Abstract
Aims:
To test a commercially available enriched chicken bone broth (ECBB) product for its potential anti-inflammatory properties and to evaluate its ability to reduce nociception and expression of protein kinase A (PKA) in a clinically relevant model of temporomandibular disorder (TMD) caused by prolonged jaw opening in rats.
Methods:
The potential of the ECBB and of a homemade broth was investigated using the Folin–Ciocalteu reagent and percent inhibition of cyclooxygenase-2 (COX-2) activity, which was determined using a commercially available kit. Additionally, the effect of ECBB and homemade broth on nocifensive head withdrawal responses to mechanical stimulation in male Sprague-Dawley rats subjected to prolonged jaw opening was evaluated. Differences were considered significant at P < .025. Changes in PKA expression in the medullary dorsal horn region of the spinal trigeminal nucleus associated with prolonged jaw opening were assessed using immunofluorescence, and these changes were considered significant at P < .05. Behavioral data were analyzed by using multiple nonparametric tests, and immunohistochemistry data were analyzed by using one-way analysis of variance with Games-Howell post hoc tests in SPSS software.
Results:
ECBB exhibited greater reducing potential and inhibition of COX-2 activity compared to homemade broth. Near maximal jaw opening was sufficient to induce sustained nocifensive responses to mechanical stimuli for 7 days. This increased sensitivity was correlated with elevated levels of the active form of PKA. Importantly, dietary inclusion of ECBB, but not of homemade broth, for 2 weeks prior to jaw opening was sufficient to reduce nocifensive behaviors and PKA expression.
Conclusion:
Findings from this study provide evidence that ECBB attenuates nociception and expression of the pro-inflammatory protein PKA and thus may be beneficial as a nutraceutical supplement to manage inflammatory pain associated with TMD.
Keywords: antioxidant, joint, temporomandibular disorder (TMD), trigeminal
Temporomandibular disorders (TMD) are one of the most prevalent orofacial pain conditions for which patients seek treatment and are more prevalent in women than in men.1,2 TMD are characterized by pain or tenderness of the jaw joint or muscle during or after mastication and by jaw clicking, limited jaw movements, ringing of the ear, and headache. TMD may develop due to strain on the temporomandibular joint (TMJ), trauma to the joint and related structures, excessive clenching and grinding (bruxism), unresolved stress, systemic autoimmune diseases, and/or prolonged jaw opening.3 Damage to the connective tissues of the TMJ and associated muscles can promote sensitization and activation of orofacial primary nociceptive afferents.4 Following activation, trigeminal neurons release pro-inflammatory molecules that mediate neurogenic inflammation, which is characterized by increased vasodilation, extravasation of plasma proteins, and recruitment of immune cells to the injured tissues.5 Activation also causes secretion of neuropeptides, including calcitonin gene-related peptide (CGRP), that promote activation of second-order nociceptive neurons in the spinal trigeminal nucleus, leading to central sensitization and a heightened pain response.4,6 CGRP is known to initiate and maintain activation and sensitization of peripheral and central nociceptive neurons and glial cells implicated in chronic pain states via modulation of ion channels, receptors, and inflammatory gene expression.7,8 Binding of CGRP to receptors localized on neurons and glial cells in the trigeminal ganglion and spinal trigeminal nucleus results in increased signaling of protein kinase A (PKA), which promotes development of peripheral and central sensitization. Prolonged activation of the CGRP/PKA pathway is implicated in several prolonged pain conditions, including TMD and migraine.9,10
Traditionally, chronic pain disorders such as TMD are very difficult to treat and can lead to a decrease in quality of life and even depression.11 Treatment options for TMD are targeted to relieve or eliminate pain, restore normal function, and reduce the possibility for future injury.12 Therapies often include patient education and increasing disease awareness that involves resting the inflamed muscles, pharmaceutical treatments, physical therapy, medical apparatuses, psychological therapy, alternative treatments, and, in extreme cases, surgical interventions.13 Patients with more extreme and complex TMD cases often seek and participate in multiple forms of treatment or team management. However, there is yet to be a specific USA Food and Drug Administration (FDA)–approved therapy for the treatment or management of TMD. Thus, the use of novel nutraceuticals should be considered a potential management option for addressing the symptoms of this debilitating disease.
Chicken broth, which is used as a base for many soups, is a liquid, water-based food solution that is traditionally prepared by slowly simmering a combination of chicken bones, meat, and sometimes vegetables. For centuries, chicken soups made from broths have been offered as remedies for colds and other inflammatory conditions of the respiratory system.14,15 Chicken broth has been shown to increase nasal mucus velocity and inhibit neutrophil chemotaxis in in vitro assays.16,17 Given the almost universal notion of the health-promoting benefits of chicken broth and soups, it is somewhat surprising that there is little scientific evidence to support this claim. Furthermore, studies providing evidence for its use in non–respiratory related pathologies have never been conducted. Thus, the aims of this study were to test a commercially available enriched chicken bone broth product (ECBB) for its potential anti-inflammatory properties and to evaluate its ability to reduce nociception and PKA expression in a clinically relevant model of TMD caused by prolonged jaw opening.
Materials and Methods
Preparation of Chicken Broths
The ECBB, which was provided by International Dehydrated Foods, was prepared by mixing finely ground raw chicken bones with approximately two parts water and then pressure cooking at a minimum of 15 pounds per square inch gauge (130°C) for at least 8 hours. The broth was separated from solids and fat, evaporated, and spray dried in a commercial dryer with no additives. The home style (homemade) broth was prepared by chopping raw chicken carcasses (after removal of legs, breast meat, skin, and wings) into small pieces and adding 1.5 parts water in a pan. Components were brought to a boil and cooked at a light boil for 45 minutes. The product then simmered for 1.25 hours. The broth was separated from solids by pouring through a screen, and visible fat was removed after chilling.
Antioxidant Potential and Cyclooxygenase Inhibition
The Folin–Ciocalteu reagent method18,19 was used to determine relative antioxidant activity in broths, reported using gallic acid as the standard. Gallic acid (Sigma) and the broths were reconstituted in 40% aqueous ethanol at a final concentration of 0.6 mg/mL. The diluted broth samples and the gallic acid standards were incubated with 0.25 N Folin–Ciocalteu reagent (Sigma) for 3 minutes at room temperature. Sodium carbonate (1 N) was mixed into each sample and incubated at room temperature for 2 hours. After incubation, absorbance of samples and standards was measured at 760 nm.5 Average optical density values obtained from ECBB and homemade broth were compared to the values obtained from the standard curve to determine the gallic acid equivalent (GAE). Results are reported as the μg/mL GAE.
An in vitro cyclooxygenase (COX) Inhibitor Screening Assay Kit (Cayman Chemical) was used to investigate the inhibitory effect of ECBB and homemade broth on COX activity according to the manufacturer’s instructions. All reagents and inhibitors (assayed in triplicate) were added to the plate and incubated for 18 hours at room temperature. The plate was washed five times with provided wash buffer, and Ellman’s reagent was added to each well and incubated for 90 minutes in a dark room. A Spectramax Plus 384 plate reader (Molecular Devices) set at 420 nm was used to measure absorbance. Average absorbance values were obtained from the standard curve and plotted as a four-parameter logistic fit. Results are reported as the percentage of inhibition, which was calculated by subtracting each sample value from the 100% initial activity sample, dividing by the 100% initial activity sample, and then multiplying by 100.
Experimental Animals
This study was conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee at Missouri State University and were in compliance with all guidelines established in the Animal Welfare Act, National Institutes of Health, and Animal Research: Reporting of In Vivo Experiments. Adult male Sprague-Dawley rats (200 to 300 g, Charles River) were placed in clean cages with unrestricted access to food and water and a 12-hour light/dark cycle. For the nociception experiments, a small effect size (Cohen’s f = 0.20) was anticipated. The study included a factorial design of 6 (groups) × 5 (time points). The groups included a group that was provided water without any additional experimental conditions (naïve), a group that received 0.5% w/v ECBB (0.5% ECBB), a group that received 0.1% w/v homemade broth (0.1% homemade), a group that received water and was subjected to near maximal jaw opening (jaw opening), a group that received 0.5% w/v ECBB and was subjected to near maximal jaw opening (jaw opening + 0.5% ECBB), and a group that received 0.1% w/v homemade broth and was subjected to near maximal jaw opening (jaw opening + 0.1% homemade). A power of 0.80 at α = 0.05 was employed, and based on the study design, the minimum sample size to detect the expected main effect and interactions would be 10 in each group.
For the immunohistochemical analyses, a similar effect size was expected based on similar previously published work (f = 1.53).20 Employing a power of 0.80 where α = .05, the minimum sample size to detect the expected main effect and interactions would require a minimum of three animals for each condition. However, to reduce the number of animals used in this study, tissues for immunohistochemical studies were acquired from animals completing the behavioral nociceptive studies. Therefore, a total of 60 adult male Sprague-Dawley rats (10 for each experimental group) were used in this study.
Dietary Inclusion of Chicken Broths
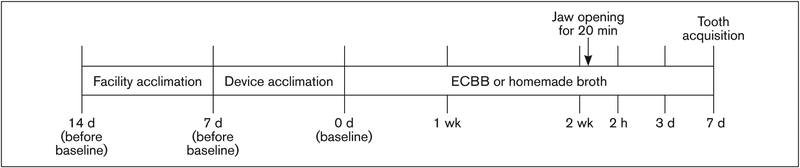
A much higher concentration of solids was realized during the industrial manufacturing of the ECBB (8%, w/v) compared to the technique used to make the homemade broth (1.66%). Broths were compared as the products that resulted from different cooking methods prior to drying processes. Based on the recommended human dose, which is equivalent to two tablespoons of powder per day, and the liquid intake of the animals in this study, ECBB was diluted 1:16 in filtered water, resulting in a 0.5% (w/v) final solution for feeding. Similarly, homemade broth (1.66%) was also diluted 1:16 in filtered water and fed at a final 0.1% (w/v) concentration. Broths were provided to the animals for 2 weeks prior to jaw opening and then daily until the end of the study (Fig 1).
Fig 1.
Timeline of experimental design. For 2 weeks prior to prolonged jaw opening, some animals were fed either 0.5% ECBB or 0.1% homemade broth. Baseline nociception measurements were obtained (prior to feeding), and additional assessments were collected at 2 hours and 3 and 7 days after the prolonged jaw opening. Animals receiving either broth were allowed to consume the product for 1 week after jaw opening (for a total of 3 weeks of broth consumption).
TMD Model and Assessment of Nocifensive Behavior
The technique of prolonged near maximal jaw opening was performed as described in a previously published study.21 While the animal was anesthetized (with 5% isoflurane), a surgical retractor was placed around the bottom and top incisors and the jaw opening maintained at 22 mm for 20 minutes. Nocifensive testing to mechanical stimulation was performed on unanesthetized animals as described previously.20,22,23 Baseline mechanical nocifensive withdrawal thresholds to a von Frey filament were determined in response to increasing force applied to the cutaneous area over the superficial masseter muscle. The 100-g filament was chosen since this force elicited a mean response of less than one out of five filament applications at baseline measurements. To remove bias, a researcher removed water bottles and cage cards from the cage prior to entry into a designated behavioral testing room, where the researcher responsible for identifying a positive response was located. Items were returned upon conclusion of testing once the animal was returned to general housing rooms. A positive response, defined by head withdrawal before the bending of the filament, was recorded by a second researcher. Each filament was applied five times, and the data reported as the average number of responses obtained from five applications of each specific calibrated filament. Results are reported as the average number of nocifensive head withdrawal responses ± SEM (out of 5). Any animals that elicited responses detected as outliers by SPSS Statistics 21 software (IBM), using 3 as a multiplier, were removed from the study (11 animals). Animals that responded 2.5 or more times out of 5 to the 100-g filament over the masseter were considered responders, and animals that responded less than these parameters were considered nonresponders. The percentage of responders in each group is also reported.
Tissue Acquisition and Immunohistochemistry
Tissues containing the spinal trigeminal nucleus were obtained 7 days after the jaw-opening session, and immunohistochemical studies were conducted as previously described.6,20,24 Tissues were mounted in Tissue-Tek Optimal Cutting Temperature media (Sakura Finetek), and 14-μm serial sections taken between −4.5 and −4.0 mm from the obex were positioned on Fisherbrand Superfrost Plus Microscope slides. Tissues were incubated with mouse antibodies against the active form of rat PKA (BD Biosciences; 1:500 dilution, 3 hours, room temperature). Tissues were then incubated in donkey anti-mouse IgG Alexa 488 (Invitrogen; 1:200 dilution, 1 hour, room temperature) and mounted in Vectashield fluorescent media with 4,6-diamidino-2-phenylindole (DAPI). Images from the right medullary dorsal horn region of the spinal trigeminal nucleus were obtained with a Zeiss Z1 microscope equipped with an apotome. Zen 2011 software (Carl Zeiss Microscopy) was utilized to evenly balance the background of each image prior to analysis. Grayscale jpeg images were opened in ImageJ software (NIH, Stapleton), four non-overlapping regions of interest with an area of equal size were placed in areas representative of protein expression in laminae 1–3 of the medullary dorsal horn in each image, and the integrated pixel densities were measured. Background intensities were also acquired through a similar procedure from acellular regions and averaged. The average background intensity acquired from each image was then subtracted from each integrated density value from the areas of interest. Subtracted integrated densities were averaged, and fold changes were calculated as the average change ± SEM from naïve level, which was set equal to 1.
Statistical Analysis
To determine normality of behavioral data sets, a Shapiro-Wilk test was utilized and a Levene’s test was used to determine equal or unequal variance. The data sets were determined to violate these assumptions; therefore, nonparametric statistical analysis was used to determine significance in changes. The following statistical tests were completed using SPSS software: Kruskal-Wallis analysis of variance (ANOVA), Mann-Whitney U with Bonferroni correction (α altered = .05/2, P < .025), and Friedman ANOVA followed by a Wilcoxon signed-rank test with Bonferroni correction (α altered = .05/4, P < .013). For the immunohistochemical analyses, a Shapiro-Wilk test was used to determine the normality of the data set, while a Levene’s test was used to determine equal or unequal variance. Data sets were found to adhere to the assumption of normality; however, not equal variance. Therefore, statistical differences were determined by using one-way ANOVA, supported by both Welch and Brown-Forsyth tests and with Games-Howell post hoc tests, and were considered to be significant when P < .05. All statistical tests were conducted utilizing SPSS software.
Results
Reducing Potential of Chicken Broths
Initially, the relative reducing potential of the chicken broths was investigated by the GAE Method and reduction of COX enzyme activity. ECBB exhibited a GAE of 4,828.80 μg/mL, and the homemade broth a GAE of 687.78 μg/mL. Incubation with ECBB greatly reduced COX-2 activity (42.40%) while exhibiting no effect on COX-1 activity (–2.30%). In contrast, the homemade broth had limited or no effect on COX-2 activity (3.5%) or COX-1 activity (–5.2%). These findings provide evidence that ECBB contains compounds with antioxidant properties and is thus likely to reduce sensitization and activation of trigeminal neurons.
Nocifensive Reponses to Mechanical Stimuli
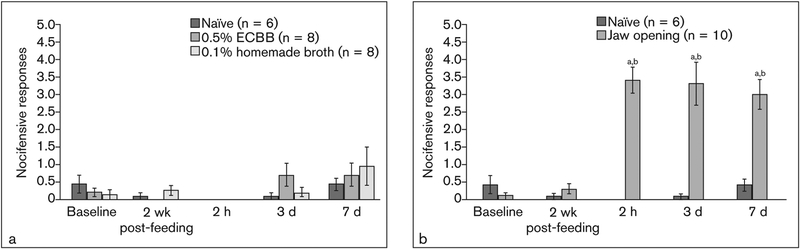
To test nocifensive responses to mechanical stimuli, the effect of dietary inclusion of ECBB or homemade broth for 2 weeks prior to prolonged jaw opening was evaluated. No significant differences in head withdrawal response to mechanical stimulation were observed in any of the groups at baseline compared to baseline naïve animals (naïve: 0.42 ± 0.26, 0% responders; jaw opening: 0.10 ± .08, 0% responders, P = .313; 0.5% ECBB: 0.19 ± 0.14, 0% responders, P = .491; 0.1% homemade: 0.13 ± 0.13, 0% responders, P = .282; jaw opening + 0.5% ECBB: 0.25 ± 0.09, 0% responders, P = .852; jaw opening + 0.1% homemade: 0.67 ± 0.31, 0% responders, P = .689; Figs 2 and 3a). Nocifensive responses of naïve animals did not significantly change compared to baseline values (2 weeks postfeeding: 0.08 ± 0.09, 0% responders, P = .102; 2 hours: 0.00 ± 0.00, 0% responders, P = .102; 3 days: 0.08 ± 0.09, 0% responders, P = .257; 7 days: 0.42 ± 0.16, 0% responders, P = 1.000; Figs 2a and 3b). Furthermore, consumption of ECBB alone did not alter nociception or the number of responders at any point compared to baseline (2 weeks postfeeding: 0.00 ± 0.00, 0% responders, P = .180; 2 hours: 0.00 ± 0.00, 0% responders, P = .180; 3 days: 0.69 ± 0.32, 13% responders, P = .109; 7 days: 0.69 ± 0.33, 0% responders, P = .221; Figs 2a and 3b), nor did ingestion of the homemade broth alone (2 weeks postfeeding: 0.25 ± 0.14, 0% responders, P = .414; 2 hours: 0.00 ± 0.00, 0% responders, P = .317; 3 days: 0.19 ± 0.14, 0% responders, P = .785; 7 days: 0.94 ± 0.54, 25% responders, P = .223; Figs 2a and 3b). Consumption of ECBB or homemade broth alone also did not significantly alter nociceptive responses compared to the naïve group at any time (2 weeks postfeeding: P = .662, P = .491, respectively; 2 hours: P = 1.000 for both; 3 days: P = .108, P = .775; 7 days: P = .950, P = .950; Fig 2a).
Fig 2.
Prolonged jaw opening caused an increase in the average number of nocifensive head withdrawal responses to mechanical stimulation. (a) Average number of withdrawal responses compared to baseline in naïve animals, animals consuming ECBB, and animals consuming homemade broth. (b) Change in average number of withdrawals in animals subjected to prolonged jaw opening compared to naïve animals. aSignificant from naïve. bSignificant from baseline.
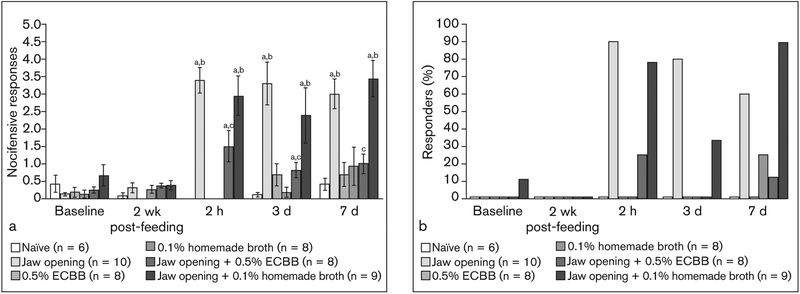
Fig 3.
ECBB reduced the number of nocifensive withdrawal responses to mechanical stimulation. (a) Average number of withdrawal responses in animals subjected to prolonged jaw opening and consumption of ECBB or homemade broth compared to baseline and naïve animals. (b) Change in average percentage of responders to mechanical stimuli. aSignificant from naïve. bSignificant from baseline. cSignificant from jaw opening alone.
In contrast, animals that were subjected to near maximal jaw opening alone exhibited a significantly increased number of nocifensive responses 2 hours post–jaw opening (3.40 ± 0.37, P < .001, 90% responders) and at days 3 (3.30 ± 0.62, P = .002, 80% responders) and 7 (3.00 ± 0.43, P < .001, 60% responders) compared to naïve animals (Figs 2b and 3b). Responses observed in these animals were also found to be significantly different from the average number of responses observed at baseline (2 hours: P = .005; 3 days: P = .008; 7 days: P = .005; Fig 2b). However, animals subjected to prolonged jaw opening and allowed to consume the ECBB exhibited significantly lower numbers of nocifensive responses to mechanical stimuli at 2 hours after jaw opening (1.50 ± 0.46, P = .006, 25% responders) and at days 3 (0.81 ± 0.23, P = .006, 0% responders) and 7 (1.00 ± 0.28, P = .001, 13% responders; Figs 3a and 3b) compared to the jaw-opening group. The number of responses observed at 2 hours after jaw opening and at day 3 were still found to be significantly higher than those observed in the naïve group (P = .020; P = .013, respectively), but were not significantly different at day 7 (P = .181). Furthermore, nocifensive responses observed in jaw opening + ECBB animals were not significantly different from baseline values at any time point (2-week postfeeding: P = .157; 2 hours: P = .027; 3 days: P = .066; 7 days: P = .039; Fig 3a). In contrast, animals consuming the homemade broth had a similar level of response to mechanical stimuli 2 hours post–jaw opening (2.94 ± 0.58, P = .661, 78% responders) and at days 3 (2.39 ± 0.80, P = .604, 33% responders) and 7 after jaw opening (3.44 ± 0.53, P = .278, 89% responders) compared to jaw-opening animals (Figs 3a and 3b). Additionally, the number of responses observed in jaw-opening + homemade-fed animals at 2 hours after jaw opening and at days 3 and 7 were also found to be significantly higher than those observed in the naïve group (P = .003; P = .018; P = .008) and significantly different from baseline nocifensive responses observed at day 7 after jaw opening (P = .012; Fig 3a).
PKA Expression in the Medullary Dorsal Horn
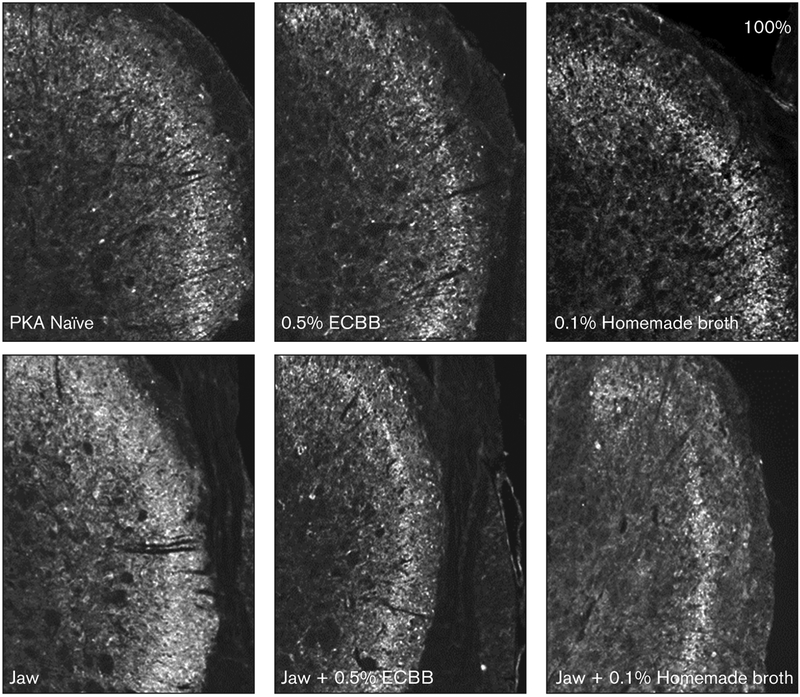
To determine if ECBB was mediating changes at the cellular level within the spinal trigeminal nucleus, the expression of the pro-inflammatory signaling protein PKA was investigated in neurons and glial cells in the medullary dorsal horn. As seen in Fig 4, the intensity of immunostaining for PKA in the outer laminae of the medullary dorsal horn obtained from animals subjected to near-maximal jaw opening (2.38 ± 0.10) was significantly increased over the naïve levels (1.00 ± 0.04, P < .001). Dietary inclusion of either ECBB (0.83 ± 0.05) or the homemade broth (1.28 ± 0.09) did not significantly alter PKA expression from naïve animals (P = .147; P = .051, respectively). In agreement with the nociception results, animals consuming ECBB prior to jaw opening (1.00 ± 0.13, P < .001) had significantly lower levels of PKA expression vs animals subjected to the jaw-opening procedures alone, and these levels were comparable to naïve levels (P = 1.00). In contrast, animals receiving the homemade broth prior to jaw opening still exhibited a significant increase in the levels of PKA expression (1.52 ± 0.14, P = .029) compared to levels detected in naïve samples.
Fig 4.
ECBB reduced the expression of PKA in the spinal trigeminal nucleus in response to prolonged jaw opening. Images of PKA expression in the medullary dorsal horn at ×100 magnification are shown.
Discussion
A major finding of this study was that dietary inclusion of an enriched bone broth for 14 days in male rats was sufficient to reduce pain-like behavior in response to mechanical stimulation and expression of a pro-inflammatory protein in response to prolonged jaw opening. The ECBB is an enriched bone broth produced by International Dehydrated Foods from the bony structures remaining from other products. The biologically active compounds and components in chicken broth are directly related to the starting ingredients and cooking process. The ECBB material is cooked at extreme temperatures and pressure cooked for up to 8 hours. The broth is allowed to separate, and the liquid layer containing the broth is removed, spray dried, and packaged. Initial biochemical characterization of the ECBB and homemade broth revealed that ECBB was greatly enriched in compounds exhibiting reducing potential, as determined by the GAE (~7-fold higher), when compared to homemade broth. The assay used to measure antioxidant properties was the GAE method, which is typically used to determine the total reducing capacity of foods and supplements in food science studies.25 Because this method is not selective for determining levels of phenolic compounds only but rather determines antioxidant potential, it is likely that multiple compounds or components contained in the ECBB contribute to the total reducing capacity. Products possessing antioxidant properties are reported to be beneficial in the management of joint pain and inflammation, including that of the TMJ, by minimizing oxidative stress.24,26–28
In addition, ECBB, but not homemade broth, was able to significantly reduce COX-2 activity while not affecting COX-1 activity. The COX family of enzymes is responsible for the synthesis of prostanoids such as prostaglandins,29 which are known to promote tissue inflammation and sensitization of trigeminal neurons.30,31 Specifically, results from a human study of synovial fluid collected from TMD patients has provided evidence for a direct correlation between prostaglandin E2 and TMJ inflammation and pain levels.32,33 While both enzymes stimulate production of prostaglandins that promote inflammation, pain, and fever, only COX-1 produces prostaglandins that activate platelets and protect the stomach and intestinal lining. Thus, compounds that reduce COX-2 activation while not affecting COX-1 are of particular interest due to their ability to block inflammation while not causing adverse side effects that are commonly reported when COX-1 activity is blocked. Based on these results, the attenuating effect of ECBB on nociception is likely to be mediated, at least in part, by its antioxidant and anti-inflammatory properties.
Results from this study provide evidence that dietary inclusion of ECBB (but not a homemade broth) was sufficient to reduce the average number of nocifensive head withdrawal responses caused by prolonged jaw opening. Importantly, the effect of ECBB supplementation was observed 2 hours after jaw opening and was sustained to day 7, which was the longest time point examined in this study. Prolonged jaw opening, which can occur during routine visits to the dentist or dental specialist, is reported to lead to sustained mechanical sensitization of trigeminal nociceptive neurons.21 TMD are thought to involve the development of peripheral and central sensitization of trigeminal nociceptive neurons4; for example, following TMJ injury, trigeminal neurons that provide sensory innervation to the tissues involved in mastication become activated and release CGRP and other inflammatory mediators that promote neurogenic inflammation and cause development of peripheral sensitization, lowering the activation threshold of those neurons. Peripheral sensitization is thought to contribute to the development of central sensitization, which is characterized by a lower activation threshold of nociceptive neurons located in the spinal trigeminal nucleus.4 In the TMD model used in the present study, a sustained increase in expression of PKA in the medullary dorsal horn region of the spinal trigeminal nucleus was observed. PKA functions as a pro-inflammatory signaling protein to induce a prolonged state of central sensitization characterized by allodynia.20,34 Importantly, dietary inclusion of ECBB, but not of the homemade broth, resulted in reduction of elevated PKA levels in the medullary dorsal horn mediated by prolonged jaw opening. This finding is in agreement with the attenuating effects of the trigeminal nociceptive pathways and development of sensitization observed with inclusion of cocoa or grape-seed extract as a dietary supplement.24,26,27 Similar to the ECBB, both cocoa and grape-seed extract are known to be enriched in antioxidant compounds and thus have the potential to reduce synthesis of pro-inflammatory prostaglandins reported to enhance and help sustain central sensitization of nociceptive neurons.35,36
Conclusions
In summary, findings from this study provide the first evidence that inclusion of ECBB as a dietary supplement can reduce nociception resulting from prolonged jaw opening that may occur during routine visits to the dentist or dental specialist. Based on results from the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study, prolonged jaw opening should be considered a risk factor for the development of chronic TMD.3 It is important to note that there are few therapeutic options for TMD patients, so the finding that ECBB can reduce facial nocifensive behavior provides evidence to support its use in the management of TMD. Based on the finding that ECBB reduced trigeminal nociception, ECBB might also be expected to similarly reduce pain-like behavioral responses associated with other diseases involving trigeminal nerve activation, including migraine, rhinosinusitis, trigeminal neuralgia, and orthodontic pain. In addition, given the effects observed in PKA expression in the spinal trigeminal nucleus, dietary inclusion of ECBB may be beneficial in reducing pain signaling and development of central sensitization caused by hyperextension of other joints (eg, the knee and ankle). When compared to homemade broth, ECBB is enriched in antioxidant molecules associated with joint health and decreasing inflammation. Thus, these findings support the notion that dietary inclusion of ECBB should be beneficial in decreasing trigeminal sensitization and nociception associated with TMD.
Table 1.
Protein Kinase A Expression in Medullary Dorsal Horn
| Group | Average ± SEM | P from naïve |
|---|---|---|
| Naïve | 1.00 ± 0.04 | |
| 0.5% ECBB | 0.83 ± 0.05 | .147 |
| 0.1% homemade broth | 1.28 ± 0.09 | .051 |
| Jaw opening | 2.38 ± 0.10 | < .001 |
| Jaw opening + 0.5% ECBB | 1.00 ± 0.13 | 1.00 |
| Jaw opening + homemade broth | 1.52 ± 0.14 | .029 |
Levels of expression are reported as fold changes compared to average mean of naïve levels, which was set equal to 1. P values are reported compared to naïve levels.
Acknowledgments
This study received financial support from International Dehydrated Foods. The authors of this study are co-authors of current registered patents with International Dehydrated Foods (US 20150011500 A1, WO215006287 A3, EP 3019187 A2) Funding for this study was also provided by NIH (DE024629).
References
- 1.Furquim BD, Flamengui LM, Conti PC. TMD and chronic pain: A current view. Dental Press J Orthod 2015;20:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case control study. J Pain 2011;12(suppl):T61–T74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohrbach R, Fillingim RB, Mulkey F, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12(suppl):T27–T45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 2011;97:179–206. [DOI] [PubMed] [Google Scholar]
- 5.Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Neuropeptides in the arthritic TMJ and symptoms and signs from the stomatognathic system with special consideration to rheumatoid arthritis. J Orofac Pain 1995;9:215–225. [PubMed] [Google Scholar]
- 6.Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain 2011;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durham PL. Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: Modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr Pain Headache Rep 2016;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol 2009;(194):451–491. [DOI] [PubMed] [Google Scholar]
- 9.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 2013;53:1230–1244. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Reyes M, Pardi V, Akerman S. A potent and selective calcitonin gene-related peptide (CGRP) receptor antagonist, MK-8825, inhibits responses to nociceptive trigeminal activation: Role of CGRP in orofacial pain. Exp Neurol 2015;271:95–103. [DOI] [PubMed] [Google Scholar]
- 11.Tjakkes GH, Reinders JJ, Tenvergert EM, Stegenga B. TMD pain: The effect on health related quality of life and the influence of pain duration. Health Qual Life Outcomes 2010;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Reyes M, Uyanik JM. Orofacial pain management: Current perspectives. J Pain Res 2014;7:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieckiewicz M, Boening K, Wiland P, Shiau YY, Paradowska-Stolarz A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J Headache Pain 2015;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosner F Therapeutic efficacy of chicken soup. Chest 1980;78:672–674. [DOI] [PubMed] [Google Scholar]
- 15.Rosner F Medical writings of Moses Maimonides. N Y State J Med 1973;73:2186–2189. [PubMed] [Google Scholar]
- 16.Saketkhoo K, Januszkiewicz A, Sackner MA. Effects of drinking hot water, cold water, and chicken soup on nasal mucus velocity and nasal airflow resistance. Chest 1978;74:408–410. [DOI] [PubMed] [Google Scholar]
- 17.Rennard BO, Ertl RF, Gossman GL, Robbins RA, Rennard SI. Chicken soup inhibits neutrophil chemotaxis in vitro. Chest 2000;118:1150–1157. [DOI] [PubMed] [Google Scholar]
- 18.Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem 2003;51:7292–7295. [DOI] [PubMed] [Google Scholar]
- 19.Abbey MJ, Patil VV, Vause CV, Durham PL. Repression of calcitonin gene-related peptide expression in trigeminal neurons by a Theobroma cacao extract. J Ethnopharmacol 2008;115:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelison LE, Hawkins JL, Durham PL. Elevated levels of calcitonin gene-related peptide in upper spinal cord promotes sensitization of primary trigeminal nociceptive neurons. Neuroscience 2016;339:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkins JL, Durham PL. Prolonged jaw opening promotes nociception and enhanced cytokine expression. J Oral Facial Pain Headache 2016;30:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrett FG, Hawkins JL, Overmyer AE, Hayden JB, Durham PL. Validation of a novel rat-holding device for studying heat- and mechanical-evoked trigeminal nocifensive behavioral responses. J Orofac Pain 2012;26:337–344. [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins JL, Denson JE, Miley DR, Durham PL. Nicotine stimulates expression of proteins implicated in peripheral and central sensitization. Neuroscience 2015;290:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cady RJ, Durham PL. Cocoa-enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res 2010;1323:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br J Nutr 2015;113(suppl):s68–s78. [DOI] [PubMed] [Google Scholar]
- 26.Cady RJ, Hirst JJ, Durham PL. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol Pain 2010;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cady RJ, Denson JE, Durham PL. Inclusion of cocoa as a dietary supplement represses expression of inflammatory proteins in spinal trigeminal nucleus in response to chronic trigeminal nerve stimulation. Mol Nutr Food Res 2013;57:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiguchi K, Takehana S, Shibuya E, et al. Resveratrol attenuates inflammation-induced hyperexcitability of trigeminal spinal nucleus caudalis neurons associated with hyperalgesia in rats. Mol Pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Consalvi S, Biava M, Poce G. COX inhibitors: A patent review (2011 – 2014). Expert Opin Ther Pat 2015;25:1357–1371. [DOI] [PubMed] [Google Scholar]
- 30.Levy D, Zhang XC, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: Inhibition by naproxen. Eur J Neurosci 2008;27:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 2011;152:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh K, Ogura N, Akutsu M, et al. Expression of cyclooxygenase-1 and −2 in IL-1beta-induced synovitis of the temporomandibular joint. J Oral Pathol Med 2009;38:584–590. [DOI] [PubMed] [Google Scholar]
- 33.Basi DL, Velly AM, Schiffman EL, et al. Human temporomandibular joint and myofascial pain biochemical profiles: A case-control study. J Oral Rehabil 2012;39:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 2004;92:2859–2866. [DOI] [PubMed] [Google Scholar]
- 35.Schuh CD, Brenneis C, Zhang DD, et al. Prostacyclin regulates spinal nociceptive processing through cyclic adenosine monophosphate-induced translocation of glutamate receptors. Anesthesiology 2014;120:447–458. [DOI] [PubMed] [Google Scholar]
- 36.Meves H The action of prostaglandins on ion channels. Curr Neuropharmacol 2006;4:41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]