Abstract
Background and Purpose:
To test the hypothesis that covert brain infarcts (CBI) are more likely to be located in non-eloquent brain areas compared to clinical strokes, and that CBI etiological subtypes carry a differential risk of vascular events compared to people without CBI.
Methods:
We used brain MRIs from 1290 stroke-free participants in the Northern Manhattan Study to evaluate for CBI. We classified CBI as cardioembolic (i.e. known atrial fibrillation), large artery atherosclerosis (LAA, extra- and intra-cranial), penetrating artery disease, and cryptogenic (no apparent cause). CBI localized in the non-motor areas of the right hemisphere were considered non-eloquent. We then evaluated risk of events by CBI subtype with adjusted Cox proportional models.
Results:
At the time of MRI, 236 participants (18%) had CBI (144 [61%] distal cryptogenic, 29 [12%] distal cardioembolic, 26 [11%] LAA, and 37 [16%] penetrating artery disease). Smaller (per mm, OR 0.8, 0.8–0.9) and non-brain stem infarcts (OR 0.2, 0.1–0.6) were more likely to be covert. During the follow-up period (10.4 ± 3.1 years), 398 (31%) died (162 [13%] of vascular death) and 117 (9%) had a stroke (99 (85%) were ischemic. Risks of events varied by CBI subtype, with the highest risk of stroke (HR 2.2, 1.3–3.7) and vascular death (HR 2.24, 1.29–3.88) noted in participants with intracranial LAA-related CBI.
Conclusions:
CBI can be classified into subtypes that have differential outcomes. Certain CBI subtypes such as those related to intracranial LAA have a high risk of adverse vascular outcomes and could warrant consideration of treatment trials.
Keywords: stroke, brain infarction, risk factor, intracranial stenosis, atherosclerosis
Subject terms: Magnetic Resonance Imaging (MRI), Ischemic stroke, Risk Factors
INTRODUCTION:
In older populations, the three most common identifiable causes of stroke include atrial fibrillation, large artery stenosis (LAA, intracranial and extracranial) and small artery disease.1, 2 In addition to etiology-specific interventions stroke subtypes also indicate different natural histories. For example, high degree stenosis due to intracranial LAA has a risk of recurrence > 10% per year, while small artery disease averages 2–3% per year.3 Although extensive data suggest that individuals with covert brain infarct (CBI) carry a higher risk of stroke than those without CBI,4 the underlying etiologies ascribed to clinical strokes are rarely applied to CBI. It is likely that CBI have similar etiologies to clinically symptomatic strokes, with the likely exception that small infarcts or those located in non-eloquent areas are more likely to remain subclinical.
Based on the knowledge gap related to CBI subtypes and informed by clinical practice, we hypothesize that CBI are more likely to be located in non-eloquent areas (e.g. right hemisphere, excluding the primary motor cortex) and/or to be smaller than infarcts causing symptoms, and that the risk of clinical stroke and vascular events among individuals with CBI will differ by etiological subtype.
METHODS:
The Northern Manhattan Study (NOMAS) is a population-based cohort of adults ≥ 40 years at the time of initial enrollment in 1993 (≥55 years old only after 1998), selected via random telephone dialing in zip codes representing Northern Manhattan.2 In 2003, surviving participants were invited to participate in a brain MRI substudy if they remained stroke-free. Written informed consent was obtained from all participants. The study was approved by the Institutional Review Boards at Columbia University and the University of Miami. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Covariates at the time of MRI:
Demographic data were self-reported. Prevalent hypertension, diabetes, and hypercholesterolemia were defined by either self-reported diagnoses, medication use to treat these vascular risks, or blood pressure and/or laboratory evidence, as previously reported.5, 6 Smoking status and cigarettes smoked per day were self-reported.
Covariates during post-MRI follow-up:
Participants were followed annually with standardized telephone interviews and/or in-person visit if a participant screened positive for a pre-defined outcome with < 2% loss to follow up. During the telephone interview, participants were asked about new diagnoses of vascular risks and whether they had started a new treatments, including antiplatelets and/or anticoagulation. Each medication name and class were recorded.
We calculated exposure time (in years) to medications for treating vascular risks by multiplying the time interval between visits (in years) by the number of visits that a given participant reported using the medications or not. Similarly, years of smoking was calculated for participants who reported continuing smoking. We arbitrarily categorized participants as having “regular primary care follow-up” if participants declared seeing their doctor > 50% of the follow-up post MRI visits.
MRI CBI definitions:
Imaging was performed on a dedicated 1.5-T research MRI system (Philips Medical Systems, please see http://stroke.ahajournals.org for detailed protocol). We used a combination of FLAIR rim hyperintensity, shape, size and anatomical location to attribute a probabilistic diagnosis informed by pathology-derived MRI parameters.7 We revised the old NOMAS definition of CBI, incorporating pathology-derived MRI parameters to systematically segregate large perivascular spaces (> 5 mm in axial diameter) from CBI. The importance of such effort is justified by the fact that large perivascular spaces do not carry a higher risk of vascular events.8 Some of the more notable changes defining CBI with a pathology-informed method included voids in the brainstem being considered infarcts regardless of whether a hyperintense flair rim encircles them9 or voids > 5 mm in axial diameter in the subinsular cortex and infraputaminal region being considered perivascular spaces unless a clear and thick flair rim surrounds them.9, 10 Cerebellar voids had to have a clear flair rim to be considered infarcts as opposed to perivascular spaces. Nonetheless, 87% of the CBI in this study had a thick FLAIR hyperintensity surrounding a parenchymal void, which approximates our definition of CBI to the definition used by others.
To derive CBI size, we obtained the longest axial diameter, a diameter perpendicular to that, and a vertical diameter (multiplying the number of slices by slice thickness [1.3 mm in this case]). The volume of each CBI was calculated using the ABC/2 formula, and we derived the volume-based diameter for analyses.11 The measurement were carried out with 3D Slicer open source software version 4.3.1. This method has moderate to good reliability.5, 6 Penetrating artery territories included the brainstem and basal ganglia, including the lenticular nucleus, caudate head, thalamus, and the anterior limb and genu of the internal capsule. The subinsular cortex, the periventricular white matter, and the cerebellum were not considered penetrating artery territories. The white matter tracts outside the basal ganglia were considered medullary artery territories (Figure 1, panel A, yellow arrows). Cerebellar and supratentorial cortical CBI were considered distal, non-penetrating artery CBI (Figure 1, panel B). We created a library with images of each CBI. Two vascular neurologists (JG and MSVE) evaluated each CBI separately and attributed the vascular territory by artery and by location into cortical, medullary or penetrating arteries, with an agreement > 95%.
Figure 1. Vascular territories and examples of covert brain infarct subtypes.
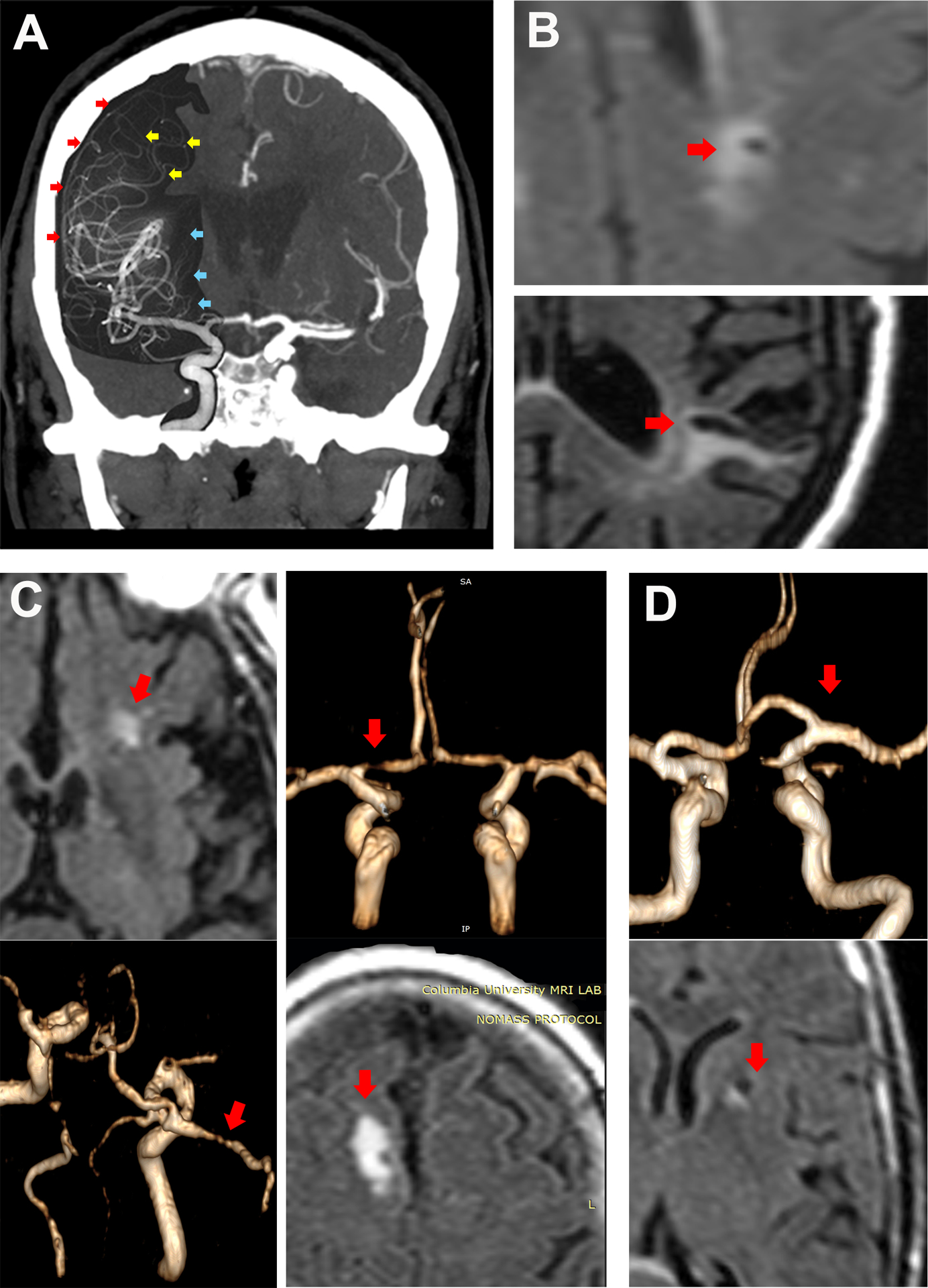
By superimposing a digital conventional angiogram to a brain computed tomographic angiogram from the same patient one can observe the brain territories supplied by penetrating arteries (panel a, blue arrows), cortical arteries (panel a, red arrows) and medullary arteries (panel a, yellow arrows). An embolus reaching the convexity surface of the brain may cause a subcortical infarct in the territory of the medullary arteries or cortical surface (panel b, top and bottom). High degree intracranial stenoses may cause brain infarction either by occluding the ostia of penetrating arteries (i.e. branch occlusive disease) or by causing artery-to-artery emboli (panel c). Lacunar infarcts (panel d) are more likely caused by small artery disease in the absence of ipsilateral high degree stenosis.
CBI etiology Subtype Classification:
In addition to the brain MRI, we used 3D reconstruction of the circle of Willis MRA and carotid Doppler data performed concomitantly to the MRI. Cortical, cerebellar and medullary artery CBI were considered distal and more likely embolic. If a distal CBI had ipsilateral extracranial carotid stenosis > 60% (by Doppler) or intracranial stenosis > 50% (by time-of-flight MRA), the etiology was presumed to be artery-to-artery embolism as none appeared to have a watershed distribution that would suggest a hemodynamic etiology. If a participant reported history of atrial fibrillation and there was evidence of a CBI, this CBI was classified as cardioembolic regardless of the territory and/or mixed pathologies given preponderance of atrial fibrillation in guiding stroke preventive measures. A distal CBI in participants without atrial fibrillation but who reported a history of congestive heart failure or valvulopathy and had no ipsilateral large artery stenosis > 50% was considered cardioembolic. The etiology of penetrating artery CBI with luminal irregularities or stenoses in the parent artery was considered due to branch occlusive disease. Branch occlusive disease and distal artery-to-artery infarcts were combined into intracranial LAA CBI (Figure 1, panel c). Penetrating artery CBI with no cardioembolic source and no ipsilateral large artery stenoses or luminal irregularities were considered isolated penetrating artery CBI (Figure 1, panel d). A distal CBI with no identified cardiac or arterial etiology was considered cryptogenic. We used the term “clinical stroke” as synonyms of a brain infarct that presented with clinical symptoms attributable to a vascular syndrome as per the vascular neurologist involved in the study blinded to their baseline brain MRI.
Outcomes assessment
Participants were screened annually with standardized telephone interviews and/or in-person visits to assess for clinical stroke, MI, and death (including cause of death). The majority (>60%) of NOMAS participants are admitted to / New York Presbyterian/ Columbia University Irving Medical Center in case of major vascular outcomes such as stroke or MI. In-house records or records from outside hospitals were available to the study investigators for outcome ascertainment. Stroke subtypes were adjudicated independently by two study vascular neurologists (blinded to the baseline MRI) and MI was adjudicated by a study cardiologist using established criteria.12 Any vascular event was defined as a composite of vascular death, any stroke or MI, as described before.13
Statistical analysis:
We report sample characteristics from the time of MRI by CBI subtypes. Differences in continuous variables were tested with Student t-tests and differences in categorical variables were assessed with Chi squared tests. First, we investigated difference in infarct size, site and subtype by clinical status in participants with either CBI at the time of MRI or clinical strokes during follow up. For the purpose of this analysis, each infarct was given a row. The columns consisted of infarct size, site, subtype, clinical status (covert versus clinical). Among participants with CBI at baseline who developed a clinical stroke during follow-up, we considered the CBI and the clinical stroke as independent events for the purpose of evaluating infarct characteristics that predict clinical versus covert status.
In the second part of the analysis, we investigated crude incidence rates and their 95% confidence intervals (CI) with Poisson regression by CBI subtype. We graphed the clinical outcome survival plots by CBI subtype. We then built risk models to obtain hazard ratios (HR) and their 95% CI with Cox proportional hazards regressions with robust sandwich error variance, adjusting for demographics, prevalent and incident vascular risk factors, and for competing risks between death and the other outcomes.14 For cryptogenic CBI, we ran interaction models with pure cortical location, multifocal locations, and with mixed penetrating artery disease to evaluate for greater discrimination in identifying stroke subtype risk during post MRI follow up. We also tested whether an interaction existed between CBI subtype and years of exposure to antihypertensives, hypoglycemics, statins, antiplatelets and anticoagulants. A P value of < 0.05 was considered statistically significant for primary outcome analyses and a P value of < 0.10 was considered significant for interactions. The statistical analysis was carried out with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS:
We included 1290 NOMAS participants. The sample characteristics are described in table 1. At baseline, 236 NOMAS participants (18%) had CBI (144 [61%] distal cryptogenic, 29 [12%] distal cardioembolic, 26 [11%] LAA, and 37 [16%] isolated penetrating artery disease). Because only three participants had extracranial LAA CBI, we focused subsequently on intracranial LAA only. Participants were followed on average for 10.4 ± 3.1 years after baseline (range 0.5–14.8, IQR 9.5–12.5 years). During the follow-up period, 398 (31%) died (162 [13%] of vascular death), 60 (5%) had a myocardial infarction, and 117 (9%) had a stroke, of which 99 (85%) were ischemic (Figure 2).
Table 1:
Characteristics of the study participants by covert infarct subtype (N=1290)
| No covert infarct N=1054 |
Any covert infarct (N=236) | P value | ||||
|---|---|---|---|---|---|---|
| Distal cryptogenic N=144 |
Distal cardioembolic N=29 |
Intracranial large artery stenosis N=23 |
Isolated penetrating artery N=37 |
|||
| Age (in years) ± SD | 70±9 | 74±9 | 75±9 | 77±9 | 74±9 | <0.001 |
| Male sex (%) | 38 | 45 | 66 | 43 | 32 | 0.019 |
| Non-white (%) | 84 | 86 | 86 | 83 | 91 | 0.799 |
| Hypertension | ||||||
| At MRI (%) | 76 | 86 | 93 | 96 | 86 | 0.020 |
| Post MRI (%) | 14 | 9 | 7 | 4 | 8 | |
| Antihypertensives at MRI (%) | 58 | 67 | 83 | 86 | 70 | <0.001 |
| Mean number of antihypertensives at MRI | 0.8±0.7 | 0.8±0.7 | 1.2±0.7 | 0.8±0.5 | 0.8±0.5 | 0.021 |
| Mean SBP at MRI (in mmHg) | 136±18 | 139±17 | 133±14 | 141±18 | 138±19 | 0.125 |
| Mean DBP at MRI (in mmHg) | 78±10 | 79±17 | 76±10 | 79±12 | 79±19 | 0.392 |
| Mean PP at MRI (in mmHg) | 58±15 | 60±14 | 57±13 | 61±15 | 60±16 | 0.450 |
| Mean MAP at MRI (in mmHg) | 97±11 | 99±11 | 95±9 | 100±13 | 94±11 | 0.129 |
| Diabetes | ||||||
| At MRI (%) | 25 | 26 | 41 | 30 | 24 | 0.511 |
| Post MRI (%) | 15 | 10 | 10 | 13 | 16 | |
| Hypoglycemics at MRI (%) | 19 | 19 | 35 | 17 | 22 | 0.372 |
| Fasting glucose at MRI | 101±33 | 102±30 | 113±61 | 98±28 | 107±42 | 0.280 |
| Dyslipidemia | ||||||
| At MRI (%) | 90 | 92 | 79 | 96 | 94 | 0.578 |
| Post MRI (%) | 5 | 3 | 10 | 4 | 3 | |
| Statin use at MRI (%) | 25 | 24 | 41 | 35 | 32 | 0.177 |
| LDL at MRI (md/dl) | 116±35 | 116±33 | 89±33 | 122±44 | 112±37 | 0.001 |
| HDL at MRI (md/dl) | 53±17 | 54±18 | 51±20 | 59±20 | 54±15 | 0.488 |
| Triglycerides at MRI (md/dl) | 129±81 | 118±61 | 108±70 | 121±66 | 121±45 | 0.379 |
| Smoking at MRI | 11 | 12 | 10 | 17 | 22 | 0.343 |
| Packs-per-day at MRI | 0.6±0.5 | 0.7±0.7 | 0.4±0.3 | 1.3±0.80 | 0.73±0.6 | 0.075 |
| Antiplatelet use at MRI (%) | 39 | 42 | 55 | 48 | 43 | 0.364 |
| Atrial fibrillation (%) | ||||||
| At MRI | 4 | 0 | 72 | 0 | 0 | <0.001 |
| Post MRI | 10 | 10 | 10 | 13 | 14 | 0.968 |
| Anticoagulation at MRI | 2 | 1 | 17 | 0 | 3 | <0.001 |
| Coronary heart disease at MRI | 23 | 21 | 79 | 43 | 35 | <0.001 |
| Primary care doctor during follow-up | 95 | 92 | 90 | 86 | 92 | 0.086 |
Note: Units for fasting glucose, LDL, HDL and triglycerides in mg/dl.
Figure 2.
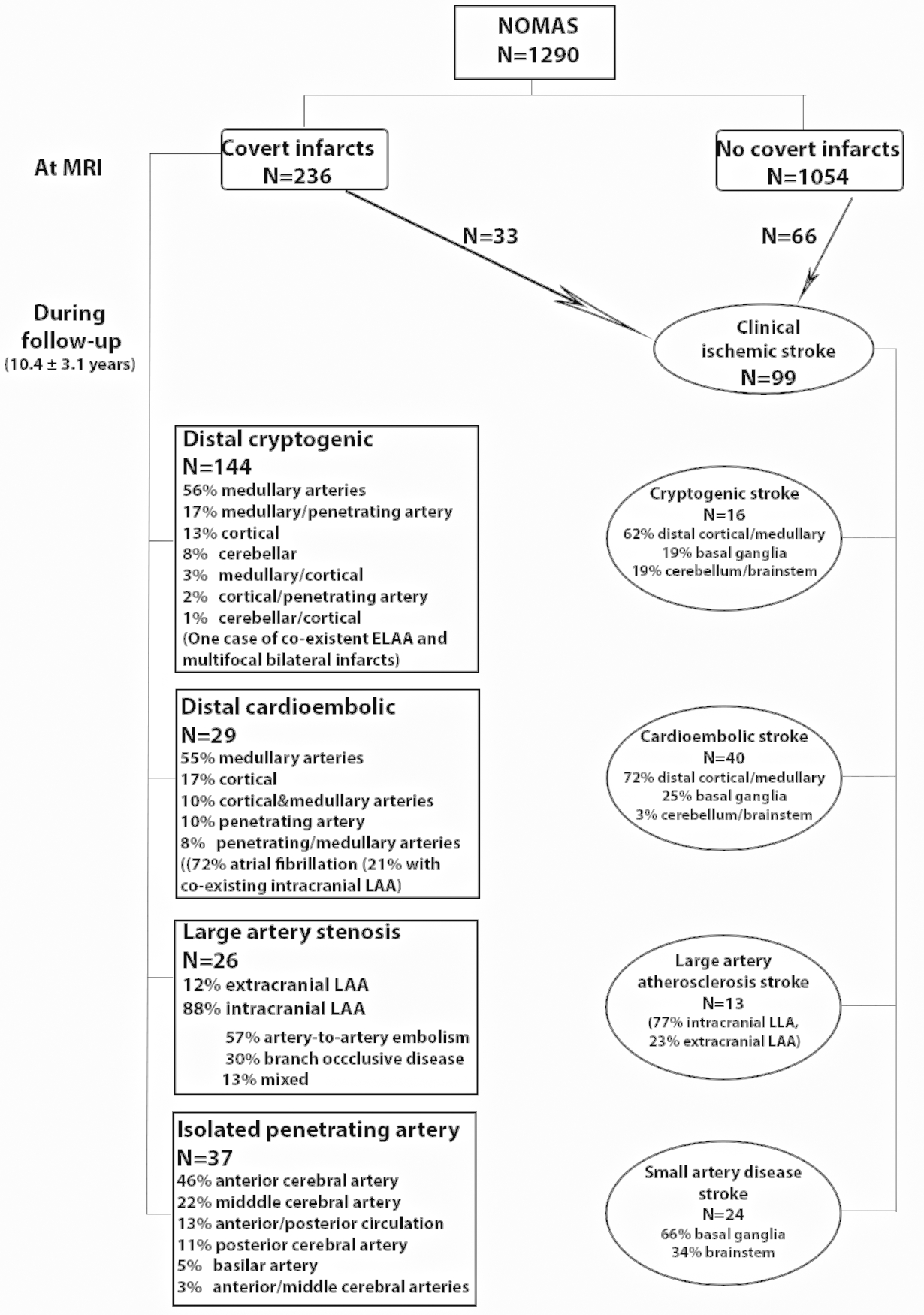
Flow diagram of the NOMAS MRI cohort.
Of the 283 NOMAS participants who had brain infarcts, 185 had CBI only, 67 had clinical strokes, and 31 had both CBI at baseline and clinical stroke during follow-up. Among participants with either CBI or stroke, right-sided non-motor supratentorial infarcts were more likely to be covert (60 vs. 48%, P=0.044) whereas left-sided supratentorial infarcts were equally likely to be covert or clinical strokes (58 vs. 61 %, P=0.68). Infarcts were more likely to present as clinical stroke if located in the brainstem (14 vs. 4%, P<0.001) or had a larger size (13 mm versus 5 mm, P<0.001). Larger size as a predictor of a clinical stroke persisted within each subtype comparison. In a logistic regression including size, side, and infarct subtype, smaller infarcts (per each mm, OR 0.80, 0.75–0.86), non-brain stem infarcts (OR 0.19, 0.06–0.62), and cryptogenic infarcts (OR 7.49, 95% CI 2.48–22.61) were more likely to be covert.
The crude incidence rates of events varied by CBI subtype (Table 2).
Table 2:
Crude incidence rate (per 1,000 person-year) of outcome vascular events by covert infarct subtype
| Any death | Vascular death | Myocardial Infarction | Any stroke | Ischemic stroke | Cryptogenic stroke | Cardioembolic stroke | Intracranial arterial disease (small and large) stroke | |
|---|---|---|---|---|---|---|---|---|
| Incidence rate, 95% Confidence Interval | ||||||||
|
No covert infarct
N=1057 |
25.2, 22.4–28.3 |
9.4, 7.8–11.3 |
4.0, 3.0–5.4 |
7.2, 5.8–9.0 |
6.0, 4.7–7.6 |
0.9, 0.5–1.7 |
2.1, 1.4–3.2 |
2.2, 1.5–3.3 |
| Distal cryptogenic N=144 |
48.1, 37.7–61.4 |
25.2, 18.0–35.2 |
4.7, 2.1–10.4 |
15.5, 10.0–24.1 |
13.1, 8.2–21.1 |
3.7, 1.6–8.9 |
4.5, 2.0–10.0 |
3.8, 1.6–9.1 |
| Distal cardioembolic N=29 |
98.5, 64.2–151.1 |
56.3, 32.0–99.1 |
24.1, 9.1–64.3 |
29.9, 13.4–66.6 |
29.9, 13.4–66.6 |
- | 29.9, 13.4–66.6 |
- |
| Intracranial large artery sstenosis
N=23 |
69.0, 40.8–116.5 |
29.6, 13.3–65.8 |
32.3, 13.4–77.5 |
27.3, 11.4–65.5 |
21.8, 8.2–58.1 |
- | 10.6, 2.6–42.2 |
5.0, 0.7–35.4 |
| Isolated penetrating artery N=37 |
39.1, 21.1–65.9 |
11.2, 29.74 |
6.0, 1.5–24.1 |
20.5, 9.8–42.9 |
17.4, 7.8–38.7 |
2.8, 0.4–19.9 |
5.6, 1.4–22.4 |
8.6, 2.8–26.8 |
Participants with cardioembolic and intracranial LAA CBI had an overall higher rate of death and vascular events. Survival plots (Figure 3) demonstrate that the higher risk of vascular death among participants with distal cardioembolic CBI is constant throughout the follow-up period. Conversely, among participants with intracranial LAA CBI, the risk of myocardial infarction, ischemic stroke, and intracranial (large and small) artery disease clinical strokes was more pronounced in the first 5 years of follow-up. Among participants with isolated penetrating artery CBI, the risk of ischemic stroke specifically intracranial (large and small) artery disease strokes, was more pronounced after year 5 of follow-up. The lowest risk of vascular death, myocardial infarction, and stroke was noted among participants with no CBI.
Figure 3.
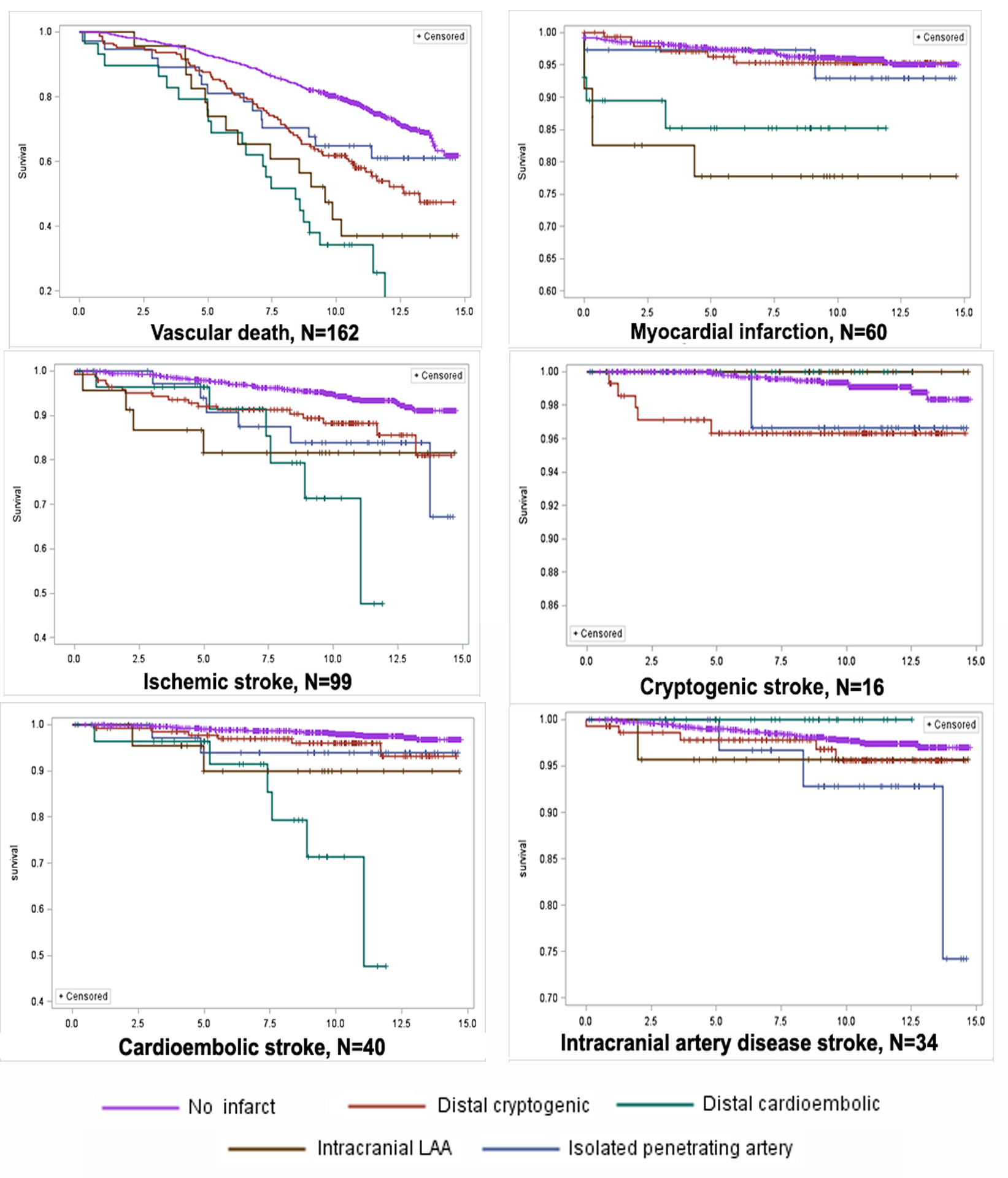
Survival plots for vascular outcomes by covert brain infarct subtypes.
We assessed the risk of events by CBI subtype with models adjusted for demographics, vascular risk, the time of MRI, and variations in exposure to treatment of vascular risk factors during follow-up, health care utilization, and competing risk of death. The mean time-to-event was shorter for participants with cardioembolic (6.9 years) and intracranial LAA CBI (7.9 years) than among participants with isolated penetrating artery CBI (9.0 years) or cryptogenic CBI (9.3 year, P < 0.01 for all comparisons). Risks of death and vascular events were persistently higher among participants with intracranial LAA and cryptogenic CBI (Table 3). The risk of ischemic stroke was higher across all CBI subtypes compared to those without CBI (Table 3). Participants with cryptogenic and intracranial LAA CBI were at a higher risk of cryptogenic, intracranial artery disease, and cardioembolic strokes, whereas those with isolated penetrating artery disease were only at risk of intracranial artery disease strokes. Among participants with cryptogenic CBI, further subdiving them by strict cortical or multifocal locations, or excluding those with mixed penetrating artery disease infarct did not help discriminating the future stroke subtype (P > 0.20 for all interactions).
Table 3:
Risk of vascular events during follow-up time (10.4 ± 3.1 year, range 0.5–14.8) by covert infarct subtype
| Covert infarcts at the time of MRI Hazard ratios, 95% Confidence Interval |
|||||
|---|---|---|---|---|---|
| Any N=236 |
Distal cryptogenic N=144 |
Distal cardioembolic N=29 |
Intracranial large artery stenosis N=23 |
Isolated penetrating artery N=37 |
|
| Any death (N=398) | 1.42, 1.12–1.80 |
1.43, 1.08–1.90 |
1.40, 0.81–2.44 |
2.24, 1.29–3.88 |
1.53, 0.88–2.65 |
| Vascular death (N=−162) | 1.48, 1.18–1.87 |
1.43, 1.08–1.90 |
1.41, 0.81–2.45 |
2.24, 1.29–3.88 |
1.53, 0.88–2.65 |
|
Myocardial Infarction
(N=60) |
1.48, 1.18–1.84 |
1.40, 1.07–1.85 |
1.65, 0.97–2.81 |
2.34, 1.41–3.92 |
1.37, 0.81–2.33 |
| Any stroke (N=117) | 1.50, 1.21–1.86 |
1.44, 1.11–1.89 |
1.51, 0.92–2.47 |
2.16, 1.27–3.67 |
1.61, 0.98–2.65 |
|
Any vascular events
(N=288) |
1.47, 1.18–1.80 |
1.37, 1.05–1.78 |
1.78, 1.10–2.89 |
2.22, 1.35–3.66 |
1.39, 0.84–2.27 |
| Ischemic stroke (N=99) | 1.49, 1.20–1.86 |
1.44, 1.10–1.89 |
1.48, 0.90–2.42 |
2.21, 1.30–3.75 |
1.63, 1.00–2.68 |
|
Cryptogenic stroke
(N=16) |
1.48, 1.18–1.87 |
1.45, 1.10–1.92 |
1.36, 0.78–2.36 |
2.16, 1.25–3.74 |
1.51, 0.87–2.61 |
|
Cardioembolic stroke
(N=40) |
1.49, 1.19–1.87 |
1.39, 1.05–1.84 |
1.59, 0.95–2.65 |
2.35, 1.38–4.01 |
1.52, 0.88–2.62 |
|
Intracranial [small and large] arterial disease stroke
(N=34, 24 small & 12 large) |
1.49, 1.19–1.86 |
1.45, 1.10–1.91 |
1.34, 0.78–2.28 |
2.07, 1.19–3.57 |
1.68, 1.03–2.80 |
Analytic notes:
-Models adjusted for age, sex, ethnicity, prevalent and incident hypertension, diabetes and hypercholesterolemia, average number of antihypertensives at MRI, Hypoglycemics and cholesterol-lowering medications at MRI, post MRI exposure (in years) to antihypertensives, hypoglycemics, statins, pack-per-year of smoking, antiplatelets and anticoagulation, and follow-up with primary care during follow-up.
-Three participants had carotid stenosis > 60% by Doppler and ipsilateral infarcts and were considered within the “any infarct” category but not in the infarct subcategories.
We found a statistical interaction between greater exposure to antihypertensives and lower risk of any stroke among participants with cryptogenic CBI (P 0.007) or with isolated penetrating artery CBI (P 0.10) and a lower risk of ischemic stroke among those with cardioembolic CBI (P 0.03). We also found a statistical interaction between exposure to statins and risk of intracranial arterial disease stroke (P=0.07) in participants with isolated penetrating artery disease CBI (please see http://stroke.ahajournals.org).
Discussion:
With the results presented here, we confirmed the hypothesis that smaller and/or non-eloquent right-sided brain infarcts are more likely to be covert whereas larger and/or brainstem infarcts are more likely to present as clinical stroke. Furthermore, we confirmed that the risks of vascular events and death vary by CBI subtype similar to the differences in risk among individuals who present with clinical stroke. Based on this, we conclude that the risks conferred by CBI and clinical strokes may be similar. Given the high risk of vascular death and vascular events among individuals with CBI, the presence of CBI may be an opportunity to review and aggressively control vascular risks. Moreover, this study supports the need for data to determine the prognostic value of prolonged holter monitoring and large artery imaging to uncover cardioembolic or large artery lesions that may underlie these subtypes of CBI.
The results presented here recapitulate risk profiles and natural histories of individuals who present with clinical strokes. Intracranial LAA has a high risk of stroke recurrence, and the risk is proportional to the severity of the stenosis and to the timing of the symptomatic events, with the risk being higher in the first 4 weeks after stroke.3, 15 Compared to the general population, the rates of ischemic stroke and intracranial artery disease stroke seem much higher in participants with CBI attributable to intracranial LAA than those with other subtypes of CBI,1, 16 which suggests that these participants have a more severe disease that carries an inherently higher risk of stroke recurrence. The association between higher risk of non-cerebral vascular events and intracranial LAA has been reported in stroke-only samples,17 and is probably related to co-existence between cerebral and coronary atherosclerosis.18, 19 Furthermore, this study is consistent with the notion that penetrating artery disease and LAA represent different stages of the same pathophysiological process, in which penetrating artery disease represents an early stage as opposed to the more advanced stage represented by intracranial LAA. Participants with isolated penetrating artery disease had a higher risk of intracranial artery disease and non-lacunar stroke, similar to what other studies have reported.20 The statistically significant interactions between years of exposure to antihypertensives and statins for stroke risk among participants with isolated penetrating artery CBI reported in the supplemental data also suggest that earlier exposure to these therapeutic measures could decrease the subsequent risk of events to a greater extent than exposure to the same measures when the disease is advanced. The overlap in genetic variants for small and large artery disease also supports the validity of “intracranial artery disease” as a phenotype.21 There is evidence, however, of a differential effect in the treatment for stroke recurrence. Among individuals with intracranial LAA who present with ipsilateral strokes, dual antiplatelet may be more effective than among those with isolated artery disease,22 especially early in the disease course.23, 24 The difference in antiplatelet benefit may be related to the pathophysiology of atherosclerosis-related strokes, which is attributed to plaque rupture and platelet-driven in-situ thrombosis25 whereas the pathophysiology related to small artery disease relates more to endothelial dysfunction.26
Distal cryptogenic strokes are the most numerous of all CBI subtypes, and perhaps offer the greatest opportunities to uncover the underlying etiology that may, or not, have a specific treatment. The more obvious possible hidden etiology may relate to occult atrial fibrillation. Some of the participants with distal cryptogenic silent infarcts had subsequent cardioembolic strokes, most caused by atrial fibrillation. Prior data has supported the value of cortical infarcts to identify individuals at a higher risk of cardioembolic stroke, but we have expanded this definition by including subcortical infarcts in the distal end of medullary arteries.
Covert infarcts are often segregated by whether they are cortical or deep, but subcortical infarcts in the distal territories of the medullary arteries are lumped together or labelled small artery disease (lacunes),27 when the more likely mechanism is embolic given that these arteries dive in from the brain surface toward the ventricular wall and have no anastomoses (Figure 1, panel A).28 Therefore, the term lacune as a qualifier of an infarct should be restricted to a brain infarction in the territory of a penetrating artery and not just any small artery.29 The unique flow pattern and geometric properties of the penetrating arterial ostia are a pathophysiological justification for preserving the specificity of the “lacunar” term for such infarcts.30
The access to carotid and brain arterial imaging concomitant to brain MRI is a strength of this cohort. The negligible loss to follow-up and thorough stroke adjudication by vascular neurologists increases the certainty that clinical stroke subtypes represent real-life clinical outcomes. The TOAST classification system is not explicit about branch occlusive disease, and it is possible that some strokes classified as small artery disease may in fact be due to intracranial LAA. The lack of systemic screening for atrial fibrillation, as stated above, may underestimate atrial fibrillation as cause of stroke. Other less common stroke pathologies such as hypercoagulable state, dissections or drug abuse are less likely in this aged cohort. The higher proportion of cryptogenic CBI as compared with rates of cryptogenic clinical strokes reported in this sample and in most population-based studies suggests that clinical workup of CBI may reveal etiologies and possibly lead to specific therapies for stroke prevention.
In summary, the data presented here suggest that CBI have a similar pathophysiology to their clinical counterparts, and that the chances of a given infarct presenting with clinical symptoms is mostly due to infarct size and site and not due to an underlying difference in risk or pathophysiology. Subclassifying CBI by their presumed etiology disclosed variability of the future risk of vascular events that resembles the differential risk of vascular events among people with stroke. This data offers a theoretical background to investigate whether treating individuals with CBI similarly to those with stroke may reduce the risk of infarct recurrence (silent or clinical) similarly to people who present with clinical stroke.
Supplementary Material
STUDY FUNDING:
Supported by NIH (5R01NS029993, R37 NS029993, K24 NS 062737-03, 1R01AG057709).
Footnotes
CONFLICT OF INTEREST:
Jose Gutierrez, MD, MPH: Dr Gutierrez receives compensation for providing consultative services for Pfizer and Prophase.
Clinton B Wright, MD, MS: Dr. Wright receives royalties from UpToDate.com for two chapters on vascular dementia.
Mitchell SV Elkind, MD, MS: Dr. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, and Sanofi-Regeneron Partnership; receives compensation for serving as an expert witness in litigation for BMS-Sanofi (Plavix), Merck/Organon (Nuvaring), and Hi-Tech Pharmaceuticals (dimethylamylamine); serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from UptoDate for chapters related to stroke.
Dr Sacco received institutional support from Boehringer Ingelheim for work on the Exec Committee of a secondary Stroke trial
No other author report conflict of interests related to this work.
REFERENCES:
- 1.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, et al. Ischemic stroke subtypes: A population-based study of incidence rates among blacks and whites. Stroke 2004;35:1552–1556 [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and hispanic residents of an urban community - the northern manhattan stroke study. American Journal of Epidemiology 1998;147:259–268 [DOI] [PubMed] [Google Scholar]
- 3.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright CB, Dong C, Perez EJ, De Rosa J, Yoshita M, Rundek T, et al. Subclinical cerebrovascular disease increases the risk of incident stroke and mortality: The northern manhattan study. Journal of the American Heart Association 2017;6. doi: 10.1161/JAHA.116.004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: The northern manhattan study. J Hypertens 2015;33:2115–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez J, Rundek T, Ekind MS, Sacco RL, Wright CB. Perivascular spaces are associated with atherosclerosis: An insight from the northern manhattan study. AJNR Am J Neuroradiol 2013;34:1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: The leukoaraiosis and disability study. Stroke 2008;39:1414–1420 [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez J, Elkind MSV, Dong C, Di Tullio M, Rundek T, Sacco RL, et al. Brain perivascular spaces as biomarkers of vascular risk: Results from the northern manhattan study. AJNR Am J Neuroradiol 2017;38:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged virchow-robin spaces: A magnetic resonance imaging and pathological study. J Neurol 1998;245:116–122 [DOI] [PubMed] [Google Scholar]
- 10.Jungreis CA, Kanal E, Hirsch WL, Martinez AJ, Moossy J. Normal perivascular spaces mimicking lacunar infarction: Mr imaging. Radiology 1988;169:101–104 [DOI] [PubMed] [Google Scholar]
- 11.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The abcs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–1305 [DOI] [PubMed] [Google Scholar]
- 12.Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid research clinics coronary primary prevention trial. JAMA 1994;271:999–1003 [DOI] [PubMed] [Google Scholar]
- 13.Boden-Albala B, Roberts ET, Bazil C, Moon Y, Elkind MSV, Rundek T, et al. Daytime sleepiness and risk of stroke and vascular disease: Findings from the northern manhattan study (nomas). Circulation: Cardiovascular Quality and Outcomes 2012;5:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.So Y, Lin G, Johnston G. Using the phreg procedure to analyze competing-risks data. SAS Global Forum https://support.sas.com/rnd/app/stat/papers/2014/competingrisk2014.pdf. Accessed February 20, 2019.
- 15.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555–563 [DOI] [PubMed] [Google Scholar]
- 16.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and hispanics: The northern manhattan study. Circulation 2005;111:1327–1331 [DOI] [PubMed] [Google Scholar]
- 17.Turan TN, Makki AA, Tsappidi S, Cotsonis G, Lynn MJ, Cloft HJ, et al. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke 2010;41:1636–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer CM, Gore I, Okabe N, White PD. Atherosclerosis of the carotid and vertebral arteries-extracranial and intracranial. Journal of Neuropathology & Experimental Neurology 1965;24:455–476 [Google Scholar]
- 19.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008;39:1142–1147 [DOI] [PubMed] [Google Scholar]
- 20.Khan A, Kasner SE, Lynn MJ, Chimowitz MI. Risk factors and outcome of patients with symptomatic intracranial stenosis presenting with lacunar stroke. Stroke 2012;43:1230–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holliday EG, Traylor M, Malik R, Bevan S, Falcone G, Hopewell JC, et al. Genetic overlap between diagnostic subtypes of ischemic stroke. Stroke 2015;46:615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: A subgroup analysis of socrates, a randomised, double-blind, controlled trial. Lancet Neurol 2017;16:301–310 [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–1717 [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez J, Lekic T. Letter re: Risks and benefits of clopidogrel-aspirin in minor stroke or tia: Time course analysis of chance. Neurology 2017;89:2121. [DOI] [PubMed] [Google Scholar]
- 25.Majidi S, Sein J, Watanabe M, Hassan AE, Van de Moortele PF, Suri MF, et al. Intracranial-derived atherosclerosis assessment: An in vitro comparison between virtual histology by intravascular ultrasonography, 7t mri, and histopathologic findings. AJNR Am J Neuroradiol 2013;34:2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deplanque D, Lavallee PC, Labreuche J, Gongora-Rivera F, Jaramillo A, Brenner D, et al. Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: A case-control study. Int J Stroke 2013;8:413–421 [DOI] [PubMed] [Google Scholar]
- 27.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated virchow-robin spaces is associated with age, blood pressure, and mri markers of small vessel disease: A population-based study. Stroke 2010;41:2483–2490 [DOI] [PubMed] [Google Scholar]
- 28.Mohr J Neurological complications of cardiac valvular disease and cardiac surgery including systemic hypotension. Handbook of clinical neurology 1979;38:143–171 [Google Scholar]
- 29.Fisher CM. Lacunes: Small, deep cerebral infarcts. Neurology 1965;15:774–784 [DOI] [PubMed] [Google Scholar]
- 30.Noren D, Palmer HJ, Frame MD. Predicted wall shear rate gradients in t-type arteriolar bifurcations. Biorheology 2000;37:325–340 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


