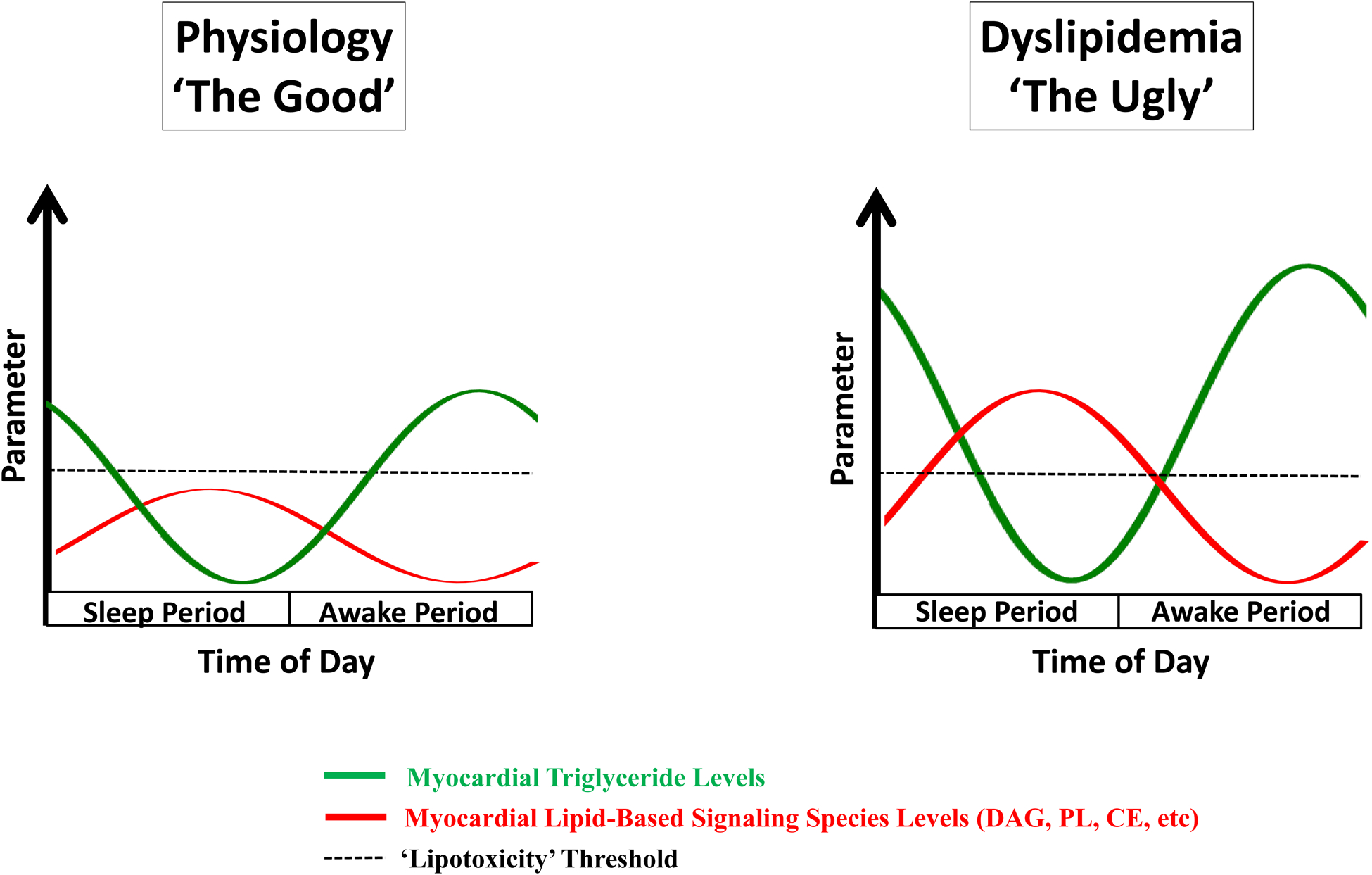
Evidence-based model for time-of-day fluctuations of myocardial fatty acid metabolism during physiologic (A) and dyslipidemic (B) states. A) Under physiologic conditions, fatty acids (FA) in excess of β-oxidation requirements are utilized cardiomyocyte triglyceride (TAG) synthesis during the awake period, whereas synthesis of FA-derived signaling molecules (e.g., diacylglycerol [DAG], cholesterol esters [CE], phospholipids [PL]) is low at this time; during the sleep period, rates of lipolysis increase, releasing FA from the TAG pool, which are utilized for both β-oxidation and signaling molecule synthesis. B) Under conditions of FA excess (i.e., dyslipidemia), there is a dramatic expansion of the TAG pool during the awake period (due to clock control of TAG synthesis); during the sleep period, lipolysis of the expanded TAG pool occurs, resulting in excessive synthesis of FA-derived signaling molecules, and subsequent cellular dysfunction (i.e., ‘lipotoxicity’). Illustration depicting 24-hr fluctuations in myocardial levels TAG and lipid-based signaling species during physiologic and dyslipidemic states (C).
Cellular Signaling
The Good.
Neural activity (e.g., sympathetic tone), autocrine/paracrine/endocrine signals (e.g., cortisol), and shear stresses (e.g., blood pressure) all fluctuate as a function of time-of-day, and elicit cellular responses through numerous signaling pathways.4, 6, 68 Equally important to the level of the stimulus is the sensitivity of the target tissue, which is also subject to daily oscillations; fluctuations in the stimulus and target tissue sensitivity collectively impact activation state of a signaling pathway at different times of the day. Changes in absolute levels of signaling cascade components (via protein turnover), as well as posttranslational modifications (e.g., phosphorylation, acetylation, O-GlcNAcylation, etc.) and pathway endpoints (e.g., transcriptional, metabolic, and functional outcomes) have been described over a 24-hr period in various tissues, including many that are critical to cardiovascular function.22, 52, 68–72
In the heart, phosphorylation and/or activation status of signaling components such as AMPK (AMP-activated protein kinase; energetic/nutritional sensor), AKT (protein kinase B; influences cell survival, growth, and metabolism), GSK3β (glycogen synthase kinase β; cell survival and metabolism modulator), mTOR (mammalian target of rapamycin; nutrient and growth factor sensor), PKA (protein kinase A; mediator of β-adrenergic signaling), ERK (extracellular signal-regulated kinase; involved in stress and growth factor response), and calcineurin (calcium-regulated protein phosphatase) exhibit time-of-day fluctuations.53–55, 59, 73 Increased PKA activity at the beginning of the active period has been proposed to promote contractility (through, for example, increased phospholamban phosphorylation and subsequent SERCA2 activity), while increased calcineurin activity at the awake-to-sleep transition appears to reverse PKA-mediated effects.73 Interestingly, key components of this system are regulated by the cardiomyocyte circadian clock; 24-hr oscillations in both calcineurin and the regulatory subunit of PKA are lost in CCM hearts.25 Such observations, as well as reports that β-adrenergic receptor density exhibits time-of-day fluctuations in the heart74, suggest that factors intrinsic and extrinsic to the heart collaboratively mediate 24-hr rhythms in this signaling system.
The cardiomyocyte circadian clock has also been implicated as a significant modulator of insulin signaling in the heart. Time-of-day fluctuations in multiple insulin signaling components are disrupted in both CCM and CBK hearts.39, 54 One example includes the regulatory subunit of PI3K (p85α), a direct BMAL1/CLOCK target gene.39 Interestingly, cardiac insulin sensitivity is higher at the beginning of sleep phase, a time at which circulating insulin levels are usually low.54, 68 When mice are subjected to prolonged continuous fasting (thus chronically lowering insulin levels), the phosphorylation status of downstream insulin signaling components (mTOR and S6) still fluctuate, with peak levels at the awake-to-sleep transition.55 This suggests that the cardiomyocyte circadian clock augments insulin signaling at this time, regardless of feeding status. It has been suggested that this occurs to promote protein synthesis during the reparative sleep phase. It is noteworthy that circadian governance of cardiac sensitivity to neurohumoral influences that result in differential transcriptional responses have been reported; for example, cardiac transcriptional response to both fatty acids and thyroid hormone is augmented during the awake period.75, 76
The Bad.
As highlighted above, 24-hr fluctuations in signaling are the product of changes in both the extracellular milieu (e.g., neurohumoral factors and shear stress) and intracellular sensitivity, both of which can be mediated by circadian clocks. When stimulus and sensitivity rhythms are in alignment, mesor-to-peak differences (i.e., amplitude) in the activation status of a signaling cascade are augmented. Conversely, when 24-hr rhythms of these two parameters are antiphase, the amplitude of signaling cascade activation is attenuated. Hypothetically, both scenarios could present benefit for homeostasis, by maximizing performance in response to an anticipated stimulus (e.g., heart rate increase during exercise) and/or ensuring tolerance to a stress (e.g., limiting hypertrophic growth in response to a daily rise in blood pressure). Therefore, depending on the nature of circadian governance, loss of normal rhythms will result in either chronic activation or inactivation of a signaling cascade. A number of examples exist. Genetic disruption of CLOCK and/or BMAL1 specifically in cardiomyocytes leads to loss of time-of-day fluctuations in the phosphorylation status of numerous kinases; for some, phosphorylation is chronically high (e.g., AKT, mTOR, ULK1, and ERK1/2), while for others it is chronically low (e.g., AMPK, GSK3β).53, 54, 59 Many of these signaling components have been implicated in adverse cardiac remodeling. For example, chronic activation of the PI3K/AKT/mTOR axis (as observed in CCM and CBK hearts) is typically associated with hypertrophic growth. Circadian disruption at the whole body level also leads to aberrant AKT signaling in the heart and vasculature.77, 78 In the latter, perturbations in AKT were associated with impaired day-night differences in eNOS and endothelial function (e.g., acetylcholine-mediated vasodilation).78
The Ugly.
Daily fluctuations in cellular signaling undoubtedly have the potential to contribute towards known time-of-day fluctuations in both the onset of, and susceptibility to, cardiovascular pathology. Cardiac ischemia/reperfusion (I/R) tolerance is influenced by a host of secondary messengers (e.g., cAMP, cGMP, Ca2+) and posttranslational modifications (e.g., phosphorylation, acetylation, O-GlcNAcylation, sumoylation, nitrosylation), as well as numerous signaling cascade components (e.g., PKA, PKG, PKC, PKB/AKT, GSK3β, calcineurin).79, 80 Almost all of these factors have been reported to be circadian regulated. However, it is noteworthy that time-of-day fluctuations may differ between basal and stressed states. Calcineurin serves as an excellent example. In the basal state, calcineurin activity (which is known to decrease I/R tolerance81) peaks in the heart at the beginning of the sleep phase (which is inconsistent with time-of-day fluctuations in cardiac I/R tolerance).73, 82 However, both pharmacological and genetic inhibition of calcineurin attenuate I/R-induced damage only at the beginning of the active period (with no effect at the beginning of the sleep phase), suggesting that I/R-induced activation of calcineurin contributes to day-night differences in I/R tolerance.82 Such observations would be explained by greater induction of calcineurin activators (e.g., Ca2+, calpain) and/or greater repression of calcineurin inhibitors (e.g., MCIP) in response to I/R at the beginning of the active phase.82 Daily fluctuations in the sensitivity of the cardiovascular system to stresses/stimuli may also help explain why inappropriately stressing the heart during the sleep phase (e.g., shift work, sleep apnea, non-dipping hypertension) is associated with greater risk of cardiovascular mortality. A steep rise in susceptibility to cardiac arrhythmias is also observed at the sleep-to-wake transition, a time at which PKA is more active, leading to speculation that increased PKA activity at this time may play a contributing role (e.g., through promotion of Ca2+ sparks).41, 73, 83 Such a concept will be discussed further in the Electrophysiology subsection below.
Electrophysiology
The Good.
Twenty-four hour rhythms in cardiac electrophysiology are firmly established. In humans, heart rate increases sharply during the morning, associated with changes in cognitive and physical activity status.3, 4 Consistent with circadian governance, studies by Hu et al in 2004 revealed that when healthy volunteers were forced to live a 28-hour day (i.e., forced dyssynchrony), a 24-hr rhythm in heart rate persisted.14 The endogenous 24-hr rhythm peaked at a time corresponding to 10am, and had an amplitude of approx. 11%;14 a normal daily rhythm (i.e., that observed in volunteers experiencing a normal 24-hr day) has an amplitude of approx. 50%.4 Time-of-day fluctuations have also been reported in humans for heart rate variability (HRV) and QT interval, which are increased during the early morning and night, respectively;84, 85 24-hr rhythms in these parameters persist when environmental/behavioral parameters are modulated, again suggesting mediation (at least in part) by an intrinsic timekeeping mechanism.86, 87
The use of genetically modified mouse models reveals a complex contribution of circadian clocks. For example, day-night differences in heart rate are severely attenuated in both germline BMAL1 knockout and CLOCKΔ19 mutant mice; in both cases, the mesor (daily average) heart rate is lower (i.e., bradycardia).23 However, germline deletion of the CLOCK paralogue NPAS2 also leads to bradycardia, but does not influence day-night differences in heart rate.23 Given that germline disruption of circadian clock components can impact both behaviors and systemic factors36, cell type specific models have been employed. Cardiomyocyte-specific CLOCK mutant mice exhibit a reduced amplitude of day-night differences in heart rate (with bradycardia again being observed); the amplitude was partially attenuated (by approximately one third).25 In contrast, cardiomyocyte-specific inducible Bmal1 knockout mice exhibit normal day-night differences in heart rate.88 These observations suggest that circadian regulation of extra-cardiac influences (such as autonomic tone) likely play an important role in mediating time-of-day fluctuations in heart rate, with a lower contribution of the cardiomyocyte circadian clock. Interestingly, deletion of PPARγ (a putative regulator of BMAL1) in either endothelial or vascular smooth muscle cells diminishes day-night differences in heart rate, suggesting a complex interplay between autonomic control, the cardiomyocyte circadian clock, and additional local oscillators.89
Multiple K+, Na+, and Ca2+ ion channels are circadian regulated in the heart. These include KCHIP2, KCNA5, KCND2, KCNH2, KCNK3, SCN5a, and CACNA1D.59, 88, 90–93 Additional channel subunits have also been identified through omics approaches, but have yet to be validated. In several instances, daily rhythms in ion channel components are associated with predicted day-night differences in cardiomyocyte electrophysiological properties. For example, steady state current (IKur) density was greater in rat myocytes during the active period (relative to the sleep period), a time at which KCNA5 protein levels are higher.93 In many instances, rhythms in these ion channel subunits are lost following disruption of circadian clocks (both whole body and cardiomyocyte-specific) and/or autonomic blockade. CACNA1D, encoding for a subunit in L-type voltage gated ion channels, is rhythmic at both mRNA and protein expression levels in wild-type hearts, but not CCM hearts.59 Another subunit of this channel, CACNA1C, may also be regulated by the cardiomyocyte clock, not only at the level of expression, but also post-translationally via the PI3K/AKT signaling axis (which is clock controlled; see Signaling sub-section).94 Together, these studies illustrate that the electrophysiologic properties of the heart are markedly different depending on the time-of-day, which likely play an important role in accommodating daily fluctuations in contractile demands.
The Bad.
As highlighted in the preceding subsection, genetic disruption of the central circadian clock components (i.e., BMAL1, CLOCK, and/or NPAS2) in either germline, cardiomyocyte specific, and/or constitutive/inducible manners, results in a bradycardia phenotype.23 In addition, Schroder et al have reported that cardiomyocyte-specific inducible Bmal1 knockout mice exhibit an increased QTc interval (particularly during the sleep period), which is known to increase arrhythmia risk in humans.88 Consistent with this, many of these mouse models exhibit pro-arrhythmic phenotypes. For example, ECG recording in cardiomyocyte-specific inducible Bmal1 knockout mice revealed increased episodes of pauses that were not observed prior to deletion of Bmal1.88 Moreover, an increased preload challenge ex vivo results in greater arrhythmia incidence in the cardiomyocyte-specific inducible Bmal1 knockout hearts.88 Studies by Jeyaraj et al suggest that the transcription factor Klf15 serves as a mechanistic link between the circadian clock and KCIP2 (transient outward potassium current subunit); genetic deletion of KLF15 abolishes time-of-day fluctuations in both KCIP2 and QTc interval.92 Importantly, QTc interval was elevated throughout the 24-hr period in KLF15 knockout mice, associated with increased susceptibility to pacing-induced arrhythmias.92 Thus, impairment of normal circadian governance over critical ion channels in the heart can increase arrhythmia risk. Notably, cardiac arrhythmia susceptibility is also increased secondary to morphological (e.g., hypertrophy, fibrosis) and conduction (e.g., connexin) abnormalities; genetic deletion of circadian clock components adversely affects these parameters. For example, CCM and CBK mice both exhibit increased cardiomyocyte size and fibrosis, which is exaggerated with age.39, 60, 61 Connexin-40 is circadian regulated in the atria, being chronically repressed in CCM hearts; interestingly, both connexin-40 null and CCM mice exhibit bradycardia.25 Collectively, these observations suggest that circadian disruption may lead to increased arrhythmia susceptibility through a host of potential mechanisms.
The Ugly.
The incidence of sudden cardiac death has a striking time-of-day fluctuation, rising sharply in the early hours of the morning, and remaining elevated throughout most of the awake period.41, 95 Such observations have led to suggestions that pro-arrhythmic triggers, such as increased workload and adrenergic stimulation (which are themselves subject to circadian governance), potentially play a significant role in the dependency of sudden cardiac death to the time-of-day.41 This leads to questions regarding why these triggers do not precipitate arrhythmias on a daily basis in all individuals. Numerous possibilities exist. One is that in healthy individuals, protective mechanisms are present during the awake period, thus reducing arrhythmogenesis susceptibility at this time. For example, HRV (a strong anti-arrhythmic ‘mechanism’) normally increases in the early hours of the morning, thus affording protection (Figure 3).84 However, during ischemic heart disease, time-of-day fluctuations in HRV are severely attenuated, and are chronically low, resulting in a loss of protection.96 It is noteworthy that the heart exhibits increased sensitivity to distinct ion channel inhibitors (e.g., oubain97) at certain times of the day, leading to speculation that time-of-day fluctuations in ion channel activity may make the heart more susceptible to elevated stresses and/or arrhythmic triggers present upon awakening during disease states.
Figure 3.
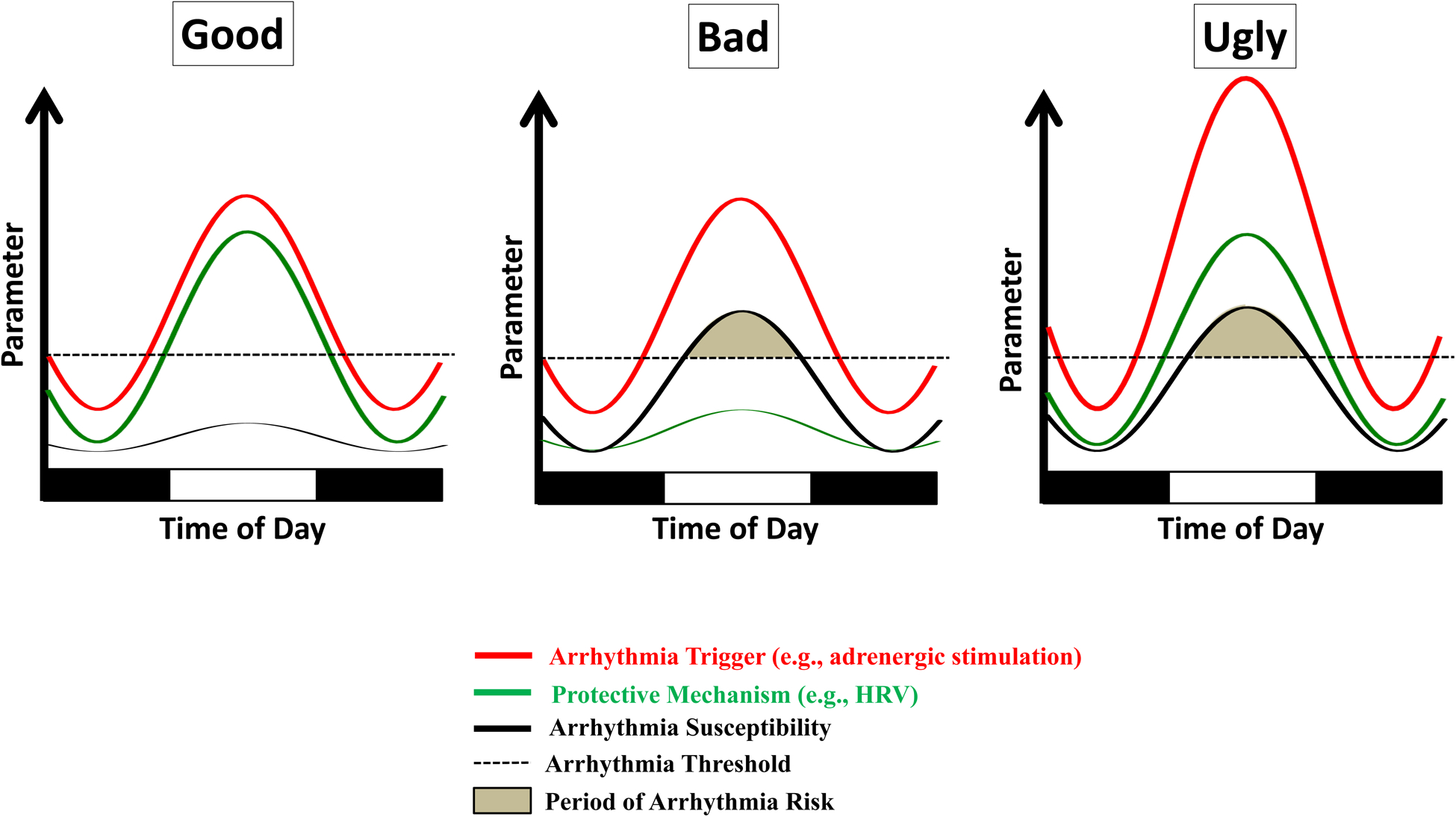
Hypothetical circadian influences on arrhythmia risk. During physiologic conditions, oscillations in factors that have the potential to trigger arrhythmias (e.g., adrenergic stimulation) are paralleled with oscillations in protective mechanisms (e.g., heart rate variability; HRV), such that arrhythmia susceptibility is low (the ‘good’). An attenuation in protective mechanisms (e.g., decreased HRV following myocardial infarction) increases arrhythmia susceptibility above the threshold when arrhythmia triggers are present (the ‘bad’). Augmentation of arrhythmia trigger oscillations also have the potential to increase arrhythmia susceptibility above the threshold (the ‘ugly’).
Aberrant circadian governance of ion channels in specific disease states is also possible. For example, in preceding sections, we hypothesized that lipid-based signaling species likely accumulate in the heart during the sleep phase, which have the potential to impair electrophysiology-based cardioprotective mechanisms in dyslipidemic subjects upon awakening. Moreover, if temporal regulation of cardiac ion channels become phase shifted, pro-arrhythmic triggers and susceptibility would become aligned. Interestingly, circadian clocks (and therefore clock controlled processes) are phase shifted through environmental exposures (e.g., light) and behaviors (e.g., food intake), as well as during numerous disease states (e.g., diabetes mellitus).16, 28–31 Another possibility involves factors that increase arrhythmia susceptibility independent of the time-of-day, such as structural/morphological impairments (e.g., fibrosis).98 In some instances, a combination of these distinct mechanisms has the potential to contribute (e.g., a diabetic patient with ischemic heart disease may exhibit chronically low HRV, a phase shift in the circadian clock, and adverse cardiac remodeling).
Extracellular Matrix
The Good.
The extracellular matrix (ECM) has a diverse array of functions, ranging from maintenance of tissue integrity, to modulation of platelet aggregability, interaction with inflammatory cells, and serving as a reservoir for numerous paracrine signals.99 Although often perceived as relatively static in certain tissues (e.g., skin) and following injury (e.g., scars), the ECM is fairly dynamic in many tissues, during physiologic conditions. In the heart, approximately 20% of collagen (a primary ECM component) undergoes daily turnover (compared to only 5% in skin).100 Evidence is emerging that the ECM is circadian regulated, such that processes involved in turnover are more active at one time of the day relative to another.
Recent studies investigating collagen synthesis/secretion in tendons serve as an excellent example. In tendons, the size of collagen fibrils vary as a function of time-of-day, associated with increased collagen-1 protein levels at the beginning of the sleep phase.101 Through the use of complementary molecular and biochemical studies, Chang et al revealed increased collagen synthesis during the active period, which was subsequently secreted towards the active-to-sleep phase transition.101 Circadian regulation of multiple mediators/ regulators of collagen-1 translation and secretion was identified, including Sec61a2 (Sec61 translocon alpha 2 subunit; involved in transfer of collagen into endoplasmic reticulum [ER]), Mia3 (melanoma inhibitory activity family, member 3; involved in cargo loading of collagen in ER), Pde4d (phosphodiesterase 4d; modulates phosphorylation status of transport machinery components via protein kinase A), and Vps33b (vacuolar protein sorting 33 homolog B; involved in vesicle transport).101 Secretion of new collagen at the beginning of the sleep phase potentially aids in tendon repair and strengthening prior to awakening (and physical stressors associated with muscle activity).
Circadian governance of the ECM also includes fibroblast activation in response to injury (critical for generation of new ECM and subsequent fibrosis); here we will consider skin laceration as a physiologic injury (as this can occur as a consequence of normal daily physical activities). Hoyle et al reported approximate 2-fold faster wound healing following laceration during the active period (compared to the sleep period) in both mice and humans, secondary to greater mobilization and activation of fibroblasts.102 Use of genetically modified mouse models confirmed mediation of this daily rhythm by the intrinsic fibroblast circadian clock, leading to speculation that temporal governance occurs in anticipation of greater likelihood of injury during the awake period (while animals forage for food and avoid predation).
Unbiased transcriptomic studies support the concept that the ECM is also regulated in a circadian fashion within cardiovascular tissues (such as heart and aorta).103, 104 For example, multiple isoforms of collagen (COL; e.g., COL1A1, COL3A1, COL4A1, COL4A2), matrix metalloproteases (MMP; e.g., MMP13), tissue inhibitors of MMPs (TIMPs; e.g., TIMP3), and secretory pathway components (e.g., VSP33b), exhibit time-of-day fluctuations in wild-type hearts, which are abolished in CBK hearts, suggesting temporal governance by the cardiomyocyte circadian clock.39 Further studies are required to characterize fully the extent to which ECM in cardiovascular tissues is circadian regulated, and whether temporal control is critical for anticipation of daily fluctuations in parameters such as shear stress and contractile demand.
The Bad.
Both germline and tissue-specific manipulation of circadian clock components result in increased fibrosis in multiple tissues. For example, mouse tendons isolated from germline CLOCKΔ19 mutant mice exhibit abnormally large collagen deposits.101 This same mouse model exhibits increased sensitivity to bleomycin-induced pulmonary fibrosis, as well as augmented age-onset fibrosis in the heart.77, 105 The latter fibrotic phenotype may be secondary to disruption of local oscillators (as opposed to systemic effects). Indeed, similar to CLOCKΔ19 mutant mice, CCM mice exhibit augmented age-onset cardiac fibrosis.60 Moreover, modest fibrosis is also observed in CBK mouse hearts, which worsens with age.39, 61 Given that CCM and CBK mice are models of temporal suspension at the beginning of the sleep phase, a time at which collagen secretion is predicted to be increased (based on studies in the tendon), it is conceivable that continuous collagen secretion throughout the day leads to this pro-fibrotic state. Consistent with this concept, expression of multiple circadian-regulated collagen isoforms are elevated in CBK hearts.61
Questions arise regarding how dysfunction of the cardiomyocyte circadian clock could cause myofibroblast activation. Prior studies suggest that factors such as TGFβ, AngII, and miRNAs (packaged within exosomes) act in a paracrine manner, transmitting signals from cardiomyocytes to fibroblasts (and vice versa);106, 107 circadian regulation of these factors has been proposed in numerous tissues. Interestingly, the phosphorylated form of SMAD2 is dramatically elevated in CBK hearts, consistent with augmented TGFβ signaling and subsequent fibrosis.61 Not only does circadian disruption lead to cardiac fibrosis, but so too does circadian misalignment. The most compelling evidence comes from studies investigating tau mutant hamsters, which due to a spontaneous mutation in the CK1ε gene, have an intrinsic circadian clock with a periodicity of 22 hours.108 When these mutant hamsters are housed in a normal 12-hr light 12-hr dark cycle (i.e., misalignment between the intrinsic 22-hr clock and the extrinsic 24-hr environment) they develop cardiac (and renal) fibrosis. However, fibrosis is prevented when tau mutant hamsters are housed in an 11-hr light 11-hr dark cycle, thus re-establishing circadian alignment.108 The potential translational importance of these observations is underscored when considering shift workers, who routinely experience circadian-related stresses (and have increased CVD risk). The circadian environment is also disrupted when patients are admitted to the critical care unit, during which patients are exposed to irregular lighting, noise, sleep, and nutritional patterns. Recent studies by Alibhai et al reported that disrupting the light/dark cycle following an MI was sufficient to increase infarct expansion and fibrosis in mice (associated with a worsening of contractility).109
The Ugly.
Fibroblast activation and fibrosis following an adverse event (such as myocardial infarction; MI) is a double-edged sword: too little fibrosis results in a scar with poor structural integrity (increasing rupture susceptibility), whereas excessive fibrosis contributes to tissue/organ dysfunction (through, for example, myocardial stiffening and/or promotion of prolonged inflammation). This leads to the question whether circadian governance over the ECM and/or fibroblast activation impacts tolerance of the heart to adverse stress. Here, we will consider ischemic events, non-dipping hypertension, and arrhythmias. Observational studies indicate that in addition to an increased incidence of MI in the morning, MI tolerance appears to be low at this time (resulting in greater myocardial damage).41, 110 Similar studies in rodents confirm lowest tolerance of the heart to MI or I/R at the beginning of the active period; ischemic events at this time lead to augmented fibrosis.82, 111 As mentioned above, fibroblast mobilization and activation potential is increased during the awake period, raising the possibility that hyperactivation of myofibroblasts in the morning results in excessive fibrosis, thereby exacerbating myocardial damage.
Cardiac fibrosis is also a hallmark of hypertension, likely playing a significant role in the etiology of contractile dysfunction.112 Prolongation of elevated blood pressure during the sleep period in non-dipping hypertensives predisposes to worse clinical outcomes, such as left ventricular hypertrophy.63 As highlighted above, the sleep phase appears to be a reparative period, including replacement of the ECM; persistence of shear and hemodynamic stresses during sleep could potentially augment collagen secretion, thereby worsening fibrosis. A similar scenario would be hypothesized during sleep apnea, during which inappropriate sympathetic tone during sleep is associated with cardiac fibrosis.113 Finally, arrhythmia incidence increases dramatically upon awakening (persisting throughout the active period).41 Arrhythmia risk is increased by numerous mechanisms (discussed in the Electrophysiology subsection), one of which includes fibrosis; excess ECM interferes with transmission of electrical impulses across the myocardium, thus precipitating arrhythmogenesis.98 This raises the possibility that excess ECM remodeling during the sleep period, in combination with ‘event triggering substrates’ during the active period, contribute towards greater arrhythmia risk at this time.
Clotting
The Good.
Blood clotting is a normal physiologic process involving a complex cascade of enzymes that facilitate fibrin polymerization and platelet activation.114 Any mismatch between coagulation and fibrinolytic activity can result not only in thrombotic events (e.g., thromboembolism, myocardial infarction), but also in hemorrhagic events (e.g., intracerebral, subarachnoid). The balance between coagulation and fibrinolysis is under strict circadian governance.115, 116 Plasminogen activator inhibitor-1 (PAI-1; inhibits fibrinolytic activity through inhibition of tissue plasminogen activator [tPA]) exhibits time-of-day fluctuation in numerous tissues.117 In humans, circulating PAI-1 levels peak in the early morning (approximately 0630); these oscillations persist during dyssynchrony protocols (as do rhythms in platelet activation), suggesting mediation by endogenous circadian clocks.118 Indeed, the CLOCK/BMAL1 heterodimer directly regulates human PAI-1 transcription in endothelial cells, by binding to E-boxes in the PAI-1 gene promoter.119 Likewise, fibrinolytic activity also exhibits 24-hr rhythms, with lowest activity in the morning hours.120 Platelets play an important role in hemostasis and thrombosis, and are also under strict circadian governance.121–123 In healthy adults, platelet function is enhanced during the early morning hours (8am-noon), along with increased levels of fibrinogen and factor VIII activity during the wake phase.124 Furthermore, human platelet activation is subject to robust endogenous circadian oscillations, evidenced by a corresponding morning peak (~0900hr) in platelet surface activated glycoproteins (GP) IIb-IIIa, GPIb, and P-selectin (required for platelet aggregation).125 Collectively, these observations support greater clotting potential upon awakening, hypothetically in anticipation of increased likelihood of lacerations at this time.
The Bad.
Shift work leads to disruption of circadian governance of multiple critical biological processes in humans. Interestingly, a single bout of night shift work is sufficient to lead to platelet hypersensitivity, as evidenced by augmented cyclooxygenase-mediated eicosanoid synthesis.126 Moreover, meta-analysis of 34 unique studies revealed that shift workers have an approximate 25% increased risk of MI (increased stroke risk was also observed).127 Shift workers exhibit impaired time-of-day fluctuations in PAI-1 levels, presenting with a biphasic pattern (peaks at noon and midnight).128 Obstructive sleep apnea (OSA) also leads to circadian clock disruption29, 129, 130; OSA patients exhibit increased plasma PAI-1 activity along with decreased t-PA activity, coinciding with higher adverse CV event risk.131 Such observations are consistent with the concept that circadian disruption in humans leads to increased risk of adverse ischemic events, in part through dysregulated clotting. However, shift work and OSA predispose individuals to numerous CVD risk factors, including sleep restriction, dyslipidemia, inflammation, hypertension, obesity, and diabetes mellitus, suggesting that increased adverse ischemic event incidence is likely multifactorial.33, 132
In order to directly assess the impact of circadian disruption on clotting, both environmental- and genetic- based animal models have been employed. Genetic disruption of PER2 in mice resulted in decreased levels of pro-platelet formation from megakaryocytes, compromised secretion and aggregation, decreased circulating platelet counts (50% reduction), and longer bleeding times.133 Moreover, circadian control of hemostatic platelet activity has been shown to be governed by circadian genes, wherein time-of-day fluctuations in platelet activity134 and clotting time27 are lost in CLOCKΔ19 mutant mice. Moreover, BMAL1 knockout mice exhibit a pro-thrombotic phenotype, with increased circulating von Willebrand factor, fibrinogen, and PAI-1 levels135, as well as shorter clotting time.27 When mice are forced to consume food during the light (sleep) phase, dyysynchrony occurs between light- and food- based entrainment signals, associated with increased megakaryocyte ploidy levels and cell‐cycle activity.121 Collectively, these observations suggest that circadian disruption adversely impacts clotting potential.
The Ugly.
As highlighted above, increased clotting in the early hours of the morning is hypothetically in anticipation of a greater need for wound healing at this time. However, in individuals with atherosclerosis, circadian governance that once served as a selective advantage may increase risk of adverse ischemic events in the early hours of the morning. In the late 1980’s, Muller and colleagues reported an increased incidence of both myocardial infarctions and sudden cardiac death in the morning.41 When comparing the timing of adverse cardiovascular events with candidate mediators, the investigators developed a model wherein the steep rise in shear stress upon awakening potentially ruptured vulnerable plaques, at a time when platelet aggregability is heightened; temporal synchronization of these risk factors (as well as hypofibrinolysis) in susceptible individuals would create a ‘perfect storm’ for formation of an occluding thrombus.41 Circumstances may arise when this temporal synchronization is enhanced. For example, on Monday and Tuesday mornings following the one hour time change during Spring Forward, myocardial infarction risk increases; although increased risk may occur secondary to numerous factors (including sleep deprivation), one possibility is that morning surges in hemodynamic stress (driven primarily by waking) and clotting potential (driven primarily by cell-autonomous clocks, which take a couple of days to reset following the time shift) become better aligned.136, 137 Conversely, during Fall Back, myocardial infarction risk decreases for the first few days (perhaps due to temporal misalignment of these risk factors).136 The endogenous nature of circadian governance over myocardial infarction risk is reinforced by studies investigating adverse cardiovascular events in vacationers; the onset of ischemic events appear to coincide more with the early morning hours in vacationer’s home time zones, as opposed to local times.138 It is noteworthy that additional triggers for increased vulnerable plaque rupture in the morning likely exist, in addition to shear stress, given that the CONVINCE trial (which lowered the morning rise in blood pressure) failed to reduce adverse ischemic events.139 Taken together, these studies reveal that increased clotting potential in the morning confers a significant risk for adverse cardiovascular events in susceptible patients.
Inflammation
The Good.
Circadian governance applies to various aspects of the immune system, including immune cell function, trafficking and recruitment.140–142 Time-of-day fluctuations in circulating immune cells are observed in humans and mice. Circulating levels of most innate (monocytes, macrophages, neutrophils) and adaptive (B-cells and CD4+ T-cells) leukocytes peak during the resting phase and decrease during the active period.142–144 For example, in mice, circulating leukocytes and monocytes peak in the first half of the sleep phase and trough at the start of the active phase.145, 146 This rhythm is antiphase to monocyte and neutrophil recruitment to tissue (including skeletal muscle and the heart)142, 146, 147 and the homing of immune cells to the bone marrow and lymph nodes,141, 142, 146, 148, 149 which peak during the active period. This rhythmicity results from the interplay of several factors, including immune cell circadian clocks, local sympathetic nerve activity and hormonal cues (which are also mediated in part by circadian clocks), retention signals in the bone marrow (e.g., C-X-C motif chemokine ligand 12 [CXCL12]) and lymph nodes (e.g., C-C motif chemokine receptor 21 [CCL21]), as well as pro-migratory signals on endothelial cells (e.g., CCL2 and adhesion molecules).141, 146, 148–153
Endogenous molecular clocks have been demonstrated in most innate and adaptive immune cell types.141 Monocytes and macrophages exhibit a particularly robust clock, with high expression amplitude of clock genes,145, 154, 155 and temporal gating of several cell-intrinsic functions including toll-like receptor 9 (TLR9) expression,156 TLR4 responsiveness and activation of nuclear factor(NF)-κB,155, 157 cytokine and chemokine release,154, 155, 158–160 and phagocytosis.161 In contrast, clock gene expression appears to be relatively low in neutrophils, suggesting that other factors may exert greater chronobiological influence (e.g,. autocrine, paracrine, and neuroendocrine factors).162 Recent studies indicate that key inflammatory genes in macrophages are CCGs, including CCL2, CCL8, interleukin-6 (IL6), matrix metalloproteinase 9 (MMP9) and CX3C-chemokine receptor 1 (CX3CR1), with important mediatory roles for BMAL1 and REV-ERBα/β as drivers of rhythmic expression.145, 155, 158, 160, 163 REV-ERBα/β impart immunomodulatory (suppressive), in part, by regulating the epigenetic state of DNA enhancer regions and repressing enhancer RNA transcription.158, 164 This exquisite degree of circadian regulation of both physiological and molecular cell function is hypothesized to confer anticipation of the onset of inflammatory/infectious insults. More specifically, it appears that the immune system is primed to combat infections at the beginning of the awake period, whereas immune system repair and replenishment peaks at the beginning of the sleep period.165
The Bad.
Shift work and circadian misalignment, which confer cardiovascular disease risk in humans, increase systemic inflammation, as reflected by augmented circulating levels of IL-6 and hsCRP.58, 166 Chronic inflammation is a key pathophysiological factor underlying many cardiovascular diseases; innate immune cell clock disruption may contribute importantly to this response. Interestingly, while dysfunction of the core molecular clock induces dysregulation of inflammatory processes, the influence of distinct clock components on inflammatory and immune mediators appear to be somewhat heterogenous (see167 for review). Myeloid-restricted loss of Bmal1 induces a pro-inflammatory phenotype in mice, with loss of time-of-day fluctuations in blood Ly6Chi monocyte levels.145 Bmal1 deficient monocytes and macrophages exhibit exaggerated gene expression of monocyte-attractant chemokines (CCL2, CCL8, S100a8) and pro-inflammatory cytokine secretion in response to both acute inflammation and high fat feeding, which increases susceptibility to bacterial infection and chronic cardiometabolic disease.145 Similarly, genetic loss of cryptochrome (CRY) results in constitutively increased pro-inflammatory cytokine expression and NF-κB activation in macrophages.159 An analogous heightened systemic and macrophage pro-inflammatory response is also observed after experimental jet lag and circadian disruption in mice.168
An augmented inflammatory response accompanying immune cell circadian clock disruption accelerates atherosclerosis. Myeloid BMAL1 deficiency on the ApoE−/− background increased Ly6Chi monocyte trafficking to atherosclerotic lesions, their differentiation to M1 macrophages, and plaque macrophage content and lesion size.169 Similar atherosclerotic and pro-inflammatory responses were observed upon shRNA‐lentiviral hematopoietic cell knockdown of REV-ERBα in LDLR−/− mice.170 In contrast, macrophage overexpression of REV-ERBα or administration of a REV-ERBα/β agonist, SR9009, induced an anti-inflammatory phenotype, and SR9009 treatment reduced atherosclerotic plaque size in LDLR−/− mice in vivo.170, 171 Human atherosclerotic plaque exhibits reduced CRY1 expression, whereas adenoviral gene transfer of CRY1 to ApoE−/− mice reduced atherosclerotic plaque size and the expression of pro-inflammatory factors.172 In the absence of genetic modulation, short term manipulation of the light/dark cycle (which causes circadian disruption) increased cardiac macrophage infiltration (but decreased neutrophil infiltration), and concomitantly worsened LV remodeling and function after MI.109 Collectively, outcomes following distinct strategies for circadian clock modulation (genetic, pharmacologic, and environmental) are consistent with the concept that circadian disruption not only leads to a pro-inflammatory state, but also augments atherosclerotic disease progression and post-MI remodeling in animal models. The impact of immune cell clock dysfunction in other forms of cardiovascular disease (e.g., LV hypertrophy, chronic HF) remains to be studied.
The Ugly.
While circadian disruption in immune cells can promote disease, evidence exists suggesting that circadian control of the immune system may also contribute (in the appropriate context) towards cardiovascular disease pathogenesis. First, normal immune rhythms may increase susceptibility to disease progression during conditions when immune activity is physiologically enhanced. For example, a recent study demonstrated that CCL2-dependent myeloid cell recruitment and adhesion to large vessel atherosclerotic plaque peaks in the latter half of the active phase in mice, and that timed pharmacological CCR2 blockade during the active phase inhibited atherosclerosis.173 It is conceivable that this pattern may contribute to time-of-day fluctuations in plaque stability and the known bimodal diurnal distribution of MI in the early morning and evening hours.174 Oscillations in tissue recruitment of myeloid cells to the heart may also significantly impact the intensity of the inflammatory response and tissue damage to myocardial injury. Schloss et al demonstrated that neutrophil recruitment to the heart preferentially occurred at the onset of the active period, and that experimentally induced MI at that time resulted in a heightened neutrophil inflammatory response, larger infarct size, worse survival, and exaggerated post-MI LV remodeling and dysfunction.147 Targeted blockade of neutrophil inflammation at the beginning of the active period improved infarct size and cardiac function. Such time-of-day fluctuations in the immune cell infiltrative response may contribute to reduced cardiac tolerance to adverse ischemic events previously reported at the onset of the awake period in both mice and humans.41, 111 The full extent to which circadian governance of inflammatory processes plays a role in both the onset (e.g., ischemic events, arrhythmias) and progression (e.g., adverse remodeling following an MI) of cardiovascular disease is an area of translational research worthy of further exploration.
Chronotherapeutic Potential for Cardiovascular Disease
Based on evidence discussed in preceding sections, it is clear that chronobiology plays critical roles not only in cardiovascular physiology, but also in cardiovascular disease (CVD) onset and progression. However, this impact on health and disease extends beyond the examples highlighted already, and includes optimization of diagnostic and treatment strategies.175 Several routine CVD biomarkers exhibit robust time-of-day fluctuations in patients, including ANP and cTnT, leading to suggestion that the time-of-day at which specific tests are performed may influence diagnostic value (although this does not seem to be the case for cTnT).176–178 The prognostic value of blood pressure assessment is a topic of heightened awareness.64, 179 Increased accessibility of ambulatory blood pressure monitoring has highlighted the previously underappreciated incidence of non-dipping hypertension, which is notably higher in certain at risk racial groups (e.g., approximately 2.4-fold higher in African Americans compared to Caucasians).180 Given the greater risk of adverse cardiac remodeling in individuals with non-dipping hypertension63, identification of this form of circadian impairment is vital for appropriate therapeutic interventions. The latter includes chronopharmacologic strategies designed to lower blood pressure at night; a large prospective ambulatory blood pressure monitoring study (MAPEC study) reported a reduction in adverse cardiovascular events when nighttime blood pressure was specifically targeted.181
Importantly, genes encoding for many common pharmacologic targets exhibit time-of-day fluctuations in multiple cardiovascular tissues.22 These include targets for aspirin (cyclooxygenase 1) and statins (3-Hydroxy-3-Methylglutaryl-CoA Reductase); both these common drugs have now been shown to elicit greater benefit when taken at bedtime, attenuating platelet aggregability upon awakening (aspirin) and cholesterol synthesis during sleep (statins).182, 183 The angiotensin-2 receptor is also under circadian governance, such that receptor antagonists appear to exhibit greater efficacy during the evening (a time at which receptor levels are high).184
Current and/or future cardiovascular-relevant pharmacologic targets are also likely to benefit from consideration of chronobiology. One example is inflammation; as highlighted in preceding sections, inflammatory processes exhibit profound time-of-day fluctuations, strongly suggesting that strategies for inflammation attenuation/resolution should consider the optimal time for treatment. Pharmacological interventions are not the only treatment modality that is impacted by time-of-day; so too are surgical, lifestyle, and environmental interventions. For example, Montaigne et al recently reported approximate 2-fold better outcomes when aortic valve replacement surgery was performed during the afternoon, relative to the morning (correlating with established time-of-day fluctuations in myocardial ischemic tolerance).185 With regards to lifestyle, many physicians recommend improvements in diet and physical activity, to reduce CVD risk. What has emerged recently is that time-of-day should be considered, to maximize benefit. Consistent with circadian governance of metabolic homeostasis, improvements in CVD risk factors (such as weight, adiposity, glucose tolerance, and even blood pressure) are observed when the majority of calories are consumed toward the beginning of the day.186 Moreover, the majority of studies report greater cardiovascular benefits of aerobic exercise in the morning.187
Additional behavioral/environmental factors influencing circadian rhythms include sleep and light exposure; appreciation of this knowledge has led to suggestions that some hospital settings may lead to circadian disruption. This is exemplified by the critical care unit, wherein patients are often subject to light-at-night, continual nutrition, and sleep disturbance. When this is modeled in rodents (e.g., MI induction in mice, followed by light/dark disruption), clinically-relevant outcomes (e.g., infarct expansion) are worsened.109 Such observations suggest that hospital environments should be designed in a ‘circadian conscience’ manner.188 A critical question relates to whether circadian clock components are a viable target for CVD treatment. Early pre-clinical studies suggest that the answer may be yes. Indeed, pharmacological activation of REV-ERBα/β attenuates pathogenesis in murine models of atherosclerosis, pressure overload, and MI.171, 189–191 Moreover, commonly prescribed drugs, such as β-blockers and thiazolidinediones entrain circadian clocks89, 192, raising the possibility that some therapeutic benefit may be through enhancement of circadian governance.
Figure 4.
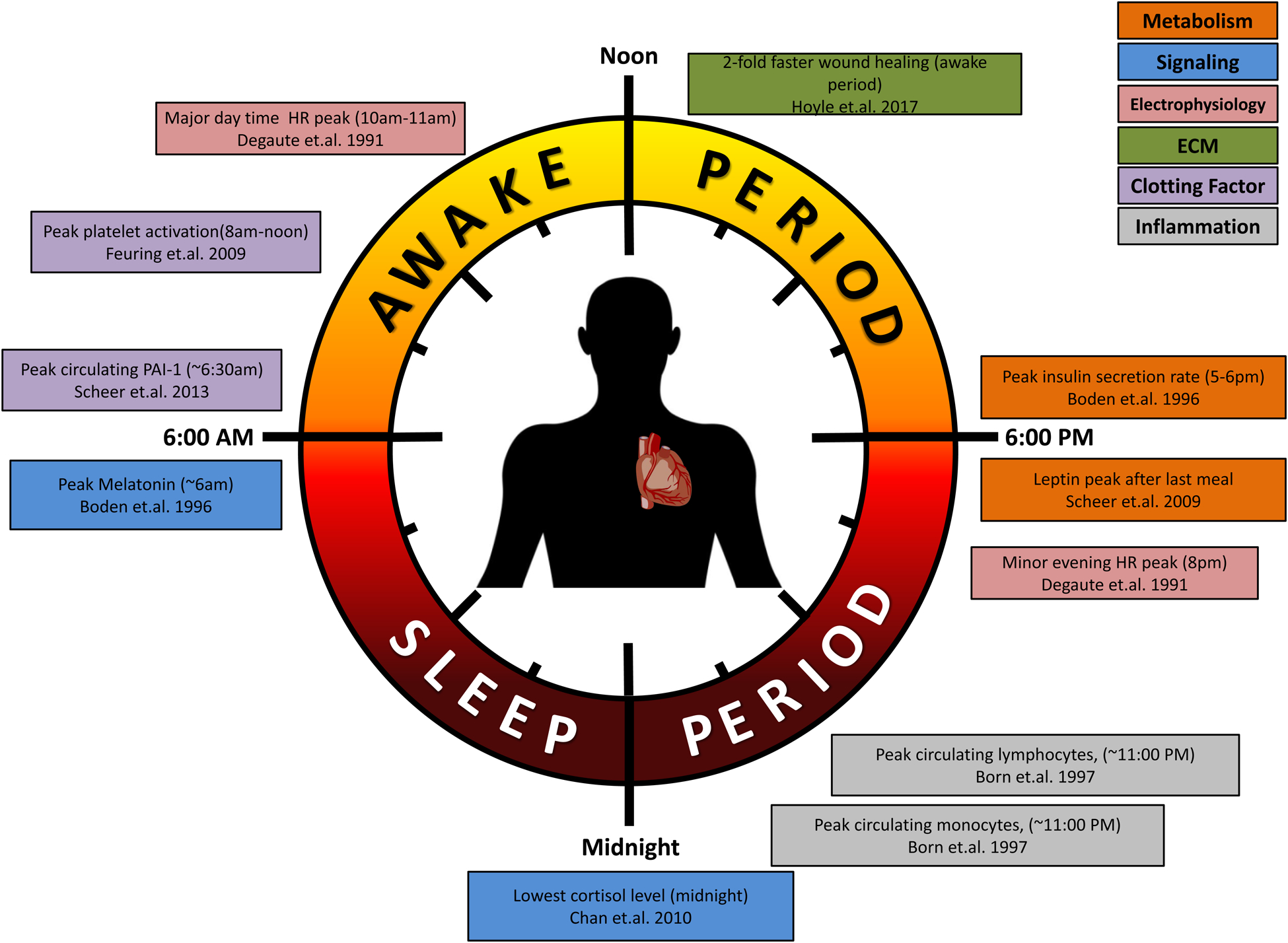
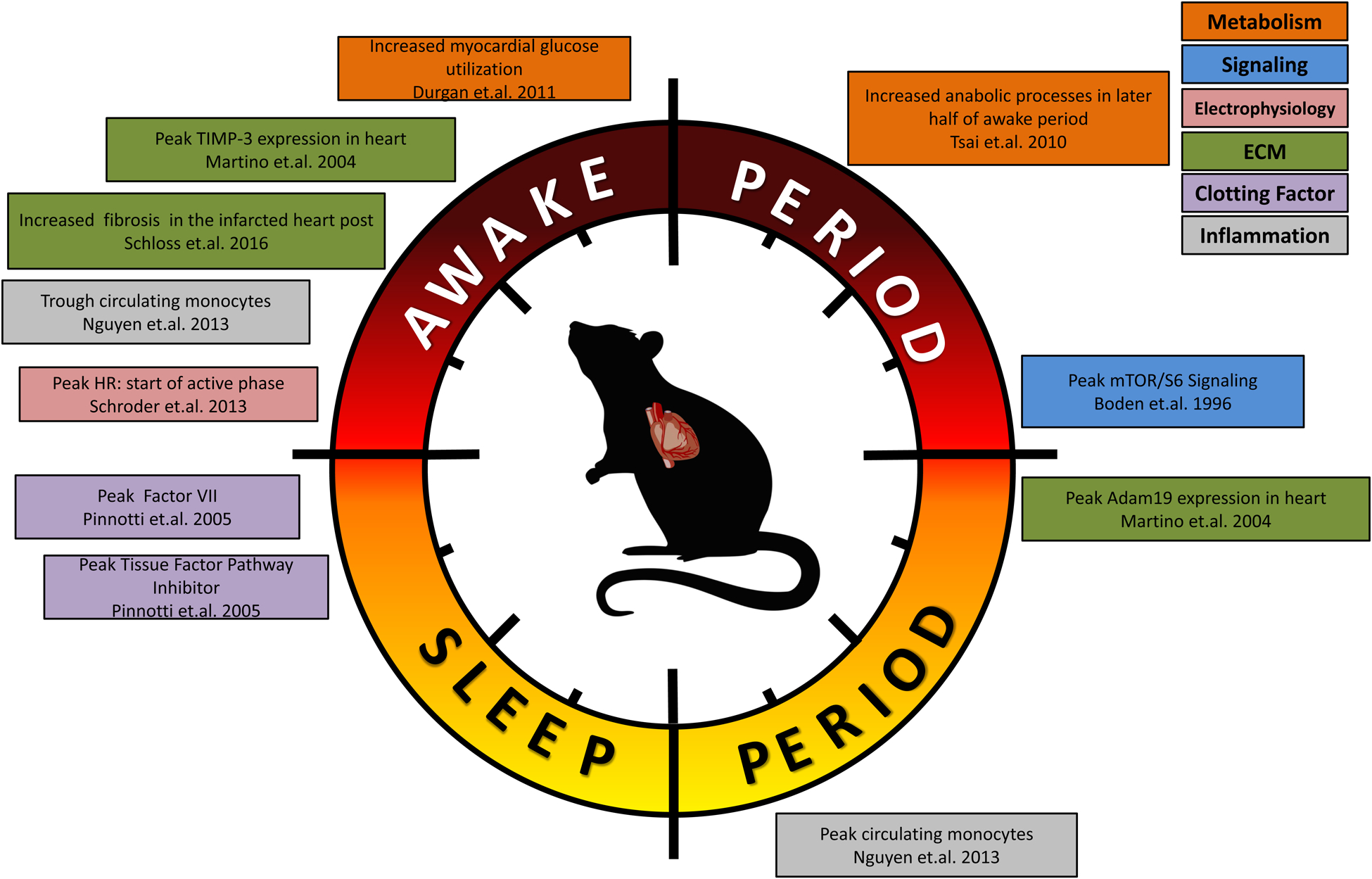
Summary of circadian influences on cardiovascular-relevant parameters in humans (A) and rodents (B).
Summary and Future Perspectives.
It is fundamentally clear that circadian rhythms critically impact cardiovascular health and disease. Metabolic pathways, signal transduction cascades, transcriptional networks, protein turnover, and more, are all temporally orchestrated, in a manner that is conducive to optimal cellular/organ function (much like a conductor synchronizes symphony players, thereby delivering an audible masterpiece, instead of random noise; The Good [Figure 4]). When circadian governance is impaired, pathology invariably ensues (The Bad). It is also increasingly apparent that persistence and/or augmentation of circadian rhythms can, in susceptible individuals, precipitate and/or intensify CVD (The Ugly). However, many critical questions remain to be addressed in this field. These include: 1) dissection of the molecular mechanisms by which circadian clocks modulate biological processes in cardiovascular-relevant cells (including cardiomyocytes, endothelial cells, smooth muscle cells, myofibroblasts, immune cells); 2) understand how cell-to-cell circadian clock synchrony is achieved in cardiovascular tissues, and whether disruption of this synchrony (e.g., misalignment of phases) contributes to CVD; 3) identify optimal lifestyle behaviors (e.g., timing of eating, exercise, etc.) and environmental conditions (e.g., lighting, temperature) that work in synchrony with circadian governance of cellular processes; and 4) develop therapeutic strategies that specifically target circadian clock components, and/or downstream mediators, for the effective treatment and/or prevention of CVD.
ACKNOWLEDGEMENTS
This work was supported by the National Heart, Lung, and Blood Institute (R01HL142216, R01HL147549, T32HL129948).
ABBREVIATIONS
- AKT
protein kinase B
- AMP
adenosine monophosphate
- AMPK
adenosine monophosphate-activated protein kinase
- AngII
angiotensin II
- ANP
atrial natriuretic peptide
- ApoE
apolipoprotein E
- ATP
adenosine triphosphate
- BMAL1
brain and muscle ARNT (arylhydrocarbon receptor nuclear translocator)-like
- CACNA1C
calcium channel, voltage-dependent, L type, alpha 1C subunit
- CACNA1D
calcium channel, voltage-dependent, L type, alpha 1D subunit
- CBK
cardiomyocyte-specific BMAL1 knockout
- CCG
clock controlled genes
- CCL2
C-C motif chemokine ligand 2
- CCM
cardiomyocyte specific CLOCK mutant
- CCR2
C-C chemokine receptor type 2
- CLOCK
circadian locomotor output cycles kaput
- CRY
cryptochrome
- cTnT
cardiac troponin T
- CV
cardiovascular
- CVD
cardiovascular disease
- CXCL
C-X-C motif chemokine ligand
- DGAT2
diacylglycerol acyltransferase 2
- DNA
deoxyribonucleic acid
- ECG
electrocardiogram
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- ERK1/2
extracellular signal regulated kinase 1/2
- FA
fatty acid
- GP
glycoproteins
- GSK3β
glycogen synthase kinase β
- HRV
heart rate variability
- hsCRP
high sensitive c-reactive protein
- I/R
ischemia/reperfusion
- IL
interleukin
- KCHIP2
potassium channel interacting protein 2
- KCIP2
potassium voltage-gated channel interacting protein 2
- KCNA5
potassium voltage-gated channel subfamily A membrane 5
- KCND2
potassium voltage-gated channel subfamily D member 2
- KCNH2
potassium voltage-gated channel subfamily H member 2
- KCNK3
potassium two pore domain channel subfamily K membrane 3
- Klf15
kruppel like factor 15
- LDLR
low density lipoprotein receptor
- LV
left ventricle
- Ly6C
lymphocyte antigen 6 complex
- MAPEC
ambulatory blood pressure monitoring in the reduction of cardiovascular events and effects of chronotherapy
- MCIP
modulatory calcineurin-interacting protein
- MI
myocardial infarction
- Mia3
melanoma inhibitory activity family, member 3
- miRNAs
micro ribonucleic acid
- MMP
matrix metalloproteases
- mTOR
mammalian target of rapamycin
- NAMPT
nicotinamide phosphoribosyltransferase
- NF
nuclear factor
- NPAS2
neuronal PAS domain protein 2
- OSA
obstructive sleep apnea
- PAI-1
plasminogen activator inhibitor-1
- Pde4d
phosphodiesterase 4d
- Per
period circadian protein
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PKB/AKT
protein kinase B
- PKC
protein kinase C
- PKG
protein kinase G
- PPARγ
peroxisome proliferator-activated receptors γ
- REV-ERB
nuclear receptor reverse-ERB
- RT-PCR
real time – polymerase chain reaction
- SCN
suprachiasmatic nucleus
- SCN5a
sodium voltage-gated channel alpha subunit 5
- SERCA2
sarcoplasmic/endoplasmic reticulum calcium ATPase 2
- SMAD2
small mother against decapentaplegic 2
- TGFβ
tumor growth factor β
- TIMP
tissue inhibitors of metalloprotease
- TLR
toll-like receptor
- tPA
tissue plasminogen activator
- UDP
uridine diphosphate
- ULK1
Unc-51 like autophagy activating kinase
- Vps33b
vacuolar protein sorting 33 homolog B
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Edery I Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi JS, Hong HK, Ko CH and McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE and Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev. 2012;16:151–66. [DOI] [PubMed] [Google Scholar]
- 4.Degaute JP, van de Borne P, Linkowski P and Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18:199–210. [DOI] [PubMed] [Google Scholar]
- 5.Richards AM, Nicholls MG, Espiner EA, Ikram H, Cullens M and Hinton D. Diurnal patterns of blood pressure, heart rate and vasoactive hormones in normal man. Clin Exp Hypertens A. 1986;8:153–66. [DOI] [PubMed] [Google Scholar]
- 6.Stern N, Beahm E, McGinty D, Eggena P, Littner M, Nyby M, Catania R and Sowers JR. Dissociation of 24-hour catecholamine levels from blood pressure in older men. Hypertension. 1985;7:1023–9. [DOI] [PubMed] [Google Scholar]
- 7.Tuck ML, Stern N and Sowers JR. Enhanced 24-hour norepinephrine and renin secretion in young patients with essential hypertension: relation with the circadian pattern of arterial blood pressure. Am J Cardiol. 1985;55:112–5. [DOI] [PubMed] [Google Scholar]
- 8.Azvolinsky A Cave Dwellers, 1938. The Scientist. 2016. [Google Scholar]
- 9.Aschoff J Circadian Rhythms in Man. Science. 1965;148:1427–32. [DOI] [PubMed] [Google Scholar]
- 10.Reinberg A, Ghata J, Halberg F, Gervais P, Abulker C, Dupont J and Gaudeau C. [Circadian rhythm of pulse, arterial blood pressure, urinary excretions of 17-hydroxycorticosteroids catecholamines and potassium in healthy adult humans active and during rest]. Annales d’endocrinologie. 1970;31:277–87. [PubMed] [Google Scholar]
- 11.Shea SA, Hilton MF, Hu K and Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108:980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF and Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107:20541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheer FA, Hilton MF, Mantzoros CS and Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu K, Ivanov P, Hilton MF, Chen Z, Ayers RT, Stanley HE and Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A. 2004;101:18223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cermakian N and Sassone-Corsi P. Multilevel Regulation of the Circadian Clock. Nat Rev Mol Cell Biol. 2000;1:59–67. [DOI] [PubMed] [Google Scholar]
- 16.Hirota T and Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoological Sci. 2004;21:359–368. [DOI] [PubMed] [Google Scholar]
- 17.Gekakis N, Staknis D, Nguyen H, Davis F, Wilsbacher L, King D, Takahashi J and Weitz C. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. [DOI] [PubMed] [Google Scholar]
- 18.Hogenesch J, Gu Y, Jain S and Bradfield C. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Aad Sci USA. 1998;95:5474–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kume K, Zylka M, Sriram S, Shearman L, Weaver D, Jin X, Maywood E, Hastings M and Reppert S. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. [DOI] [PubMed] [Google Scholar]
- 20.Shearman L, Sriram S, Weaver D, Maywood E, Chaves I, Zheng B, Kume K, Lee C, van d, Horst GT, Hastings M and Reppert S. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. [DOI] [PubMed] [Google Scholar]
- 21.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U and Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. [DOI] [PubMed] [Google Scholar]
- 22.Zhang R, Lahens NF, Ballance HI, Hughes ME and Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS and Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS and Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray M, Shaw C, Moore M, Garcia R, Zanquetta M, Durgan D, Jeong W, Tsai J, Bugger H, Zhang D, Rohrwasser A, Rennison J, Dyck J, Litwin S, Hardin P, Chow C, Chandler M, Abel E and Young M. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function; metabolism; and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. [DOI] [PubMed] [Google Scholar]
- 26.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z and Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA and FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–95. [DOI] [PubMed] [Google Scholar]
- 28.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW and Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. [DOI] [PubMed] [Google Scholar]
- 29.Durgan DJ, Crossland RF and Bryan RM Jr. The rat cerebral vasculature exhibits time-of-day-dependent oscillations in circadian clock genes and vascular function that are attenuated following obstructive sleep apnea. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37:2806–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young M, Wilson C, Razeghi P, Guthrie P and Taegtmeyer H. Alterations of the Circadian Clock in the Heart by Streptozotocin-induced Diabetes. J Mol Cell Cardiol. 2002;34:223–231. [DOI] [PubMed] [Google Scholar]
- 31.Damiola F, Le MN, Preitner N, Kornmann B, Fleury-Olela F and Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XS, Armstrong ME, Cairns BJ, Key TJ and Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med (Lond). 2011;61:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa G Shift work and health: current problems and preventive actions. Saf Health Work. 2010;1:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M and Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott EM, Carter AM and Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond). 2008;32:658–62. [DOI] [PubMed] [Google Scholar]
- 36.Turek F, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen D, Eckel R, Takahashi J and Bass J. Obesity and metabolic syndrome in Clock mutant mice. Science. 2005;308:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS and Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefta M, Campbell KS, Feng HZ, Jin JP and Esser KA. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart Circ Physiol. 2012;303:H475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, Chatham JC, Wende AR, Yang Q, Chow CW, Martino TA and Gamble KL. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms. 2014;29:257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL and Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond). 2013;37:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller J, Tofler G and Stone P. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. [DOI] [PubMed] [Google Scholar]
- 42.Manfredini R, Boari B, Salmi R, Fabbian F, Pala M, Tiseo R and Portaluppi F. Twenty-four-hour patterns in occurrence and pathophysiology of acute cardiovascular events and ischemic heart disease. Chronobiol Int. 2013;30:6–16. [DOI] [PubMed] [Google Scholar]
- 43.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria Malagoni A, Haus E and Manfredini F. Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. Chronobiol Int. 2005;22:417–53. [DOI] [PubMed] [Google Scholar]
- 44.Manfredini R, Portaluppi F, Zamboni P, Salmi R and Gallerani M. Circadian variation in spontaneous rupture of abdominal aorta. Lancet. 1999;353:643–4. [DOI] [PubMed] [Google Scholar]
- 45.Bass J and Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green CB, Takahashi JS and Bass J. The meter of metabolism. Cell. 2008;134:728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young ME. Temporal partitioning of cardiac metabolism by the cardiomyocyte circadian clock. Experimental physiology. 2016;101:1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young ME. Circadian Control of Cardiac Metabolism: Physiologic Roles and Pathologic Implications. Methodist DeBakey cardiovascular journal. 2017;13:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatham JC and Young ME. Regulation of myocardial metabolism by the cardiomyocyte circadian clock. J Mol Cell Cardiol. 2012;55:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young M, Razeghi P, Cedars A, Guthrie P and Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–1208. [DOI] [PubMed] [Google Scholar]
- 51.Durgan D, Moore M, Ha N, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw C, Bray M, Nannegari V, Hickson-Bick D, Heird W, Dyck J, Chandler M and Young M. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am J Physiol Heart Circ Physiol. 2007;293:H2385–H2393. [DOI] [PubMed] [Google Scholar]
- 52.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL Jr., Dyck JR, Bray MS, Gamble KL, Chatham JC and Young ME. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem. 2011;286:44606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS and Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2010;285:2918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGinnis GR, Tang Y, Brewer RA, Brahma MK, Stanley HL, Shanmugam G, Rajasekaran NS, Rowe GC, Frank SJ, Wende AR, Abel ED, Taegtmeyer H, Litovsky S, Darley-Usmar V, Zhang J, Chatham JC and Young ME. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. J Mol Cell Cardiol. 2017;110:80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewer RA, Collins HE, Berry RD, Brahma MK, Tirado BA, Peliciari-Garcia RA, Stanley HL, Wende AR, Taegtmeyer H, Rajasekaran NS, Darley-Usmar V, Zhang J, Frank SJ, Chatham JC and Young ME. Temporal partitioning of adaptive responses of the murine heart to fasting. Life Sciences. 2018;197:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodwin G, Taylor C and Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. [DOI] [PubMed] [Google Scholar]
- 57.Russell RR 3rd, Cline GW, Guthrie PH, Goodwin GW, Shulman GI and Taegtmeyer H. Regulation of exogenous and endogenous glucose metabolism by insulin and acetoacetate in the isolated working rat heart. A three tracer study of glycolysis, glycogen metabolism, and glucose oxidation. J Clin Invest. 1997;100:2892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leproult R, Holmback U and Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko ML, Shi L, Tsai JY, Young ME, Neuendorff N, Earnest DJ and Ko GY. Cardiac-specific mutation of Clock alters the quantitative measurements of physical activities without changing behavioral circadian rhythms. J Biol Rhythms. 2011;26:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM and Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 2011;28:187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ingle KA, Kain V, Goel M, Prabhu SD, Young ME and Halade GV. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am J Physiol Heart Circ Physiol. 2015;309:H1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knutson KL, Spiegel K, Penev P and Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F and Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–36. [DOI] [PubMed] [Google Scholar]
- 64.Hermida RC, Ayala DE, Mojon A, Fernandez JR, Smolensky M and Portaluppi F. Morning surge, dipping, and sleep-time blood pressure as prognostic markers of cardiovascular risk. Hypertension. 2013;61:e3. [DOI] [PubMed] [Google Scholar]
- 65.Wende AR, Symons JD and Abel ED. Mechanisms of lipotoxicity in the cardiovascular system. Current hypertension reports. 2012;14:517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L and Unger R. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma S, Adrogue J, Golfman L, Uray I, Lemm J, Youker K, Noon G, Frazier O and Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. [DOI] [PubMed] [Google Scholar]
- 68.Gamble KL, Berry R, Frank SJ and Young ME. Circadian clock control of endocrine factors. Nature reviews Endocrinology. 2014;10:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP and Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–15. [DOI] [PubMed] [Google Scholar]
- 70.Robles MS, Humphrey SJ and Mann M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 2017;25:118–127. [DOI] [PubMed] [Google Scholar]
- 71.Grimaldi B, Nakahata Y, Kaluzova M, Masubuchi S and Sassone-Corsi P. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK and SIRT1. Int J Biochem Cell Biol. 2009;41:81–6. [DOI] [PubMed] [Google Scholar]
- 72.Dallmann R, Viola AU, Tarokh L, Cajochen C and Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, Copeland V, Oh M, Bush E, Shelton JM, Bibb JA, Hill JA and Rothermel BA. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart. Circ Res. 2011;108:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witte K, Parsa-Parsi R, Vobig M and Lemmer B. Mechanisms of the circadian regulation of beta-adrenoceptor density and adenylyl cyclase activity in cardiac tissue from normotensive and spontaneously hypertensive rats. J Mol Cell Cardiol. 1995;27:1195–202. [DOI] [PubMed] [Google Scholar]
- 75.Durgan D, Trexler N, Egbejimi O, McElfresh T, Suk H, Petterson L, Shaw C, Hardin P, Bray M, Chandler M, Chow C and Young M. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. [DOI] [PubMed] [Google Scholar]
- 76.Peliciari-Garcia RA, Bargi-Souza P, Young ME and Nunes MT. Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol Int. 2018;35:147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alibhai FJ, LaMarre J, Reitz CJ, Tsimakouridze EV, Kroetsch JT, Bolz SS, Shulman A, Steinberg S, Burris TP, Oudit GY and Martino TA. Disrupting the key circadian regulator CLOCK leads to age-dependent cardiovascular disease. J Mol Cell Cardiol. 2017;105:24–37. [DOI] [PubMed] [Google Scholar]
- 78.Shang X, Pati P, Anea CB, Fulton DJ and Rudic RD. Differential Regulation of BMAL1, CLOCK, and Endothelial Signaling in the Aortic Arch and Ligated Common Carotid Artery. J Vasc Res. 2016;53:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pagliaro P, Moro F, Tullio F, Perrelli MG and Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxidants & redox signaling. 2011;14:833–50. [DOI] [PubMed] [Google Scholar]
- 80.Heusch G Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–99. [DOI] [PubMed] [Google Scholar]
- 81.van Rooij E, Doevendans PA, Crijns HJ, Heeneman S, Lips DJ, van Bilsen M, Williams RS, Olson EN, Bassel-Duby R, Rothermel BA and De Windt LJ. MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction. Circ Res. 2004;94:e18–26. [DOI] [PubMed] [Google Scholar]
- 82.Rotter D, Grinsfelder DB, Parra V, Pedrozo Z, Singh S, Sachan N and Rothermel BA. Calcineurin and its regulator, RCAN1, confer time-of-day changes in susceptibility of the heart to ischemia/reperfusion. J Mol Cell Cardiol. 2014;74:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–27. [DOI] [PubMed] [Google Scholar]
- 84.Boudreau P, Dumont G, Kin NM, Walker CD and Boivin DB. Correlation of heart rate variability and circadian markers in humans. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2011;2011:681–2. [DOI] [PubMed] [Google Scholar]
- 85.Bexton RS, Vallin HO and Camm AJ. Diurnal variation of the QT interval--influence of the autonomic nervous system. Br Heart J. 1986;55:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boudreau P, Yeh WH, Dumont GA and Boivin DB. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int. 2012;29:757–68. [DOI] [PubMed] [Google Scholar]
- 87.Hilton MF, Umali MU, Czeisler CA, Wyatt JK and Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- 88.Schroder EA, Lefta M, Zhang X, Bartos DC, Feng HZ, Zhao Y, Patwardhan A, Jin JP, Esser KA and Delisle BP. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am J Physiol Cell Physiol. 2013;304:C954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE and Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tong M, Watanabe E, Yamamoto N, Nagahata-Ishiguro M, Maemura K, Takeda N, Nagai R and Ozaki Y. Circadian expressions of cardiac ion channel genes in mouse might be associated with the central clock in the SCN but not the peripheral clock in the heart. Biol Rhythm Res. 2013;44:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schroder EA, Burgess DE, Zhang X, Lefta M, Smith JL, Patwardhan A, Bartos DC, Elayi CS, Esser KA and Delisle BP. The cardiomyocyte molecular clock regulates the circadian expression of Kcnh2 and contributes to ventricular repolarization. Heart Rhythm. 2015;12:1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS and Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT and Watanabe H. Circadian variation of cardiac K+ channel gene expression. Circulation. 2003;107:1917–22. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y, Zhu D, Yuan J, Han Z, Wang Y, Qian Z, Hou X, Wu T and Zou J. CLOCK-BMAL1 regulate the cardiac L-type calcium channel subunit CACNA1C through PI3K-Akt signaling pathway. Canadian journal of physiology and pharmacology. 2016;94:1023–32. [DOI] [PubMed] [Google Scholar]
- 95.Ni YM, Rusinaru C, Reinier K, Uy-Evanado A, Chugh H, Stecker EC, Jui J and Chugh SS. Unexpected shift in circadian and septadian variation of sudden cardiac arrest: the Oregon Sudden Unexpected Death Study. Heart Rhythm. 2019;16:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malik M, Farrell T and Camm AJ. Circadian rhythm of heart rate variability after acute myocardial infarction and its influence on the prognostic value of heart rate variability. Am J Cardiol. 1990;66:1049–54. [DOI] [PubMed] [Google Scholar]
- 97.Nelson W, Kupferberg H and Halberg F. Dose-response evaluations of a circadian rhythmic change in susceptibility of mice to ouabain. Toxicology and applied pharmacology. 1971;18:335–9. [DOI] [PubMed] [Google Scholar]
- 98.Pellman J, Zhang J and Sheikh F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: Mechanisms and model systems. J Mol Cell Cardiol. 2016;94:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spinale FG, Frangogiannis NG, Hinz B, Holmes JW, Kassiri Z and Lindsey ML. Crossing Into the Next Frontier of Cardiac Extracellular Matrix Research. Circ Res. 2016;119:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mays PK, McAnulty R and Laurent GJ. Age-related changes in total protein and collagen metabolism in rat liver. Hepatology. 1991;14:1224–9. [PubMed] [Google Scholar]
- 101.Chang J, Garva R, Pickard A, Chloé Yeung C-Y, Mallikarjun V, Swift J, Holmes DF, Calverley B, Lu Y, Adamson A, Raymond-Hayling H, Jensen O, Shearer T, Meng QJ and Kadler KE. Circadian control of the secretory pathway is a central mechanism in tissue homeostasis. bioRxiv. 2019:304014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, Bray LK, Thomas JM, Dunn K, Blaikley J and O’Neill JS. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Science translational medicine. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M and Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004;82:256–64. [DOI] [PubMed] [Google Scholar]
- 104.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB and FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–24. [DOI] [PubMed] [Google Scholar]
- 105.Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, Yamamoto M, Chan J, van der Horst GT, Fukada Y and Meng QJ. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28:548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jourdan-Lesaux C, Zhang J and Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci. 2010;87:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dorn GW, 2nd. MicroRNAs: redefining mechanisms in cardiac disease. J Cardiovasc Pharmacol. 2010;56:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR and Sole MJ. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–83. [DOI] [PubMed] [Google Scholar]
- 109.Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O’Sullivan ML, Pyle WG, Sole MJ and Martino TA. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res. 2014;114:1713–22. [DOI] [PubMed] [Google Scholar]
- 110.Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C and Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97:970–6. [DOI] [PubMed] [Google Scholar]
- 111.Durgan D, Pulinilkunnil T, Villegas-Montoya C, Garvey M, Frangogiannis N, Michael L, Chow C, Dyck J and Young M. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kong P, Christia P and Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cellular and molecular life sciences : CMLS. 2014;71:549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baguet JP, Barone-Rochette G, Tamisier R, Levy P and Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nature reviews Cardiology. 2012;9:679–88. [DOI] [PubMed] [Google Scholar]
- 114.Smith SA, Travers RJ and Morrissey JH. How it all starts: Initiation of the clotting cascade. Critical reviews in biochemistry and molecular biology. 2015;50:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takeda N and Maemura K. Circadian clock and the onset of cardiovascular events. Hypertens Res. 2016;39:383–90. [DOI] [PubMed] [Google Scholar]
- 116.Montagnana M, Salvagno GL and Lippi G. Circadian variation within hemostasis: an underrecognized link between biology and disease? Seminars in thrombosis and hemostasis. 2009;35:23–33. [DOI] [PubMed] [Google Scholar]
- 117.Naito Y, Tsujino T, Kawasaki D, Okumura T, Morimoto S, Masai M, Sakoda T, Fujioka Y, Ohyanagi M and Iwasaki T. Circadian gene expression of clock genes and plasminogen activator inhibitor-1 in heart and aorta of spontaneously hypertensive and Wistar-Kyoto rats. J Hypertens. 2003;21:1107–15. [DOI] [PubMed] [Google Scholar]
- 118.Scheer FA and Shea SA. Human circadian system causes morning peak in pro-thrombotic plasminogen activator inhibitor-1 (PAI-1) independent of sleep/wake cycle. Blood. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schoenhard JA, Smith LH, Painter CA, Eren M, Johnson CH and Vaughan DE. Regulation of the PAI-1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J Mol Cell Cardiol. 2003;35:473–81. [DOI] [PubMed] [Google Scholar]
- 120.Jovicic A and Mandic S. Circadian variations of platelet aggregability and fibrinolytic activity in healthy subjects. Thromb Res. 1991;62:65–74. [DOI] [PubMed] [Google Scholar]
- 121.Hartley PS, John Sheward W, French K, Horn JM, Holmes MC and Harmar AJ. Food-entrained rhythmic expression of PER2 and BMAL1 in murine megakaryocytes does not correlate with circadian rhythms in megakaryopoiesis. Journal of thrombosis and haemostasis : JTH. 2008;6:1144–52. [DOI] [PubMed] [Google Scholar]
- 122.Hartley PS, Sheward J, Scholefield E, French K, Horn JM, Holmes MC and Harmar AJ. Timed feeding of mice modulates light-entrained circadian rhythms of reticulated platelet abundance and plasma thrombopoietin and affects gene expression in megakaryocytes. Br J Haematol. 2009;146:185–92. [DOI] [PubMed] [Google Scholar]
- 123.Packham MA. Role of platelets in thrombosis and hemostasis. Canadian journal of physiology and pharmacology. 1994;72:278–84. [DOI] [PubMed] [Google Scholar]
- 124.Feuring M, Wehling M, Ruf A and Schultz A. Circadian variation of platelet function measured with the PFA-100. Platelets. 2009;20:466–70. [DOI] [PubMed] [Google Scholar]
- 125.Scheer FA, Michelson AD, Frelinger AL 3rd, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR and Shea SA. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6:e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakao T, Yasumoto A, Tokuoka S, Kita Y, Kawahara T, Daimon M and Yatomi Y. The impact of night-shift work on platelet function in healthy medical staff. J Occup Health. 2018;60:324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G and Hackam DG. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peternel P, Stegnar M, Salobir U, Keber D and Vene N. Shift work and circadian rhythm of blood fibrinolytic parameters. Fibrinolysis. 1990;4:113–115. [Google Scholar]
- 129.Burioka N, Koyanagi S, Endo M, Takata M, Fukuoka Y, Miyata M, Takeda K, Chikumi H, Ohdo S and Shimizu E. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2008;32:105–12. [DOI] [PubMed] [Google Scholar]
- 130.Canales MT, Holzworth M, Bozorgmehri S, Ishani A, Weiner ID, Berry RB, Beyth RJ and Gumz M. Clock gene expression is altered in veterans with sleep apnea. Physiol Genomics. 2019;51:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bagai K, Muldowney JA 3rd, Song Y, Wang L, Bagai J, Artibee KJ, Vaughan DE and Malow BA. Circadian variability of fibrinolytic markers and endothelial function in patients with obstructive sleep apnea. Sleep. 2014;37:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bradley T and Floras J. Sleep apnea and heart failure: Part I: Obstructive sleep apnea. Circulation. 2003;107:1671–1678. [DOI] [PubMed] [Google Scholar]
- 133.Zhao Y, Zhang Y, Wang S, Hua Z and Zhang J. The clock gene Per2 is required for normal platelet formation and function. Thromb Res. 2011;127:122–30. [DOI] [PubMed] [Google Scholar]
- 134.Ohkura N, Oishi K, Sudo T, Hayashi H, Shikata K, Ishida N, Matsuda J and Horie S. CLOCK regulates circadian platelet activity. Thromb Res. 2009;123:523–7. [DOI] [PubMed] [Google Scholar]
- 135.Somanath PR, Podrez EA, Chen J, Ma Y, Marchant K, Antoch M and Byzova TV. Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype. J Cell Physiol. 2011;226:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janszky I and Ljung R. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008;359:1966–8. [DOI] [PubMed] [Google Scholar]
- 137.Manfredini R, Fabbian F, Cappadona R, De Giorgi A, Bravi F, Carradori T, Flacco ME and Manzoli L. Daylight Saving Time and Acute Myocardial Infarction: A Meta-Analysis. Journal of clinical medicine. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Couch RD. Travel, time zones, and sudden cardiac death. Emporiatric pathology. Am J Forensic Med Pathol. 1990;11:106–11. [DOI] [PubMed] [Google Scholar]
- 139.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH Jr., Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A and Anders RJ. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. Jama. 2003;289:2073–82. [DOI] [PubMed] [Google Scholar]
- 140.Labrecque N and Cermakian N. Circadian Clocks in the Immune System. Journal of biological rhythms. 2015;30:277–90. [DOI] [PubMed] [Google Scholar]
- 141.Scheiermann C, Gibbs J, Ince L and Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18:423–437. [DOI] [PubMed] [Google Scholar]
- 142.Scheiermann C, Kunisaki Y and Frenette PS. Circadian control of the immune system. Nature reviews Immunology. 2013;13:190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, Simpson R, Shaw J and Wong TY. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–9. [DOI] [PubMed] [Google Scholar]
- 144.Born J, Lange T, Hansen K, Molle M and Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. Journal of immunology. 1997;158:4454–64. [PubMed] [Google Scholar]
- 145.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS and Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M and Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O and Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med. 2016;8:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H and Scheiermann C. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity. 2017;46:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Suzuki K, Hayano Y, Nakai A, Furuta F and Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med. 2016;213:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dimitrov S, Benedict C, Heutling D, Westermann J, Born J and Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mendez-Ferrer S, Lucas D, Battista M and Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. [DOI] [PubMed] [Google Scholar]
- 152.Prendergast BJ, Cable EJ, Patel PN, Pyter LM, Onishi KG, Stevenson TJ, Ruby NF and Bradley SP. Impaired leukocyte trafficking and skin inflammatory responses in hamsters lacking a functional circadian system. Brain Behav Immun. 2013;32:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.He W, Holtkamp S, Hergenhan SM, Kraus K, de Juan A, Weber J, Bradfield P, Grenier JMP, Pelletier J, Druzd D, Chen CS, Ince LM, Bierschenk S, Pick R, Sperandio M, Aurrand-Lions M and Scheiermann C. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity. 2018;49:1175–1190 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A and Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW and Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silver AC, Arjona A, Walker WE and Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, Xue C, Geiger SS, Hokamp K, Reilly MP, Coogan AN, Vigorito E, FitzGerald GA and O’Neill LA. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, Lee CY, Watt A, Grossman TR, Rosenfeld MG, Evans RM and Glass CK. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S and Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H and Kizaki T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–17. [DOI] [PubMed] [Google Scholar]
- 161.Oliva-Ramirez J, Moreno-Altamirano MM, Pineda-Olvera B, Cauich-Sanchez P and Sanchez-Garcia FJ. Crosstalk between circadian rhythmicity, mitochondrial dynamics and macrophage bactericidal activity. Immunology. 2014;143:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ella K, Mocsai A and Kaldi K. Circadian regulation of neutrophils: Control by a cell-autonomous clock or systemic factors? Eur J Clin Invest. 2018:e12965. [DOI] [PubMed] [Google Scholar]
- 163.Hayashi M, Shimba S and Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–6. [DOI] [PubMed] [Google Scholar]
- 164.Oishi Y, Hayashi S, Isagawa T, Oshima M, Iwama A, Shimba S, Okamura H and Manabe I. Bmal1 regulates inflammatory responses in macrophages by modulating enhancer RNA transcription. Sci Rep. 2017;7:7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Curtis AM, Bellet MM, Sassone-Corsi P and O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–86. [DOI] [PubMed] [Google Scholar]
- 166.Morris CJ, Purvis TE, Hu K and Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carter SJ, Durrington HJ, Gibbs JE, Blaikley J, Loudon AS, Ray DW and Sabroe I. A matter of time: study of circadian clocks and their role in inflammation. J Leukoc Biol. 2016;99:549–60. [DOI] [PubMed] [Google Scholar]
- 168.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT and Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. Journal of immunology. 2010;185:5796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huo M, Huang Y, Qu D, Zhang H, Wong WT, Chawla A, Huang Y and Tian XY. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017;31:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma H, Zhong W, Jiang Y, Fontaine C, Li S, Fu J, Olkkonen VM, Staels B and Yan D. Increased atherosclerotic lesions in LDL receptor deficient mice with hematopoietic nuclear receptor Rev-erbalpha knock- down. J Am Heart Assoc. 2013;2:e000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sitaula S, Billon C, Kamenecka TM, Solt LA and Burris TP. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem Biophys Res Commun. 2015;460:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang L, Chu Y, Wang L, Wang Y, Zhao X, He W, Zhang P, Yang X, Liu X, Tian L, Li B, Dong S and Gao C. Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-kappaB pathway. Int Immunopharmacol. 2015;28:525–30. [DOI] [PubMed] [Google Scholar]
- 173.Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, Winter J, Drechsler M, de Jong R, Immler R, Sperandio M, Hristov M, Zeller T, Nicolaes GAF, Weber C, Viola JR, Hidalgo A, Scheiermann C and Soehnlein O. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018;28:175–182 e5. [DOI] [PubMed] [Google Scholar]
- 174.Ridker PM, Manson JE, Buring JE, Muller JE and Hennekens CH. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation. 1990;82:897–902. [DOI] [PubMed] [Google Scholar]
- 175.De Giorgi A, Mallozzi Menegatti A, Fabbian F, Portaluppi F and Manfredini R. Circadian rhythms and medical diseases: does it matter when drugs are taken? European journal of internal medicine. 2013;24:698–706. [DOI] [PubMed] [Google Scholar]
- 176.Portaluppi F, Montanari L, Bagni B, degli Uberti E, Trasforini G and Margutti A. Circadian rhythms of atrial natriuretic peptide, blood pressure and heart rate in normal subjects. Cardiology. 1989;76:428–32. [DOI] [PubMed] [Google Scholar]
- 177.Richards AM, Tonolo G, Fraser R, Morton JJ, Leckie BJ, Ball SG and Robertson JI. Diurnal change in plasma atrial natriuretic peptide concentrations. Clinical science. 1987;73:489–95. [DOI] [PubMed] [Google Scholar]
- 178.Vogiatzis I Circadian rhythm of cardiac troponins. Does it really exist? International journal of cardiology. 2018;270:72–73. [DOI] [PubMed] [Google Scholar]
- 179.Drawz PE, Pajewski NM, Bates JT, Bello NA, Cushman WC, Dwyer JP, Fine LJ, Goff DC Jr., Haley WE, Krousel-Wood M, McWilliams A, Rifkin DE, Slinin Y, Taylor A, Townsend R, Wall B,Wright JT and Rahman M. Effect of Intensive Versus Standard Clinic-Based Hypertension Management on Ambulatory Blood Pressure: Results From the SPRINT (Systolic Blood Pressure Intervention Trial) Ambulatory Blood Pressure Study. Hypertension. 2017;69:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ and Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hermida RC, Ayala DE, Mojon A and Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–51. [DOI] [PubMed] [Google Scholar]
- 182.Bonten TN, Saris A, van Oostrom MJ, Snoep JD, Rosendaal FR, Zwaginga J, Eikenboom J, van der Meer PF and van der Bom JG. Effect of aspirin intake at bedtime versus on awakening on circadian rhythm of platelet reactivity. A randomised cross-over trial. Thromb Haemost. 2014;112:1209–18. [DOI] [PubMed] [Google Scholar]
- 183.Wallace A, Chinn D and Rubin G. Taking simvastatin in the morning compared with in the evening: randomised controlled trial. BMJ. 2003;327:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hermida RC, Ayala DE, Chayan L, Mojon A and Fernandez JR. Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int. 2009;26:61–79. [DOI] [PubMed] [Google Scholar]
- 185.Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P and Staels B. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet. 2018;391:59–69. [DOI] [PubMed] [Google Scholar]
- 186.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K, American Heart Association Obesity Committee of the Council on L, Cardiometabolic H, Council on Cardiovascular Disease in the Y, Council on Clinical C and Stroke C. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Seo DY, Lee S, Kim N, Ko KS, Rhee BD, Park BJ and Han J. Morning and evening exercise. Integr Med Res. 2013;2:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Reitz CJ and Martino TA. Disruption of Circadian Rhythms and Sleep on Critical Illness and the Impact on Cardiovascular Events. Current pharmaceutical design. 2015;21:3505–11. [DOI] [PubMed] [Google Scholar]
- 189.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM and Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang L, Zhang R, Tien CL, Chan RE, Sugi K, Fu C, Griffin AC, Shen Y, Burris TP, Liao X and Jain MK. REV-ERBalpha ameliorates heart failure through transcription repression. JCI insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stujanna EN, Murakoshi N, Tajiri K, Xu D, Kimura T, Qin R, Feng D, Yonebayashi S, Ogura Y, Yamagami F, Sato A, Nogami A and Aonuma K. Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS One. 2017;12:e0189330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ushijima K, Maekawa T, Ishikawa-Kobayashi E, Ando H, Shiga T and Fujimura A. Influence of beta-blockers on the myocardial mRNA expressions of circadian clock- and metabolism-related genes. Journal of the American Society of Hypertension : JASH. 2013;7:107–17. [DOI] [PubMed] [Google Scholar]


