Abstract
PURPOSE
To identify effective and less toxic therapy for children with acute myeloid leukemia, we introduced clofarabine into the first course of remission induction to reduce exposure to daunorubicin and etoposide.
PATIENTS AND METHODS
From 2008 through 2017, 285 patients were enrolled at eight centers; 262 were randomly assigned to receive clofarabine and cytarabine (Clo+AraC, n = 129) or high-dose cytarabine, daunorubicin, and etoposide (HD-ADE, n = 133) as induction I. Induction II consisted of low-dose ADE given alone or combined with sorafenib or vorinostat. Consolidation therapy comprised two or three additional courses of chemotherapy or hematopoietic cell transplantation. Genetic abnormalities and the level of minimal residual disease (MRD) at day 22 of initial remission induction determined final risk classification. The primary end point was MRD at day 22.
RESULTS
Complete remission was induced after two courses of therapy in 263 (92.3%) of the 285 patients; induction failures included four early deaths and 15 cases of resistant leukemia. Day 22 MRD was positive in 57 of 121 randomly assigned evaluable patients (47%) who received Clo+AraC and 42 of 121 patients (35%) who received HD-ADE (odds ratio, 1.86; 95% CI, 1.03 to 3.41; P = .04). Despite this result, the 3-year event-free survival rate (52.9% [44.6% to 62.8%] for Clo+AraC v 52.4% [44.0% to 62.4%] for HD-ADE, P = .94) and overall survival rate (74.8% [67.1% to 83.3%] for Clo+AraC v 64.6% [56.2% to 74.2%] for HD-ADE, P = .1) did not differ significantly across the two arms.
CONCLUSION
Our findings suggest that the use of clofarabine with cytarabine during remission induction might reduce the need for anthracycline and etoposide in pediatric patients with acute myeloid leukemia and may reduce rates of cardiomyopathy and treatment-related cancer.
INTRODUCTION
Intensification of therapy, refinements in supportive care, and more precise risk classification have contributed to improvements in outcome for children with acute myeloid leukemia (AML), with overall survival rates now exceeding 70%.1 However, additional intensification of induction regimens has not yielded superior results.2 In addition, late cardiotoxicity related to anthracycline exposure remains a significant problem.3-5 Clofarabine, a second-generation purine nucleoside analog, is safe and active when given alone or together with cytarabine in adults and children with relapsed AML.6-8 Because of the paucity of targeted agents for childhood AML, we sought to improve the clinical outcome and reduce the risks of cardiotoxicity and secondary malignancy by replacing daunorubicin and etoposide with clofarabine during the first course of induction therapy. Pediatric patients with AML were randomly assigned to receive clofarabine and cytarabine (Clo+AraC) or high-dose cytarabine, daunorubicin, and etoposide (HD-ADE) as their initial course of induction therapy. Subsequent treatment was based on presenting features and sequential evaluation of minimal residual disease (MRD). A subset of high-risk patients received vorinostat and a subset of standard-risk patients received infusions of natural killer cells, the results of which will be reported elsewhere.
PATIENTS AND METHODS
Patients
Patients with untreated AML who were younger than 22 years old and who did not have Down syndrome or acute promyelocytic leukemia were eligible for the AML08 trial (ClinicalTrials.gov identifier: NCT00703820). Those with normal creatinine for age, serum bilirubin less than or equal to 1.5 times the upper limit of normal, and aspartate transaminase and alanine transaminase less than or equal to 2.5 times the upper limit of normal were randomly assigned to the Clo+AraC or the HD-ADE arm of the trial. Patients who did not meet these criteria were treated according to the HD-ADE arm. From August 2008 through March 2017, 285 patients were enrolled at eight centers. The protocol was approved by the review boards of all participating institutions, and written informed consent and assent was obtained from patients or their guardians or parents.
Risk Classification and Definitions of Treatment Response
Genetic and morphologic features as well as response to therapy as assessed by flow cytometric studies of MRD level determined risk classification. Patients with core binding factor (CBF) leukemia [t(8;21)(q22;q22)/RUNX1-RUNX1T1, inv(16) or t(16;16)(p13.1;q22)/CBFβ-MYH11] and MRD less than 0.1% at day 22 of induction therapy were considered low risk. High-risk criteria included t(6;9)(p22;q34), t(8;16)(p11.2;p13.3), t(16;21)(q24;q22), -7, -5, or 5q-; French-American-British (FAB) classification M0 or M6; FAB M7 without t(1;22)(p13;q13); treatment-related AML; FLT3-internal tandem duplication (ITD) and MRD greater than or equal to 0.1% at day 22 regardless of NPM1 and WT1 status; and MRD greater than or equal to 5% at day 22 or greater than or equal to 0.1% after induction II. All other patients were classified as having standard-risk AML.
Complete remission was defined as trilineage hematopoietic recovery with less than 5% blasts in the marrow; induction failure as the presence of greater than or equal to 5% leukemia at the end of induction II; early death as death before achievement of remission; and treatment-related mortality as death during postremission treatment. MRD was determined by flow-cytometric assessment of leukemia-associated immunophenotypes that were identified in diagnostic bone marrow specimens. Marker combinations that allowed detection of 10 leukemia cells per 10,000 mononuclear bone marrow cells were applied to subsequent samples; at least 100,000 viable mononuclear cells were analyzed in each sample. Results were reported as the percentage of cells expressing the leukemia-associated immunophenotype, and MRD positivity was defined as greater than or equal to 0.1%.
Therapy
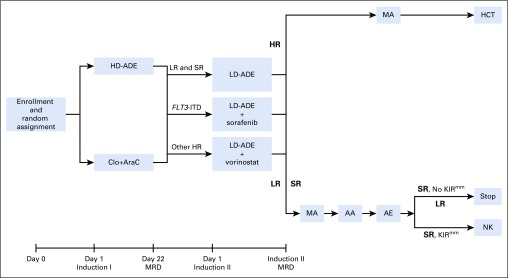
Patients with adequate organ function were randomly assigned at the time of enrollment to receive clofarabine (provided by Sanofi Genzyme; 52 mg/m2 on days 1 to 5) and cytarabine (1 g/m2 on days 1 to 5) or high-dose cytarabine (3 g/m2 every 12 hours on days 1, 3, 5), daunorubicin (50 mg/m2 on days 2, 4, 6), and etoposide (100 mg/m2 on days 2 to 6) during induction I (Fig 1). During induction II, low-dose cytarabine (100 mg/m2 every 12 hours on days 1 to 8), together with the same doses of daunorubicin and etoposide as those during induction I (LD-ADE) were given to low-risk and standard-risk patients, with the addition of sorafenib (400 mg/m2 per day starting on day 9 for 21 doses) to patients with FLT3-ITD, or vorinostat (300 mg/m2 per day on days −2, −1, and 0) to high-risk patients who lacked FLT3-ITD. Sorafenib was also given to FLT3-ITD–positive patients after each course of consolidation therapy, but not after hematopoietic cell transplantation (HCT). Low-risk patients then received chemotherapy, standard-risk patients received chemotherapy with or without natural killer cell therapy,9 and high-risk patients were eligible for HCT. Initially, three courses of consolidation therapy were administered. However, on the basis of the results of the MRC AML12 trial,10 the protocol was amended to remove the final course of therapy, reducing the total number of chemotherapy courses from five to four. Dexrazoxane was allowed but not routinely used.
FIG 1.
AML08 treatment schema. Patients with adequate organ function were randomly assigned to receive clofarabine and cytarabine (Clo+AraC; clofarabine 52 mg/m2 per day on days 1 to 5 and cytarabine 1 g/m2 per day on days 1 to 5) or high-dose cytarabine, daunorubicin, and etoposide (HD-ADE; cytarabine 3 g/m2 every 12 hours on days 1, 3, and 5, daunorubicin 50 mg/m2 on days 2, 4, and 6, and etoposide 100 mg/m2 per day on days 2 to 6) during induction I. Minimal residual disease (MRD) was assessed at day 22. During induction II, low-dose cytarabine (100 mg/m2 every 12 hours on days 1 to 8), daunorubicin, and etoposide (LD-ADE) were given alone to low-risk (LR) and standard-risk (SR) patients, with sorafenib to patients with FLT3–internal tandem duplication (ITD), and with vorinostat to high-risk patients who lacked FLT3-ITD. Induction II MRD was assessed at the time of count recovery. LR patients then received chemotherapy, SR patients received chemotherapy with or without subsequent natural killer (NK) cell therapy, and high-risk (HR) patients were eligible for hematopoietic cell transplantation (HCT). Postinduction chemotherapy initially included MA (mitoxantrone 12 mg/m2 on days 3 to 5 and cytarabine 1 g/m2 every 12 hours on days 1to 4), AA (cytarabine 3 g/m2 every 12 hours on days 1, 2, 8, 9 and l-asparaginase 6000 Units/m2 3 hours after the fourth and eighth doses of cytarabine), and AE (cytarabine 1 g/m2 every 12 hours on days 1 to 5 and etoposide 150 mg/m2 on days 1 to 5). The protocol was amended to remove AE. KIRmm, killer immunoglobulin–like receptor–human leukocyte antigen-mismatched donors.
Patients without CNS disease received a single dose of age-adjusted intrathecal methotrexate, hydrocortisone, and cytarabine before each course of therapy, for a total of four or five doses. Patients with CNS leukemia at diagnosis also received weekly intrathecal therapy until the cerebrospinal fluid was clear of leukemic cells.
Statistical Design and Analytic Methods
The primary objective was to compare the rates of MRD negativity on day 22 of induction I among patients randomly assigned to receive Clo+AraC with that of patients randomly assigned to HD-ADE. East software (Cytel; Cambridge, MA) was used to develop the design, and simulations in R (with 1,000,000 independent replications) were used to compute its statistical properties. The design specified enrollment of 240 randomly assigned patients who were evaluable for MRD and included four interim analyses (each evaluated at two-sided α = .005) and one final analysis (evaluated with two-sided α = .0429) according to the Haybittle-Peto method.11 Each interim analysis and the final analysis were performed using an exact Cochran-Mantel-Haenzel test stratified by initial risk assignment that was determined by disease characteristics at diagnosis. The design provides 80% power to declare statistical significance if the odds of MRD positivity in the Clo+AraC arm are 2.5 times that of the HD-ADE arm (corresponding to overall MRD positive rates of 55% and 37.4% in the Clo+AraC and HD-ADE arms, respectively). In addition, the design provides 83% power to detect the setting in which the odds of MRD positivity in the Clo+AraC arm are 0.4 times that of the HD-ADE arm (corresponding to MRD positive rates of 22.6% and 37.4% in the Clo+AraC and HD-ADE arms, respectively). The design was based on the MRD positive rate of 37.4% observed in the AML02 trial (ClinicalTrial.gov identifier: NCT02440568) at the time that AML08 was designed. At each interim analysis, event-free survival and overall survival were monitored for inequality between arms, and treatment-related death was monitored for excessive deaths compared with our previous trial. A futility analysis was performed at the fourth interim analysis.
Zelen’s block-randomization method was used to assign patients in a stratified manner to Clo+AraC or HD-ADE.12 For patients for whom karyotype was available, the random assignment was stratified by inv(16) versus t(8,21) versus 11q23 versus M7 versus others. For other patients, the random assignment was stratified by M2 with Auer rods versus M4Eo versus M5 versus M7 versus others. The results of the treatment assignment were provided to the treating physician, the participants, and the data analysts; no masking was performed.
Event-free survival was defined as the time from study enrollment (or date of MRD evaluation for post-MRD event-free survival) to induction failure, relapse, second malignancy, refusal of therapy, removal from therapy because of unacceptable toxicity, or death, with patients who had not experienced any of these events censored at last follow-up. Overall survival was defined as the time from study enrollment (or date of MRD evaluation) to death, with living patients censored at last follow-up. The Kaplan-Meier method13 was used to estimate event-free and overall survival rates, and the log-rank test was used to compare survival curves. Survival estimates are reported with SEs determined by the method of Peto and Pike.14
Cox proportional hazards regression models were used to explore the associations of MRD levels collected at baseline, at day 22, and at the end of Induction II as a piecewise-constant time-varying covariate, and other clinical features as fixed covariates with event-free and overall survival.15 Gray’s method16 was used to estimate and compare the cumulative incidence of major adverse events (relapse, death in remission, induction failure) while adjusting for competing risks. Cumulative incidence estimates are reported with SEs. Fisher’s exact test was used to compare categorical features and outcomes with one another. All analyses were performed with R software (www.r-project.org), Windows version 3.3.3, using the base, stats, survival, KMsurv, and cmprsk packages. Except where explicitly stated otherwise, results with a P value < .05 are deemed statistically significant.
RESULTS
Primary End Point and Outcome According to Induction Random Assignment
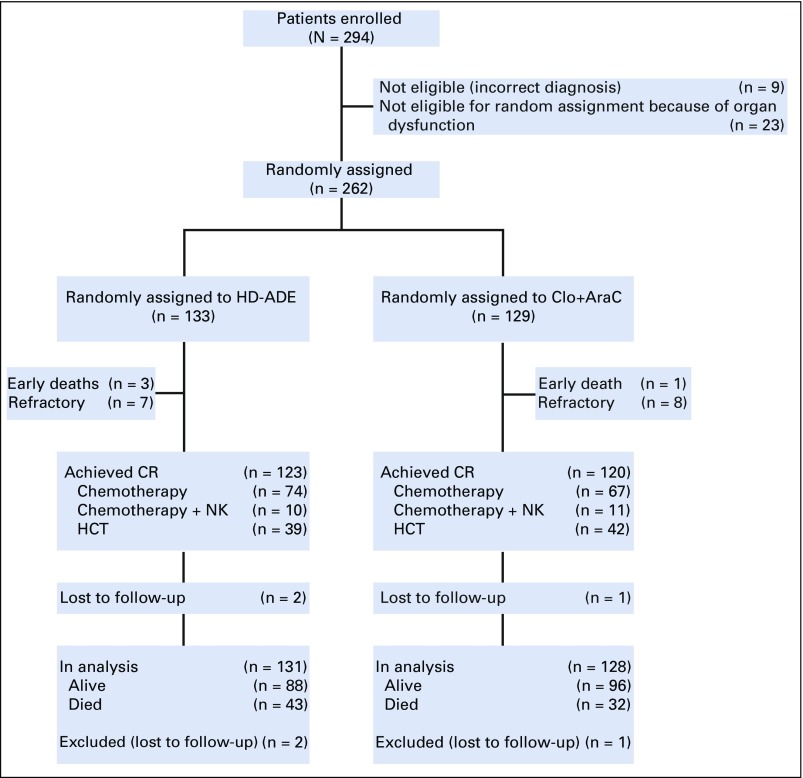
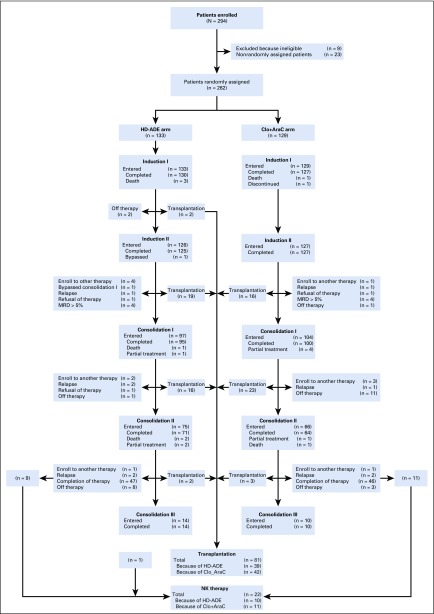
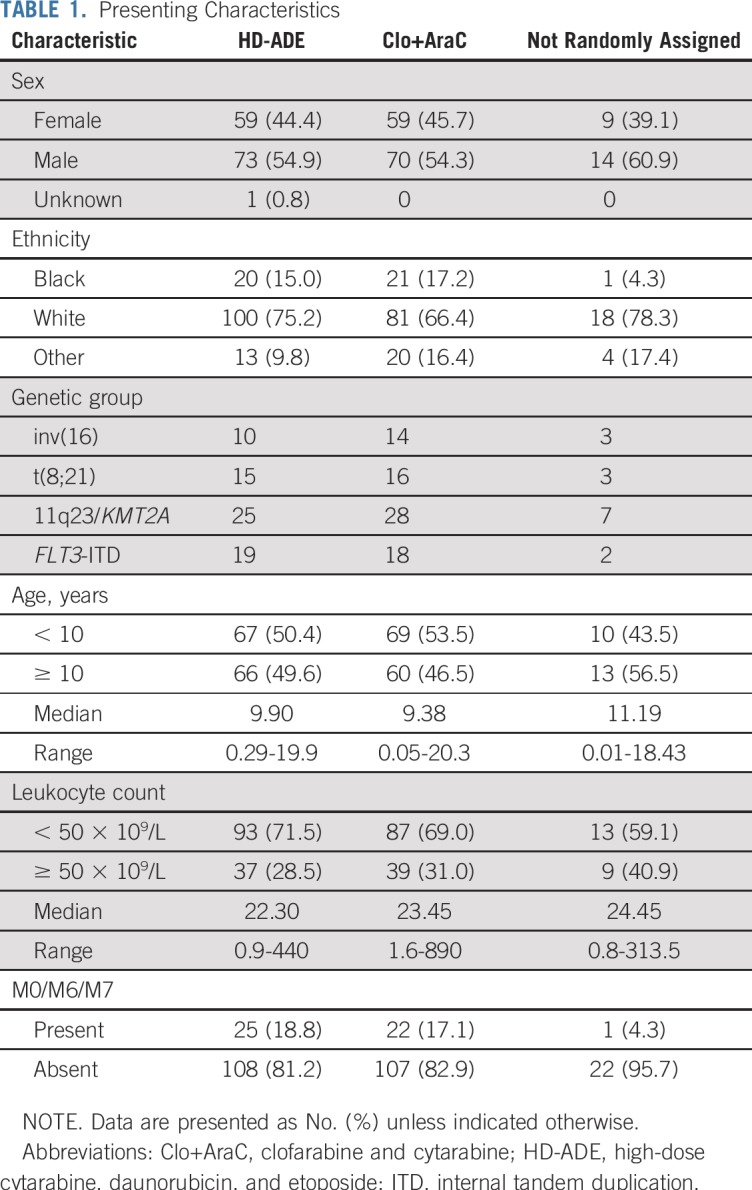
Among 285 eligible patients enrolled, 129 were randomly assigned to Clo+AraC and 133 to HD-ADE; 23 were nonrandomly assigned to HD-ADE because of elevated creatinine, bilirubin, aspartate transaminase, or alanine transaminase and are not included in subsequent analyses (Fig 2 and Appendix Fig A1, online only). There were no significant differences in presenting characteristics among patients in the three treatment groups (Table 1).
FIG 2.
Consort diagram. Clo+AraC, clofarabine 52 mg/m2 per day on days 1 to 5 and cytarabine 1 g/m2 per day on days 1 to 5; CR, complete remission; HCT, hematopoietic cell transplantation; HD-ADE, cytarabine 3 g/m2 every 12 hours on days 1, 3, and 5, daunorubicin 50 mg/m2 on days 2, 4, and 6, and etoposide 100 mg/m2 per day on days 2 to 6; NK, natural killer.
TABLE 1.
Presenting Characteristics
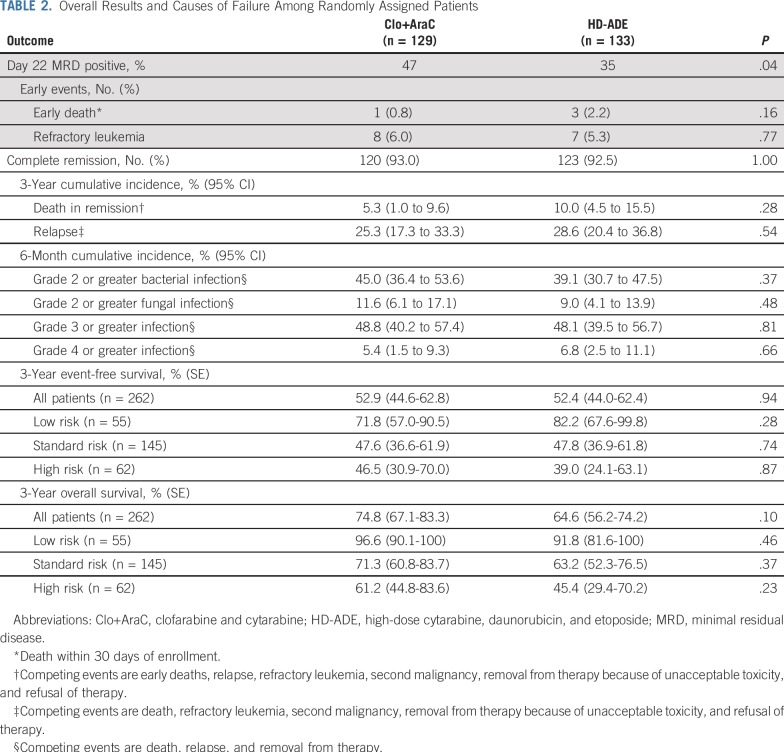
Day 22 MRD was positive in 57 (47%) of 121 evaluable patients assigned to Clo+AraC and in 42 (35%) of 121 evaluable patients assigned to HD-ADE; thus, Clo+AraC patients were 1.86 (1.03 to 3.41) times more likely to be MRD positive on day 22 than were HD-ADE patients (P = .04; Appendix Table A1, online only). However, after completion of induction II, 24 (20%) of 119 patients who received Clo+AraC and 16 (14%)of 115 patients who received HD-ADE were MRD positive, so the two arms did not differ significantly in the MRD positive rate (odds ratio, 1.62 [0.72 to 3.67]; P = .26). In addition, the number of patients whose risk classification changed because of MRD or poor morphologic response was similar in the treatment arms (12 of 129 patients who received Clo+AraC v 13 of 133 patients who received HD-ADE). There were no statistically significant differences in the number of early deaths, cases of refractory leukemia, cumulative rates of deaths in remission, or any grade bacterial or fungal infection between the two treatment arms (Table 2).
TABLE 2.
Overall Results and Causes of Failure Among Randomly Assigned Patients
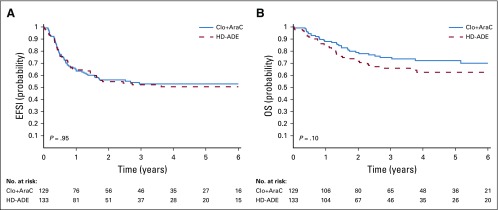
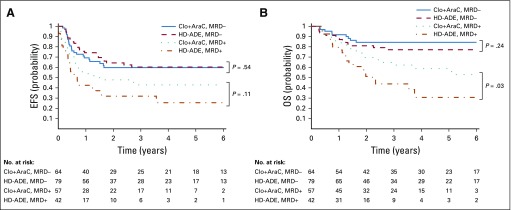
Despite the greater prevalence of MRD on day 22 among patients treated in the Clo+AraC arm compared with those treated on the HD-ADE arm, the complete remission rate after two courses of induction therapy (93.0% [87.2% to 96.8%] v 92.5% [86.6% to 96.3%]; P = 1.0) did not differ significantly between treatment groups (Table 2). Similarly, with median follow-up times of 1,378 and 1,064 days for survivors treated in the Clo+AraC and HD-ADE arms, respectively, there were no significant differences in 3-year event-free survival rate (52.9% [44.6% to 62.8%] v 52.4% [44.0% to 62.4%]; P = .95), 3-year cumulative incidence of relapse (25.3% [17.3% to 33.3%] v 28.6% [20.4% to 36.8%]; P = .54), or 3-year overall survival rate (74.8% [67.1% to 83.3%] v 64.6% [56.2% to 74.2%]; P = .1) between treatment groups (Table 2; Fig 3). Among patients who were MRD negative at day 22 of induction, subsequent event-free and overall survival rates were similar between treatment groups (Appendix Fig A2, online only). However, among day-22 MRD-positive patients, those who received Clo+AraC had a better subsequent 3-year overall survival rate than did those who received HD-ADE (62.2% [49.9% to 77.6%] v 42.7% [28.9% to 63.0%]; P = .03; Appendix Fig A2). Similarly, among patients who remained MRD positive after induction II, those who received Clo+AraC had a markedly better postinduction II 3-year overall survival rate than did those who received HD-ADE (47.9% [30.4% to 75.4%] v 6.2% [0.9% to 41.7%]; P < .01).
FIG 3.
Outcome according to treatment arm. (A) Probability of event-free survival (EFS) according to treatment. (B) Probability of overall survival (OS) according to treatment.
Importantly, the presence or absence of MRD, although having an appreciable effect on outcome in both treatment groups, exerted its greatest impact among patients randomly assigned to receive HD-ADE. In this group, the 3-year event-free survival rate for MRD-negative patients was 61.5% (50.8% to 74.5%), compared with 30.8% (19.3% to 49.1%) for those with detectable disease (P < .001), whereas the corresponding results for patients treated in the Clo+AraC arm were 59.7% (48.4% to 73.8%) and 42.9% (31.3% to 58.8%; P = .06).
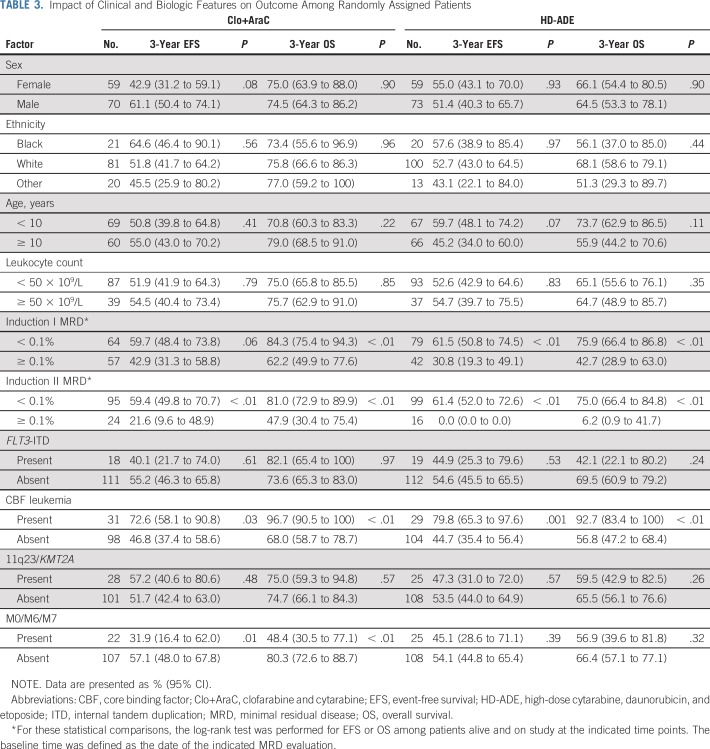
Association of Clinical Characteristics With Event-Free and Overall Survival
Given the differential association of MRD with event-free and overall survival across treatment arms, we evaluated the association of clinical characteristics with event-free and overall survival separately in each arm (Table 3). In the Clo+AraC arm, induction I MRD positivity, induction II MRD positivity, and the presence of FAB types M0, M6, or M7 were significantly associated with a worse outcome (P < .05; Table 3), whereas the presence of CBF leukemia was significantly associated with a better outcome (P < .05; Table 3). In the HD-ADE arm, induction I MRD positivity and induction II MRD positivity were significantly associated with a worse outcome, and the presence of the CBF karyotype was significantly associated with a better outcome.
TABLE 3.
Impact of Clinical and Biologic Features on Outcome Among Randomly Assigned Patients
We also evaluated the association of clinical characteristics with event-free and overall survival in a multiple-predictor Cox model fit separately to each arm. The characteristics included as predictor variables have been reported previously as associated with event-free and overall survival: age, leukocyte count at diagnosis, MRD, FLT3-ITD, CBF leukemia, KMT2A fusions, and presence of the M0, M6, or M7 FAB types. In the HD-ADE arm, all characteristics were significantly (P < .05) or marginally (P < .10) associated with event-free (Appendix Table A2, online only) and/or overall (Appendix Table A3, online only) survival in directions consistent with previous reports. The Clo+AraC arm had a strikingly different pattern of associations of clinical characteristics with event-free and overall survival—CBF karyotype was marginally associated with event-free and significantly associated with overall survival; no other characteristics had a significant or marginal association with either event-free or overall survival (Appendix Tables A2 and A3).
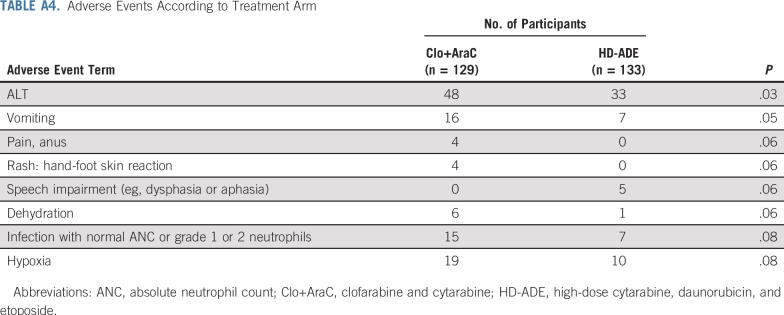
Adverse Events
A detailed tabulation of adverse events in this trial has been reported to ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT00703820). Appendix Table A4 (online only) lists the results for adverse event terms that showed significant differences in their rates across the two arms at the P = .10 level.
DISCUSSION
In adults with AML, the addition of low-dose clofarabine to induction therapy was associated with better outcomes for intermediate-risk patients.17 Similarly, the substitution of three courses of clofarabine plus cytarabine for high-dose cytarabine was associated with a lower incidence of relapse and a higher relapse-free survival rate among adults with intermediate-risk AML who did not undergo HCT.18 In the current trial, the combination of clofarabine and cytarabine was less effective at inducing MRD negativity compared with HD-ADE, but was nevertheless associated with excellent results, with a 3-year overall survival rate of 75%, which is comparable to the best outcomes of other trials for childhood AML.2,10,19-23 Clofarabine-based induction therapy, which included one course of Clo+AraC and one course of LD-ADE, was well tolerated, with rates of early death, death in remission, fungal infection, and bacterial infection similar to those of HD-ADE-based induction.
Consistent with our previous study,2 the initial response to therapy as assessed by MRD, which provides a distinction between residual leukemia and normal regenerating hematopoiesis, was significantly associated with outcome. However, one must use caution when applying MRD after one course of therapy as a surrogate end point for determining superiority between treatment regimens. In the current study, higher rates of day-22 MRD positivity after Clo+AraC compared with HD-ADE suggested that Clo+AraC–based induction was inferior, but similar MRD positivity rates after two courses of induction therapy, as well as similar event-free and overall survival rates between treatment arms, suggest that a suboptimal response to Clo+AraC may be overcome more easily than such a response to HD-ADE. The explanation for the excellent outcome among patients treated in the Clo+AraC arm despite high day-22 MRD levels is multifactorial and may include small differences in treatment-related mortality, outcome after HCT, and salvage rates after relapse.
Importantly, the substitution of clofarabine for daunorubicin during induction I resulted in lower cumulative anthracycline exposure, as well as lower exposure to epipodophyllotoxins. Although anthracyclines are an essential component of AML therapy, they are associated with dose-dependent late cardiotoxicity, leading to significant morbidity and mortality in AML survivors. Recent studies indicate that the incidence of late subclinical cardiotoxicity ranges from 8.1% to 15.3% with late clinical cardiotoxicity diagnosed in as many as 9.3% of survivors, suggesting that a reduction of anthracycline exposure of the magnitude tested in the current trial would improve the quality of life of these patients.3-5 Using the validated conversion factors of 0.5 for daunorubicin, 5 for idarubicin, and 4 for mitoxantrone,24 patients treated in the Clo+AraC arm received 219 mg/m2 of doxorubicin equivalents, compared with 294 mg/m2 for patients who received HD-ADE. These exposures are much lower than the Children's Oncology Group AAML0531 trial (ClinicalTrials.gov identifier: NCT 00372593) in which patients received 342 mg/m2,20 the AML-Berlin-Frankfurt-Muenster (BFM) 98 trial (ClinicalTrials.gov identifier: NCT00111345) in which patients received 400 mg/m2,25 the Associazione Italiana di Ematologia e Oncologia Pediatricia (AIEOP) AML 2002/01trial in which patients received 380 mg/m2,22 AML12 (ClinicalTrials.gov identifier: NCT00002658) in which patients received 350 or 392 mg/m2,10 and AML99 (ClinicalTrials.gov identifier: NCT01716793) in which patients received 540 mg/m226.. Although data regarding late cardiotoxicity is not yet available in this trial, it is intriguing that all five patients who suffered grade 3 acute ventricular dysfunction during treatment were treated in the HD-ADE arm. It should be noted, however, that late effects will not only reflect the primary chemotherapy received, but may also be related to salvage chemotherapy given for relapse as well as late toxicity secondary to HCT.
Our results indicate that clofarabine should be further studied to determine if it can reduce the cumulative anthracycline and etoposide exposure without compromising survival rates in children with AML. It must be noted, however, that the trial was powered to detect differences in induction I MRD, not in event-free or overall survival. In addition, it was not powered to identify specific patient subgroups that benefited from treatment with clofarabine or with higher doses of anthracyclines. Nevertheless, it is intriguing that the negative impact of older age, higher leukocyte count, and presence of FLT3-ITD among patients treated with HD-ADE was abrogated by treatment with Clo+AraC, suggesting that the benefit of clofarabine may extend across subgroups.
The overall survival rates of 70% for the entire group of patients and 75% for those treated with clofarabine remind us that new approaches to the treatment of AML are urgently needed. In the current study, approximately one half of all relapses occurred in patients who were MRD-negative, indicating that the MRD methodology used lacked sufficient sensitivity to identify patients with persistent but low levels of leukemia. The use of newly described leukemia-associated profiles that allow universal monitoring of MRD at a sensitivity of 0.01% and the use of molecular techniques may help identify patients who are at a high risk of relapse and candidates for novel therapy.27,28 However, the development of new agents is still needed. Toward this end, we are testing a selective inhibitor of nuclear export (selinexor; ClinicalTrials.gov identifier: NCT02212561),29 a histone deacetylase inhibitor (panobinostat; ClinicalTrials.gov identifier: NCT02676323),30 and a BCL2 inhibitor (venetoclax; ClinicalTrials.gov identifier: NCT03194932)31 in children with relapsed or refractory AML.
ACKNOWLEDGMENT
We thank John Gilbert for expert scientific editing, Kathy Jackson for data collection, and Elizabeth Stevens for preparing the figures. We also thank Lei Shi and Xueyuan Cao for preparing Data Safety Monitoring Board reports and for performing other statistical monitoring activities for the study.
APPENDIX
FIG A1.
Detailed patient flowchart. Clo+AraC, clofarabine and cytarabine; HD-ADE, high-dose cytarabine, daunorubicin, and etoposide; MRD, minimal residual disease; NK, natural killer.
FIG A2.
Outcome according to treatment arm and minimal residual disease positive (MRD+) or negative (MRD−). (A) Probability of event-free survival (EFS) according to induction I MRD and treatment. Patients alive and on study at the time of MRD evaluation were included in this analysis. In addition, date of MRD evaluation was defined as baseline time. (B) Probability of overall survival (OS) according to induction I MRD and treatment. Patients alive and on study at the time of MRD evaluation were included in this analysis. In addition, date of MRD evaluation was defined as baseline time. Clo+AraC, clofarabine and cytarabine; HD-ADE, high-dose cytarabine, daunorubicin, and etoposide.
TABLE A1.
Day-22 Induction I MRD Positivity According to Subgroup
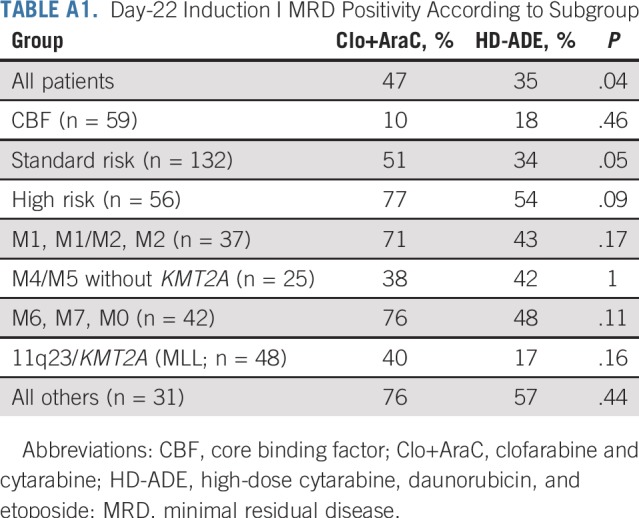
TABLE A2.
Multivariate Analysis of Prognostic Factors for Event-Free Survival
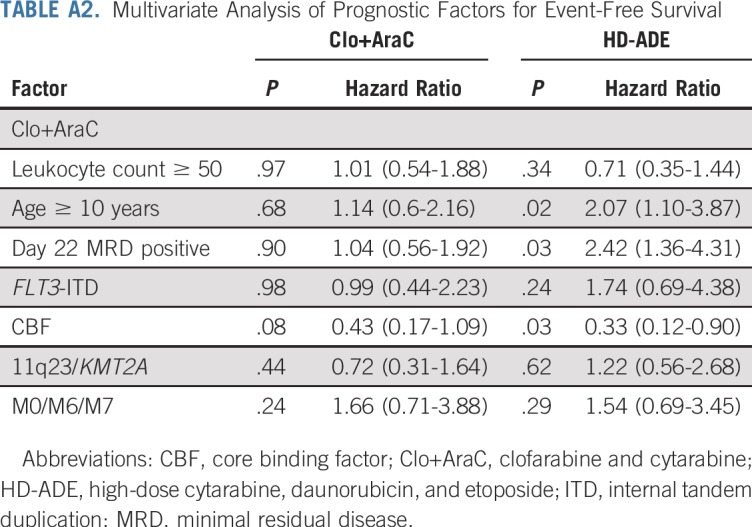
TABLE A3.
Multivariate Analysis of Prognostic Factors for Overall Survival
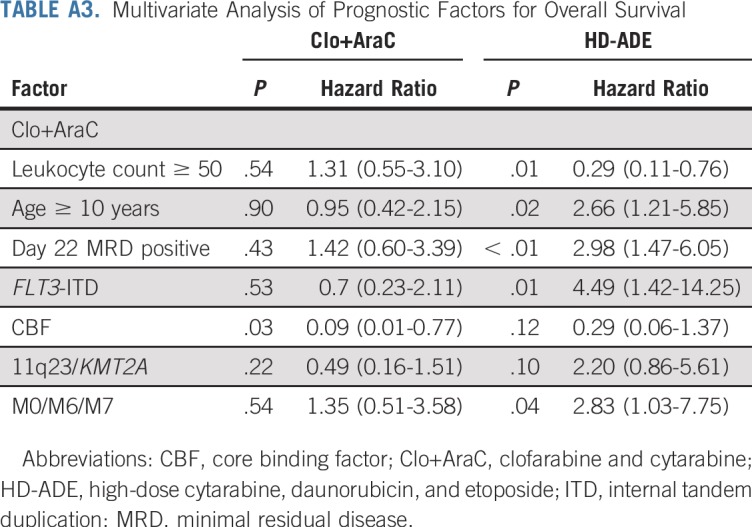
TABLE A4.
Adverse Events According to Treatment Arm
Footnotes
Supported by Cancer Center Support Grant P30 CA021765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities.
Clinical trial information: NCT00703820.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey E. Rubnitz, Norman J. Lacayo, Hiroto Inaba, Kenneth Heym, Raul C. Ribeiro, Jeffrey Taub, Barbara Degar, Allen Eng-JuhYeoh, Elaine Coustan-Smith, Stanley Pounds, Ching-Hon Pui
Administrative support: Allen Eng-JuhYeoh, Ching-Hon Pui
Collection and assembly of data: Jeffrey E. Rubnitz, Norman J. Lacayo, Hiroto Inaba, Kenneth Heym, Barbara Degar, Deborah Schiff, Allen Eng-Juh Yeoh, Brandon Triplett, Susana C. Raimondi, John Choi
Provision of study material or patients: Jeffrey E. Rubnitz, Norman J. Lacayo, Hiroto Inaba, Kenneth Heym, Raul C. Ribeiro, Jeffrey Taub, Jennifer McNeer, Barbara Degar, Deborah Schiff, Allen Eng-Juh Yeoh, Brandon Triplett, Ching-Hon Pui
Data analysis and interpretation: Jeffrey E. Rubnitz, Norman J. Lacayo, Kenneth Heym, Raul C. Ribeiro, Jennifer McNeer, Barbara Degar, Deborah Schiff, Allen Eng-Juh Yeoh, Elaine Coustan-Smith, Lei Wang, Brandon Triplett, Jeffery Klco, Stanley Pounds, Ching-Hon Pui
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Clofarabine Can Replace Anthracyclines and Etoposide in Remission Induction Therapy for Childhood Acute Myeloid Leukemia: The AML08 Multicenter, Randomized Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Hiroto Inaba
Research Funding: Servier
Elaine Coustan-Smith
Stock and Other Ownership Interests: Unum Therapeutics (I), Nkarta Therapeutics (I), Medisix Therapeutics (I)
Honoraria: GlaxoSmithKline (I)
Consulting or Advisory Role: Medisix Therapeutics (I)
Patents, Royalties, Other Intellectual Property: Juno Therapeutics/Celgene (I), Unum Therapeutics (I), Nkarta Therapeutics (I), Medisix Therapeutics (I), Patent and patent application
Travel, Accommodations, Expenses: GSK (I), Celgene (I), Nkarta Therapeutics (I)
Brandon Triplett
Travel, Accommodations, Expenses: Miltenyi Biotec
Ching-Hon Pui
Consulting or Advisory Role: Adaptive Biotechnologies
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33:2949–2962. doi: 10.1200/JCO.2015.62.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlogis V, Auquier P, Bertrand Y, et al. Late cardiomyopathy in childhood acute myeloid leukemia survivors: A study from the L.E.A. program. Haematologica. 2015;100:e186–e189. doi: 10.3324/haematol.2014.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarfelt M, Kujacic V, Holmgren D, et al. Exercise echocardiography reveals subclinical cardiac dysfunction in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:835–840. doi: 10.1002/pbc.21289. [DOI] [PubMed] [Google Scholar]
- 5.Temming P, Qureshi A, Hardt J, et al. Prevalence and predictors of anthracycline cardiotoxicity in children treated for acute myeloid leukaemia: Retrospective cohort study in a single centre in the United Kingdom. Pediatr Blood Cancer. 2011;56:625–630. doi: 10.1002/pbc.22908. [DOI] [PubMed] [Google Scholar]
- 6.Cooper TM, Alonzo TA, Gerbing RB, et al. AAML0523: A report from the Children’s Oncology Group on the efficacy of clofarabine in combination with cytarabine in pediatric patients with recurrent acute myeloid leukemia. Cancer. 2014;120:2482–2489. doi: 10.1002/cncr.28674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: Results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeha S, Razzouk B, Rytting M, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Oncol. 2009;27:4392–4397. doi: 10.1200/JCO.2008.18.8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with acute myeloid leukaemia: Medical Research Council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 11.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44:793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 12.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox DR: Regression models and life-tables. J Royal Stat Soc Series B 34:187-202, 1972. [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Löwenberg B, Pabst T, Maertens J, et al. Therapeutic value of clofarabine in younger and middle-aged (18-65 years) adults with newly diagnosed AML. Blood. 2017;129:1636–1645. doi: 10.1182/blood-2016-10-740613. [DOI] [PubMed] [Google Scholar]
- 18.Thomas X, de Botton S, Chevret S, et al. Randomized phase II study of clofarabine-based consolidation for younger adults with acute myeloid leukemia in first remission. J Clin Oncol. 2017;35:1223–1230. doi: 10.1200/JCO.2016.70.4551. [DOI] [PubMed] [Google Scholar]
- 19.Creutzig U, Zimmermann M, Bourquin JP, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: Results from study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- 20.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura T, Iwamoto S, Kanai R, et al. Outcome in 146 patients with paediatric acute myeloid leukaemia treated according to the AML99 protocol in the period 2003-06 from the Japan Association of Childhood Leukaemia Study. Br J Haematol. 2012;159:204–210. doi: 10.1111/bjh.12030. [DOI] [PubMed] [Google Scholar]
- 22.Pession A, Masetti R, Rizzari C, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 2013;122:170–178. doi: 10.1182/blood-2013-03-491621. [DOI] [PubMed] [Google Scholar]
- 23.Tierens A, Bjørklund E, Siitonen S, et al. Residual disease detected by flow cytometry is an independent predictor of survival in childhood acute myeloid leukaemia; Results of the NOPHO-AML 2004 study. Br J Haematol. 2016;174:600–609. doi: 10.1111/bjh.14093. [DOI] [PubMed] [Google Scholar]
- 24.Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33:3774–3780. doi: 10.1200/JCO.2015.61.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: Results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 26.Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: The AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- 27.Coustan-Smith E, Song G, Shurtleff S, et al. Universal monitoring of minimal residual disease in acute myeloid leukemia. JCI Insight. 2018;3:98561. doi: 10.1172/jci.insight.98561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klco JM, Miller CA, Griffith M, et al. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA. 2015;314:811–822. doi: 10.1001/jama.2015.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander TB, Lacayo NJ, Choi JK, et al. Phase I study of selinexor, a selective inhibitor of nuclear export, in combination with fludarabine and cytarabine, in pediatric relapsed or refractory acute leukemia. J Clin Oncol. 2016;34:4094–4101. doi: 10.1200/JCO.2016.67.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie C, Drenberg C, Edwards H, et al. Panobinostat enhances cytarabine and daunorubicin sensitivities in AML cells through suppressing the expression of BRCA1, CHK1, and Rad51. PLoS One. 2013;8:e79106. doi: 10.1371/journal.pone.0079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. doi: 10.1016/S1470-2045(18)30010-X. [DOI] [PubMed] [Google Scholar]