Abstract
PURPOSE
Therapeutic radiation in childhood cancer has decreased over time with a concomitant increase in chemotherapy. Limited data exist on chemotherapy-associated subsequent malignant neoplasm (SMN) risk.
PATIENTS AND METHODS
SMNs occurring > 5 years from diagnosis, excluding nonmelanoma skin cancers, were evaluated in survivors diagnosed when they were < 21 years old, from 1970 to 1999 in the Childhood Cancer Survivor Study (median age at diagnosis, 7.0 years; median age at last follow-up, 31.8 years). Thirty-year SMN cumulative incidence and standardized incidence ratios (SIRs) were estimated by treatment: chemotherapy-only (n = 7,448), chemotherapy plus radiation (n = 10,485), radiation only (n = 2,063), or neither (n = 2,158). Multivariable models were used to assess chemotherapy-associated SMN risk, including dose-response relationships.
RESULTS
Of 1,498 SMNs among 1,344 survivors, 229 occurred among 206 survivors treated with chemotherapy only. Thirty-year SMN cumulative incidence was 3.9%, 9.0%, 10.8%, and 3.4% for the chemotherapy-only, chemotherapy plus radiation, radiation-only, or neither-treatment groups, respectively. Chemotherapy-only survivors had a 2.8-fold increased SMN risk compared with the general population (95% CI, 2.5 to 3.2), with SIRs increased for subsequent leukemia/lymphoma (1.9; 95% CI, 1.3 to 2.7), breast cancer (4.6; 95% CI, 3.5 to 6.0), soft-tissue sarcoma (3.4; 95% CI, 1.9 to 5.7), thyroid cancer (3.8; 95% CI, 2.7 to 5.1), and melanoma (2.3; 95% CI, 1.5 to 3.5). SMN rate was associated with > 750 mg/m2 platinum (relative rate [RR] 2.7; 95% CI, 1.1 to 6.5), and a dose response was observed between alkylating agents and SMN rate (RR, 1.2/5,000 mg/m2; 95% CI, 1.1 to 1.3). A linear dose response was also demonstrated between anthracyclines and breast cancer rate (RR, 1.3/100 mg/m2; 95% CI, 1.2 to 1.6).
CONCLUSION
Childhood cancer survivors treated with chemotherapy only, particularly higher cumulative doses of platinum and alkylating agents, face increased SMN risk. Linear dose responses were seen between alkylating agents and SMN rates and between anthracyclines and breast cancer rates. Limiting cumulative doses and consideration of alternate chemotherapies may reduce SMN risk.
INTRODUCTION
Subsequent malignant neoplasms (SMNs) occurring after a childhood cancer diagnosis are associated with significant morbidity and mortality. Previous reports of childhood cancer survivors have described the incidence of SMNs, as well as associated risk factors.1-3 Therapeutic radiation has been associated consistently with the highest risk for SMNs.1 Treatment with radiation among patients who had childhood cancer steadily decreased from the 1970s through the 1990s4-6 and concurrent with this reduction, an increasing proportion of patients received alkylating agents, anthracyclines, epipodophyllotoxins, and platinum-based drugs.3 The cumulative incidence of and risk for SMNs decreased with advancing treatment decades, which was at least partially attributable to decreases in therapeutic radiation exposure.3 Despite temporal decreases in SMN risk among survivors, they continue to have increased risk compared with expected cancer rates in the general population.3
Multiple investigations have described associations between chemotherapeutic agents and the risk of specific SMNs, including: alkylating agents and epipodophyllotoxins with leukemia7,8; anthracyclines and alkylating agents with sarcoma9-11; procarbazine, platinum-based and alkylating agents with GI cancers11-13; alkylating agents with carcinomas14; alkylating agents and anthracyclines with breast cancer15; and cisplatin-based therapy with solid tumors after testicular nonseminomas.16 However, the generalizability of these findings is limited because they have typically included individuals who were treated with both radiation and chemotherapy, have focused on specific primary cancers, or have focused on specific SMN types. Risk for SMNs among patients receiving chemotherapy alone requires additional study, particularly because the types and doses of chemotherapies have changed over recent treatment eras. Using the large and heterogeneous population of pediatric cancer survivors in the North American Childhood Cancer Survivor Study (CCSS) cohort, we evaluated associations between chemotherapy and SMNs among nonirradiated, long-term survivors.
MATERIALS AND METHODS
Population
The CCSS cohort includes 5-year childhood cancer survivors diagnosed between January 1, 1970, and December 31, 1999, at one of 28 participating centers in North America. Participants were diagnosed at < 21 years of age with leukemia, Hodgkin lymphoma, non-Hodgkin lymphoma, CNS tumor, Wilms tumor, neuroblastoma, soft-tissue sarcoma (STS), or bone cancer. Participating centers received human subjects committee approval before subject recruitment and participants, or parents of children < 18 years old, provided informed consent. Participants completed a baseline questionnaire and up to five follow-up questionnaires and were censored at the date of death or most recent contact before November 30, 2016. CCSS study design and methods have been described previously.17,18
Treatment Exposures and SMN Identification
Chemotherapy agents and cumulative doses from 5 years after diagnosis were abstracted from medical records of consenting participants. Cumulative alkylating agent doses are reported as cyclophosphamide equivalent dose (CED),19 cumulative anthracycline doses are based on doxorubicin isotoxic equivalent dose,20 and cumulative platinum doses were calculated using the formula described by Travis et al.21,22 Maximum radiation treatment dose was calculated for eight body regions for each patient.
SMNs were initially identified through self or proxy report, or death certificate, and were validated through pathology report review or, if a pathology report was unavailable, by review of death certificate, medical records, or both. SMNs were independently reviewed by a pathologist and an oncologist. SMNs are defined as neoplasms histologically unique from the childhood cancer, classified as behavior code 3 in the International Classification of Diseases for Oncology, Third Edition23 excluding nonmelanoma skin cancers. Recurrences of primary cancers or SMNs were excluded. Only SMNs occurring ≥ 5 years after childhood cancer diagnosis were included and individuals not reporting an SMN were assumed to have no SMN.
Statistical Methods
CCSS participant follow-up began 5 years from childhood cancer diagnosis and continued through the date of the last completed questionnaire or death. Analyses were weighted to account for undersampling of survivors of acute lymphoblastic leukemia between 1987 and 1999, with weights of 1.21 for age 0 or 11 to 20 years at diagnosis and 3.63 for those aged 1 to 10 years. Survivors were divided into four mutually exclusive groups on the basis of on childhood cancer treatment: (1) treatment with chemotherapy and no radiation, subsequently referred to as chemotherapy only; (2) treatment with chemotherapy plus radiation; (3) treatment with radiation and no chemotherapy; and (4) treatment with no chemotherapy and no radiation therapy. The cumulative incidence and the cumulative burden, assessed using the summarization of all events occurring in the cohort over the defined time,24 of SMNs were estimated using time since diagnosis as the time scale and treating death as a competing risk event. Cumulative incidence curves were estimated and compared between treatment groups using the Gray K-sample test.25
Standardized incidence ratios (SIRs) and absolute excess risk (AER) per 1,000 person-years were calculated using age-, sex-, race-, and calendar-year–specific US cancer incidence rates from the SEER program to determine the expected number of events.26 Multivariable piecewise exponential models were used to estimate the relative rates (RRs) of SMN incidence, adjusting for sex, attained age, age at diagnosis, 5-year treatment eras, history of splenectomy, and cumulative dose levels of chemotherapy classes (alkylating agents, anthracyclines, epipodophyllotoxins, and platinum-based agents), with date of last survey or death considered the termination time point of the at-risk period. Chemotherapy doses were categorized to evaluate the dose-response relationship of each chemotherapy with risk of all SMNs and risk of SMN subtypes. When applicable, a linear dose-response model was fit to estimate the RR of SMN per chemotherapy dose increment. Because primary cancer diagnosis and treatment are highly interrelated and colinear, only chemotherapy, and not primary cancer, was adjusted for in the multivariable models. Study methods were in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for observational studies (Data Supplement).27
RESULTS
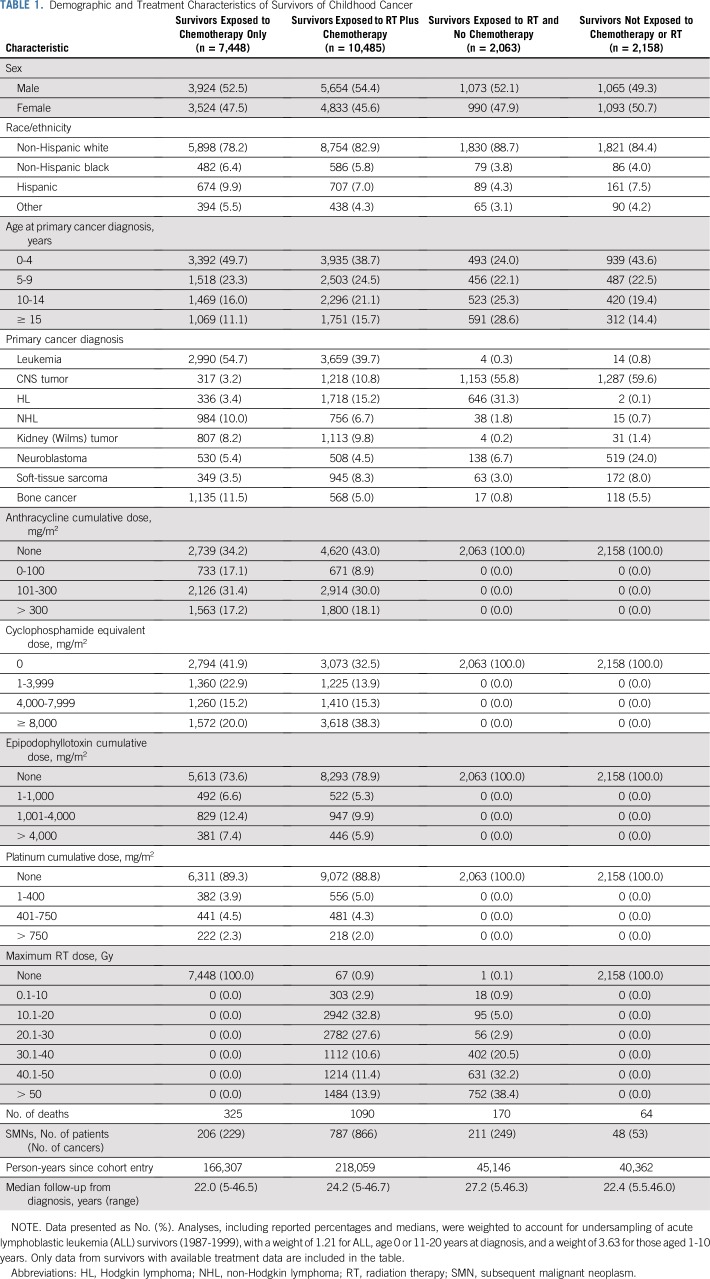
Treatment data were available for 22,154 survivors (median age at diagnosis, 7.0 years, range, 0 to 20.9 years; age at last follow-up, 31.9 years, range, 5.6 to 65.9 years), including 7,448 treated with chemotherapy only, 10,485 with chemotherapy plus radiation, 2,063 with radiation and no chemotherapy, and 2,158 with neither chemotherapy nor radiation (Table 1; Data Supplement). Among survivors treated with chemotherapy only, 48% were female and approximately half were diagnosed with childhood cancer before age 5 years. The most common initial diagnoses were leukemia, bone cancer, and non-Hodgkin lymphoma, and median follow-up was 22.0 years (range, 5 to 46.5 years).
TABLE 1.
Demographic and Treatment Characteristics of Survivors of Childhood Cancer
Cumulative Incidence and Cumulative Burden of SMNs
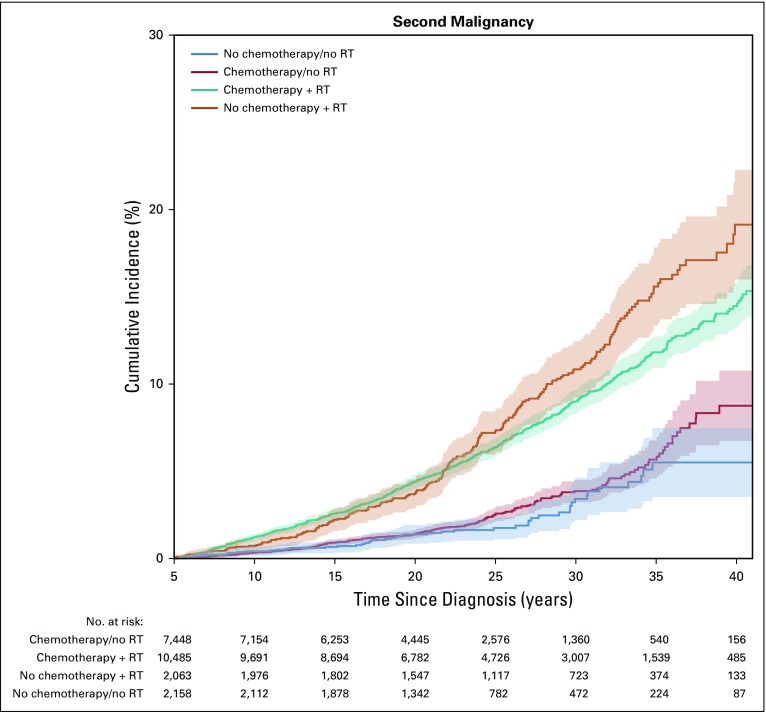
With 166,307 person-years of follow-up for chemotherapy-only survivors, 229 SMNs were identified among 206 survivors. The most frequently observed SMNs were breast (n = 51), thyroid (n = 36), melanoma (n = 18), and STS (n = 14). In the chemotherapy-only subgroup, 28% of SMNs were among osteosarcoma survivors (12% of survivors; Data Supplement). The 30-year SMN cumulative incidence was 3.9% (95% CI, 3.2% to 4.5%; Fig 1) and was highest among survivors of Hodgkin lymphoma (11.2%; 95% CI, 5.5% to 16.9%) and osteosarcoma (7.0%; 95% CI, 4.9% to 9.0%; Data Supplement). Among other treatment subgroups, the SMN cumulative incidence for survivors treated with chemotherapy and radiation, radiation and no chemotherapy, and neither chemotherapy nor radiation was 9.0% (95% CI, 8.3% to 9.7%), 10.8% (95% CI, 9.2% to 12.5%), and 3.4% (95% CI, 2.2% to 4.6%), respectively (Fig 1). Piecewise exponential models adjusted for attained age, sex, years since diagnosis, and diagnosis, comparing SMN rates in the chemotherapy-only and no chemotherapy or radiation groups at different time points showed similar rates 5 to 10 years after childhood cancer diagnosis (RR, 0.9; 95% CI, 0.4 to 2.3) but a nearly two-fold higher rate in the chemotherapy-only group at 10 to 25 years (RR, 1.9; 95% CI, 1.0 to 3.5). Thirty-year estimated cumulative burden was 4.4, 9.8, 12.2, and 3.7 per 100 individuals for chemotherapy-only, chemotherapy plus radiation, radiation but no chemotherapy, and no chemotherapy or radiation treatment, respectively (Data Supplement).
FIG 1.
Cumulative incidence of second malignancy, by childhood cancer treatment. RT, radiation therapy.
Risk and Risk Factors for SMNs
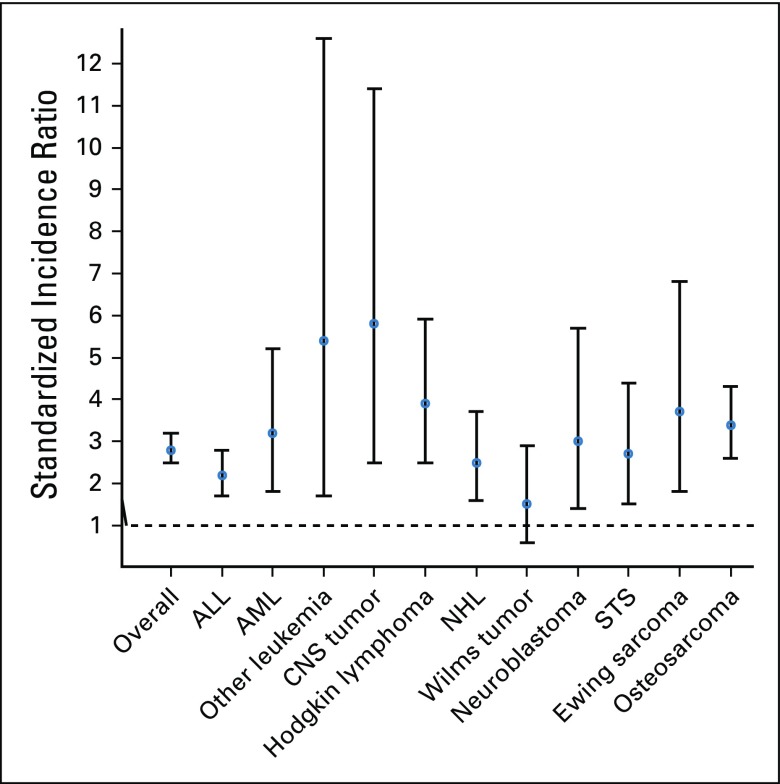
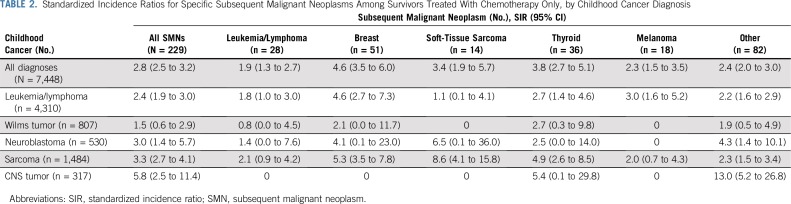
The SMN rate among survivors treated with chemotherapy only was nearly three-fold greater than that of the general population (SIR, 2.8; 95% CI, 2.5 to 3.2), and the AER per 1,000 person-years was 1.0 (95% CI, 0.8 to 1.2; Fig 2; Data Supplement). The SMN rate relative to the general population was highest among survivors of CNS tumors (SIR, 5.8), driven by subsequent, nonrecurrent CNS tumors. Rates were also elevated for survivors of sarcoma (SIR, 3.3), neuroblastoma (SIR, 3.0), and leukemia or lymphoma (SIR, 2.4; Fig 2). For survivors of sarcomas who received chemotherapy only, the SMN rate was increased and greatest for subsequent, nonrecurrent STS (SIR, 8.6), breast cancer (SIR, 5.3), and thyroid cancer (SIR, 4.9). Survivors of leukemia or lymphoma were at increased risk for subsequent breast cancer (SIR, 4.6), melanoma (SIR, 3.0), and thyroid cancer (SIR, 2.7; Table 2). Elevated SIRs for survivors not treated with chemotherapy or radiation were noted after CNS tumors (SIR, 1.7) and osteosarcoma (SIR, 2.5; Data Supplement).
FIG 2.
Standardized incidence ratios and 95% CIs for subsequent malignancy, by childhood cancer diagnosis among chemotherapy-only group. ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; NHL, Non-Hodgkin lymphoma; STS, soft-tissue sarcoma.
TABLE 2.
Standardized Incidence Ratios for Specific Subsequent Malignant Neoplasms Among Survivors Treated With Chemotherapy Only, by Childhood Cancer Diagnosis
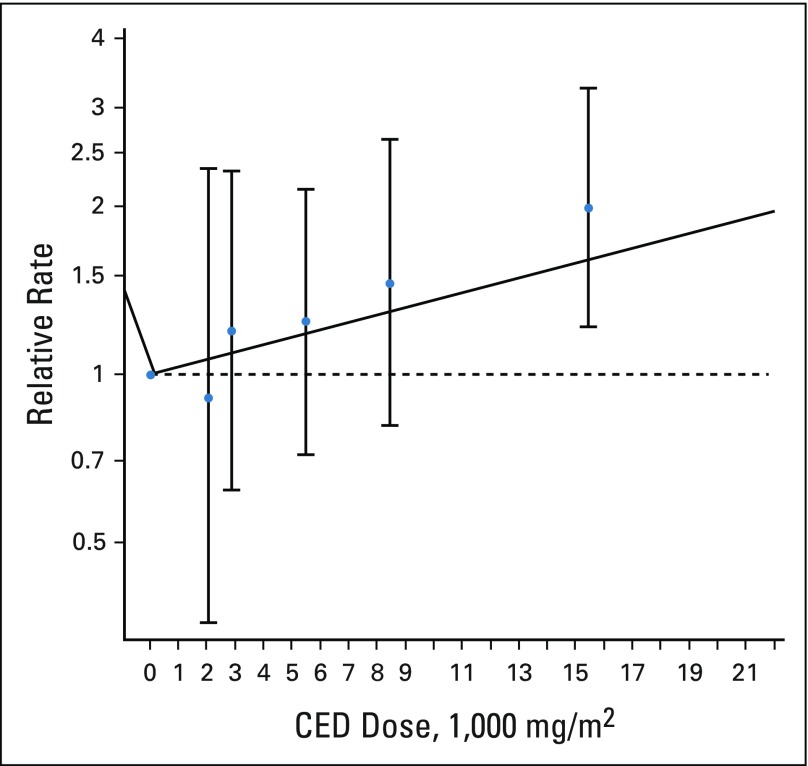
Within the group of chemotherapy-only survivors, the SMN rate was associated with female sex (RR, 1.8; 95% CI, 1.3 to 2.6), high cumulative alkylating agent exposure (CED > 10,000 mg/m2; RR v no alkylating agents, 2.0; 95% CI, 1.2 to 3.3), and high cumulative platinum exposure (> 750 mg/m2; RR v no platinum, 2.7; 95%, CI 1.1 to 6.5; Table 3). Neither anthracycline nor epipodophyllotoxin exposure at any dose level was associated with increased SMN rates among chemotherapy-only survivors. A linear dose response was found between cumulative alkylating agent exposure and the SMN rate (RR, 1.2/5,000 mg/m2; 95% CI, 1.1 to 1.3; Fig 3). No association was found between SMN rate and 5-year treatment era.
TABLE 3.
Multivariable Model for Subsequent Malignancy Relative Rates for Survivors Treated With Chemotherapy Only
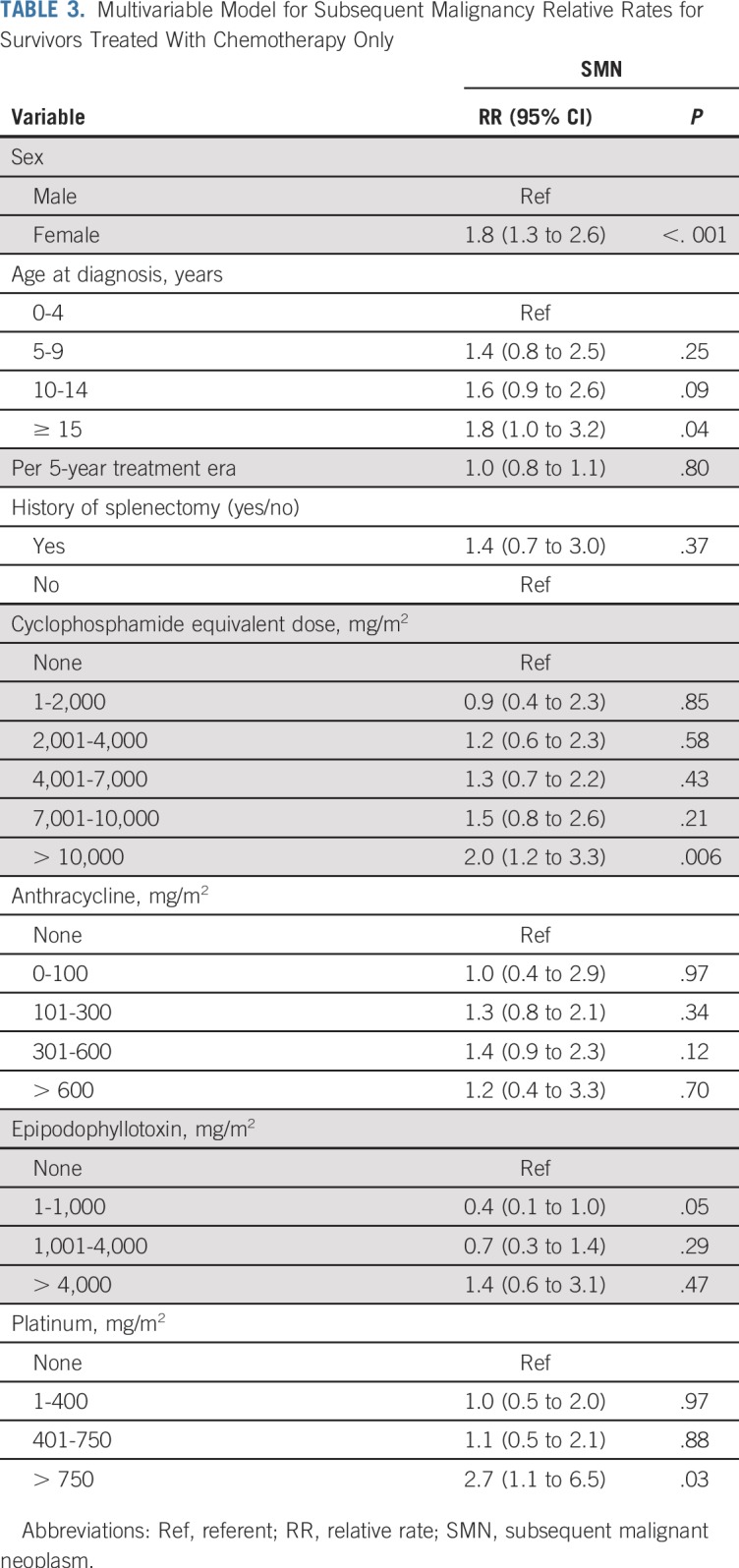
FIG 3.
Subsequent malignant neoplasm dose-response model for cumulative alkylating agent dose. Blue circles represent rate ratios from a categorical model (error bars represent 95% CI) for the median dose for the category: median (× 1,000 mg/m2) category (95% CI): 2.077 (0.1 to 2), 2.882 (2.01 to 4), 5.504 (4.01 to 7), 8.455 (7.01 to 10), and 15.451 (10.01 to 144.67). Linear model log (relative rate) = 0.153 × dose per 5,000 mg/m2 (P < .001). The horizontal dashed line corresponds to a relative rate of 1.0. CED, cyclophosphamide equivalent dose.
To explore whether chemotherapies were associated with risk of specific SMN types (ie, breast, thyroid, STS, melanoma) among chemotherapy-only survivors, multivariable analyses were also performed. Increased subsequent breast cancer rate was associated with anthracycline exposure > 300 mg/m2 and a linear dose response was seen (RR, 1.3/100 mg/m2; 95% CI, 1.2 to 1.6; P < .001; Data Supplement). The multiple comparison adjustment was applied for four chemotherapy classes and four SMN subtypes, and this association remained below the multiple comparison-corrected P value of .003. Associations were not identified with other chemotherapies. No other associations were found with specific SMN types relative to chemotherapy classes or cumulative dose (Data Supplement).
Among survivors exposed to therapeutic radiation alone or in combination with chemotherapy, SIRs and AERs were higher than those in the chemotherapy-only group. Although survivors not exposed to chemotherapy or radiation had the lowest SMN rates, they still had an increased risk (SIR, 1.8; 95% CI, 1.4 to 2.4; Data Supplement) compared with the general population. Multivariable analysis performed among survivors treated with both chemotherapy and radiation showed the SMN rate was also associated with female sex (RR, 1.6; 95% CI, 1.4 to 1.9) and high-dose alkylating agent exposure (RR, 1.4; 95% CI, 1.1 to 1.9). Platinum exposure was not associated with SMN rate in this group.
DISCUSSION
Therapeutic radiation use has declined over time because of long-term health complications, and the types and doses of prescribed chemotherapies have shifted as well. As cancer treatments have changed, there have been documented declines in SMN risk among childhood cancer survivors.3 Few studies have focused on whether chemotherapies, in the absence of therapeutic radiation, are associated with SMN risk.11,12,15 Here, in a large, well-characterized cohort of childhood cancer survivors, we demonstrated that survivors treated with chemotherapy alone are at increased risk for developing an SMN compared with the general population, though risk and cumulative incidence were approximately half of what was observed in survivors exposed to radiation plus chemotherapy. We also showed that survivors treated with higher cumulative doses of alkylating agents and/or platinum-based drugs experienced increased rates of SMNs, and there is a linear dose-response relationship between alkylating agent cumulative dose and SMN relative rate. We built on previous findings that breast cancer rates are associated with anthracycline exposure,11,15 identifying a linear dose-response relationship among chemotherapy-only survivors. Despite decreased SMN risk over time for the overall survivor population,3 an association between 5-year treatment era and SMN rates in patients treated with chemotherapy only was not identified.
The increased SMN rates associated with high-dose alkylating agent and platinum exposure identified in this study are unique from previous work in that few studies have looked at survivors treated with chemotherapy alone. A report from the Dutch Childhood Cancer Oncology Group-Long-Term Effects After Childhood Cancer (DCOG-LATER) cohort described chemotherapy-associated solid SMN risk.11 Solid tumor, and specifically breast cancer, risk was associated with anthracycline exposure, and consistent with our findings, the association between breast cancer risk and anthracyclines was maintained among women not exposed to chest or total body irradiation. They also reported an association between cyclophosphamide exposure and sarcoma risk; however, this association was not evident when only nonirradiated survivors were considered. Overall solid SMN risk in chemotherapy-only survivors was not reported.11 A SEER analysis of survivors of testicular nonseminomas treated with platinum-based chemotherapy alone showed an increased risk for solid SMNs, including cancers of the thyroid, kidney, and soft tissue.16
The association identified here between higher cumulative dose of anthracycline and breast cancer rates in chemotherapy-only survivors validates previous work from the aforementioned DCOG-LATER cohort and from the CCSS.11,15 Henderson et al15 examined subsequent breast cancer risk among female childhood cancer survivors not treated with chest radiation and found a four-fold increased risk for breast cancer compared with the general population, similar to findings in this study; risk was associated with dose-dependent alkylating agent and anthracycline exposure. The anthracycline association is consistent with what is reported here; however, we did not replicate the alkylating agent findings. The absence of this finding in the current analysis is likely multifactorial. The previous analysis included only CCSS participants diagnosed between 1970 and 1986 and included individuals who had been exposed to radiation outside of the chest field, including radiation to the pelvis, which is established to modify breast cancer risk, thus representing a different population from what is presented here.
Within the CCSS, a recent analysis identified increased SMN risk associated with both moderate- to high-dose alkylating agent and platinum exposure.3 In the present analysis, the association with alkylating agents was evident with and without radiation exposure, whereas the association with platinum was only among the chemotherapy-only subgroup of survivors. It is possible that the platinum association within our study is a proxy for the high proportion of Li Fraumeni–associated malignancies, because many treatment regimens for CNS tumors and sarcomas include platinum therapy. The CCSS has also reported associations between high-dose procarbazine and platinum exposure and GI SMNs12; however, within the previous analysis and ours, these associations were not seen among nonirradiated survivors, likely due to small numbers and limited power. Other significant associations between chemotherapy and sarcoma and renal SMNs have been identified in CCSS studies, but all have been among irradiated survivors.9,10,28 In aggregate, these studies affirm the importance of more detailed investigation of chemotherapeutic risk factors for the development of SMNs, particularly as childhood cancer therapies evolve.
This analysis has important limitations. SMNs occurring < 5 years from diagnosis are excluded, thus reducing identification of epipodophyllotoxin-associated early leukemias. The CCSS relies on initial self or proxy report of SMNs, which may lead to underestimation of SMN incidence and risk. Only treatments received within 5 years of childhood cancer diagnosis are included and radiation exposure from routine imaging studies is not addressed. In addition, the CCSS does not include survivors treated with novel chemotherapeutic agents introduced after 1999, which may affect future risk for SMNs. It is essential for studies to evaluate these risks as well as those of specific multimodal therapy combinations. Although we identified significant associations between alkylating and platinum agents and SMN risk, the sample size for individual chemotherapeutic agent exposures or combinations of agents limited our power to identify more specific associations, and small numbers of many SMN types limited our ability to evaluate associations between rates of specific SMNs and chemotherapy exposures. We identified a difference in SMN rates between individuals treated with chemotherapy only and individuals not treated with chemotherapy or radiation; however, given the limited number of SMNs in the no-treatment group (Data Supplement), it is difficult to address the clinical relevance of this difference. It is possible that surveillance bias was present among those exposed to chemotherapy, making them more likely to undergo cancer screenings, potentially leading to increased rates of identified SMNs. This may be evident in the time beyond 35 years from diagnosis, when the cumulative incidence curves for the chemotherapy-only and nontreated individuals diverge (cohort characteristics at 35 years are shown in the Data Supplement). Surveillance and screening practices are at the discretion of the survivor and their medical providers and likely vary by primary cancer diagnosis and treatment type. Collaboration with other large cohorts of childhood cancer survivors would increase the likelihood of identifying associations between chemotherapy agents and specific SMN types and identifying whether clinically important differences in SMN risk between chemotherapy-treated and nontreated survivors exist.
We were also unable to assess genetic cancer predisposition, which partially contributes to SMN risk29 across all survivor treatment groups. Although this study supports a role for chemotherapy in increasing SMN risk, SMN risk also could be attributable to genetic cancer susceptibility. Within the chemotherapy-only group, 28% of SMNs occurred in survivors of osteosarcoma, and the most frequent SMNs included breast cancers, thyroid cancers, leukemia/lymphomas, melanomas, and STSs, which may be consistent with Li Fraumeni syndrome.30,31 Similarly, for survivors with primary CNS tumors, increased risk for CNS SMNs may be attributable to a subpopulation of survivors with neurofibromatosis, type 1.
In summary, within the CCSS cohort, we showed that survivors exposed to chemotherapy only are at increased risk for SMNs and that these elevated risks are associated with higher-dose alkylating agent or platinum exposure. We also showed that anthracycline exposure is associated with subsequent breast cancer rates in a linear dose-response relationship. These findings inform risk-based counseling and support the need for surveillance for early detection of SMNs among individuals treated with chemotherapy, particularly higher cumulative doses of alkylating agents and platinum and no radiotherapy. Additional studies are needed to fully inform chemotherapy dose thresholds for surveillance recommendations. Important next steps include additional evaluation of genetic susceptibility for SMN development, as well as collaboration among childhood cancer survivor cohorts to elucidate whether individual chemotherapeutic exposures increase SMN risk and to better understand SMN types that are increased with individual or combination chemotherapy exposures.
Footnotes
Portions of this study were presented as an oral abstract at the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018.
Supported by the National Cancer Institute (Grant No. CA55727 [G.T.A.]; Grant No. CA234232 [L.M.T.]). Support to St Jude Children’s Research Hospital was provided by the Cancer Center Support (Grant No. CA21765) and the American Lebanese-Syrian Associated Charities.
The study sponsors had no role in the design or conduct of the study.
AUTHOR CONTRIBUTIONS
Conception and design: Lucie M. Turcotte, Tara O. Henderson, Wendy Leisenring, Gregory T. Armstrong, Leslie L. Robison, Joseph P. Neglia
Financial support: Gregory T. Armstrong, Leslie L. Robison, Joseph P. Neglia
Administrative support: Wendy Leisenring, Leslie L. Robison, Joseph P. Neglia
Provision of study material or patients: Gregory T. Armstrong, Leslie L. Robison, Joseph P. Neglia
Collection and assembly of data: Lucie M. Turcotte, Michael A. Arnold, Gregory T. Armstrong, Rebecca M. Howell, Leslie L. Robison, Joseph P. Neglia
Data analysis and interpretation: Lucie M. Turcotte, Qi Liu, Yutaka Yasui, Todd M. Gibson, Wendy Leisenring, Michael A. Arnold, Daniel M. Green
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chemotherapy and Risk of Subsequent Malignant Neoplasms in the Childhood Cancer Survivor Study Cohort
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tara O. Henderson
Research Funding: Seattle Genetics
Other Relationship: Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2319. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 3.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA. 2017;317:814–824. doi: 10.1001/jama.2017.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60:1083–1094. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins MM, Wilson LM, Stovall MA, et al. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ. 1992;304:951–958. doi: 10.1136/bmj.304.6832.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pui CH, Ribeiro RC, Hancock ML, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325:1682–1687. doi: 10.1056/NEJM199112123252402. [DOI] [PubMed] [Google Scholar]
- 9.Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2012;84:224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teepen JC, van Leeuwen FE, Tissing WJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER Study cohort: Role of chemotherapy. J Clin Oncol. 2017;35:2288–2298. doi: 10.1200/JCO.2016.71.6902. [DOI] [PubMed] [Google Scholar]
- 12. doi: 10.1059/0003-4819-156-11-201206050-00002. Henderson TO, Oeffinger KC, Whitton J, et al: Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann Intern Med 156:757-66, W-260, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nottage K, McFarlane J, Krasin MJ, et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;30:2552–2558. doi: 10.1200/JCO.2011.37.8760. [DOI] [PubMed] [Google Scholar]
- 14.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 15.Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34:910–918. doi: 10.1200/JCO.2015.62.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung C, Fossa SD, Milano MT, et al. Solid tumors after chemotherapy or surgery for testicular nonseminoma: A population-based study. J Clin Oncol. 2013;31:3807–3814. doi: 10.1200/JCO.2013.50.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339(dec08 1):b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 22. doi: 10.1016/0305-7372(85)90019-2. Ozols RF, Behrens BC, Ostchega Y, et al: High dose cisplatin and high dose carboplatin in refractory ovarian cancer. Cancer Treat Rev 12:59-65, 1985 (Suppl A) [DOI] [PubMed] [Google Scholar]
- 23.Fritz AG. International Classification of Diseases for Oncology: ICD-O. Geneva, Switzerland, : World Health Organization; 2000. [Google Scholar]
- 24.Dong H, Robison LL, Leisenring WM, et al. Estimating the burden of recurrent events in the presence of competing risks: The method of mean cumulative count. Am J Epidemiol. 2015;181:532–540. doi: 10.1093/aje/kwu289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RJ. A class of $K$-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 26. National Cancer Institute: Previous version: SEER Cancer Statistics Review, 1975-2012. https://seer.cancer.gov/archive/csr/1975_2012/
- 27.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 28.Wilson CL, Ness KK, Neglia JP, et al. Renal carcinoma after childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2013;105:504–508. doi: 10.1093/jnci/djt014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Wilson CL, Easton J, et al. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2018;36:2078–2087. doi: 10.1200/JCO.2018.77.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amadou A, Waddington Achatz MI, Hainaut P. Revisiting tumor patterns and penetrance in germline TP53 mutation carriers: Temporal phases of Li-Fraumeni syndrome. Curr Opin Oncol. 2018;30:23–29. doi: 10.1097/CCO.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 31.Valdez JM, Nichols KE, Kesserwan C. Li-Fraumeni syndrome: A paradigm for the understanding of hereditary cancer predisposition. Br J Haematol. 2017;176:539–552. doi: 10.1111/bjh.14461. [DOI] [PubMed] [Google Scholar]