Abstract
Two major questions about addictive behaviors need to be explained by any worthwhile neurobiological theory. First, why do people seek drugs in the first place? Second, why do some people who use drugs seem to eventually become unable to resist drug temptation and so become “addicted”? We will review the theories of addiction that address negative-reinforcement views of drug use (i.e., taking opioids to alleviate distress or withdrawal), positive-reinforcement views (i.e., taking drugs for euphoria), habit views (i.e., growth of automatic drug-use routines), incentive-sensitization views (i.e., growth of excessive “wanting” to take drugs as a result of dopamine-related sensitization), and cognitive-dysfunction views (i.e., impaired prefrontal top-down control), including those involving competing neurobehavioral decision systems (CNDS), and the role of the insula in modulating addictive drug craving. In the special case of opioids, particular attention is paid to whether their analgesic effects overlap with their reinforcing effects and whether the perceived low risk of taking legal medicinal opioids, which are often prescribed by a health professional, could play a role in the decision to use. Specifically, we will address the issue of predisposition or vulnerability to becoming addicted to drugs (i.e., the question of why some people who experiment with drugs develop an addiction, while others do not). Finally, we review attempts to develop novel therapeutic strategies and policy ideas that could help prevent opioid and other substance abuse.
Keywords: opioid abuse, decision making, incentive sensitization, insula
People use psychoactive drugs for many different reasons. Some may use alcohol or opioids for relief of boredom, stress, anxiety, or pain. Others may use drugs for more positive types of pleasure associated with feelings of relaxation, visceral sensations that they find intensely gratifying, or “euphoria.” Others may use stimulants, such as amphetamines, to achieve heightened alertness and endurance. Still others may use drugs, at least initially, because of social group pressure to conform. One thing that all these motives have in common is that the use of drugs somehow achieves a purpose for the user, whether by producing pleasure, by relieving displeasure, or by winning approval from peers. However, in most of these instances, the repeated use of these drugs could lead to several types of negative consequences, including physical, psychological, or social consequences. Most users have the “willpower” or the decision-making capacity to moderate their frequency of use to avoid these negative consequences, regardless of the reward that these drugs may bring in the short term. (We do not use the term willpower to refer to a dimension of morality; see below.) Although the use of psychoactive drugs has occurred in almost every society in human history, and many drug users have needed help, the scale of the more recent wave of heroin and other opioid fatalities is unprecedented. In recent years, more Americans have died of overdoses than from car accidents (Seth, Scholl, Rudd, & Bacon, 2018). Hence, understanding the neurobiological mechanisms underlying these drug-seeking behaviors is vital; it is necessary to find evidence-based recommendations for educational and policy campaigns that could help prevent opioid use in particular and substance abuse in general.
To address this need, here we (a) provide an overview of the different theories of addiction that have been proposed over the years; (b) address the topic of opioid analgesia versus reinforcement/reward and the potential biological mechanisms that lead to opioid addiction; (c) examine the role of willpower and decision making, with reference to the role of the prefrontal cortex in resisting the lure of drug reward, as well as the role of the insula in mediating the urge or craving to take drugs; (d) discuss mechanisms and therapeutic opportunities inspired from the field of neuroeconomics to treat or prevent opioid misuse in particular and substance abuse in general.
Addiction Theories
While reasons for initial use of drugs are diverse, only relatively few of those who take drugs recreationally go on to become addicted, in the sense of persistent and potentially near-compulsive levels of craving. After successfully giving up drugs for months or even years, these individuals may remain vulnerable to relapse, even if strongly motivated to remain drug-free. Therefore, theoretical explanations have been offered for why drug use becomes so compelling and hard for some individuals to resist in addiction.
These addiction theories fall into several major categories (see also Bickel et al., 2018):
Traditional pleasure and withdrawal explanations of addiction emphasize the euphoria of drugs, or the opposite need to hedonically self-medicate the unpleasantness of withdrawal or other life stresses.
Habit explanations emphasize repetition and automaticity of drug use, traditionally as a product of basic stimulus–response (S-R) learning, and the emergence of drug-use routines often performed without reflection.
Incentive-sensitization explanations emphasize the excessive intensity of addictive cravings in those who remain vulnerable to relapse, even after withdrawal ends, as a result of long-term changes caused by drugs, specifically to brain mesolimbic “wanting” systems.
Cognitive-dysregulation explanations emphasize loss of self-control as a result of damage or disruption in cortical systems of cognitive control and the resulting imbalance between top-down control and bottom-up impulses.
Each explanation has adherents in psychology and neuroscience, and all may apply to a degree. Yet some may be better than others for explaining why addictive behaviors become so difficult to break for so many.
Opponent-process/pleasure-withdrawal/allostasis model of addiction
Drugs of abuse are typically pleasant, and the most commonly held explanation for addiction is simply the traditional view that drugs are first taken for pleasure or to gain peer approval, and the behavior is maintained by the pleasant experiences. Traditionally, psychologists called this a positive-reinforcement explanation. Over time, however, unpleasant withdrawal experiences and other bad consequences begin to dominate the affective life of addicts, and they begin to take drugs for the additional reason of reducing the unpleasantness of those experiences. This has been called a negative-reinforcement explanation, as an act performed to escape an unpleasant stimulus.
An elegant motivational theory that captured and built on this pleasure/withdrawal view is the opponent-process theory, originated by the psychologist Richard Solomon (Solomon & Corbit, 1973, 1974). This theory has been extended to potential brain mechanisms by addiction neuroscientists (George, Le Moal, & Koob, 2012; Koob & Le Moal, 1997; Koob & Volkow, 2010; Volkow, Koob, & McLellan, 2016). These opponent-process theories have also sometimes been called allostasis, hedonic homeostasis, or hedonic-dysregulation models of addiction.
In all opponent-process theories, a drug first activates a dose-dependent pleasurable A process in brain reward circuits. This is experienced initially as a pure “A state,” or peak euphoria, unopposed by any other process. The neural basis of the A process was originally thought to involve stimulation of mesocorticolimbic dopamine release (Koob & Le Moal, 1997), although later evidence indicated that pleasure generation may be restricted to other neural candidates, such as stimulation of μ opioid or δ opioid, endocannabinoid, or orexin receptors stimulation, and a few other neuronal events in hedonic hotspots of limbic structures specialized for generating pleasure (Berridge & Kringelbach, 2015; George et al., 2012).
However, drug activation of a pleasant A process itself triggers in turn counteractivation of an unpleasant B process, which opposes the A process. In the person’s experience, the B process is essentially subtracted from the A process, so that the A state diminishes, producing tolerance. With repeated drug use, the aversive B process gradually strengthens further. However, the A process either remains unchanged, according to the original opponent-process theory (Solomon & Corbit, 1974), or the A process declines with tolerance, according to later neuroscience versions (George et al., 2012; Koob & Le Moal, 1997; Volkow et al., 2016). Whether the A process declines or remains unchanged, the B process is posited by all opponent-process theories to grow disproportionately to the A process. So if drug use continues, the B process continues to grow, while the A process does not, until the B process dwarfs the A process. Further, the strengthened B process may persist for hours or days after the drug wears off and the A process fades. The persistence of the B process after the drug, no longer opposed by any A process, produces withdrawal syndromes as an unpleasant opponent “B state.”
Neuroscience versions of the opponent-process theory originally assigned the reduction of the A process to down-regulation of neuronal dopamine D2 receptors (George et al., 2012; Koob & Le Moal, 1997; Koob & Volkow, 2010). D2 receptors are one of the two major families of dopamine receptors, and the one most readily measured in neuroimaging studies. Their down-regulation refers to a gradual loss in the number of D2 receptors in nucleus accumbens or ventral striatum, dorsal striatum that lies above the nucleus accumbens, and other reward structures produced as a compensatory consequence of being bombarded by the high levels of dopamine that are released by drugs and is thought to be a mechanism of tolerance (George et al., 2012; Koob & Le Moal, 1997; Koob & Volkow, 2010). Conversely, growth of the unpleasant B process has been suggested by the same models to be due to release of corticotropin-releasing factor (CRF), a major stress neurotransmitter, in the amygdala and related brain structures (George et al., 2012; Koob & Le Moal, 1997). κ opioid receptor stimulation in nucleus accumbens is another neural candidate that has been suggested recently to mediate distress-related drug use (Massaly, Moron, & Al-Hasani, 2016), given that κ opioid receptor stimulation can be aversive in many brain sites.
However, if an addict becomes drug abstinent, the B process gradually begins to decay as long as drugs are not taken again (Solomon & Corbit, 1974). Eventually, over weeks or months, the withdrawal symptoms and drug tolerance gradually disappear. In terms of therapy implications, the opponent-process theory requires an addict to go completely “cold turkey” and give up drugs completely in order for the B process to disappear completely. But, on the bright side, it implies that once the B process has returned to normal, the person would no longer be addicted.
The strength of opponent-process/pleasure-withdrawal theories is that they recognize withdrawal and distress to be important reasons why people often take drugs. However, a problem for these withdrawal-based views has been the recognition that neither addiction nor vulnerability to relapse of drug use go away even after withdrawal symptoms and tolerance have disappeared (Lewis, 2011). Vulnerability to relapse often persists for many months or years afterward for recovering addicts. This requires a major effort by the still-recovering addict to successfully abstain from drugs for a prolonged period. This vulnerability also requires a different theoretical explanation to account for why addiction so often persists in the absence of withdrawal or other major distress.
S-R habit and reinforcement theories of addiction
Habit explanations for addiction have been offered by psychologists for more than a century (James, 1890). Habits were traditionally viewed as sequences of action that become automatic after repeated practice and were able to be performed without voluntary attention and sometimes even triggered unknowingly during momentary inattention, which became established through a form of S-R learning in which drugs “stamped in” S-R habits.
The detachment of S-R habits from attention was stressed by William James: “habit diminishes the conscious attention with which our acts are performed” (p. 114). James proposed that habits are formed by learning linked S-R associations that compose a chain of sequential action:
habit soon brings it about that each event calls up its own appropriate successor without any alternative offering itself, and without any reference to the conscious will, until at past the whole chain, A, B, C, D, E, F, G, rattles itself off as soon as A occurs.
(p. 114)
Being detached from attention, habits were thought by early psychologists to surface especially at moments of inattention, such as when people are distracted or thinking of something else. As James described,
Very absent-minded persons in going to their bedroom to dress for dinner have been known to take off one garment after another and finally to get into bed, merely because that was the habitual issue of the first few movements when performed at a later hour.
(p. 115)
Habit theories became psychologists’ preferred explanation of addiction during the behaviorist decades of 1900 through the 1950s, keeping to features of action automaticity and freedom from needs for attention or voluntary control. Addictive S-R habits were therefore viewed as especially likely to surface as automatic chains of action during attentional lapses. For example, the behaviorist psychologist Edwin Guthrie (1935) described the habit account of smoking relapse: “We resolve to stop smoking. … We suddenly find ourselves smoking. Some cue which we had not alienated has taken us unawares and had its usual response” (p. 102).
Some modern neuroscience theories of addictive habits have proposed neural substrates to account for why habits become automatic and relatively detached from consequences. One suggests that the neural control of drug use shifts dorsally in the brain from reward circuitry in nucleus accumbens to motor and habit circuitry in neostriatum (Everitt & Robbins, 2005, 2016). This suggestion is based in part on anatomical evidence for ascending neural loops that convey signals from nucleus accumbens to midbrain and eventually to the top layer of dorsal neostriatum (Haber, Fudge, & McFarland, 2000). The dorsal neostriatum (especially the lateral half) is thought especially to control movements, movement sequences, and learned habits (Everitt et al., 2008; Smith & Graybiel, 2016). Their habit hypothesis suggests that neural migration of control from nucleus accumbens to dorsolateral neostriatum makes drug use habitual, automatic, and resistant to its consequences.
The strength of S-R habit theories of addiction is that they can readily explain the automaticity of drug-use rituals and the ability of drug-use routines to play out on a daily basis without need of attention and without requiring self-reflection or any thinking about goals. Habit theories are also excellent for explaining how drug-use behaviors might surface during inattention lapses or at moments of distraction. However, habit theories run into difficulties when faced with motivationally compulsive features of addiction or the emergence of new behaviors or flexible strategies when needed to obtain drugs. Another problem concerns attention: Attending to a habit can usually help suppress or alter the undesired habit, but attending directly to an addictive craving is unlikely to help resist it and may instead exacerbate the urge to take drugs. Even focusing the attention of a recovering addict on a competing desire to abstain may not be very effective in resisting relapse (Gollwitzer, 2014; Köpetz, Lejuez, Wiers, & Kruglanski, 2013). That is, addiction typically is a problem of controlling powerful motivational urges, not merely a matter of automatic habitual responding that surfaces during inattention.
Some habit accounts do recognize the motivational power of drug addiction. For example, recent versions of the neostriatum-habit theory add the postulate that migration to dorsal striatum control gives habitual actions a “must do!” quality (Everitt & Robbins, 2005, 2016), which essentially adds a strong motivational component to a habit. That motivational component in turn requires explanation by one of the motivational theories of addiction to account for why the habitual action “must!” be done. Evidence indicates that the dorsal striatum also participates in motivational functions, including the magnification of reward incentive salience or “wanting,” in addition to movement and habit functions. Recognition that motivation adds a “must!” urge to addictive action shifts the major explanatory burden back to identifying the nature of that motivational function and understanding how it becomes magnified by addiction.
Incentive-sensitization theory: excessive motivational “wanting”
The incentive-sensitization theory of addiction posits that “wanting” to take drugs, especially when triggered by drug cues, becomes excessive in addicts because of drug-induced changes in brain reward circuitry (Berridge & Robinson, 2016; Robinson & Berridge, 1993, 2003). Conversely, “liking” for the drug need not grow proportionally and may even decline with tolerance. The intensity of cue-triggered “wanting” to take drugs becomes especially exacerbated during emotional states that heighten mesocorticolimbic reactivity to drug cues. Finally, the brain changes underlying sensitization of wanting remain long after drug tolerance and withdrawal have disappeared, whether or not the drug is still liked very much, and even if a recovering addict has been completely drug abstinent for a long time. The incentive-sensitization theory is not intended to explain drug use that is motivated simply to reduce withdrawal or other distress. Rather, this motivational theory specifically explains why addictive cravings with a compulsion-like intensity can emerge in some drug users and continue to cause vulnerability to relapse during drug-abstinent recovery long after withdrawal symptoms end.
The incentive-sensitization theory originated out of two discoveries (Berridge & Robinson, 2016; Robinson & Berridge, 1993, 2003): First, brain mesocorticolimbic dopamine systems do not actually mediate the pleasure or “liking” of rewards; rather, they mediate only the psychological function of incentive salience or “wanting” those rewards that is often triggered by related learned cues. Second, addictive drugs (cocaine, amphetamine, heroin, alcohol, nicotine, etc.) produce permanent neural incentive-sensitization or cue-triggered hyperreactivity in the mesocorticolimbic dopamine systems of vulnerable individuals.
The psychological form of wanting—or incentive salience mediated by dopamine-related mesocorticolimbic systems, which become sensitized in addiction—is different from the ordinary sense of wanting as a cognitive desire, which is mediated by more cortically weighted brain systems (Berridge & Robinson, 2016; Robinson & Berridge, 1993, 2003). Incentive salience is tightly linked to triggering percepts, reward cues, and vivid imagery. In some situations, incentive salience can detach from conscious declarative goals. Thus an individual suffering from addiction can intensely want to take drugs, even while cognitively wanting to abstain or after recognizing that the drug will not produce much pleasure. Incentive salience attribution makes drug cues attention grabbing and attractive, and it triggers urges to seek and consume the drug. The intensity of a cue-triggered urge can vary depending on the individual’s state of the moment, including states of stress, emotional excitement, relevant appetites, or intoxication because those states modulate the reactivity of mesocorticolimbic brain systems to particular reward cues (Berridge, 2012). State-dependent amplification of incentive salience is a reason why recovering addicts can be suddenly surprised and overwhelmed by the higher intensity of a cue-triggered urge to take drugs, even if they have often successfully resisted the same cues in the past. State-dependent amplification is also a mechanism that makes it so hard for a recovering addict to take “just one” drink or hit and then stop. This state-dependent process is also a reason why emotional stresses—even happy ones such as a promotion—can promote vulnerability to cue-triggered wanting and to relapse in addiction.
Incentive sensitization produces mesocorticolimbic hyperreactivity that causes drug cues to trigger higher pulses of dopamine signals in nucleus accumbens and neostriatum, which interact with corticolimbic glutamate signals to generate more intense motivational urges (Leyton & Vezina, 2014; Paulson & Robinson, 1995; Steketee & Kalivas, 2011; Wolf, 2016). Mesocorticolimbic sensitization changes neuronal functions in several structures in the mesocorticolimbic circuit: ventral tegmentum, nucleus accumbens and neostriatum, and limbic regions of cortex, which all combine to make the circuitry hyperreactive to drug cues. Drug-induced sensitization also can change the anatomical structure of neurons in these brain structures, such as either increasing or decreasing (depending on type of drug) the density of spines on dendrites of neurons in nucleus accumbens or prefrontal cortex, which act as receiving antennae for incoming neurotransmitter signals (Robinson & Kolb, 2004).
Neural sensitization involves brain changes that are essentially the functional opposite of drug tolerance but proceed through different molecular mechanisms, even inside some of the same cells. A sensitized dopamine-related system is not hyperactive constantly; rather, it is momentarily hyperreactive to particular cues and events (Berridge & Robinson, 2016; Robinson & Berridge, 1993, 2003). Sensitization and tolerance can happen together in mesocorticolimbic dopamine systems because they are mediated by independent sets of molecular events in neurons (Leyton & Vezina, 2013; Steketee & Kalivas, 2011; Wolf, 2010). In the short run, tolerance and withdrawal may mask sensitization, but if drug use stops, mesocorticolimbic sensitization remains after tolerance and withdrawal have faded (Boileau et al., 2006; Paulson & Robinson, 1995). Consequently, sensitized wanting does not diminish when drug use stops. Instead, if anything, it grows stronger over weeks or months of abstinence, which has been called incubation of craving (Lu, Grimm, Hope, & Shaham, 2004).
Incentive-sensitization theory has been extended in recent years beyond drugs to a variety of behavioral addictions, such as food addiction, gambling addiction, sex addiction, and internet addiction (Davis & Carter, 2009; Gearhardt et al., 2011; Hartston, 2012; Linnet et al., 2012; Ray et al., 2012; Voon, Mole, et al., 2014). These applications to behavioral addictions are justified partly by evidence from neuroimaging studies that individuals with these conditions exhibit brain signatures of incentive sensitization, such as mesocorticolimbic hyperreactivity elicited specifically by their addictive reward cues. The possibility is further supported by evidence, including from animal studies, that neural sensitization of mesocorticolimbic dopamine-related systems can be induced without drugs in some individuals. Finally, additional evidence that supports a common incentive-sensitization bridge between dopamine-related mechanisms of drug addiction and behavioral addictions are reports that compulsive motivations for gambling, shopping, sex, and so forth have been induced in some medicated patients with Parkinson’s by high doses of “direct agonist” medications that directly stimulate dopamine receptors (Callesen, Scheel-Kruger, Kringelbach, & Moller, 2013; Friedman & Chang, 2013; Maloney, Djamshidian, & O’Sullivan, 2017; Ondo & Lai, 2008; Politis et al., 2013; Wu, Politis, et al., 2014).
The Special Case of Opioids: Their Analgesic and Reinforcing Properties
Opioids are any drugs that act on any of the different types of opioid receptors (i.e., μ opioid, δ opioid, or κ opioid receptors) throughout the brain. These receptors are the target of endogenous opioids—typically endorphins, dynorphins, and enkephalins. The most well-known class of endogenous opioids is endorphins; they are released during exercise, excitement, orgasm, and pain (Koneru, Satyanarayana, & Rizwan, 2009). Exogenous opioid drugs, such as heroin, morphine, fentanyl, and oxycodone, bind to opioid receptors, especially μ opioid receptors, and produce intense feelings of euphoria. While the analgesic properties of opioids are linked to pain pathways in the brain, their addictive properties—including subjective pleasure—are attributed to their effects that lead to an increase in mesocorticolimbic dopamine (Herz, 1997; Tanda, Pontieri, & Di Chiara, 1997).
More specifically, opioids bind to μ opioid receptors, which inhibit γ-aminobutyric acid (GABA)-producing neurons that normally suppress dopaminergic cell firing in the ventral tegmental area. This inhibition of GABA neurons leads to an increase in the firing rate of these now disinhibited dopaminergic cells in the ventral tegmental area (Wise, 1996). The increased dopaminergic neuron firing in the ventral tegmental area directly increases dopamine release in the nucleus accumbens (Spanagel, Herz, & Shippenberg, 1992). Receptor types other than μ opioid receptors do not demonstrate this same effect on mesocorticolimbic dopamine: In fact, some evidence indicates that κ opioid receptors (KOPRs) may act in opposition to μ opioid receptors via down-regulation of dopamine production at many sites in the nucleus accumbens (Spanagel et al., 1992; but, for one nucleus accumbens site at which stimulation of κ opioid receptors can have rewarding effects similar to those of stimulation of μ opioid receptors, see Berridge, 2012; Berridge & Kringelbach, 2015). Besides differences in dopaminergic output, these receptors also produce different feelings and subjective experiences. Opioid-receptor activation also occurs directly on neurons in nucleus accumbens to cause increases in liking, as well as wanting feelings. People report feelings of euphoria when δ opioid and μ opioid receptors are activated but feelings of dysphoria when κ opioid receptors are activated (Spanagel et al., 1992). For people who are addicted to opioids, environmental cues can trigger craving similar to that triggered by stimulants such as cocaine and amphetamine (Sell et al., 2000). Functional neuroimaging studies reveal that when current heroin users are shown drug-related cues, there is increased blood flow to mesocorticolimbic brain regions that are the location of dopaminergic signaling; this indicates that the brain is preemptively expecting and desiring to receive heroin and could be a major factor in relapse (Sell et al., 2000). In short, although opioids have their own pharmacological systems and exert their own pharmacological effects, including potent increases in liking, they share with other drugs of abuse a common denominator: the mesocorticolimbic dopamine system (i.e., the wanting or incentive salience that becomes sensitized in addiction).
Use of opioids for pain treatment
Opioid analgesics constitute a wide range of medicinal products that typically share the ability to relieve acute severe pain through their action on the μ opioid receptor—the major analgesic opioid receptor expressed throughout the nervous system. The number of opioid analgesics has progressively increased, although they differ in their chemical composition, route of administration, uptake, distribution, type/rate of elimination, and ability to bind to opioid receptors. Certain of these drugs have ultrashort durations of action uniquely suited to providing analgesia as a component of a balanced surgical anesthetic. Others have very long durations of action, either because of the intrinsic properties of the opioid molecule or because of the pharmaceutical formulation, releasing the drug at a predictable rate into a patient’s body. An additional feature of these medications contributing to their clinical utility is the availability of oral, intravenous, transdermal, intranasal, epidural, and intrathecal preparations.
Opioids have long been used successfully to treat acute postsurgical and postprocedural pain. Opioids have been found to be more effective than placebo for nociceptive pain (response to a specific stimulus, such as pain from extreme temperature, inflammation, chemicals, or physical injury) and neuropathic pain (pain that is not due to a specific stimulus, which indicates that the nervous system is not working properly) of less than 16 weeks’ duration (Furlan, Chaparro, Irvin, & Mailis-Gagnon, 2011). Although evidence exists for the use of opioids for the treatment of acute and subacute pain, evidence of efficacy for chronic, long-standing pain is very limited (Chou et al., 2015; Dowell, Haegerich, & Chou, 2016). The few randomized controlled trials showing opioid efficacy have small numbers and rarely have data that extend past 3 months, the length of time after which pain is considered to be chronic. The average pain reduction ascribed to long-term use of opioids has been found to be approximately 30% (Kalso, Edwards, Moore, & McQuay, 2004). Data on functional improvement is limited. A Danish epidemiological study evaluating the effects of long-term (> 6 months) opioids in more 10,000 patients with chronic noncancer pain failed to show improvement in any of the items in the Short Form Health Survey (SF)-36 (Tarlov et al., 1989) scoring of health-related quality of life, relative to time zero and to people who do not use opioids for pain (Eriksen, Sjøgren, Bruera, Ekholm, & Rasmussen, 2006). Some evidence suggests that return to work is more often delayed than expedited for patients receiving opioids for the long term (Von Korff, 2013). Today, despite the existence of a number of opioid compounds and formulation, no evidence exists that suggests one opioid analgesic is superior to another in its ability to manage acute or chronic pain.
Patients who have been on opioids longer than 90 days are at risk of continuing on opioids chronically; this also increases the likelihood of developing a substance-use disorder (Krashin, Murinova, & Sullivan, 2016). In addition to substance-use disorder, morbidity related to opioid therapy for chronic pain includes reduced testosterone, cardiac abnormalities, fractures, and immunosuppression, among other adverse outcomes (Chou et al., 2015). A 2015 systematic review of opioid misuse in chronic pain estimated the prevalence of opioid misuse (i.e., use of opioids in ways other than prescribed despite adverse effects) in the United States to be between 21.7% and 29.3% and the prevalence of addiction or continued use despite harm to be between 7.8% and 11.7% (Vowles et al., 2015). In the elderly and other patients with higher risk of cognitive impairment, opioids may result in further impairment in cognition and executive function (Schiltenwolf et al., 2014). Overdose from these drugs is a risk factor that results from opioid-induced respiratory depression (Chou et al., 2015).
Of the many consequences of long-term opioid use, tolerance and opioid-induced hyperalgesia (OIH; increased sensitivity to pain) are commonly cited as reasons for the waning therapeutic effect over time, and they may persist for days or weeks after drug discontinuation. These tolerance-related changes are also consistent with the opponent-process models of addiction outlined earlier. Laboratory evidence is very clear that some of these phenomena occur after exposure to even short periods or to large doses of opioids (Angst & Clark, 2006; Trang et al., 2015; P. Yi & Pryzbylkowski, 2015). Likewise, tolerance and OIH have been demonstrated in opioid addicts, and abnormal pain sensitivity in addicts is associated with drug craving (Ren, Shi, Epstein, Wang, & Lu, 2009). On the other hand, OIH has been shown after short-term exposure to potent, rapidly eliminated opioids such as remifentanil in human volunteers (Angst & Clark, 2006; Eisenach, Tong, & Curry, 2015). Correspondingly, when remifentanil is incorporated into patients’ surgical anesthetic, they seem to have higher postoperative pain levels or opioid requirements consistent with either tolerance or OIH (de Hoogd et al., 2016; Fletcher & Martinez, 2014).
However, the rapidity, severity, duration, and pervasiveness of tolerance and OIH are very poorly defined in chronic pain populations, as are possible differences between opioids in causing these adverse consequences. The situation is made more problematic by difficulties in assessing tolerance and OIH in clinical settings. Rapid dose escalation with worsening pain and the spread of painful symptoms have been suggested as indicators of tolerance and OIH, but few well-validated clinical methods for quantifying tolerance and OIH exists for patients with chronic pain (Mao, 2002). One of the U.S. Food and Drug Administration’s required postmarketing studies for extended-release/long-acting opioid analgesics is a clinical trial to estimate risk for the development of hyperalgesia following long-term use (i.e., for at least 1 year) of these drugs to treat chronic pain.
Although all prescription opioids interact with opioid receptors, some more recently developed agents possess additional pharmacological activities, and even newer agents have been engineered to interact with opioid receptors in ways that may enhance analgesic benefits and minimize side effects (Dahan, 2016). Therefore, additional opioid drugs are likely to be developed, for a wide range of painful conditions, with properties perhaps superior in important ways to those of existing drugs. Nevertheless, these new drugs are likely to rely at least in part on the activation of the μ opioid receptor, a structure closely linked to important other effects of opioids, including respiratory depression and euphoria. Thus, the propensity of new opioid medications to cause overdose and abuse, as a result of their addictive properties—including euphoria and subjective pleasure—is likely to continue to be a concern.
Opioid treatment in patients with opioid-use disorders
Patients with histories of substance-use disorders also commonly report chronic pain. For example, more than 40% of patients receiving methadone maintenance treatment have chronic pain (Dunn, Finan, Tompkins, Fingerhood, & Strain, 2015; Voon, Callon, et al., 2014). One concern is that use of opioids to treat opioid-use disorders (OUD) in fact supports chronic pain. For example, patients with OUD maintained on methadone are more likely to experience pain than are former opioid misusers (Peles et al., 2015). In addition, patients receiving methadone and buprenorphine maintenance treatments have measurably lower pain thresholds and tolerances than control subjects who are not receiving opioids (Compton, Canamar, Hillhouse, & Ling, 2012; Compton, Charuvastra, & Ling, 2001). Likewise, cross-sectional studies of populations of patients managed with opioids frequently identify a significant percentage with substance-use disorders. Furthermore, persistent pain may lead individuals to use prescription opioids in patterns different from what their prescribing physician initially intended, resulting in opioid abuse or dependence (Blanco et al., 2016). The percentage of such patients in a treatment population is dependent on risk factors such as younger age and higher overall opioid dosage (Palmer, Ji, & Stephens, 2014).
A history of substance-use disorder is a risk factor for aberrant opioid use while being treated for pain (Chou et al., 2009). Opioid risk-assessment tools often take this characteristic into account, and such risk assessment is advocated in the U.S. Centers for Disease Control (CDC) “Guideline for Prescribing Opioids for Chronic Pain” (Dowell et al., 2016). However, additional risk-assessment tools, such as those for prefrontal cortex functions and dysfunctions as discussed later, should also be considered when prescribing opioids for pain.
The impact of pain on opioid-use disorder
Pain and reward are considered opposite processes but are processed within overlapping brain structures. Rewarding stimuli can decrease pain sensitivity (Leknes & Tracey, 2008), whereas pain can impair reward processing and lead to an anhedonic state (Elman, Borsook, & Volkow, 2013). Few studies have examined disruption of this circuitry caused by pain and whether the dopaminergic system contributes to the aversive component of ongoing persistent pain (Navratilova et al., 2015; Navratilova et al., 2012). Furthermore, how the presence of pain modifies the reinforcing properties of natural rewards or opioids is unknown. The mesocorticolimbic pathway is a critical brain circuit that is altered in opioid addiction, making it an ideal system to investigate the mechanistic basis for opioid abuse in the presence of pain (Cui et al., 2014; Fields & Margolis, 2015).
The neurobiology of the intersection between pain and opioid-use disorder
The brain does not passively receive nociceptive information from the body but instead actively regulates nociception by way of interactions between descending pain modulatory systems (Heinricher, Tavares, Leith, & Lumb, 2009; Reynolds, 1969) and corticocortical networks (Rainville, 2002). The descending pain modulatory system exerts influences on nociceptive input from the spinal cord through a network of cortical, subcortical, and brainstem structures, including prefrontal cortex, anterior cingulate cortex, insula, amygdala, hypothalamus, periaqueductal gray, rostral ventromedial medulla, and dorsolateral pons (Tracey & Mantyh, 2007). The descending pain modulatory system has been construed as the means by which the central nervous system inhibits nociceptive signals at the spinal outputs (Heinricher et al., 2009). Endogenous and exogenous opioids relieve pain by targeting the descending pain-modulatory system (Besson, 1999), most notably in the periaqueductal gray, a brain region involved in processing the placebo analgesic effect (Tracey, 2010). In addition, acute administration of single-dose opioids in healthy individuals exerts direct analgesic effects by reducing sensory evaluation processes evidenced by reduced activation in brain regions corresponding to processing lower level afferent processes (i.e., primary and secondary somatosensory cortex, thalamus; Wagner et al., 2007; Wise et al., 2002) and by modulating neurotransmission in the substantia gelatinosa of the dorsal horn of the spine (Le Bars, Rivot, Dickenson, Chaouch, & Besson, 1980; Yaksh, 1987).
Given the complexity of pain, the fact that opioid analgesia operates through both neuropharmacologic and psychological mechanisms is not surprising. In addition to attenuating sensory aspects of pain, opioids may alleviate the affective dimensions of pain. In that regard, among healthy individuals, analgesia induced through acute opioid administration operates, in part, through the modulation of neural circuits involved in the regulation of attention, emotion, and neurovisceral integration (Thayer & Lane, 2009). Opioids, like all drugs of abuse, also stimulate mesocorticolimbic dopamine reward systems (S. W. Johnson & North, 1992). Opioid-induced dopamine release in the nucleus accumbens associated with positive mood and reward may promote pain management. However, much of what is known about the psychobiological mechanisms of opioid-induced analgesia comes from studies of healthy individuals exposed to laboratory pain inductions. However, the development of co-occurring chronic pain and opioid-use disorders over time may alter the neurobiological response to opioids in clinically significant ways.
The decreased positive reinforcement associated with the presence of chronic pain is well documented (Cahill et al., 2013; Hipólito et al., 2015; Leitl, Onvani, et al., 2014; Leitl, Potter, et al., 2014; Martin, Buechler, Kahn, Crews, & Eisenach, 2004; Shippenberg, Stein, Huber, Millan, & Herz, 1988). This chronic pain-induced alteration has been linked to a decrease in reinforcer-induced dopaminergic transmission (Hipólito et al., 2015; Loggia et al., 2014; McDougle, Bond, & Taylor, 2015; Niikura, Narita, Butelman, Kreek, & Suzuki, 2010). Despite this evidence, few studies have assessed the impact of pain on opioid intake in preclinical studies. Most studies have used a conditioned place-preference paradigm to test the reinforcing properties of opioids in rodents undergoing neuropathic or chronic pain (Cahill et al., 2013; Narita et al., 2005; Ozaki et al., 2002; A. M. W. Taylor et al., 2015). Wu, Na, and colleagues (2014) revealed that the known reinforcing doses of morphine were unable to induce a place preference under painful conditions. However, animals exposed to chronic pain developed a clear preference for the morphine-paired side when the dose of morphine was increased (Wu, Na, et al., 2014).
In line with these findings, there was a decrease in low-dose drug consumption among rodents that self-administered opioids while experiencing pain (compared with control rodents; Hipólito et al., 2015; Lyness, Smith, Heavner, Iacono, & Garvin, 1989; Martin & Ewan, 2008; A. M. W. Taylor et al., 2015; Wade et al., 2013). This opioid consumption was increased, however, when high doses were accessible (Hipólito et al., 2015). Together these important results suggest a rightward-shift in the dose response for opioid consumption in conditions of chronic pain, which may correlate with modifications in dopaminergic transmission from the ventral tegmental area to the nucleus accumbens (Hipólito et al., 2015). Furthermore, inflammatory pain induces a desensitization of μ opioid receptors in the ventral tegmental area (Hipólito et al., 2015). These changes in opioid receptor function lead to decreases in dopamine release induced by DAMGO ([D-Ala2,N-MePhe4,Gly-ol]-enkephalin) and heroin in the nucleus accumbens. In addition, recent findings point to another opioid system, the KOPR, which may also be involved in these changes in dopamine release. Evidence points toward a role for the KOPR system in many of the changes induced by chronic pain (Cahill et al., 2014).
In conjunction with the data showing that inflammatory pain decreases morphine- and heroin-induced nucleus accumbens dopamine release and impairs the rewarding effects of morphine (Hipólito et al., 2015; Narita et al., 2005), Narita and colleagues (2005) showed that pain-induced attenuation in place preference can be reversed by systemic or local nucleus accumbens blockade of KOPR using norbinaltorphimine (NorBNI), a highly selective antagonist for KOPR. The aversive component of exogenous KOPR stimulation, measured by place-preference conditioning, is also suppressed when animals are experiencing inflammatory pain conditions (Shippenberg et al., 1988), suggesting the presence of a κ opioid activation during painful conditions that induces a sustained dysphoric state.
The role of the KOPR system in regulating the reinforcing properties of rewards during pain is controversial. Some studies have shown that KOPR antagonism during pain did not reverse the pain-induced decrease in intracranial self-stimulation of mesocorticolimbic pathway in rats (Leitl, Onvani, et al., 2014; Leitl, Potter, et al., 2014). These discrepancies could be possibly explained by the presence of hot and cold spots, such as two distinct areas in the nucleus accumbens shell in which activation of KOPR: One drives reward processes and one drives aversive processes (Al-Hasani et al., 2015; Castro & Berridge, 2014). Taking drugs that are KOPR antagonists systemically affects the whole body and targets both of these discrete areas simultaneously to potentially produce mixed valence effects, whereas microinjections of KOPR agonists or antagonists into subregional sites in the nucleus accumbens reveal site-specific roles and different potential interpretations.
Finally, we acknowledge the important role of other brain regions (besides the ventral tegmental area and the nucleus accumbens) critical in the regulation of pain, stress, and reward responses. The amygdala is very much involved in the processing of both positive and negative valence (Janak & Tye, 2015). Specifically, the basolateral amygdala and the central nucleus of the amygdala play important roles in affective pain in addition to better studied roles in the processing of mood, fear disorders, and reinforcement (Pare & Duvarci, 2012; Veinante, Yalcin, & Barrot, 2013). More recently the habenula-to-nucleus-accumbens dopaminergic neurons have been shown to drive inhibitory antireward processes during stress and pain conditions (Lee & Goto, 2011). The lateral hypothalamus, a region critical to positive reinforcement, also plays a role in the pain response through sensory mechanisms (Ezzatpanah, Babapour, Sadeghi, & Haghparast, 2015). These structures contribute to increases in norepinephrine, vasopressin, hypocretin, and substance P, driving a stress-like emotional state.
In summary, pain and reward are processed by overlapping brain structures. Positive reinforcement is decreased in the presence of chronic pain. Much of our knowledge of the mechanisms underlying opioid analgesia and reward comes from studies from healthy individuals. Research among people experiencing chronic pain is needed. Indeed, preclinical studies show that pain promotes opioid dose escalation in animals with a prior history of opioid intake.
Willpower, Decision Making, and Urge to Use Drugs: Roles of the Prefrontal Cortex and Insula
Willpower (1999), as defined by the Encarta World English Dictionary, is a combination of determination and self-discipline that enables somebody to do something despite the difficulties involved. This is one mechanism that enables one to endure sacrifices now to obtain benefits later. From this perspective, deciding to take, or not to take, drugs is beyond the ability to control an impulse. Duckworth, Milkman, and Laibson (2018) provide a detailed discussion of “willpower” in life situations beyond addiction, and the potential strategies that could reduce self-control failures (Duckworth et al., 2018). Each theoretical explanation discussed earlier, including the case of opioids and their use as analgesics, accounts for at least some aspects of addictive drug-seeking and drug use, particularly the question of why people seek drugs in the first place. The theories about changes in brain circuitry involving dopamine projections from ventral tegmental area to nucleus accumbens and rest of the striatum may be relevant also to arguments about addiction as a “brain disease” (Berridge, 2017; Hall, Carter, & Forlini, 2015; Leshner, 1997; Lewis, 2017; Pickard, 2017; Volkow & Koob, 2015). When cognitive dysfunction exists, is induced, or exacerbated by drugs, the problem of self-control becomes more severe. Thus, the central features of addiction are embedded in additional contributing factors that altogether power excessive pursuit and consumption of drugs.
Moreover, evidence suggests that—in some addicts, at least—cognitive function may preexist, may be induced, or may be exacerbated by cumulative drug effects on prefrontal cortex. Indeed, the discovery of abnormalities in the frontostriatal brain systems of stimulant-dependent individuals, some of which are also shared by their biological siblings who have no history of chronic drug abuse, support the idea of an underlying neurocognitive endophenotype for stimulant drug addiction (Ersche, Jones, et al., 2012). Together, these studies have brought the prefrontal cortex to the fore-front of discussion on the neurobiology of addictive substances. That is, the ventromedial prefrontal cortex and the orbitofrontal cortex play an important role in long-term goal setting, decision making, and impulse control (Schoenbaum, Roesch, & Stalnaker, 2006; Schoenbaum & Shaham, 2008; Volkow & Fowler, 2000). Deficits in such processes (e.g., goal setting, decision making, inhibition of impulses, and implicit cognition) have direct implications for addiction and contribute to the continuation of drug use despite a desire to abstain from using these substances.
Another recent line of research showed that the insula also plays a major role in substance addictions (Droutman, Read, & Bechara, 2015; Naqvi & Bechara, 2009). The human insula is hidden from view, buried in the lateral surface of the frontal cortex. Historically, the insula has been known to receive interoceptive signals from the body, and it has been found to be important in the experience of emotion and self-awareness, such as pain and disgust, but also in positive emotions (Craig, 2009, 2010). The insula has been studied in relation to substance addictions, with enhanced understanding of individual subregions of the insula (Droutman et al., 2015). The insula can be engaged either by homeostatic imbalance, such as withdrawal from opioids, or by reward cues, such as an environmental context that predicts drug use (Naqvi & Bechara, 2009). Emergence of the role of the insula in drug craving and addiction came to light from studies of patients who were heavy smokers and suffered a stroke that damaged the insula. The insula might be viewed to translate bottom-up, interoceptive signals into what subjectively may be experienced as an urge or craving. These signals (which could remain subconscious or be subjectively experienced as feelings of urge and craving) then potentiate the activity of the “impulsive” system, which intensifies the “wanting” to take drugs and impulsive behaviors while weakening or hijacking the goal-driven cognitive resources that are necessary for normal operation of the prefrontal cortex necessary for exerting willpower and self-control to resist drug use.
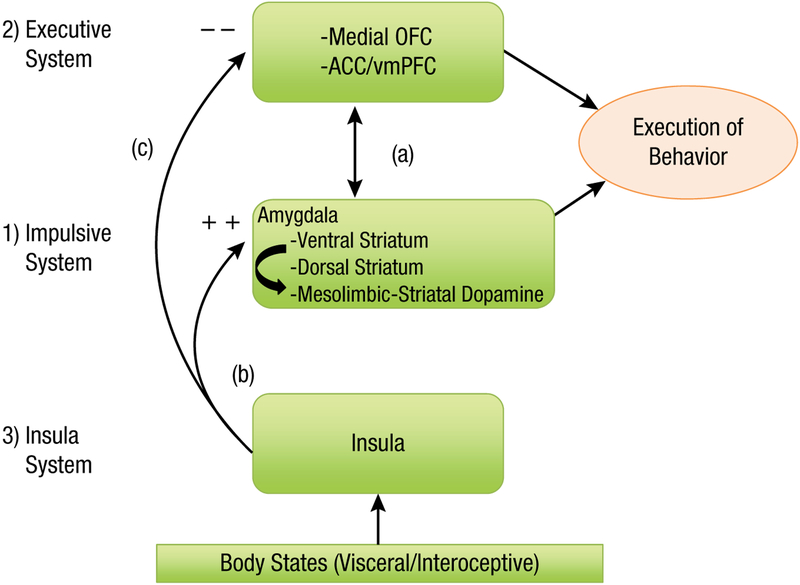
Thus, addiction could be a result of an imbalance between two systems—reward seeking and self-control (i.e., the dual systems models of addiction)—but the role of the insula in influencing this balance may add a third system, which interacts to influence the balance between the others. In sum, abnormal functioning in one or more of these three neurocognitive systems: the amygdala–striatum (which we have referred to as the “impulsive” system in previous publications), the prefrontal cortex (which has been referred to as the “executive” system), and the insula may all act together to influence addiction (Noël, Brevers, & Bechara, 2013). Treatment strategies could be directed at correcting abnormalities in any one or more of these neurocognitive systems (Fig. 1).
Fig. 1.
A schematic neurocognitive model illustrating a proposed functional role for three key neural systems in addiction: The first two systems include the competing-neurobehavioral-decision-systems (CNDS) model, which consists of an impulsive system that includes the amygdala and its connections to the ventral and dorsal striatum, and their associated mesolimbic dopamine systems. This system mediates, at least in part, the motivational wanting and habitual/compulsive seeking of drug reward. The executive system includes the prefrontal in general (i.e., dorsal and medial sectors), but in particular, the regions more concerned with decision making and “willpower”: the medial orbitofrontal/ventromedial prefrontal cortex (OFC/vmPFC), and adjacent anterior cingulate cortex (ACC). This system forecasts the future consequences of a behavior such as seeking drugs, and normally exerts control over the impulsive system (a). The functional role of the insula system emerged more recently, and it seems to play a role in modulating the functions of the impulsive and executive systems in response to perturbances in the viscera and homeostasis. More specifically, internal factors associated with deprivation states (e.g., withdrawal or pain) or emotional states (e.g., anxiety and stress) are viewed as a “gate” that determines how effective the incentive input is in exciting the motivational circuits that “pull” and “steer” the person toward the appropriate goal object. This process depends on the insula. Interoceptive signals arising from the body, reflecting the status of the viscera and homeostasis, and mediated through the insula, will adjust the strengths of the conflicting signals, thereby increasing the influence of the impulsive system (b), and potentially overriding the inhibitory control of the executive system (c). An additional possibility is that insula signals may subvert the decision-making processes of the reflective system that supports planning actions to seek and procure drugs.
The prefrontal cortex and impaired “willpower”
Although more than 30 years of research yielded remarkable success in understanding the subcortical neural mechanisms that motivate behaviors toward reward, especially drugs, very little attention was paid to the importance of the prefrontal cortex in the decision to take drugs in the first place. In the case of humans, the initiation and escalation of drug use, including the analgesic use of opioids, is often done with extensive prior knowledge of the negative consequences associated with continued drug use. An addicted person continues to pursue drugs at the cost of incurring problems with their social status, financial stability, and family relations. The prefrontal cortex is important for self-regulation and the ability to predict consequences of behavior. When the prefrontal cortex is compromised, exerting top-down cognitive control of urges and inhibition of automatic and habitual behaviors is difficult.
The importance of a loss of top-down control from the prefrontal cortex in the escalation of drug use began to emerge from early theoretical accounts that discussed how these top-down control mechanisms make habits become compulsive (Clark & Robbins, 2002; Everitt, Dickinson, & Robbins, 2001; Everitt & Robbins, 2005; Jentsch & Taylor, 1999). Early functional neuroimaging studies began to link heavy substance use, especially cocaine, to abnormal activities within the prefrontal cortex (London, Ernst, Grant, Bonson, & Weinstein, 2000; Volkow & Fowler, 2000). Later neuropsychological studies in humans began to link addictive behaviors to abnormal mechanisms of decision making associated with dysfunction of the prefrontal cortex (Grant, Contoreggi, & London, 2000; Rogers et al., 1999). Perhaps the earliest study linking substance-use disorders to impaired decision making and abnormal prefrontal cortex is one that compared the performance of amphetamine and opioid users, together with patients with frontal lesions, on the Cambridge Gambling Task (Rogers et al., 1999), an explicit decision-making task. Several recent theories of addiction propose that impaired inhibitory control and poor decision making are due to a dysfunctional prefrontal cortex (Bechara, 2005; Bickel, Snider, Quisenberry, Stein, & Hanlon, 2016; Ersche, Jones, et al., 2012; Goldstein & Volkow, 2011; Jentsch & Taylor, 1999; Liu, Matochik, Cadet, & London, 1998; Noel, Jaafari, & Bechara, 2017; Schoenbaum, Chang, Lucantonio, & Takahashi, 2016; Zilverstand, Huang, Alia-Klein, & Goldstein, 2018).
Numerous studies have shown that individuals with substance-use disorders, including opioids, have poor self-control or impulse control and poor decision-making (Bickel, Jarmolowicz, Mueller, Gatchalian, & McClure, 2012; Bickel, Jarmolowicz, Mueller, Koffarnus, & Gatchalian, 2012). Self-control can be estimated by an individual’s capacity to inhibit prepotent motor responses using the stop-signal or go/no-go tasks, and people with a substance-use disorder show behavioral impairments in both tasks (Noël et al., 2013). These studies do not tell whether this poor inhibitory control precedes, or follows, the substance-use disorder. However, considerable evidence suggests that this trait precedes substance use in many individuals, and may in fact be a predisposing factor for acquiring the disorder (e.g., see Ersche, Jones, et al., 2012; He et al., 2010; also see section Does Prefrontal Cortex Dysfunction Precede or Follow Drug Use?).
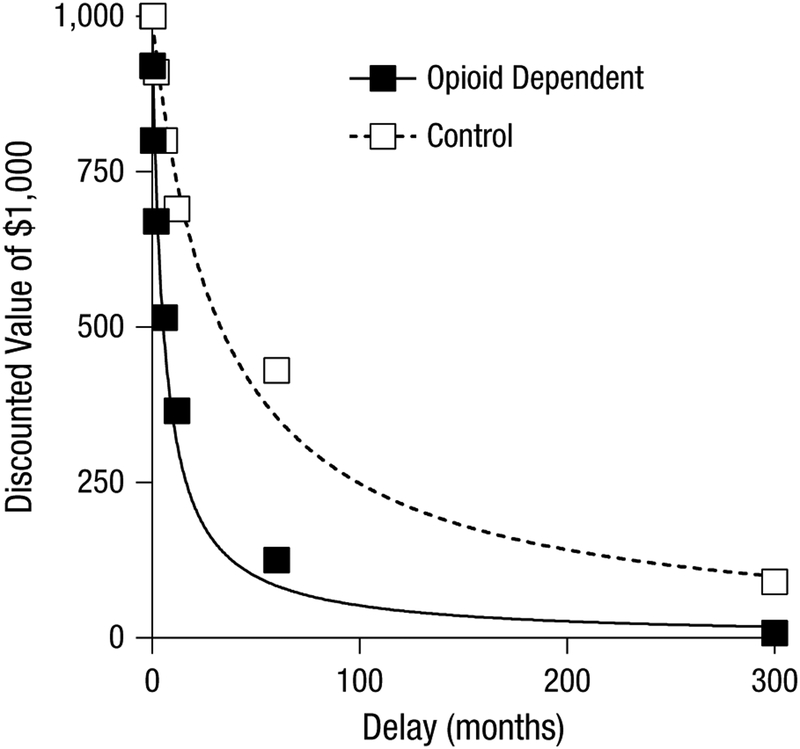
Decisions that are costly in the short term but that yield long-term benefits (e.g., remaining sober while mourning a death in the family) are also impaired in people with a substance-use disorder. Delayed discounting is a procedure that measures the degree of an immediacy bias: $5 now is better than $7 a week later. Individuals with an addiction systematically devalue delayed rewards at a faster rate than control subjects: They are more likely to act on impulse (Kirby, Petry, & Bickel, 1999). Impaired working memory, an executive function important for decision making and mediated by the prefrontal cortex (particularly the dorsolateral sector), may explain the findings with delayed-discounting behaviors in people with substance-use disorders (Hinson, Jameson, & Whitney, 2003; Wesley & Bickel, 2014). Another behavioral measure of decision making in populations with an addiction is the Iowa Gambling Task (IGT). A person must make decisions with uncertainty in the context of punishment and reward: Some of the choices yield positive short-term results (high reward) but predict poor long-term results (high punishments that wipe out accrued rewards), whereas others produce small short-term rewards (low reward) but predict long-term positive results (low punishments that are offset by small, steady rewards). To perform well, participants must forgo short-term benefits for long-term benefits—something that persons suffering from addiction, or persons with bilateral damage to the ventromedial prefrontal cortex, are characteristically unable to do successfully (Bechara, 2004, 2005). Participants with an addiction who perform the IGT demonstrate preferences for short-term high rewarding stimuli, with the end result being a net loss (Bechara, 2004, 2005).
Decreased gray-matter volume in prefrontal cortical regions, including the orbitofrontal cortex, ventromedial prefrontal cortex, and anterior cingulate, is reported in at least some subpopulations of methamphetamine users (Thompson et al., 2004), heroin users (Yuan et al., 2009), alcohol users (Mechtcheriakov et al., 2007), and cocaine users (Fein, Di Sclafani, & Meyerhoff, 2002). Some of these abnormalities may precede substance use (Ersche, Jones, et al., 2012), and they do not seem to recover even after a long period of abstinence from chronic drug use (He et al., 2018). The orbitofrontal cortex has connectivity to subcortical structures—notably the amygdala and nucleus accumbens, which both play a role in associative learning (Schoenbaum et al., 2006) and cue-triggered motivation for rewards. Because of these connections, the orbitofrontal cortex can “predict” future outcomes on the basis of associations: It can use the value of expected versus actual outcomes to guide executive functions, including decision making.
Functional neuroimaging studies show hypoactivation in brain regions associated with error detection, namely the anterior cingulate cortex, indicating a lack of prefrontal cortex activity associated with the prediction of “bad” future outcomes in people with a substance-use disorder (Hester & Garavan, 2004). This hypoactivation in prefrontal cortical circuits of decision making could exacerbate the problems that arise from hyperactivation of the subcortical amygdala-striatal (dopamine linked) system that powers craving. The prefrontal self-control and subcortical reward circuits are competing systems, and in sensitized people with an addiction, the subcortical circuits of cue-triggered craving may additionally become hyperresponsive to drug cues as the prefrontal circuits simultaneously become weaker (Bickel et al., 2016; Droutman et al., 2015; Zilverstand et al., 2018): This imbalance is likely to compromise the self-control of individuals who are attempting to remain abstinent in a range of substance-use disorders, including nicotine (Krishnan-Sarin et al., 2007), cocaine (Garavan & Hester, 2007), and alcohol (Bowden-Jones, McPhillips, Rogers, Hutton, & Joyce, 2005). Combining stronger desires with weaker self-control would produce a particularly vulnerable pack-age of drug-induced psychological and neurobiological changes that could trap people in addictive behaviors and raise their risk of relapse when they try to give up drugs.
In summary, dysfunction in the prefrontal cortex system could lead to impaired response inhibition and heightened salience attribution toward high short-term rewards in individuals with a substance-use disorder (Goldstein & Volkow, 2011). Note that people with an addiction are not simply left with a nonfunctional prefrontal cortex; considerable planning and coordinating are required for a person to get his or her next dose, and people who are daily users can maintain this detailed schedule of seeking, finding, purchasing, and then using with a high degree of success despite the barriers set out by law enforcement, family, friends, and jobs. All these behaviors require a functional prefrontal cortex. Therefore, in the case of drug addiction, the prefrontal cortical system is likely “hijacked” such that people cease to make advantageous decisions and instead divert their cognitive capacities toward thinking relentlessly of drugs and the cycle of seeking, finding, purchasing, and using.
The metaphor of drugs hijacking the brain fits well in terms of diverting cognitive resources away from future-oriented goals and toward immediate goals. Put differently, addictive drugs may subvert the decision-making processes and abilities of the prefrontal cortex away from “normal functioning” and into formulating plans for action to seek and procure drugs (Fig. 1). These changes make it difficult for addicts to deploy their executive capacities for “good” behavior. The data suggest that the insula and other limbic circuitry recruited in drug use and addiction subvert the ability of prefrontal cortex to direct behavioral strategies toward beneficial actions that would correct this homeostatic imbalance. Indeed some connotations of the word willpower may be misleading when applied to addiction (i.e., that addiction is simply a failure of will or a moral weakness; National Academies of Science, Engineering, and Medicine, 2019). In short, evidence from brain studies reveal that the roots of failed willpower or purported “moral” weakness is often a chronic brain disease involving dysfunction in the ventromedial prefrontal cortex. Indeed, patients with lesions to the ventromedial prefrontal cortex show impairments when judging moral situations (Koenigs et al., 2007; Sobhani & Bechara, 2011; Young et al., 2010).
The insular cortex and “urge” to seek reward
The insular cortex (insula) has emerged as a neural structure that plays a key role in interoceptive representations generated from drug-related cues (e.g., smoking). Activity in the insular cortex elicited by homeostatic imbalance, such as deprivation states, withdrawal, pain, stress, or reward cues, serves to sensitize the motivational circuits that propel individuals toward reward and to hijack the prefrontal cortical system, preventing it from using the cognitive resources necessary to exert self-control to resist reward (Fig. 1; Naqvi & Bechara, 2009; Naqvi, Rudrauf, Damasio, & Bechara, 2007).
The first reported evidence linking damage of the insula to cessation of an addictive behavior came from patients who were avid smokers before suffering a middle-cerebral-artery stroke; if their brain damage included the insula, they dropped the habit immediately and effortlessly (Naqvi et al., 2007). This quitting does not occur in every patient with an insular lesion, but the percentage of patients with disruption of smoking addiction after insula damage was significantly higher than that after a lesion anywhere else in the brain, with an odds ratio of more than 136 for successfully quitting smoking after insula lesion (Naqvi et al., 2007). Detailed analyses of these lesions revealed that the disruption of smoking addiction did not depend on whether it involved the anterior or posterior regions of the insula, and it did not matter whether the lesion was on the left or the right side of the brain (Naqvi et al., 2007). Another study found that insular stroke increased odds of quitting smoking (within a year of the stroke) fivefold compared with noninsular stroke (Suner-Soler et al., 2012).
Subsequent studies revealed that this disruption of smoking addiction is not exclusive to insula damage; damage to adjacent basal ganglia structures (e.g., the striatum) can also lead to smoking cessation (Gaznick, Tranel, McNutt, & Bechara, 2014). However, when basal ganglia damage is combined with insula damage, the disruption of smoking addiction is intensified, as reflected by the higher number of patients who quit smoking after stroke (compared with patients with only striatal lesions) and by a more sustained quitting over time (Gaznick, Tranel, et al., 2014). Additional studies suggested that sex-related differences could exist regarding the side of insula lesions (i.e., right versus left) and the disruption of an addictive behavior. That is, in patients with left-insula damage, quit rates were significantly higher in men than in women, whereas in patients with right-insula damage, quit rates were higher in women than in men, but this trend did not reach statistical significance (Gaznick, Bechara, & Tranel, 2014). (One study that examined the effects of brain lesions on smoking behavior reported that increased likelihood of smoking cessation after insular lesion was not significant; Bienkowski, Zatorski, Baranowska, Ryglewicz, & Sienkiewicz-Jarosz, 2010.) However, a likely explanation for this discrepancy could relate to cultural differences in the perception of the long-term harms of smoking (Gaznick, Bechara, & Tranel, 2014).
Research on the insula in recent years has uncovered its role in communicating interoceptive cues to the brain, which play a role in the subjective experience of emotional states (Craig, 2009). Interoceptive cues may be the basis for the experience of craving and the urge to use a drug (Gray & Critchley, 2007). Interviews with people who have an addiction reveal that they often feel the need to use in order to feel normal (Khantzian, 1987). This could be related to interoceptive cues signaling a homeostatic imbalance.
Despite the commonality that drugs of abuse share—an increase in mesocorticolimbic dopamine—each ritual associated with individual drugs has a distinct effect on the body that can contribute to its subjective emotional meaning. Smoking cigarettes produces its own set of interoceptive cues: Increased heart rate, autonomic effects, sensory effects on the airway, and the taste of tobacco combine to form the unique experience of smoking a cigarette or vaping. Injecting heroin produces a different set of cues: piercing the skin, decreased respiration, and subjective feelings of warmth. Snorting cocaine produces a bitter taste, increased heart rate, and a harsh sensation in the nose and throat. Each drug changes homeostasis and leads to increased activation in the insular cortex, which translates these interoceptive state changes into conscious feelings (Gasquoine, 2014). These interoceptive signals are sent from different areas throughout the body and up to the thalamus, which then relays this information to the insular cortex where awareness begins. The hedonic information that accompanies these cues is not currently thought to be dependent on the insula, although the mechanism for how that information is assigned is not yet known. The insula likely sends signals to the amygdala and the orbitofrontal cortex to attach corresponding positive and negative valuation to interoceptive signals (Kringelbach, 2005). The insula is functionally connected to both mesocorticolimbic brain structures and prefrontal cortices. This functional position is critical for its ability to influence the amygdala–striatum (mesocorticolimbic) and prefrontal cortex systems. Activity in the insular cortex is correlated with subjective ratings of the magnitude of urges to use substances, including cigarettes, alcohol, heroin, and cocaine (Naqvi & Bechara, 2010; Schmidt et al., 2014).
In the case of opioids, the interoceptive signals received by the insula may also be due to pain. The insula not only drives motivation toward the pleasurable effects of the drug but also plays a role in avoiding punishment. The insula is involved in the emotional experience of pain, which is present in addiction through the negative interoceptive cues associated with drug use (Singer, Critchley, & Preuschoff, 2009). The motivation to avoid pain is a contributor to the maintenance of addiction, primarily via withdrawal symptoms. Withdrawal is a compilation of negative interoceptive cues associated with the lack of drugs in the body and brain. For some drugs, it is not only painful but also can even be lethal if not treated appropriately (Carlson et al., 2012). Functional neuroimaging also provides supporting evidence that the insula is involved in the motivation to avoid unpleasant stimuli (Samanez-Larkin, Hollon, Carstensen, & Knutson, 2008; Seymour, Daw, Dayan, Singer, & Dolan, 2007). Altogether, these results are consistent with the conclusion made earlier that pain and reward are processed by overlapping brain structures.
Addressing the role of the insula complements prior work and advances our efforts to find novel therapeutic approaches for breaking the cycle of addiction, including opioids. Stimulation of future research on the insula has a number of practical implications for clinical studies. The most obvious is that therapeutically modulating the function of the insula may make it easier to overcome addiction and perhaps combat the current opioid crisis. This could be accomplished through the design of new pharmacological therapies that target receptors within the insula. Invasive techniques such as deepbrain stimulation are also an option. Because of the risks associated with invasive methods, noninvasive methods such as repetitive transcranial magnetic stimulation (TMS) should be investigated. However, risks associated with addiction may outweigh those associated with invasive techniques.
Individual variability: is the prefrontal cortex dysfunctional in all substance users?
Although individuals with substance-use disorders reveal abnormal prefrontal cortex functions on various neuropsychological tasks when examined as a large group, looking at individual differences reveals that not all addicted individuals are the same. Some studies compared (a) patients with bilateral damage (lesions) to their ventromedial prefrontal cortex (but who were not substance abusers) and (b) individuals diagnosed with substance dependence (DSM–IV terminology), but who had no brain lesions (Bechara & Damasio, 2002; Bechara et al., 2001; Bechara, Dolan, & Hindes, 2002). Only some multisubstance users were found to match the profiles of the patients with ventromedial prefrontal cortex lesions; many others did not. The authors suggested that the particular individuals who more closely match patients with ventromedial prefrontal cortex lesions are characterized by insensitivity to future consequences—they are oblivious to future consequences, positive and negative, and are strongly guided by immediate prospects. These results indicate that a key problem in this subgroup is a dysfunction in the prefrontal cortex. Another subgroup of multisubstance users matched patients with ventromedial prefrontal cortex lesions only partially in their behavioral and physiological profiles, and they were considered hypersensitive to reward, so that the prospect of a reward outweighs the prospect of punishment (Bechara & Damasio, 2002; Bechara et al., 2001; Bechara et al., 2002). The key problem in this subgroup may be a hyper-active mesocorticolimbic dopamine or “impulsive” system, which overwhelms the capacity of the ventromedial prefrontal cortex to resist the temptation of an immediate reward. In light of the more recent research on the insula, this reward hypersensitivity could also be attributed to abnormal insula function.
These differences may have implications for prognosis, and they provide testable hypotheses that can be addressed in future research: Individuals with substance-use disorders who closely match (rather than only partially match) patients with ventromedial prefrontal cortex lesions may have a harder time recovering from addiction and remaining abstinent. One subgroup of individuals with multisubstance use appeared normal and showed no behavioral or physiological signs of decision-making deficits. This suggests that not every drug user has impaired decision making. We have described these individuals as functional addicts because a closer inspection of their everyday lives showed that they have suffered minimal social and psychological harm as a consequence of their drug use, (i.e., the ability to keep their job). This subgroup of functional addicts can be viewed as those who will seek drugs and plan out how and when to use but who will tend to seek drugs within what society might deem reasonable means. This subgroup is characterized by a certain threshold for drug use—a point at which procuring or using the drug is no longer worth the cost (economically, socially, or psychologically).
Does prefrontal cortex dysfunction precede or follow drug use?
A more intriguing finding is that when a large sample of the “normal” population on the IGT was tested, a small subgroup achieved scores that were comparable with those of patients with lesions to the ventromedial prefrontal cortex. This raises the question of whether these individuals are predisposed to (or at higher risk for) addiction than individuals with normal decision-making capabilities. One underlying basis for this predisposition or vulnerability may be poor mechanisms of decision making and impulse control. This suggestion is reasonable in light of the evidence that heritability is a risk factor for addiction, and genes (e.g., the serotonin transporter gene) can act in a general fashion to predispose individuals to multiple drug addictions as opposed to specific drug addictions (Goldman, Oroszi, & Ducci, 2005). The evidence suggesting an underlying neurocognitive endophenotype for stimulant-drug addiction (Ersche, Jones, et al., 2012) also supports this view. Other studies revealed that decision making in healthy subjects, as measured by the IGT, is linked to the serotonin transporter-linked polymorphic region (5-HTTLPR; He et al., 2010). Specifically, after researchers controlled for intellectual and memory abilities, subjects homozygous for the short allele had lower IGT scores than carriers of the long allele (He et al., 2010). Evidence for possible gene-environment interaction on affective decision making, as measured by the IGT, was also found in a large healthy sample in relation to the catechol-O-methyl transferase gene (COMT) associated with the metabolism of dopamine in the prefrontal cortex region (He et al., 2012). Other longitudinal studies in adolescents showed that lower IGT scores predicted higher binge-drinking problems (C. A. Johnson et al., 2008; Xiao et al., 2009) and higher smoking behavior (Xiao et al., 2008) 1 year later (after controlling for demographic and other cognitive variables).
These results support the view that lower decision-making capacity in some individuals is genetically linked and that poor decision making may be an important determinant of future substance use. Perhaps future research using functional imaging methods that focus on relationships among (a) genotypes related to specific neurotransmitter systems (e.g., serotonin transporter gene), (b) level of neural activity in specific neural circuits, and (c) quality of choice on complex laboratory tasks of decision-making could reveal whether genetic factors lead to suboptimal function in specific neural systems, especially those involving decision-making, that are then expressed in real life as a variety of behaviors reflecting poor decision-making, including the decision to use substances. Genetic links do not imply that decision making cannot be improved or that such traits cannot be harnessed for the good of the individual and society. Indeed, understanding genetic dispositions—and their interactions with modifiable factors—is an important step toward changing life outcomes.
Moreover, not all risk factors are necessarily genetic; other factors could be environmental or could be the product of gene–environment interactions. The potential for harm in the form of brain development remains relatively high if an individual abuses drugs during adolescence. Evidence suggests that the functions of the prefrontal cortex may not develop fully until the age of 21; until that time, the development of neural connections that underlie decision making and the ability to control powerful temptations is still taking place (Crews, He, & Hodge, 2007). Therefore, exposing the prefrontal cortex to drugs before its maturity could be harmful to decision making, akin to exposing a fetus to drugs during pregnancy. Beyond neurotoxicity to the brain from drug use, an important risk factor for addiction is the incidence of a traumatic brain injury (TBI). People who use substances are at a higher risk of experiencing a TBI, and research indicates that TBI may be a risk factor for the later onset of substance-use disorders, although the evidence is still limited at this point (Bjork & Grant, 2009; Graham & Cardon, 2008). Early life emotional distress, such as child abuse, also plays a role in abnormal development of the prefrontal cortex. During stress, orchestration of the brain’s response patterns switches from thoughtful PFC regulation to the rapid emotional responses of the amygdala, insula, and related subcortical structures (Arnsten, 2009). Loss of self-control associated with chronic stress has been linked to a number of substance-use disorders, such as smoking, drinking alcohol, and drug addiction, including opioids (Sinha, 2008). Finally, one longitudinal study of 192 Chinese 10th graders suggested that parenting styles could play a role in the development of decision-making capacities, as measured by the IGT, and its subsequent influence on substance use (Xiao et al., 2011). In brief, compared with adolescents whose parents made decisions for them, adolescents allowed by their parents to engage in everyday decision making, such as spending money, leisure activities, or curfew time, showed significant improvement in IGT scores and significantly less binge drinking 1 year later (Xiao et al., 2011). Although such differences could reflect parental causation, they might also reflect selection effects; for example, parents who allowed adolescents to make decisions may differ in other ways (or the adolescents differed in other ways) that were responsible for outcomes. Only experiments can resolve causation.
In sum, although chronic drug use, especially use in early life, can cause neural dysfunction of the prefrontal cortex, several lines of evidence also suggest that this dysfunction might predate the initiation of drug use, which renders individuals more predisposed or vulnerable to succumbing to addiction if conditions are met. Subsequent drug use may exacerbate this prefrontal cortex dysfunction. Although recovery of neuropsychological functions are expected after a long period of drug abstinence, the persistence of a decision-making impairment has been intriguing (He et al., 2018). For example, alcoholics who maintained long-term abstinence persisted in showing deficits in decision making as measured by the IGT (Fein, Klein, & Finn, 2004). Neuroimaging studies in former chronic cocaine users with long-term abstinence (from 1 year up to 30 years) also suggest that neural recovery occurs mostly in neural systems related to reward, craving, and simple inhibitory control but to a lesser extent in neural systems related to decision making (i.e., the ventromedial prefrontal cortex; He et al., 2018). These results suggest that drug users are indefinitely at a higher risk for deficits in judgment and decision making leading to possible relapse, if reward salience and craving become more intense. In other words, poor decision making linked to the ventromedial prefrontal cortex reflects a vulnerability or predisposing factor to substance-use disorders that persists with abstinence.
The rise of the opioid crisis: risk perception and societal influences
Evidence suggests that the perceived risk of a drug affects its use among the population (Bachman, Johnston, & O’Malley, 1990). A substance that is perceived to be too risky to take may alert the PFC to resist the temptation, whereas a substance perceived as low risk may pass the PFC screening test. Fifty years ago, heroin users were primarily young adolescent males in minority groups who lived in low-income urban areas. Today, users are more likely to be White, middle-class, either male or female, and in their early 20s (Cicero, Ellis, Surratt, & Kurtz, 2014). Part of this change has been attributed to the marked increase in the abuse of prescription drugs such as oxycodone (Compton & Volkow, 2006; Grau et al., 2007). The perceived risk of prescription drug use is lower than that of illicit substances. People tend to feel that prescription abuse is low risk because doctors and the U.S. Food and Drug Administration have deemed prescribed opioids safe for human use (Daniulaityte, Falck, & Carlson, 2012; but, for recent changes, see National Academies of Sciences, Engineering, and Medicine, 2017). Among people in treatment for drug abuse, 75% of heroin users stated that their first regular opioid was a prescription drug, in contrast to 50 years ago, when more than 75% of people in treatment stated that heroin was the first opioid they used regularly (Cicero et al., 2014). Pharmaceutical companies have tried to reduce the addictive liability of these drugs by making them more difficult to snort, inject, and break down. However, instead of quitting the substance, many users turned to heroin, which created an entirely new problem (Cicero et al., 2014). Heroin and intravenous drug use has increased the risk for needle-related infections. Moreover, lethal and nonlethal overdoses from opioids are rising as more potent synthetic opioids, such as fentanyl and carfentanil, become more widely available.
Currently, the United States is facing an unprecedented number of deaths involving opioids; the National Institute on Drug Abuse (NIDA) reported more than 30,000 opioid-related fatal overdoses in 2015 alone (see also National Academies of Sciences, Engineering, and Medicine, 2017). Public-policy recommendations include targeted programs to improve addiction care, as well as improving access and availability of harm-reduction programs. Opioids may seem to be the most addictive substance in history. However, when we look back at the early 1980s, we see that cocaine was the most widely used illicit substance in the United States, and a 1986 Gallup Poll indicated that people felt that crack cocaine and other forms of cocaine were the most serious problem that society was facing compared with alcohol, heroin, and other drugs of abuse (Miech, Chilcoat, & Harder, 2005). However, this problem was not equally prevalent throughout the population: Blue-collar workers, men, and minorities were at a higher risk for mortality related to drug use—indicating a relationship among public policies, social factors, and the severity of problems caused by drug use (Harlow, 1990). Cocaine and methamphetamine had traditionally been drugs used mostly by the upper social class, but the introduction of crack cocaine (a cheaper and more potent substrate) resulted in a historical period effect wherein lower social classes began to use the drug at a much higher rate (Miech et al., 2005). During this time, public attitude shifted, and the perceived risk of cocaine began to rise. Use began to decline in the late 1980s and early 1990s and, according to NIDA, has continued to drop as recently as 2013 (NIDA, 2016). To further highlight the role of risk perception in the rise or fall of substance use is the use of marijuana, which has been steadily on the rise since 2007 and is the drug that first-time users are most likely to try (Lipari, Ahrnsbrak, Pemberton, & Porter, 2017). New state laws legalizing marijuana because of its perceived low risk are likely to further increase the use of recreational marijuana, even as it remains federally illegal.
While public opinion, risk perception, drug policies, and public health could help drive down the use of certain drugs, such as cocaine, the clinical use of opioids remains necessary for analgesic purposes, and indiscriminate restriction of their clinical use can inadvertently punish those who legitimately need them. Therefore, in addition to the current opioid risk assessment tools advocated in the “CDC Guideline for Prescribing Opioids for Chronic Pain” (Dowell et al., 2016), one could consider the use of additional risk-assessment tools, such as those that test prefrontal-cortex functions. Patients with chronic pain who have signs of prefrontal-cortex dysfunction could be at higher risk for transforming their opioid use from pain management to opioid addiction. Naturally, before clinical use, assessments should achieve acceptable levels of predictive validity (Enkavi et al., 2019).
Opioid Dependence: From Mechanisms to Therapeutic Opportunities
Opioid dependence, as outlined above, is a challenging disorder that should be both understood and treated. Ideally, the understanding of the mechanisms and the resulting treatment should be related to each other, if not directly derived from each other (Bickel et al., 2018). Fortunately, the field of neuroeconomics, which incorporates neuroscience, economics, and decision sciences, has provided a useful model, referred to as the competing-neurobehavioral-decision-systems (CNDS) approach, that can link mechanism and treatment in addiction (Bechara, 2005; Bickel et al., 2007; Bickel et al., 2016; Jentsch & Taylor, 1999). The CNDS is a dual-system model, and such models have been employed in diverse areas of psychology (Evans & Stanovich, 2013; Strack & Deutsch, 2004), applied to addiction (Bechara, 2005; Bickel et al., 2007; Bickel et al., 2016; Jentsch & Taylor, 1999; McClure & Bickel, 2014), and have recently been expanded to a tripartite system (Turel & Bechara, 2016). Here we focus on the two original components of the dual system, which have a longer scientific history. This conceptual model posits that choices result from two interactive decision systems: the impulsive-decision system, which is embodied in the limbic and paralimbic brain regions and functions to obtain biologically important reinforcers, and the executive-decision system, which is embodied in the prefrontal cortices and functions to consider longer-term consequences of decisions. For those who can manage their appetitive behavior, the CNDS posits that the impulsive-decision system and the executive-decision system are in regulatory balance. Among those with addiction, however, the CNDS posits that the impulsive-decision system exhibits relatively greater control (i.e., it is hyperactive) and the executive-decision system exhibits relatively less control (i.e., it is hypoactive; Bickel et al., 2007; Bickel et al., 2016; Crews & Boettiger, 2009).
Research into this dual-system model was further advanced by the recognition that the behavioral process of delay discounting, which measures the decline in value of a reinforcer as a function of delay to its receipt, also indicates the relative control expressed by these two decision systems. Delay discounting is measured by examining choices between smaller, immediate reinforcers and larger, delayed reinforcers. The role of the dual decision systems in delay discounting was discovered in a study that used functional MRI to examine the neural correlates of delay discounting in healthy adults (McClure, Laibson, Loewenstein, & Cohen, 2004). That study reported relatively greater activity of the limbic and paralimbic brain regions when participants chose the smaller, immediate reinforcer. In contrast, relatively greater activity in the prefrontal cortices was detected when the larger, delayed reinforcer was chosen (McClure et al., 2004). Neuroeconomic and related studies have further implicated the dorsolateral prefrontal cortex, posterior parietal cortex, anterior cingulate cortex, and anterior insular cortex in a wide range of substance-use disorders (Amlung, Sweet, Acker, Brown, & MacKillop, 2014; Amlung, Vedelago, Acker, Balodis, & MacKillop, 2017; Boettiger et al., 2007; Claus, Kiehl, & Hutchison, 2011; Clewett et al., 2014; Wesley & Bickel, 2014).
Note that the results of delay-discounting experiments among those suffering from addiction might be interpreted in the light of the findings of McClure et al. (2004). Consider one of the first studies of this type: Individuals who were opioid-dependent and matched control subjects completed a delay-discounting assessment in which the hypothetical monetary amount being discounted was $1,000 (Madden, Petry, Badger, & Bickel, 1997). The results of this study are depicted in Figure 2, which shows that opioid-dependent individuals discount delayed monetary rewards substantially more than did control subjects. On the basis of McClure et al.’s observations, this finding suggests that the opioid-dependent individuals’ excessive discounting results from relatively greater control from the impulsive-decision systems than the executive-decision system. This result of greater delay discounting among opioid-dependent individuals has been replicated numerous times (Kirby & Petry, 2004; Kirby et al., 1999; Odum, Madden, Badger, & Bickel, 2000).
Fig. 2.
Evidence of greater discounting in opioid users compared with demographically matched controls. Data replotted from Madden et al. (1997).
Since the initial study by Madden et al. (1997), considerable research over the past 20 years has been conducted with delay discounting (Frederick, Loewenstein, & O’Donoghue, 2002), including research among those suffering from addiction (Bickel & Marsch, 2001). The extensive research on this topic has led to meta-analyses of these observations. The first meta-analysis of delay discounting reviewed those studies in which groups exhibiting addictive behavior were compared with control subjects (MacKillop et al., 2011). This meta-analysis identified 46 studies reporting 57 comparisons for a total sample size of 3,329. The authors observed a significant aggregate effect size that was moderated by addiction severity. That is, effect-size comparisons were larger for groups meeting diagnostic criteria for substance-use disorders (Cohen’s d = .61) than for subclinical substance users (Cohen’s d = .45). A subsequent meta-analysis of existing substance users found significant positive associations between delay discounting and continuous measures of addiction severity and quantity-frequency of substance use (Amlung et al., 2017). Collectively, these studies appear to support excessive discounting of delayed outcomes as a trait-like phenomenon (Odum, 2011).
Some evidence, however, also suggests that delay discounting is a state-dependent phenomenon. Another state-dependent phenomenon that is important to opioid dependence and that may also affect delay discounting is withdrawal. Withdrawal is a process that occurs following discontinuation of opioids and is associated with a variety of signs and symptoms that are similar to a case of the flu, such as muscle ache, restlessness, running nose, sweating, and diarrhea, among others. Opioid-dependent individuals seek to avoid opioid withdrawal and, if they cannot avoid it, then they often seek to terminate the experience by finding more opioids to consume. One study examined the effects of mild opioid withdrawal on the delay discounting of opioid-dependent individuals in treatment with buprenorphine, a partial opioid agonist (Giordano et al., 2002). Specifically, delay discounting was assessed for heroin (smaller, immediate amounts of heroin vs. larger, delayed amounts of heroin) and for money (smaller, immediate amounts of money vs. larger, delayed amounts of money) after mild withdrawal induction and after buprenorphine administration (satiation). Results indicated that opioid withdrawal was associated with significantly greater discounting of delayed heroin and money compared with the satiation condition.
On the basis of the body of research reviewed above, some have proposed that delay discounting is a behavioral marker of the addiction process (Ainslie & Monterosso, 2003; Bickel, Koffarnus, Moody, & Wilson, 2014; Bickel, Moody, Eddy, & Franck, 2017). Specifically, this proposition was supported by four observations. First, delay discounting can identify individuals who are substance-dependent and can distinguish them from control subjects. Second, in longitudinal studies, delay discounting can identify those at risk of developing substance dependence (Audrain-McGovern et al., 2009; Khurana et al., 2013; Kim-Spoon, McCullough, Bickel, Farley, & Longo, 2015; Mischel, Shoda, & Peake, 1988). These findings are consistent with, and corroborate, the earlier recommendation that these delay discounting instruments, along with the IGT, could be used as additional assessment tools for evaluating patients at higher risk for becoming opioid abusers (Bickel, Moody, et al., 2017). Third, delay discounting can function as a metric of addiction severity and correlates with all stages of addiction development. Fourth, delay discounting has been shown to change with effective treatment (Black & Rosen, 2011; Landes, Christensen, & Bickel, 2012; R. Yi et al., 2008). Moreover, delay discounting appears to function as a trans-disease process operating in a variety of disease states, such as obesity and problem gambling (Bickel, Jarmolowicz, Mueller, Koffarnus, & Gatchalian, 2012).
The CNDS approach generates testable predictions relevant to the development of novel treatments for opioid and other substance use. Because this approach posits that addiction results from hypoactivity of the executive-decision system or hyperactivity of the impulsive-decision system, any intervention that restores balance to the CNDS may improve self-regulatory behavior and facilitate substance abstinence. This restoration may be accomplished either by increasing relative control of the executive-decision system (including the parietal lobe and prefrontal cortex) or by decreasing relative control of the impulsive-decision system, including limbic (e.g., midbrain, amygdala, habenular commissure, and nucleus accumbens, including mesocorticolimbic dopamine) and paralimbic (e.g., insula and dorsal striatum) brain regions (Bickel et al., 2007). Moreover, because delay discounting provides a measure of the relative control of the impulsive and executive decision systems, it may be used as behavioral evidence that an intervention is operating on one or both of the dual decision systems.
Neuroeconomics-Based Interventions
Many existing interventions have been shown to affect substance use, such as medication-assisted treatments, cognitive behavioral therapy, and contingency management (Verdejo-Garcia, Alcázar-Córcoles, & Albein-Urios, 2019), although their influence on the CNDS—and delay discounting as an integrated measure of that dual system—has yet to be fully explored. Therefore, in the following sections, we restrict our review to neuroeconomic interventions that specifically target one or both of the dual decision systems in the CNDS. Below, we review evidence from interventions that are directed at modifying the relative control of these systems.
Interventions to increase control of the executive decision system
Episodic future thinking.
Episodic future thinking is a form of prospection that involves mental simulation of future events (Atance & O’Neill, 2001). The prevailing view suggests that episodic future thinking relies on episodic memory to allow dynamic retrieval of past events to generate novel simulations of the future (Schacter & Addis, 2007; Schacter, Benoit, & Szpunar, 2017). Neural evidence demonstrates that future thinking activates frontal cortices (Benoit & Schacter, 2015; Okuda et al., 2003) associated with the executive-decision system, including the dorsolateral prefrontal cortex. Moreover, impaired episodic future thinking is associated with poor executive function (de Vito et al., 2012), opioid use (Moustafa et al., 2018), and high rates of delay discounting (Bromberg, Wiehler, & Peters, 2015).
Most importantly, enhancement of episodic future thinking using a laboratory-based intervention designed to prompt individuals to generate detailed simulations of the future and vividly imagine these events has reliably been shown to reduce both delay discounting (Bromberg et al., 2015; Daniel, Stanton, & Epstein, 2013a, 2013b; O’Donnell, Daniel, & Epstein, 2017; Peters & Buchel, 2010; Stein et al., 2016) as well as addictive behavior and related phenomena, including overeating and valuation of high-energy-density fast foods in overweight and obese populations (Daniel, Said, Stanton, & Epstein, 2015; Daniel et al., 2013a, 2013b; Sze, Stein, Bickel, Paluch, & Epstein, 2017), cigarette smoking and valuation of cigarettes in smokers (Chiou & Wu, 2017; Stein, Tegge, Turner, & Bickel, 2018; Stein et al., 2016), and valuation of alcohol in problem drinkers (Bulley & Gullo, 2017; Snider, LaConte, & Bickel, 2016). For example, Figure 3 illustrates results of a recent study in which we showed that episodic future thinking, relative to a control condition, decreases delay discounting and the number of cigarette puffs participants self-administered during a laboratory smoking task (Stein et al., 2016). These data suggest that an episodic future thinking intervention remediates deficits in the ability to engage in prospection and restores regulatory balance to the CNDS. Adaptation of this intervention for use in clinical treatment (Sze, Daniel, Kilanowski, Collins, & Epstein, 2015) may reveal its efficacy for the treatment of opioid and other substance-use disorder.
Fig. 3.
Effects of an episodic future thinking (EFT) intervention on delay discounting (left) and the number of cigarette puffs earned during a self-administration task (right) in cigarette smokers. Data replotted from Stein et al. (2016).
Transcranial magnetic stimulation
TMS is a noninvasive brain-stimulation technique allowing selective neural excitation or inhibition by altering the frequency and placement of cortical stimulation (Hallett, 2000). Excitatory effects are achieved by applying high frequency or intermittent theta-burst frequency to the cortex. When used in this way, excitatory stimulation of the executive decision system (e.g., dorsolateral prefrontal cortex) reduces craving or consumption of cocaine (Camprodon, Martinez-Raga, Alonso-Alonso, Shih, & Pascual-Leone, 2007; Politi, Fauci, Santoro, & Smeraldi, 2008), cigarettes (Amiaz, Levy, Vainiger, Grunhaus, & Zangen, 2009; Eichhammer et al., 2003; Li et al., 2013; Pripfl, Tomova, Riecansky, & Lamm, 2014), and alcohol (Mishra, Nizamie, Das, & Praharaj, 2010). Although the neurobiological mechanism of these effects have yet to be fully explored and some studies report null effects (Herremans et al., 2012), these findings suggest that activation of the executive decision system via excitatory stimulation restores regulatory balance to the CNDS and facilitates self-regulation (e.g., resistance to substance cues), perhaps by remediating preexisting deficits in executive function (Goldstein et al., 2004; Moeller et al., 2010).
Additional work suggests that TMS protocols targeting frontal and prefrontal cortices, among other areas, produce analgesic effects (Borckardt et al., 2006; Canavero et al., 2002; de Andrade, Mhalla, Adam, Texeira, & Bouhassira, 2011; Mhalla et al., 2011; J. J. Taylor, Borckardt, & George, 2012) and have further been shown to reduce patients’ postsurgical need for prescription opioids to control pain (Borckardt et al., 2006). This effect, likely mediated by endogenous opioids (de Andrade et al., 2011; J. J. Taylor et al., 2012), suggests that TMS holds promise as an alternative to prescription opioid medication for analgesia and may, therefore, reduce the incidence of opioid dependence resulting from initial use of prescription opioids for pain management. Moreover, among existing opioid users, such stimulation protocols could be used as an alternative pain-management strategy that simultaneously reduces opioid craving (Sahlem et al., 2017).
Future work must be conducted to resolve the optimal stimulation parameters (e.g., frequency and duration of treatment), the long-term efficacy of various stimulation protocols, as well as the degree to which therapeutic effects generalize to opioid use (Sahlem et al., 2017). However, existing TMS evidence supports the CNDS approach and represents a promising area for future addiction research and treatment.
Working memory training
Working memory, the ability to store and manipulate information (Cowan, 2008), is an executive function mediated by the prefrontal cortex and is involved in higher-order cognitive processes such as attention, planning, and goal-directed behavior (Engle, Tuholski, Laughlin, & Conway, 1999; Miller & Cohen, 2001; Owen, Downes, Sahakian, Polkey, & Robbins, 1990). Training designed to increase working memory capacity has been shown to produce increases in activation of the prefrontal, frontal, and parietal regions (Olesen, Westerberg, & Klingberg, 2004). Moreover, impaired working memory capacity has been demonstrated in substance-dependent populations, including opioid users (Bechara & Martin, 2004; Vo, Schacht, Mintzer, & Fishman, 2014; Yan et al., 2014). We have demonstrated that working memory training decreases delay discounting in stimulant-dependent participants (Bickel, Yi, Landes, Hill, & Baxter, 2011), thus providing support for this approach to increase executive-system functionality. In addition, among alcohol-dependent participants showing excessive delay discounting, working memory training has been shown to improve the efficacy of episodic future thinking (Snider et al., 2017). Future work should examine the replicability of these working memory training effects on delay discounting, as well as its capacity to affect measures of substance use.
Interventions to decrease control of the impulsive-decision system
TMS.
Although relatively more research has been conducted examining manipulation of executive-system function, emerging TMS research suggests that impulsive-system function may also be targeted. As mentioned previously, TMS allows selective excitation or inhibition of targeted brain regions by altering the frequency and placement of cortical stimulation. Thus, inhibition of limbic and paralimbic areas comprising the impulsive-decision system represents one method to decrease the relative control of this system. Prior work in this area suggests that such an approach is feasible (Hanlon et al., 2015). Specifically, vulnerability to drug-related cues is likely mediated by hyperactivity of the impulsive-decision system (Ersche, Turton, et al., 2012; Moeller et al., 2010; Moreno-Lopez et al., 2012). Moreover, use of continuous theta-burst stimulation to the frontal lobe has been shown to selectively decrease activation in the medial prefrontal cortex and nucleus accumbens (Hanlon et al., 2015). Because drug craving is associated with elevated striatal dopamine, reducing activation of this circuit through TMS is a potential treatment strategy for reducing drug craving.
Just as TMS protocols could be applied to the prefrontal cortex to remediate preexisting deficits in executive function, or to the orbitofrontal cortex-nucleus accumbens region to reduce reward seeking, the same protocol, which is noninvasive and has no associated risks, could be applied to the insula to down-regulate its function and reduce urges and cravings. Targeting the insula, in combination with prefrontal-cortex stimulation or inhibition of any part of the impulsive system, could work synergistically to restore regulatory balance to the CNDS, facilitate self-regulation (e.g., resistance to substance cues), and decrease craving and urges. However, future research is needed to further explore the efficacy of this approach in substance-use treatment, including opioid use and opioid analgesia.
Current Status and Future Directions
Concerns over the opioid crisis, including record-high overdose-related deaths (Rudd, Aleshire, Zibbell, & Matthew Gladden, 2016), sets the occasion for the much-needed development of novel substance-use intervention approaches. The CNDS approach has been useful in developing and interpreting interventions to affect the neuroeconomic decision making of individuals with addiction. By positing that decision making is under the control of dual decision systems, which could be modulated by a third insula-related system, the CNDS identifies targets (e.g., dorsolateral prefrontal cortex, mesocorticolimbic/impulsive system, or insula), techniques (e.g., TMS), and training (e.g., working memory) for intervention. In the near future, technology and other novel approaches will be developed to further extend TMS (Hanlon, Philip, Price, Bickel, & Downar, 2019) and other neuromodulatory techniques, including ultrasound (Tufail, Yoshihiro, Pati, Li, & Tyler, 2011; Tyler, 2011) and transcranial direct-current stimulation (Charvet et al., 2015). In addition, other novel approaches are being developed, such as the laboratory-based use of narratives to alter delay discounting and valuation of drugs of abuse (Bickel, Moody, et al., 2017; Bickel, Stein, et al., 2017), on the basis of the observation that listening to narratives activates diverse neural structures associated with the CNDS. Future translational work should be directed at adapting these narrative methods for use in clinical intervention.
Footnotes
Declaration of Conflicting Interests
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
References
- Ainslie G, & Monterosso J (2003). Hyperbolic discounting as a factor in addiction: A critical analysis. In Vuchinich RE & Heather N (Eds.), Choice, behavioural economics and addiction (pp. 35–69). doi: 10.1016/B978-008044056-9/50043-9 [DOI] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, … Bruchas MR (2015). Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron, 87, 1063–1077. doi: 10.1016/j.neuron.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, & Zangen A (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction, 104, 653–660. doi: 10.1111/j.1360-0443.2008.02448.x [DOI] [PubMed] [Google Scholar]
- Amlung M, Sweet LH, Acker J, Brown CL, & MacKillop J (2014). Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addiction Biology, 19, 743–753. doi: 10.1111/adb.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, & MacKillop J (2017). Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction, 112, 51–62. doi: 10.1111/add.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, & Clark JD (2006). Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology, 104, 570–587. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atance CM, & O’Neill DK (2001). Episodic future thinking. Trends in Cognitive Sciences, 5, 533–539. doi: 10.1016/S1364-6613(00)01804-0 [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, & Wileyto EP (2009). Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence, 103, 99–106. doi: 10.1016/j.drugalcdep.2008.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman JG, Johnston LD, & O’Malley PM (1990). Explaining the recent decline in cocaine use among young adults: Further evidence that perceived risks and disapproval lead to reduced drug use. Journal of Health and Social Behavior, 31, 173–184. doi: 10.2307/2137171 [DOI] [PubMed] [Google Scholar]
- Bechara A (2004). The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition, 55, 30–40. [DOI] [PubMed] [Google Scholar]
- Bechara A (2005). Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience, 8, 1458–1463. [DOI] [PubMed] [Google Scholar]
- Bechara A, & Damasio H (2002). Decision-making and addiction (part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia, 40, 1675–1689. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, & Nathan PE (2001). Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia, 39, 376–389. doi: 10.1016/S0028-3932(00)00136-6 [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, & Hindes A (2002). Decision-making and addiction (part II): Myopia for the future or hypersensitivity to reward? Neuropsychologia, 40, 1690–1705. [DOI] [PubMed] [Google Scholar]
- Bechara A, & Martin EM (2004). Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology, 18, 152–162. doi: 10.1037/0894-4105.18.1.152 [DOI] [PubMed] [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. doi: 10.1016/j.neuropsychologia.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2012). From prediction error to incentive salience: Mesolimbic computation of reward motivation. European Journal of Neuroscience, 35, 1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2017). Is addiction a brain disease? Neuroethics, 10, 29–33. doi: 10.1007/s12152-016-9286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Kringelbach ML (2015). Pleasure systems in the brain. Neuron, 86, 646–664. doi: 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist, 71, 670–679. doi: 10.1037/amp0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson JM (1999). The neurobiology of pain. The Lancet, 353, 1610–1615. doi: 10.1016/S0140-6736(99)01313-6 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, & McClure SM (2012). Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology, 221, 361–387. doi: 10.1007/s00213-012-2689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, & Gatchalian KM (2012). Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacology & Therapeutics, 134, 287–297. doi: 10.1016/j.pharmthera.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, & Wilson AG (2014). The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology, 76, 518–527. doi: 10.1016/j.neuropharm.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, & Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction, 96, 73–86. doi: 10.1046/j.1360-0443.2001.961736.x [DOI] [PubMed] [Google Scholar]
- Bickel WK, Mellis AM, Snider SE, Athamneh LN, Stein JS, & Pope DA (2018). 21st century neurobehavioral theories of decision making in addiction: Review and evaluation. Pharmacology Biochemistry and Behavior, 164, 4–21. doi: 10.1016/j.pbb.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, & Pitcock JA (2007). Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence, 90, S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Moody LN, Eddy CR, & Franck CT (2017). Neurocognitive dysfunction in addiction: Testing hypotheses of diffuse versus selective phenotypic dysfunction with a classification-based approach. Experimental and Clinical Psychopharmacology, 25, 322–332. doi: 10.1037/pha0000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Snider SE, Quisenberry AJ, Stein JS, & Hanlon CA (2016). Competing neurobehavioral decision systems theory of cocaine addiction: From mechanisms to therapeutic opportunities. Progress in Brain Research, 223, 269–293. doi: 10.1016/bs.pbr.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Stein JS, Moody LN, Snider SE, Mellis AM, & Quisenberry AJ (2017). Toward narrative theory: Interventions for reinforcer pathology in health behavior. Impulsivity: How Time and Risk Influence Decision Making, 64, 227–267. doi: 10.1007/978-3-319-51721-6_8 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, & Baxter C (2011). Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry, 69, 260–265. doi: 10.1016/j.biopsych.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Zatorski P, Baranowska A, Ryglewicz D, & Sienkiewicz-Jarosz H (2010). Insular lesions and smoking cessation after first-ever ischemic stroke: A 3-month follow-up. Neuroscience Letters, 478, 161–164. doi: 10.1016/j.neulet.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Bjork JM, & Grant SJ (2009). Does traumatic brain injury increase risk for substance abuse? Journal of Neurotrauma, 26, 1077–1082. doi: 10.1089/neu.2008.0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AC, & Rosen MI (2011). A money management-based substance use treatment increases valuation of future rewards. Addictive Behaviors, 36, 125–128. doi: 10.1016/j.addbeh.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Wall MM, Okuda M, Wang S, Iza M, & Olfson M (2016). Pain as a predictor of opioid use disorder in a nationally representative sample. American Journal of Psychiatry, 173, 1189–1195. doi: 10.1176/appi.ajp.2016.15091179 [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’ Esposito M, & Fields HL (2007). Immediate reward bias in humans: Fronto-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. The Journal of Neuroscience, 27, 14383–14391. doi: 10.1523/Jneurosci.2551-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, & Benkelfat C (2006). Modeling sensitization to stimulants in humans: An [11C]raclopride/positron emission tomography study in healthy men. Archives of General Psychiatry, 63, 1386–1395. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR, … George MS (2006). Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology, 105, 557–562. doi: 10.1097/00000542-200609000-00020 [DOI] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Hutton S, & Joyce E (2005). Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: A pilot study. Journal of Neuropsychiatry and Clinical Neurosciences, 17, 417–420. doi: 10.1176/appi.neuropsych.17.3.417 [DOI] [PubMed] [Google Scholar]
- Bromberg U, Wiehler A, & Peters J (2015). Episodic future thinking is related to impulsive decision making in healthy adolescents. Child Development, 86, 1458–1468. doi: 10.1111/cdev.12390 [DOI] [PubMed] [Google Scholar]
- Bulley A, & Gullo MJ (2017). The influence of episodic foresight on delay discounting and demand for alcohol. Addictive Behaviors, 66, 1–6. doi: 10.1016/j.addbeh.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Cahill CM, Taylor AMW, Cook C, Ong E, Morón JA, & Evans CJ (2014). Does the kappa opioid receptor system contribute to pain aversion? Frontiers in Pharmacology, 5, Article 253. doi: 10.3389/fphar.2014.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Xue L, Grenier P, Magnussen C, Lecour S, & Olmstead MC (2013). Changes in morphine reward in a model of neuropathic pain. Behavioural Pharmacology, 24, 207–213. doi: 10.1097/FBP.0b013e3283618ac8 [DOI] [PubMed] [Google Scholar]
- Callesen MB, Scheel-Kruger J, Kringelbach ML, & Moller A (2013). A systematic review of impulse control disorders in Parkinson’s disease. Journal of Parkinson’s Disease, 3, 105–138. doi: 10.3233/JPD-120165 [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, & Pascual-Leone A (2007). One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug and Alcohol Dependence, 86, 91–94. doi: 10.1016/j.drugalcdep.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Canavero S, Bonicalzi V, Dotta M, Vighetti S, Asteggiano G, & Cocito D (2002). Transcranial magnetic cortical stimulation relieves central pain. Stereotactic and Functional Neurosurgery, 78, 192–196. doi: 10.1159/000068965 [DOI] [PubMed] [Google Scholar]
- Carlson RW, Kumar NN, Wong-Mckinstry E, Ayyagari S, Puri N, Jackson FK, & Shashikumar S (2012). Alcohol withdrawal syndrome. Critical Care Clinics, 28, 549–585. doi: 10.1016/J.CCC.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Castro DC, & Berridge KC (2014). Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting.” The Journal of Neuroscience, 34, 4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, … Bikson M (2015). Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: Guidelines for technology and protocols. Frontiers in Systems Neuroscience, 9, Article 26. doi: 10.3389/fnsys.2015.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou WB, & Wu WH (2017). Episodic future thinking involving the nonsmoking self can induce lower discounting and cigarette consumption. Journal of Studies on Alcohol and Drugs, 78, 106–112. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, & Portenoy RK (2009). Opioids for chronic non-cancer pain: Prediction and identification of aberrant drug-related behaviors: A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. The Journal of Pain, 10, 131–146. doi: 10.1016/j.jpain.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, … Deyo RA (2015). The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Annals of Internal Medicine, 162, 276–286. doi: 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, & Kurtz SP (2014). The changing face of heroin use in the United States. JAMA Psychiatry, 71, 821–826. doi: 10.1001/jamapsychiatry.2014.366 [DOI] [PubMed] [Google Scholar]
- Clark L, & Robbins TW (2002). Decision-making deficits in drug addiction. Trends in Cognitive Sciences, 6, 361–363. doi: 10.1016/s1364-6613(02)01960-5 [DOI] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, & Hutchison KE (2011). Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcoholism: Clinical and Experimental Research, 35, 1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Luo S, Hsu E, Ainslie G, Mather M, & Monterosso J (2014). Increased functional coupling between the left fronto-parietal network and anterior insula predicts steeper delay discounting in smokers. Human Brain Mapping, 35, 3774–3787. doi: 10.1002/hbm.22436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Canamar CP, Hillhouse M, & Ling W (2012). Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. The Journal of Pain, 13, 401–409. doi: 10.1016/j.jpain.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, & Ling W (2001). Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug and Alcohol Dependence, 63, 139–146. doi: 10.1016/S0376-8716(00)00200-3 [DOI] [PubMed] [Google Scholar]
- Compton WM, & Volkow ND (2006). Abuse of prescription drugs and the risk of addiction. Drug and Alcohol Dependence, 83(Suppl. 1), S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Cowan N (2008). What are the differences between long-term, short-term, and working memory? Progress in Brain Research, 169, 323–338. doi: 10.1016/S0079-6123(07)00020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig AD (2010). The sentient self. Brain Structure and Function, 214, 563–577. doi: 10.1007/s00429-010-0248-y [DOI] [PubMed] [Google Scholar]
- Crews F, He J, & Hodge C (2007). Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior, 86, 189–199. doi: 10.1016/J.PBB.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Crews FT, & Boettiger CA (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology Biochemistry and Behavior, 93, 237–247. doi: 10.1016/j.pbb.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW, … Yang XW (2014). Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nature Neuroscience, 17, 254–261. doi: 10.1038/nn.3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A (2016). Potent opioid analgesia without respiratory depression: Could it be possible? Anesthesiology, 125, 841–843. doi: 10.1097/ALN.0000000000001321 [DOI] [PubMed] [Google Scholar]
- Daniel TO, Said M, Stanton CM, & Epstein LH (2015). Episodic future thinking reduces delay discounting and energy intake in children. Eating Behaviors, 18, 20–24. doi: 10.1016/j.eatbeh.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, & Epstein LH (2013a). The future is now: Comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite, 71, 120–125. doi: 10.1016/j.appet.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, & Epstein LH (2013b). The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychological Science, 24, 2339–2342. doi: 10.1177/0956797613488780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Falck R, & Carlson RG (2012). “I’m not afraid of those ones just ‘cause they’ve been prescribed”: Perceptions of risk among illicit users of pharmaceutical opioids. International Journal of Drug Policy, 23, 374–384. doi: 10.1016/j.drugpo.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, & Carter JC (2009). Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite, 53, 1–8. [DOI] [PubMed] [Google Scholar]
- de Andrade DC, Mhalla A, Adam F, Texeira MJ, & Bouhassira D (2011). Neuropharmacological basis of rTMS-induced analgesia: The role of endogenous opioids. Pain, 152, 320–326. doi: 10.1016/j.pain.2010.10.032 [DOI] [PubMed] [Google Scholar]
- de Hoogd S, Ahlers SJGM, van Dongen EPA, van de Garde EMW, Hamilton-Ter Brake TAT, Dahan A, … Knibbe CAJ (2016). Is intraoperative remifentanil associated with acute or chronic postoperative pain after prolonged surgery? An update of the literature. The Clinical Journal of Pain, 32, 726–735. doi: 10.1097/AJP.0000000000000317 [DOI] [PubMed] [Google Scholar]
- de Vito S, Gamboz N, Brandimonte MA, Barone P, Amboni M, & Della Sala S (2012). Future thinking in Parkinson’s disease: An executive function? Neuropsychologia, 50, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, & Chou R (2016). CDC guideline for prescribing opioids for chronic pain–United States, 2016. Journal of the American Medical Association, 315, 1624–1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V, Read SJ, & Bechara A (2015). Revisiting the role of the insula in addiction. Trends in Cognitive Sciences, 19, 414–420. doi: 10.1016/j.tics.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Milkman KL, & Laibson D (2018). Beyond willpower: Strategies for reducing failures of self-control. Psychological Science in the Public Interest, 19, 102–129. doi: 10.1177/1529100618821893 [DOI] [PubMed] [Google Scholar]
- Dunn KE, Finan PH, Tompkins DA, Fingerhood M, & Strain EC (2015). Characterizing pain and associated coping strategies in methadone and buprenorphine-maintained patients. Drug and Alcohol Dependence, 157, 143–149. doi: 10.1016/j.drugalcdep.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, & Hajak G (2003). High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. Journal of Clinical Psychiatry, 64, 951–953. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Tong C, & Curry RS (2015). Failure of intrathecal ketorolac to reduce remifentanil-induced postinfusion hyperalgesia in humans. Pain, 156, 81–87. doi: 10.1016/j.pain.0000000000000005 [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, & Volkow ND (2013). Pain and suicidality: Insights from reward and addiction neuroscience. Progress in Neurobiology, 109, 1–27. doi: 10.1016/j.pneurobio.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, & Conway AR (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 128, 309–331. [DOI] [PubMed] [Google Scholar]
- Enkavi AZ, Eisenberg IW, Bissett PG, Mazza GL, MacKinnon DP, Marsch LA, & Poldrack RA (2019). Large-scale analysis of test-retest reliabilities of self-regulation measures. Proceedings of the National Academy of Sciences, USA, 116, 5472–5477. doi: 10.1073/pnas.1818430116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Sjøgren P, Bruera E, Ekholm O, & Rasmussen NK (2006). Critical issues on opioids in chronic non-cancer pain: An epidemiological study. Pain, 125, 172–179. doi: 10.1016/j.pain.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, & Bullmore ET (2012). Abnormal brain structure implicated in stimulant drug addiction. Science, 335, 601–604. doi: 10.1126/science.1214463 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, & Robbins TW (2012). Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry, 169, 926–936. doi: 10.1176/appi.ajp.2012.11091421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JS, & Stanovich KE (2013). Dual-process theories of higher cognition: Advancing the debate. Perspectives on Psychological Science, 8, 223–241. doi: 10.1177/1745691612460685 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, & Robbins TW (2008). Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Proceedings of the Royal Society B: Biological Sciences, 363, 3125–3135. doi: 10.1098/rstb.2008.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, & Robbins TW (2001). The neuropsychological basis of addictive behaviour. Brain Research Reviews, 36, 129–138. doi: 10.1016/s0165-0173(01)00088-1 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience, 8, 1481–1489. doi: 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2016). Drug addiction: Updating actions to habits to compulsions ten years on. Annual Review of Psychology, 67, 23–50. doi: 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Ezzatpanah S, Babapour V, Sadeghi B, & Haghparast A (2015). Chemical stimulation of the lateral hypothalamus by carbachol attenuated the formalin-induced pain behaviors in rats. Pharmacology Biochemistry and Behavior, 129, 105–110. doi: 10.1016/j.pbb.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, & Meyerhoff DJ (2002). Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug and Alcohol Dependence, 68, 87–93. doi: 10.1016/S0376-8716(02)00110-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, & Finn P (2004). Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcoholism: Clinical and Experimental Research, 28, 1487–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, & Margolis EB (2015). Understanding opioid reward. Trends in Neurosciences, 38, 217–225. doi: 10.1016/j.tins.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher D, & Martinez V (2014). Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. British Journal of Anaesthesia, 112, 991–1004. doi: 10.1093/bja/aeu137 [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, & O’Donoghue T (2002). Time discounting and time preference: A critical review. Journal of Economic Literature, 40, 351–401. doi: 10.1257/002205102320161311 [DOI] [Google Scholar]
- Friedman JH, & Chang V (2013). Crack cocaine use due to dopamine agonist therapy in Parkinson disease. Neurology, 80, 2269–2270. doi: 10.1212/WNL.0b013e318296e9d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan A, Chaparro LE, Irvin E, & Mailis-Gagnon A (2011). A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic non-cancer pain. Pain Research & Management, 16, 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, & Hester R (2007). The role of cognitive control in cocaine dependence. Neuropsychology Review, 17, 337–345. doi: 10.1007/s11065-007-9034-x [DOI] [PubMed] [Google Scholar]
- Gasquoine PG (2014). Contributions of the insula to cognition and emotion. Neuropsychology Review, 24, 77–87. doi: 10.1007/s11065-014-9246-9 [DOI] [PubMed] [Google Scholar]
- Gaznick N, Bechara A, & Tranel D (2014). Hemispheric side of damage influences sex-related differences in smoking cessation in neurological patients. Journal of Clinical and Experimental Neuropsychology, 36, 551–558. doi: 10.1080/13803395.2014.915012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaznick N, Tranel D, McNutt A, & Bechara A (2014). Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine & Tobacco Research, 16, 445–453. doi: 10.1093/ntr/ntt172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, & Brownell KD (2011). Neural correlates of food addiction. Archives of General Psychiatry, 68, 808–816. doi: 10.1001/archgenpsychiatry.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, & Koob GF (2012). Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiology & Behavior, 106, 58–64. doi: 10.1016/j.physbeh.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, & Badger GJ (2002). Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology, 163, 174–182. doi: 10.1007/s00213-002-1159-2 [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, & Ducci F (2005). The genetics of addictions: Uncovering the genes. Nature Reviews Genetics, 6, 521–532. doi: 10.1038/nrg1635 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, … Volkow ND (2004). Severity of neuropsychological impairment in cocaine and alcohol addiction: Association with metabolism in the prefrontal cortex. Neuropsychologia, 42, 1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12, 652–669. doi: 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollwitzer PM (2014). Weakness of the will: Is a quick fix possible? Motivation and Emotion, 38, 305–322. doi: 10.1007/s11031-014-9416-3 [DOI] [Google Scholar]
- Graham DP, & Cardon AL (2008). An update on substance use and treatment following traumatic brain injury. Annals of the New York Academy of Sciences, 1141, 148–162. doi: 10.1196/annals.1441.029 [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, & London ED (2000). Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia, 38, 1180–1187. doi: 10.1016/s0028-3932(99)00158-x [DOI] [PubMed] [Google Scholar]
- Grau LE, Dasgupta N, Grau LE, Dasgupta N, Harvey AP, Grau LE, … Heimer R (2007). Illicit use of opioids: Is OxyContin® a “gateway drug”? American Journal on Addictions, 16, 166–173. doi: 10.1080/10550490701375293 [DOI] [PubMed] [Google Scholar]
- Gray MA, & Critchley HD (2007). Interoceptive basis to craving. Neuron, 54, 183–186. doi: 10.1016/j.neuron.2007.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie ER (1935). The psychology of learning (1st ed.). New York, NY: Harper & Brothers. [Google Scholar]
- Haber SN, Fudge JL, & McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. The Journal of Neuroscience, 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Carter A, & Forlini C (2015). The brain disease model of addiction: Is it supported by the evidence and has it delivered on its promises? The Lancet Psychiatry, 2, 105–110. doi: 10.1016/S2215-0366(14)00126-6 [DOI] [PubMed] [Google Scholar]
- Hallett M (2000). Transcranial magnetic stimulation and the human brain. Nature, 406, 147–150. doi: 10.1038/35018000 [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, & George MS (2015). What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Research, 1628, 199–209. doi: 10.1016/j.brainres.2015.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Philip NS, Price RB, Bickel WK, & Downar J (2019). A case for the frontal pole as an empirically derived neuromodulation treatment target [Letter to the editor]. Biological Psychiatry, 85, e13–e14. doi: 10.1016/j.biopsych.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow KC (1990). Patterns of rates of mortality from narcotics and cocaine overdose in Texas, 1976–87. Public Health Reports, 105, 455–462. [PMC free article] [PubMed] [Google Scholar]
- Hartston H (2012). The case for compulsive shopping as an addiction. Journal of Psychoactive Drugs, 44, 64–67. [DOI] [PubMed] [Google Scholar]
- He QH, Huang XL, Turel O, Schulte M, Huang D, Thames A, … Hser YI (2018). Presumed structural and functional neural recovery after long-term abstinence from cocaine in male military veterans. Progress in NeuroPsychopharmacology & Biological Psychiatry, 84, 18–29. doi: 10.1016/j.pnpbp.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QH, Xue G, Chen CS, Lu ZL, Chen CH, Lei XM, … Bechara A (2012). COMT Val158Met polymorphism interacts with stressful life events and parental warmth to influence decision making. Scientific Reports, 2, Article 677. doi: 10.1038/srep00677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QH, Xue G, Chen CS, Lu ZL, Dong Q, Lei XM, … Bechara A (2010). Serotonin transporter gene-linked polymorphic region (5-HTTLPR) influences decision making under ambiguity and risk in a large Chinese sample. Neuropharmacology, 59, 518–526. doi: 10.1016/j.neuropharm.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, & Lumb BM (2009). Descending control of nociception: Specificity, recruitment and plasticity. Brain Research Reviews, 60, 214–225. doi: 10.1016/j.brainresrev.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, & De Raedt R (2012). No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: Results of a naturalistic study. Drug and Alcohol Dependence, 120, 209–213. doi: 10.1016/j.drugalcdep.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Herz A (1997). Endogenous opioid systems and alcohol addiction. Psychopharmacology, 129, 99–111. doi: 10.1007/s002130050169 [DOI] [PubMed] [Google Scholar]
- Hester R, & Garavan H (2004). Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of Neuroscience, 24, 11017–11022. doi: 10.1523/jneurosci.3321-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, & Whitney P (2003). Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29, 298–306. doi: 10.1037/0278-7393.29.2.298 [DOI] [PubMed] [Google Scholar]
- Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, … Morón JA (2015). Inflammatory pain promotes increased opioid self-administration: Role of dysregulated ventral tegmental area μ opioid receptors. The Journal of Neuroscience, 35, 12217–12231. doi: 10.1523/JNEUROSCI.1053-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W (1890). Principles of psychology. New York, NY: Holt H. [Google Scholar]
- Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517, 284–292. doi: 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, & Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward- related stimuli. Psychopharmacology, 146, 373–390. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, Wei YL, … Bechara A (2008). Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia, 46, 714–726. doi: 10.1016/j.neuropsychologia.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, & North RA (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of Neuroscience, 12, 483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso E, Edwards JE, Moore RA, & McQuay HJ (2004). Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain, 112, 372–380. doi: 10.1016/j.pain.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Khantzian EJ (1987). The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence In Allen DF (Ed.), The cocaine crisis (pp. 65–74). New York, NY: Springer; (Reprinted from American Journal of Psychiatry, Vol. 142, pp. 1259–1264) [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, & Hurt H (2013). Working memory ability predicts trajectories of early alcohol use in adolescents: The mediational role of impulsivity. Addiction, 108, 506–515. doi: 10.1111/add.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, McCullough ME, Bickel WK, Farley JP, & Longo GS (2015). Longitudinal associations among religiousness, delay discounting, and substance use initiation in early adolescence. Journal of Research on Adolescence, 25, 36–43. doi: 10.1111/jora.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, & Petry NM (2004). Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction, 99, 461–471. doi: 10.1111/j.1360-0443.2003.00669.x [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, & Bickel WK (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General, 128, 78–87. doi: 10.1037/0096-3445.128.1.78 [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, & Damasio AR (2007). Damage to the prefrontal cortex increases utilitarian moral judgments. Nature, 446, 908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru A, Satyanarayana S, & Rizwan S (2009). Endogenous opioids: Their physiological role and receptors. Global Journal of Pharmacology, 3, 149–153. [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug abuse: Hedonic homeostatic dysregulation. Science, 278(5335), 52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35, 217–238. doi: 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpetz CE, Lejuez CW, Wiers RW, & Kruglanski AW (2013). Motivation and self-regulation in addiction: A call for convergence. Perspectives on Psychological Science, 8, 3–24. doi: 10.1177/1745691612457575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashin D, Murinova N, & Sullivan M (2016). Challenges to treatment of chronic pain and addiction during the “opioid crisis.” Current Pain and Headache Reports, 20(12), Article 65. doi: 10.1007/s11916-016-0596-2 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience, 6, 691–702. doi: 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, … Potenza MN (2007). Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence, 88, 79–82. doi: 10.1016/j.drugalcdep.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landes RD, Christensen DR, & Bickel WK (2012). Delay discounting decreases in those completing treatment for opioid dependence. Experimental and Clinical Psychopharmacology, 20, 302–309. doi: 10.1037/a0027391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Rivot JP, Dickenson AH, Chaouch A, & Besson JM (1980). Rôle de la sérotonine dans les contrôles inhibiteurs diffus induits par des stimulations nociceptives [Role of serotonin in the diffuse inhibitory controls induced by nociceptive stimulation]. Comptes Rendus des Séances de l'Académie Des Sciences. Série D, Sciences Naturelles, 290, 379–382. [PubMed] [Google Scholar]
- Lee Y-A, & Goto Y (2011). Neurodevelopmental disruption of cortico-striatal function caused by degeneration of habenula neurons. PLOS ONE, 6(4), Article e19450. doi: 10.1371/journal.pone.0019450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, … Negus SS (2014). Pain-related depression of the mesolimbic dopamine system in rats: Expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology, 39, 614–624. doi: 10.1038/npp.2013.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, & Negus SS (2014). Sustained pain-related depression of behavior: Effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Molecular Pain, 10. doi: 10.1186/1744-8069-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, & Tracey I (2008). A common neurobiology for pain and pleasure. Nature Reviews. Neuroscience, 9, 314–320. doi: 10.1038/nrn2333 [DOI] [PubMed] [Google Scholar]
- Leshner AI (1997). Addiction is a brain disease, and it matters. Science, 278(5335), 45–47. [DOI] [PubMed] [Google Scholar]
- Lewis M (2011). Memoirs of an addicted brain. New York, NY: PublicAffairs Perseus Books. [Google Scholar]
- Lewis M (2017). Addiction and the brain: Development, not disease. Neuroethics, 10, 7–18. doi: 10.1007/s12152-016-9293-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, & Vezina P (2013). Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neuroscience & Biobehavioral Reviews, 37(9, Pt. A), 1999–2014. doi: 10.1016/j.neubiorev.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, & Vezina P (2014). Dopamine ups and downs in vulnerability to addictions: A neurodevelopmental model. Trends in Pharmacological Sciences, 35, 268–276. doi: 10.1016/j.tips.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XB, Hartwell KJ, Owens M, LeMatty T, Borckardt JJ, Hanlon CA, & George MS (2013). Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biological Psychiatry, 73, 714–720. doi: 10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet J, Mouridsen K, Peterson E, Møller A, Doudet DJ, & Gjedde A (2012). Striatal dopamine release codes uncertainty in pathological gambling. Psychiatry Research: Neuroimaging, 204, 55–60. doi: 10.1016/j.pscychresns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Lipari RN, Ahrnsbrak RD, Pemberton MR, & Porter JD (2017, September). Risk and protective factors and estimates of substance use initiation: Results from the 2016 National Survey on Drug Use and Health. CBHSQ Data Review. Rockville, MD: Substance Abuse and Mental Health Services Administration; Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK481723 [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, & London ED (1998). Smaller volume of prefrontal lobe in polysub-stance abusers: A magnetic resonance imaging study. Neuropsychopharmacology, 18, 243–252. doi: 10.1016/S0893-133X(97)00143-7 [DOI] [PubMed] [Google Scholar]
- Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, … Napadow V (2014). Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis & Rheumatology, 66, 203–212. doi: 10.1002/art.38191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, & Weinstein A (2000). Orbitofrontal cortex and human drug abuse: Functional imaging. Cerebral Cortex, 10, 334–342. doi: 10.1093/cercor/10.3.334 [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, & Shaham Y (2004). Incubation of cocaine craving after withdrawal: A review of preclinical data, 47(Suppl. 1), 214–226. doi: 10.1016/j.neuropharm.2004.06.027 [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL, Heavner JE, Iacono CU, & Garvin RD (1989). Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sciences, 45, 2217–2224. doi: 10.1016/0024-3205(89)90062-3 [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, & Munafo MR (2011). Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology, 216, 305–321. doi: 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, & Bickel WK (1997). Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology, 5, 256–262. doi: 10.1037//1064-1297.5.3.256 [DOI] [PubMed] [Google Scholar]
- Maloney EM, Djamshidian A, & O’Sullivan SS (2017). Phenomenology and epidemiology of impulsive-compulsive behaviours in Parkinson’s disease, atypical Parkinsonian disorders and non-Parkinsonian populations. Journal of the Neurological Sciences, 374, 47–52. doi: 10.1016/j.jns.2016.12.058 [DOI] [PubMed] [Google Scholar]
- Mao J (2002). Opioid-induced abnormal pain sensitivity: Implications in clinical opioid therapy. Pain, 100, 213–217. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, & Eisenach JC (2004). Effects of laparotomy on spon-taneous exploratory activity and conditioned operant responding in the rat: A model for postoperative pain. Anesthesiology, 101, 191–203. [DOI] [PubMed] [Google Scholar]
- Martin TJ, & Ewan E (2008). Chronic pain alters drug self-administration: Implications for addiction and pain mechanisms. Experimental and Clinical Psychopharmacology, 16, 357–366. doi: 10.1037/a0013597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Moron JA, & Al-Hasani R (2016). A trigger for opioid misuse: Chronic pain and stress dysregulate the mesolimbic pathway and kappa opioid system. Frontiers in Neuroscience, 10, Article 480. doi: 10.3389/fnins.2016.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, & Bickel WK (2014). A dual-systems perspective on addiction: Contributions from neuroimaging and cognitive training. Addiction Reviews, 1327, 62–78. doi: 10.1111/nyas.12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, & Cohen JD (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306, 503–507. doi: 10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- McDougle SD, Bond KM, & Taylor JA (2015). Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. The Journal of Neuroscience, 35, 9568–9579. doi: 10.1523/JNEUROSCI.5061-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, & Marksteiner J (2007). A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. Journal of Neurology, Neurosurgery, and Psychiatry, 78, 610–614. doi: 10.1136/jnnp.2006.095869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhalla A, Baudic S, de Andrade DC, Gautron M, Perrot S, Teixeira MJ, … Bouhassira D (2011). Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain, 152, 1478–1485. doi: 10.1016/j.pain.2011.01.034 [DOI] [PubMed] [Google Scholar]
- Miech RA, Chilcoat H, & Harder VS (2005). The increase in the association of education and cocaine use over the 1980s and 1990s: Evidence for a “historical period” effect. Drug and Alcohol Dependence, 79, 311–320. doi: 10.1016/J.DRUGALCDEP.2005.01.022 [DOI] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, & Peake PK (1988). The nature of adolescent competencies predicted by preschool delay of gratification. Journal of Personality and Social Psychology, 54, 687–696. doi: 10.1037/0022-3514.54.4.687 [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, & Praharaj SK (2010). Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: A sham-controlled study. Addiction, 105, 49–55. doi: 10.1111/.1360-0443.2009.02777.x [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma LS, Liu SJ, Kjome KL, … Narayana PA (2010). Working memory fMRI activation in cocaine-dependent subjects: Association with treatment response. Psychiatry Research-Neuroimaging, 181, 174–182. doi: 10.1016/j.pscychresns.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, & Verdejo-Garcia A (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence, 125, 208–214. doi: 10.1016/j.drugalcdep.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Morris AN, Nandrino JL, Misiak B, Szewczuk-Boguslawska M, Frydecka D, & El Haj M (2018). Not all drugs are created equal: Impaired future thinking in opiate, but not alcohol, users. Experimental Brain Research, 236, 2971–2981. doi: 10.1007/s00221-018-5355-7 [DOI] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2009). The hidden island of addiction: The insula. Trends in Neurosciences, 32, 56–67. doi: 10.1016/J.TINS.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2010). The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function, 214, 435–450. doi: 10.1007/s00429-010-0268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, & Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315, 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, & Suzuki T (2005). Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology, 30, 111–118. doi: 10.1038/sj.npp.1300527 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2017). Pain management and the opioid epidemic: Balancing societal and individual benefits and risks of prescription opioid use. Washington, DC: The National Academies Press. doi: 10.17226/24781 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2019. March 20). Medications to treat opioid addiction are effective and save lives, but barriers prevent broad access and use, says new report [Press release]. Washington, DC: The National Academies Press. [Google Scholar]
- National Institute on Drug Abuse. (2016). What is the scope of cocaine use in the United States? Retrieved from https://www.drugabuse.gov/publications/research-reports/cocaine/what-scope-cocaine-use-in-united-states
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, … Porreca F (2015). Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. The Journal of Neuroscience, 35, 7264–7271. doi: 10.1523/JNEUROSCI.3862-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, … Porreca F (2012). Pain relief produces negative reinforcement through activation of mesolimbic reward–valuation circuitry. Proceedings of the National Academy of Sciences, USA, 109, 20709–20713. doi: 10.1073/pnas.1214605109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Narita M, Butelman ER, Kreek MJ, & Suzuki T (2010). Neuropathic and chronic pain stimuli down-regulate central mu-opioid and dopaminergic transmission. Trends in Pharmacological Sciences, 31, 299–305. doi: 10.1016/j.tips.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Noël X, Brevers D, & Bechara A (2013). A triadic neurocognitive approach to addiction for clinical interventions. Frontiers in Psychiatry, 4, Article 179. doi: 10.3389/fpsyt.2013.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Jaafari N, & Bechara A (2017). Addictive behaviors: Why and how impaired mental time matters? Progress in Brain Research, 235, 219–237. doi: 10.1016/bs.pbr.2017.07.011 [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Daniel TO, & Epstein LH (2017). Does goal relevant episodic future thinking amplify the effect on delay discounting? Consciousness and Cognition, 51, 10–16. doi: 10.1016/j.concog.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL (2011). Delay discounting: I’m a k, you’re a k. Journal of the Experimental Analysis of Behavior, 96, 427–439. doi: 10.1901/jeab.2011.96-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, & Bickel WK (2000). Needle sharing in opioid-dependent outpatients: Psychological processes underlying risk. Drug and Alcohol Dependence, 60, 259–266. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, … Yamadori A (2003). Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. NeuroImage, 19, 1369–1380. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, & Klingberg T (2004). Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience, 7, 75–79. doi: 10.1038/nn1165 [DOI] [PubMed] [Google Scholar]
- Ondo WG, & Lai D (2008). Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism and Related Disorders, 14, 28–32. doi: 10.1016/j.parkreldis.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, & Robbins TW (1990). Planning and spatial working memory following frontal-lobe lesions in man. Neuropsychologia, 28, 1021–1034. doi: 10.1016/0028-3932(90)90137-D [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, & Suzuki T (2002). Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: Implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. Journal of Neurochemistry, 82, 1192–1198. [DOI] [PubMed] [Google Scholar]
- Palmer P, Ji X, & Stephens J (2014). Cost of opioid intravenous patient-controlled analgesia: Results from a hospital database analysis and literature assessment. Clinicoeconomics and Outcomes Research: CEOR, 6, 311–318. doi: 10.2147/CEOR.S64077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, & Duvarci S (2012). Amygdala microcircuits mediating fear expression and extinction. Current Opinion in Neurobiology, 22, 717–723. doi: 10.1016/j.conb.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, & Robinson TE (1995). Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: A microdialysis study in behaving rats. Synapse, 19, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Sason A, Tene O, Domany Y, Schreiber S, & Adelson M (2015). Ten years of abstinence in former opiate addicts: Medication-free non-patients compared to methadone maintenance patients. Journal of Addictive Diseases, 34, 284–295. doi: 10.1080/10550887.2015.1074502 [DOI] [PubMed] [Google Scholar]
- Peters J, & Buchel C (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron, 66, 138–148. doi: 10.1016/j.neuron.2010.03.026 [DOI] [PubMed] [Google Scholar]
- Pickard H (2017). Responsibility without blame for addiction. Neuroethics, 10, 169–180. doi: 10.1007/s12152-016-9295-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, & Smeraldi E (2008). Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. American Journal on Addictions, 17, 345–346. doi: 10.1080/10550490802139283 [DOI] [PubMed] [Google Scholar]
- Politis M, Loane C, Wu K, O’Sullivan SS, Woodhead Z, Kiferle L, … Piccini P (2013). Neural response to visual sexual cues in dopamine treatment-linked hyper-sexuality in Parkinson’s disease. Brain, 136(Pt. 2), 400–411. doi: 10.1093/brain/aws326 [DOI] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, & Lamm C (2014). Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimulation, 7, 226–233. doi: 10.1016/j.brs.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Rainville P (2002). Brain mechanisms of pain affect and pain modulation. Current Opinion in Neurobiology, 12, 195–204. doi: 10.1016/S0959-4388(02)00313-6 [DOI] [PubMed] [Google Scholar]
- Ray N, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, … Strafella AP (2012). Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiology of Disease, 48, 519–525. doi: 10.1016/j.nbd.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z-Y, Shi J, Epstein DH, Wang J, & Lu L (2009). Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology, 204, 423–429. doi: 10.1007/s00213-009-1472-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DV (1969). Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science, 164, 444–445. doi: 10.1126/science.164.3878.444 [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews, 18, 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2003). Addiction. Annual Review of Psychology, 54, 25–53. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology, 47, 33–46. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, … Robbins TW (1999). Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology, 20, 322–339. doi: 10.1016/s0893-133x(98)00091-8 [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Matthew Gladden R (2016). Increases in drug and opioid overdose deaths-United States, 2000–2014. American Journal of Transplantation, 16, 1323–1327. doi: 10.1111/ajt.13776 [DOI] [PubMed] [Google Scholar]
- Sahlem GL, Breedlove J, Taylor JJ, Badran BA, Lauer A, George MS, … Hanlon CA (2017). Dorsolateral prefrontal cortex transcranial magnetic stimulation as a tool to decrease pain and craving in opiate dependent individuals: A pilot study of feasibility and effect size. Brain Stimulation, 10, 482. [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, & Knutson B (2008). Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychological Science, 19, 320–323. doi: 10.1111/j.1467-9280.2008.02087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, & Addis DR (2007). On the constructive episodic simulation of past and future events. Behavioral & Brain Sciences, 30, 331–332. doi: [DOI] [Google Scholar]
- Schacter DL, Benoit RG, & Szpunar KK (2017). Episodic future thinking: Mechanisms and functions. Current Opinion in Behavioral Sciences, 17, 41–50. doi: 10.1016/j.cobeha.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltenwolf M, Akbar M, Hug A, Pfüller U, Gantz S, Neubauer E, … Wang H (2014). Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician, 17, 9–20. [PubMed] [Google Scholar]
- Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, … Walter M (2014). Acute effects of heroin on negative emotional processing: Relation of amygdala activity and stress-related responses. Biological Psychiatry, 76, 289–296. doi: 10.1016/j.biopsych.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chang CY, Lucantonio F, & Takahashi YK (2016). Thinking outside the box: Orbitofrontal cortex, imagination, and how we can treat addiction. Neuropsychopharmacology, 41, 2966–2976. doi: 10.1038/npp.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, & Stalnaker TA (2006). Orbitofrontal cortex, decision-making and drug addiction. Trends in Neurosciences, 29, 116–124. doi: 10.1016/j.tins.2005.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, & Shaham Y (2008). The role of orbitofrontal cortex in drug addiction: A review of preclinical studies. Biological Psychiatry, 63, 256–262. doi: 10.1016/J.BIOPSYCH.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RSJ, Friston KJ, & Dolan RJ (2000). Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug and Alcohol Dependence, 60, 207–216. doi: 10.1016/S0376-8716(99)00158-1 [DOI] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, & Bacon S (2018). Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morbidity and Mortality Weekly Report, 67, 349–358. doi: 10.15585/mmwr.mm6712a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, & Dolan R (2007). Differential encoding of losses and gains in the human striatum. The Journal of Neuroscience, 27, 4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, & Herz A (1988). Motivational effects of opioids in an animal model of prolonged inflammatory pain: Alteration in the effects of kappa- but not of mu-receptor agonists. Pain, 35, 179–186. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, & Preuschoff K (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13, 334–340. doi: 10.1016/j.tics.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141, 105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, & Graybiel AM (2016). Habit formation. Dialogues in Clinical Neuroscience, 18, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Deshpande HU, Lisinski JM, Koffarnus MN, LaConte SM, & Bickel WK (2017). Working memory training improves alcohol users’ episodic future thinking: A rate-dependent analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 160–167. doi: 10.1016/j.bpsc.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, LaConte SM, & Bickel WK (2016). Episodic future thinking: Expansion of the temporal window in individuals with alcohol dependence. Alcoholism: Clinical and Experimental Research, 40, 1558–1566. doi: 10.1111/acer.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani M, & Bechara A (2011). A somatic marker perspective of immoral and corrupt behavior. Social Neuroscience, 6, 640–652. doi: 10.1080/17470919.2011.605592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD (1973). An opponent-process theory of motivation. II. Cigarette addiction. Journal of Abnormal Psychology, 81, 158–171. [DOI] [PubMed] [Google Scholar]
- Solomon RL, & Corbit JD (1974). An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review, 81, 119–145. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, & Shippenberg TS (1992). Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proceedings of the National Academy of Sciences, USA, 89, 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Tegge AN, Turner JK, & Bickel WK (2018). Episodic future thinking reduces delay discounting and cigarette demand: An investigation of the good-subject effect. Journal of Behavioral Medicine, 41, 269–276. doi: 10.1007/s10865-017-9908-1 [DOI] [PubMed] [Google Scholar]
- Stein JS, Wilson AG, Koffarnus MN, Daniel TO, Epstein LH, & Bickel WK (2016). Unstuck in time: Episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology, 233, 3771–3778. doi: 10.1007/s00213-016-4410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, & Kalivas PW (2011). Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Reviews, 63, 348–365. doi: 10.1124/pr.109.001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack F, & Deutsch R (2004). Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review, 8, 220–247. doi: 10.1207/s15327957pspr0803_1 [DOI] [PubMed] [Google Scholar]
- Suner-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Davalos A, … Serena J (2012). Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke, 43, 131–136. doi: 10.1161/strokeaha.111.630004 [DOI] [PubMed] [Google Scholar]
- Sze YY, Daniel TO, Kilanowski CK, Collins RL, & Epstein LH (2015). Web-based and mobile delivery of an episodic future thinking intervention for overweight and obese families: A feasibility study. JMIR mHealth and uHealth, 3(4), Article e97. doi: 10.2196/mhealth.4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze YY, Stein JS, Bickel WK, Paluch RA, & Epstein LH (2017). Bleak present, bright future: Online episodic future thinking, scarcity, delay discounting, and food demand. Clinical Psychological Science, 5, 683–697. doi: 10.1177/2167702617696511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, & Di Chiara G (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science, 276, 2048–2050. doi: 10.1126/SCIENCE.276.5321.2048 [DOI] [PubMed] [Google Scholar]
- Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, … Cahill CM (2015). Microglia disrupt mesolimbic reward circuitry in chronic pain. The Journal of Neuroscience, 35, 8442–8450. doi: 10.1523/JNEUROSCI.4036-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, & George MS (2012). Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain, 153, 1219–1225. doi: 10.1016/j.pain.2012.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33, 81–88. doi: 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, … London ED (2004). Structural abnormalities in the brains of human subjects who use methamphetamine. The Journal of Neuroscience, 24, 6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I (2010). Getting the pain you expect: Mechanisms of placebo, nocebo and reappraisal effects in humans. Nature Medicine, 16, 1277–1283. doi: 10.1038/nm.2229 [DOI] [PubMed] [Google Scholar]
- Tracey I, & Mantyh PW (2007). The cerebral signature for pain perception and its modulation. Neuron, 55, 377–391. doi: 10.1016/j.neuron.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, & Cahill CM (2015). Pain and poppies: The good, the bad, and the ugly of opioid analgesics. The Journal of Neuroscience, 35, 13879–13888. doi: 10.1523/JNEUROSCI.2711-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Yoshihiro A, Pati S, Li MM, & Tyler WJ (2011). Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nature Protocols, 6, 1453–1470. doi: 10.1038/nprot.2011.371 [DOI] [PubMed] [Google Scholar]
- Turel O, & Bechara A (2016). A triadic reflective-impulsive-interoceptive awareness model of general and impulsive information system use: Behavioral tests of neuro-cognitive theory. Frontiers in Psychology, 7, Article 601. doi: 10.3389/fpsyg.2016.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ (2011). Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist, 17, 25–36. doi: 10.1177/1073858409348066 [DOI] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, & Barrot M (2013). The amygdala between sensation and affect: A role in pain. Journal of Molecular Psychiatry, 1, Article 9. doi: 10.1186/2049-9256-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Alcázar-Córcoles MA, & Albein-Urios N (2019). Neuropsychological interventions for decision-making in addiction: A systematic review. Neuropsychology Review, 29, 79–92. doi: 10.1007/s11065-018-9384-6 [DOI] [PubMed] [Google Scholar]
- Vo HT, Schacht R, Mintzer M, & Fishman M (2014). Working memory impairment in cannabis- and opioid-dependent adolescents. Substance Abuse, 35, 387–390. doi: 10.1080/08897077.2014.954027 [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Fowler JS (2000). Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral Cortex, 10, 318–325. doi: 10.1093/cer-cor/10.3.318 [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Koob G (2015). Brain disease model of addiction: Why is it so controversial? Lancet Psychiatry, 2, 677–679. doi: 10.1016/S2215-0366(15)00236-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic advances from the brain disease model of addiction. The New England Journal of Medicine, 374, 363–371. doi: 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M (2013). Opioids for chronic musculoskeletal pain: Putting patient safety first. Pain, 154, 2583–2585. doi: 10.1016/j.pain.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon P, Callon C, Nguyen P, Dobrer S, Montaner J, Wood E, & Kerr T (2014). Self-management of pain among people who inject drugs in Vancouver. Pain Management, 4, 27–35. doi: 10.2217/pmt.13.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Mole TB, Banca P, Porter L, Morris L, Mitchell S, … Irvine M (2014). Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLOS ONE, 9(7), Article e102419. doi: 10.1371/journal.pone.0102419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, & van der Goes DN (2015). Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain, 156, 569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1 [DOI] [PubMed] [Google Scholar]
- Wade CL, Krumenacher P, Kitto KF, Peterson CD, Wilcox GL, & Fairbanks CA (2013). Effect of chronic pain on fentanyl self-administration in mice. PLOS ONE, 8(11), Article e79239. doi: 10.1371/journal.pone.0079239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KJ, Sprenger T, Kochs EF, Tölle TR, Valet M, & Willoch F (2007). Imaging human cerebral pain modulation by dose-dependent opioid analgesia: A positron emission tomography activation study using remifentanil. Anesthesiology, 106, 548–556. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, & Bickel WK (2014). Remember the future II: Meta-analyses and functional overlap of working memory and delay discounting. Invited meta-analyses. Biological Psychiatry, 75, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willpower. (1999). In: Soukhanov A (Ed.), Encarta World English Dictionary. New York, NY: St. Martin’s Press. [Google Scholar]
- Wise RA (1996). Neurobiology of addiction. CurrentOpinion in Neurobiology, 6, 243–251. doi: 10.1016/S0959-4388(96)80079-1 [DOI] [PubMed] [Google Scholar]
- Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, … Tracey I (2002). Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. NeuroImage, 16, 999–1014. doi: 10.1006/nimg.2002.1146 [DOI] [PubMed] [Google Scholar]
- Wolf ME (2010). The Bermuda Triangle of cocaine-induced neuroadaptations. Trends in Neurosciences, 33, 391–398. doi: 10.1016/j.tins.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME (2016). Synaptic mechanisms underlying persistent cocaine craving. Nature Reviews Neuroscience, 17, 351–365. doi: 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Politis M, O’Sullivan SS, Lawrence AD, Warsi S, Lees A, & Piccini P (2014). Problematic internet use in Parkinson’s disease. Parkinsonism and Related Disorders, 20, 482–487. doi: 10.1016/j.parkreldis.2014.01.019 [DOI] [PubMed] [Google Scholar]
- Wu Y, Na X, Zang Y, Cui Y, Xin W, Pang R, … Liu X (2014). Upregulation of tumor necrosis factor-alpha in nucleus accumbens attenuates morphine-induced rewarding in a neuropathic pain model. Biochemical and Biophysical Research Communications, 449, 502–507. doi: 10.1016/j.bbrc.2014.05.025 [DOI] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Cen S, Grenard JL, Stacy AW, Gallaher P, … Johnson CA (2008). Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th-grade Chinese adolescent smokers. Nicotine & Tobacco Research, 10, 1085–1097. doi: 10.1080/14622200802097530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Grenard J, Stacy WA, Palmer P, Wei YL, … Johnson CA (2009). Affective decision-making predictive of Chinese adolescent drinking behaviors. Journal of the International Neuropsychological Society, 15, 547–557. doi: 10.1017/s1355617709090808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Palmer PH, Trinidad DR, Wei YL, Jia Y, & Johnson CA (2011). Parent-child engagement in decision-making and the development of adolescent affective decision capacity and binge-drinking. Personality and Individual Differences, 51, 285–292. doi: 10.1016/j.paid.2010.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL (1987). Spinal opiates: A review of their effect on spinal function with emphasis on pain processing. Acta Anaesthesiologica Scandinavica, 31(Suppl. 85), 25–37. doi: 10.1111/j.1399-6576.1987.tb02667.x [DOI] [PubMed] [Google Scholar]
- Yan WS, Li YH, Xiao L, Zhu N, Bechara A, & Sui N (2014). Working memory and affective decision-making in addiction: A neurocognitive comparison between heroin addicts, pathological gamblers and healthy controls. Drug and Alcohol Dependence, 134, 194–200. doi: 10.1016/j.drugalcdep.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Yi P, & Pryzbylkowski P (2015). Opioid Induced Hyperalgesia. Pain Medicine, 16(Suppl. 1), S32–S36. doi: 10.1111/pme.12914 [DOI] [PubMed] [Google Scholar]
- Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, & Bickel WK (2008). The effects of reduced cigarette smoking on discounting future rewards: An initial evaluation. Psychological Record, 58, 163–174. doi: 10.1007/Bf03395609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, & Damasio A (2010). Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron, 65, 845–851. doi: 10.1016/j.neuron.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, … Weng X (2009). Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain and Cognition, 71, 223–228. doi: 10.1016/J.BANDC.2009.08.014 [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Huang AS, Alia-Klein N, & Goldstein RZ (2018). Neuroimaging impaired response inhibition and salience attribution in human drug addiction: A systematic review. Neuron, 98, 886–903. doi: 10.1016/j.neuron.2018.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]