Abstract
PURPOSE
Cardiovascular disease (CVD) is a major source of morbidity and mortality among breast cancer survivors. Although body mass index (BMI) is associated with CVD risk, adipose tissue distribution may better identify patients with a high risk of CVD after breast cancer.
METHODS
Among 2,943 patients with nonmetastatic breast cancer without prior CVD, we used International Classification of Diseases (9th and 10th revisions) codes to identify incidence of nonfatal stroke, myocardial infarction, heart failure, or CVD death. From clinically acquired computed tomography scans obtained near diagnosis, we measured visceral adiposity (centimeters squared), subcutaneous adiposity (centimeters squared), and intramuscular adiposity (fatty infiltration into muscle [Hounsfield Units, scored inversely]). We estimated hazard ratios (HRs) and 95% CIs per SD increase in adiposity accounting for competing risks and adjusting for demographics, smoking, cancer treatment, and pre-existing CVD risk factors.
RESULTS
Mean (SD) age was 56 (12) years. Over a median follow-up of 6 years, 328 CVD events occurred. Each SD increase in visceral or intramuscular adiposity was associated with an increase in CVD risk (HR, 1.15 [95% CI, 1.03 to 1.29] and HR, 1.21 [95% CI, 1.06 to 1.37]), respectively). Excess visceral and intramuscular adiposity occurred across all BMI categories. Among normal-weight patients, each SD greater visceral adiposity increased CVD risk by 70% (HR, 1.70 [95% CI, 1.10 to 2.62]).
CONCLUSION
Visceral and intramuscular adiposity were associated with increased CVD incidence after breast cancer diagnosis, independent of pre-existing CVD risk factors and cancer treatments. The increased CVD incidence among normal-weight patients with greater visceral adiposity would go undetected with BMI alone. Measures of adipose tissue distribution may help identify high-risk patients and tailor CVD prevention strategies.
INTRODUCTION
Improvements in early detection and treatment have improved prognosis for the more than 3.4 million women in the United States who are living with breast cancer.1 For older patients with early-stage disease, the risk of death from cardiovascular disease (CVD) is now higher than the risk of cancer recurrence.2,3 Breast cancer survivors may be at increased risk of CVD relative to women without a cancer history because of their high burden of pre-existing CVD risk factors combined with the direct (eg, radiation-induced cardiovascular injury and the cardiotoxic effects of systemic therapies) and the indirect (eg, deconditioning and physical inactivity) effects of cancer treatment.4,5
It is well known that higher body mass index (BMI) is associated with CVD mortality in the general population. However, BMI is not always an accurate proxy for individual level adiposity and does not describe adipose tissue distribution. In populations without cancer, visceral (intra-abdominal) and intramuscular (triglyceride accumulation within skeletal muscle cells) adiposity have the strongest associations with CVD,6-11 hypertension, diabetes, and metabolic syndrome.12-16 Prior research among breast cancer survivors has examined only CVD mortality and BMI or waist circumference, surrogate measures of overall and central adiposity, without measuring body composition, nonfatal CVD events, or CVD subtypes with differing etiology.17,18 Furthermore, in addition to overall adiposity, the location of the adipose tissue (eg, visceral, subcutaneous, or intramuscular) may be crucial to identifying patients with high CVD risk.
In the current study of 2,943 patients with nonmetastatic breast cancer without prior CVD, we quantified visceral, subcutaneous, and intramuscular adiposity from clinically acquired computed tomography (CT) images and examined associations with incident CVD, adjusting for pre-existing risk factors (diabetes, dyslipidemia, and hypertension) and receipt of chemotherapy or radiation. Second, we examined whether cancer treatment type (eg, anthracycline-containing chemotherapy, which has known cardiotoxic effects) modified these associations.
METHODS
The Breast, Sarcopenia, Cancer and Near-Term Survival study's population included all Kaiser Permanente Northern California (KPNC) members diagnosed from 2005 to 2013 with nonmetastatic stage I to III invasive breast cancer who had an abdominal CT scan at diagnosis and no cancer history. For all women, prospectively collected electronic medical record (EMR) data were available.19 We excluded women with any EMR-recorded International Classification of Diseases (ICD) code or history of myocardial infarction, stroke, heart failure, intracranial hemorrhage, or coronary artery revascularization before breast cancer diagnosis (n = 194) and missing smoking exposure (n = 2). The KPNC institutional review board approved the study.
CT Image Analysis
We quantified adiposity from CT scans taken within 6 months of diagnosis before chemotherapy or radiation (the median time from diagnosis to scan was 1.2 months). Two centrally trained researchers (blinded to clinical outcomes, including CVD) using SliceOmatic Software version 5.0 (TomoVision, Montreal, Quebec, Canada)9 contoured the cross-sectional area of each tissue in centimeters squared (cm2) at the third lumbar vertebra (L3), distinguishing muscle from visceral and subcutaneous adipose tissue using anatomic knowledge and tissue-specific Hounsfield unit ranges. The coefficients of variation (%) for muscle, subcutaneous adipose, and visceral adipose tissue quantifications between raters were 0.66%, 0.79%, and 6.72%, respectively. Single-slice L3 areas are well correlated with whole-body tissue volumes from magnetic resonance imaging.10 To quantify intramuscular adiposity, we computed the average radiation attenuation of skeletal muscle tissue in Hounsfield units, scaled inversely because lower muscle radiodensity indicates higher intramyocellular triglyceride.20
Covariate and Death Assessment
From KPNC’s comprehensive EMR and cancer registry, we obtained information on tumor characteristics (American Joint Committee on Cancer stage and estrogen, progesterone receptor, and human epidermal growth factor receptor 2 [HER2] status) and cancer treatment (radiation, anthracycline-containing v nonanthracycline chemotherapy, HER2-directed, and/or endocrine therapy). The KPNC EMR began in 1996 and encapsulates all care within KPNC, including diagnostic, procedural, laboratory, and radiographic data from various sources, including, but not limited to, inpatient, outpatient, claims, and referrals. We obtained information on pre-existing cardiovascular risk factors (diabetes, hypertension, and dyslipidemia) through the KPNC diabetes registry and the ICD 9th and 10th revision codes shown in the Data Supplement. The EMR also captured age, race/ethnicity (non-Hispanic white, black, Hispanic/Latino, Asian/Pacific Islander, or other) and smoking history (current, former, or never). Medical assistants measured height and weight at clinical visits. We calculated BMI from the measurements closest to the CT scan, categorized as underweight (less than 18.5 kg/m2), normal weight (18.5 to less than 25 kg/m2), overweight (25 to less than 30 kg/m2), class I obesity (30 to less than 35 kg/m2), and class II obesity (35 kg/m2 or greater). We combined mortality data from KPNC, California death records, and the Social Security Administration to determine patients’ vital status and cause of death.
CVD Outcomes
Events of interest included acute myocardial infarction, ischemic stroke, heart failure, and a composite end point that included any of these events in addition to intracranial hemorrhage, coronary artery revascularization, and deaths for which CVD was listed as a primary cause (Data Supplement).
Statistical Analysis
To facilitate comparison among exposures with different ranges and units, we treated each continuously in SD units. To create more easily interpretable risks groups, we also categorized adipose depots into tertiles.21,22 Follow-up began at breast cancer diagnosis and continued until the first date of occurrence of any event of interest (acute myocardial infarction, ischemic stroke, heart failure, intracranial hemorrhage, coronary artery revascularization, or death from CVD), disenrollment from the health plan, death from non-CVD causes, or June 30, 2018.
To account for competing risks, we used Fine and Gray’s23 extension of Cox regression that models (the hazards of) the cumulative incidence function, herein referred to as the subdistribution hazard ratio (HR) and its corresponding 95% CIs.24 The subdistribution HRs are reported in the text. The cause-specific HRs, wherein individuals who experienced competing events were censored, were similar and are reported in the Data Supplement. We assessed proportional hazards through product terms between exposures and logged follow-up time; we detected no violations. We calculated P for trend by treating adipose tissue tertiles as ordinal variables.
We examined each adipose depot in separate multivariable models adjusted for a priori covariates, including diagnosis age, height, race/ethnicity, smoking, diabetes, hypertension, dyslipidemia, and tumor and treatment characteristics. In addition to evaluating overall associations, we stratified by BMI category. To evaluate possible effect modification, we used likelihood ratio tests for the inclusion of cross-product terms of adiposity measures with radiation therapy (received or not), age at diagnosis (younger than 55 years or 55 years and older, as a proxy for menopausal status) and chemotherapy type (none, anthracycline containing, or nonanthracycline containing).
In sensitivity analyses, we examined whether results changed with adjustment for additional treatment variables (aromatase inhibitors, radiation, and HER2-directed therapy, which can increase CVD risk through altering blood lipids or direct cardiotoxic effects).
All statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC). A P value of < .05 for a two-tailed test was considered statistically significant.
RESULTS
Table 1 lists sample characteristics. At breast cancer diagnosis, mean (SD) age was 56 (12) years, and mean (SD) BMI was 28 (6) kg/m2. Pre-existing CVD risk factors were common at diagnosis, with more than a third of women having hypertension and/or dyslipidemia, and a quarter having diabetes.
TABLE 1.
Characteristics of Patients Diagnosed With Nonmetastatic Breast Cancer at Kaiser Permanente Northern California (2006-2011)
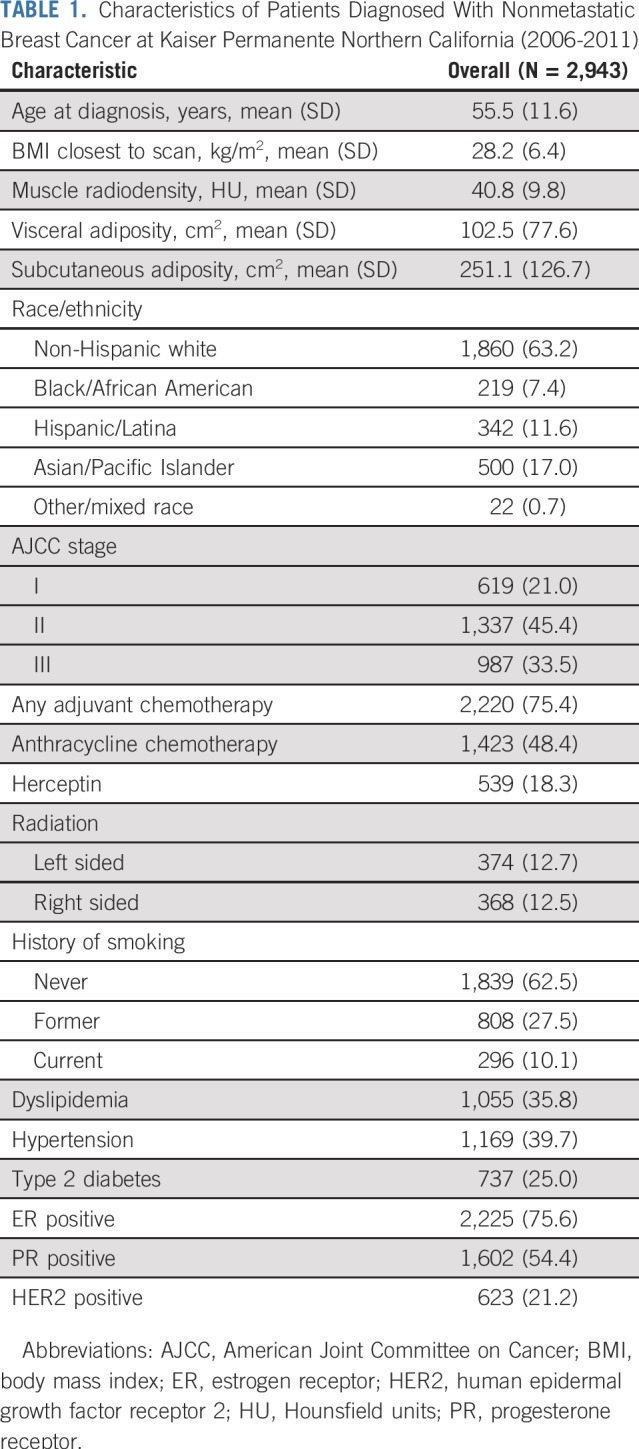
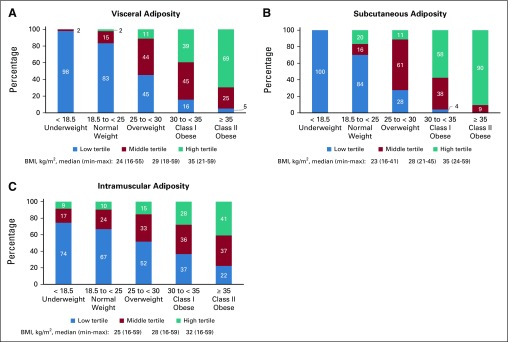
Figure 1 illustrates the distributions of visceral (Fig 1A), subcutaneous (Fig 1B), and intramuscular (Fig 1C) adiposity across BMI categories. All types of adiposity increase with BMI, but this is most pronounced for subcutaneous and least pronounced for intramuscular adiposity. For example, most patients with normal BMI have visceral and subcutaneous adiposity in the lowest tertile (83% and 84%, respectively), whereas most patients with class II obesity have visceral and subcutaneous adiposity in the highest tertile (69% and 90%, respectively). By contrast, intramuscular adiposity occurs across BMI categories, with less than one half of patients with class II obesity and more than 10% of normal-weight patients in the highest tertile.
FIG 1.
Specific adipose tissue depots within body mass index (BMI, kg/m2) categories. Both visceral (A) and subcutaneous (B) adiposity increase with BMI. However, visceral adiposity in particular occurs across the BMI spectrum (eg, 2% of normal-weight and 11% of overweight patients in the highest tertile of visceral adiposity). Meanwhile, (C) intramuscular adiposity occurs in substantial numbers of patients in every BMI group (low muscle radiodensity). This novel cardiovascular disease risk factor is occult unless imaging methods such as computed tomography are used.
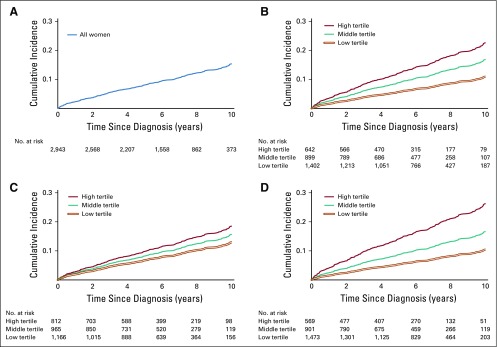
Over a median follow-up of 6 years (maximum, 13 years), 328 CVD events occurred. Four hundred sixty-six women died as a result of competing events before developing CVD, including 361 deaths as a result of breast cancer. The cumulative incidence of CVD reached 15% by year 10 of follow-up (Fig 2A; cumulative incidence, 0.15 [95% CI, 0.14 to 0.17]). Women in the highest tertiles of adiposity had the highest cumulative incidence of CVD (Figs 2B-2D).
FIG 2.
Incidence of cardiovascular events in the decade after a diagnosis of nonmetastatic breast cancer. Results from the Breast, Sarcopenia, Cancer and Near-Term Survival study (n = 2, 943). Overall, the cumulative incidence of cardiovascular disease increased steadily, exceeding 15% by year 10 of follow-up (A; cumulative incidence at year 10 = 0.15 [95% CI, 0.14 to 0.17]). Cardiovascular disease incidence was highest for women in the highest tertile of visceral (B; Gray’s test P = .01), subcutaneous (C; Gray’s test P = .01), and intramuscular (D; Gray’s test P ≤ .001) adiposity.
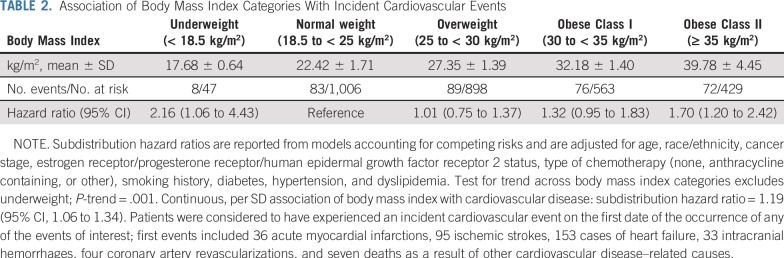
The association of BMI with CVD risk was nonlinear. Compared with normal-weight women, those who were underweight or who had class II obesity had an increased CVD risk after breast cancer diagnosis (HR, 2.16 [95% CI, 1.06 to 4.43] and HR, 1.70 [95% CI, 1.20 to 2.42], respectively; Table 2). Meanwhile, women with overweight BMI or class I obesity had no statistically significant increased risk of CVD (HR, 1.01 [95% CI, 0.75 to 1.37] and HR, 1.32 [95% CI, 0.95 to 1.83], respectively; Table 2).
TABLE 2.
Association of Body Mass Index Categories With Incident Cardiovascular Events
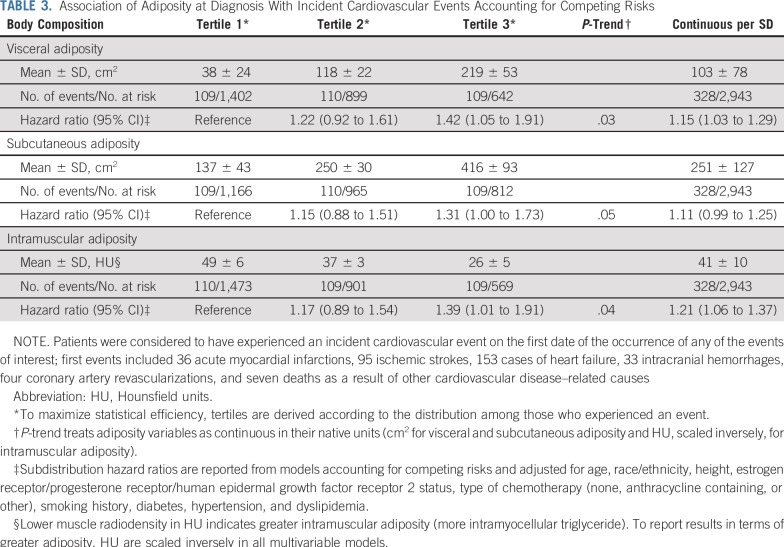
Examining specific adipose tissue depots (Table 3), we observed an increased CVD risk in the highest (v lowest) tertiles of visceral (HR, 1.42 [95% CI, 1.05 to 1.91]; P-trend = .03) and intramuscular (HR, 1.39 [95% CI, 1.01 to 1.91]; P-trend = .04) adiposity. Modeling associations continuously (linear in the log-hazard) yielded consistent results, with increases of 15% (95% CI, 1.03% to 1.29%) and 21% (95% CI, 1.06% to 1.37%) per SD increase in visceral and intramuscular adiposity, respectively. Associations were slightly weaker when comparing the highest (v lowest) tertile of subcutaneous adiposity (HR, 1.31 [95% CI, 1.00 to 1.73]), and the continuous association per SD increase (HR, 1.11 [95% CI, 0.99 to 1.25]) was statistically nonsignificant. Associations with CVD subtypes followed a similar pattern, although estimates were imprecise given the smaller numbers of events (Data Supplement).
TABLE 3.
Association of Adiposity at Diagnosis With Incident Cardiovascular Events Accounting for Competing Risks
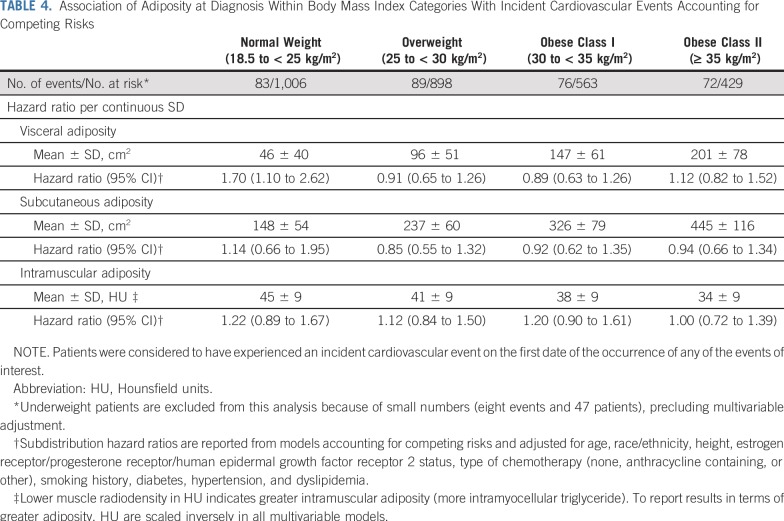
When we stratified analyses by BMI category (Table 4), we found that increasing visceral adiposity was most strongly associated with CVD risk among normal-weight women (HR, 1.70 [95% CI, 1.10 to 2.62] per SD). Effect estimates per SD subcutaneous and intramuscular adiposity among normal-weight women were similar to those in the overall sample, although CIs were wider (HR, 1.14 [95% CI, 0.66 to 1.95] per SD increase in subcutaneous adiposity and HR, 1.22 [95% CI, 0.89 to 1.67] per SD increase in intramuscular adiposity).
TABLE 4.
Association of Adiposity at Diagnosis Within Body Mass Index Categories With Incident Cardiovascular Events Accounting for Competing Risks
The association of adiposity with CVD risk did not vary by age, radiation, or chemotherapy type (Data Supplement). Additional adjustment for other treatment variables (endocrine and HER2-directed therapies) did not alter the association of adiposity with CVD (data not shown).
DISCUSSION
Among 2,943 survivors, we observed for the first time that visceral and intramuscular adiposity are associated with an increased CVD incidence after breast cancer. Furthermore, our data highlight visceral adiposity as an occult risk factor among normal-weight patients with breast cancer, who are not typically considered to have a high CVD risk: greater visceral adiposity was associated with a 70% increased CVD risk among normal-weight women independent of cancer treatments and pre-existing risk factors such as diabetes, hypertension, or dyslipidemia.
BMI was also associated with an increased risk of CVD, but this became apparent only with class II obesity. The lack of association of overweight BMI with CVD risk is likely because BMI scales weight to height without distinguishing muscle from adipose tissue or describing adipose tissue distribution.25-32 High BMI levels (eg, class II obesity) are a reasonable proxy for total adiposity. However, we found that, at lower BMI levels, body composition and therefore CVD risk is more heterogeneous. For example, 11% of normal-weight women would be reclassified as high risk after consideration of their intramuscular and/or visceral adiposity. Thus, measures of adipose tissue distribution may be necessary to identify patients with breast cancer with high CVD risk.
Studies in patients without cancer have also shown that adipose tissue distribution outperforms BMI in identifying patients with cardiometabolic dysfunction. In addition, many patients with normal BMI have excess adiposity.33-36 For example, using data from the National Health and Nutrition Examination Survey, Sahakyan et al37 reported that normal-weight central obesity was the body composition phenotype that carried the highest CVD risk: normal-weight women with a high waist-to-hip ratio (a proxy for visceral adiposity) had a higher risk of cardiovascular death than did normal-weight women without central obesity, and also a higher risk than obese women, according to BMI only. The need for precise measures of adipose tissue distribution is potentially greater among patients with breast cancer than among the general population because of aging and the high prevalence of comorbidities and deconditioning, known to influence adipose tissue distribution and to decrease BMI’s sensitivity as a measure of total adiposity.38,39 CT scans, already collected at diagnosis in many patients with breast cancer, can be used opportunistically to measure adipose tissue distribution and thereby improve the identification of those with high CVD risk. Although our analyses used trained research assistants, automated methods to measure body composition using CT scans are increasingly available and have excellent correlation and similarity metrics relative to manual analysis by a trained rater.40-42
In patients without cancer, a limited number of prospective studies have examined adipose tissue depots and incident CVD; consistent with our findings, associations with CVD were typically stronger for visceral and intramuscular adiposity than for subcutaneous adiposity.6-11 This is likely because visceral and intramuscular adiposity directly promote metabolic dysfunction and inflammation and are reflective of anatomic and functional disturbances in adipose tissue.43 Subcutaneous adipose tissue is the body’s primary energy store. In the obese state, excess energy overloads subcutaneous adipose tissue stores. Once no additional lipid can be accommodated in subcutaneous adipose tissue, excess lipid and adipose tissue accumulates in abnormal depots, such as intramuscular and visceral adipose depots.43 Intramuscular and visceral adiposity help promote a cascade of changes that increase CVD risk: lipotoxic free fatty acid delivery to nonadipose organs (eg, muscle, liver, and pancreas), insulin resistance,20 type 2 diabetes,12 and inflammation.44
Examining whether adiposity increases CVD incidence after breast cancer is clinically important because CVD is an important source of morbidity and mortality after a breast cancer diagnosis.45 Breast cancer therapies have direct and indirect effects that may promote heart failure, arrhythmias, valvular heart disease, and accelerated atherosclerosis.46 Anthracycline-containing regimens are associated with acute and long-term cardiac toxicity.47 Aromatase inhibitors can result in weight gain and altered lipid profiles,48 and there are documented declines in physical activity after breast cancer diagnosis.49 The association of adiposity with CVD risk did not vary by chemotherapy type in this study, possibly because clinicians already consider patients’ cardiovascular history when prescribing chemotherapy. Furthermore, our study was limited to women with CT scans without a history of CVD at diagnosis to avoid the influence of pre-existing CVD before diagnosis. Therefore, most women received chemotherapy (62% of these regimens contained anthracyclines). With most patients receiving chemotherapy, we may have been underpowered to detect whether the associations differed by the type of chemotherapy received.
Importantly, adipose tissue distribution is modifiable among patients with breast cancer and should be a priority target for preventive interventions regardless of weight loss. Interventions such as aerobic exercise and resistance training are effective for reducing overall, visceral, and intramuscular adiposity,50 are safe in patients with breast cancer (including those on active treatment),51 and have beneficial effects on CVD risk factors.52 For some patients, pharmacologic management of CVD risk factors should also be considered.
Our study was enabled by the comprehensive EMR, but there are also important limitations that result from our use of EMR data. For example, physical activity information was not collected routinely at clinical visits until 2006 and thus is not included as a covariate. Furthermore, we did not confirm CVD outcomes via medical record review in the current study but instead defined CVD conservatively from ICD codes and death certificates validated in prior studies.53 In addition, CT scans were not available for all women. Among otherwise eligible women diagnosed with invasive breast cancer at KPNC, 36% of stage II and 77% of stage III women had scans. However, this is unlikely to have induced substantial bias because women with (v without) scans were slightly younger (mean age, 55 years v 58 years at diagnosis), the 10-year cumulative incidence of the CVD events examined was similar, and there were no differences in BMI or race/ethnicity.
To our knowledge, this is the first study to demonstrate that adipose tissue distribution is associated with incidence of new CVD events after a breast cancer diagnosis, including among patients with a normal BMI. Body composition may be useful in identifying CVD risk in these women, who may be sedentary or have metabolic disturbances that have not yet reached clinical thresholds for dyslipidemia, hypertension, or diabetes. Prior research among breast cancer survivors has examined only CVD mortality and BMI or waist circumference, surrogate measures of overall and central adiposity, without including nonfatal CVD events or body composition data.54,55 Our study provides new evidence to help identify patients with breast cancer at high CVD risk during and after treatment and suggests that adipose tissue distribution may improve on BMI for risk stratification.
Although it has been assumed that excess adiposity increases the risk of CVD after breast cancer, this first-of-its-kind study demonstrates that adipose tissue distribution best identifies patients with breast cancer with higher CVD risk after diagnosis, including those with normal BMI. Specifically, visceral and intramuscular adiposity were associated most strongly with CVD risk. Software is now available that automatically measures body composition from clinically acquired CT scans, facilitating clinical integration. Measures of adipose tissue distribution from CT or anthropometry (eg, waist circumference) may help identify individuals with high CVD risk and tailor prevention efforts to patients' body composition.
ACKNOWLEDGMENT
We thank Sherin Fernandes and Taiwo Olobatuyi for their work in body composition analysis for this study.
Footnotes
Presented in abstract form (preliminary data) at the American Society for Preventive Oncology Conference in New York City, NY, on March 12, 2018.
Supported by National Cancer Institute Grants R01CA184953 (B.J.C.) and K01CA226155 (E.M.C.F.).
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth M. Cespedes Feliciano, Wendy Y. Chen, Patrick T. Bradshaw, Carla M. Prado, Bette J. Caan
Financial support: Elizabeth M. Cespedes Feliciano, Bette J. Caan
Administrative support: Elizabeth M. Cespedes Feliciano, Adrienne L. Castillo
Collection and assembly of data: Elizabeth M. Cespedes Feliciano, Wendy Y. Chen, Carla M. Prado, Adrienne L. Castillo
Data analysis and interpretation: Elizabeth M. Cespedes Feliciano, Wendy Y. Chen, Patrick T. Bradshaw, Carla M. Prado, Stacey Alexeeff, Kathleen B. Albers, Bette J. Caan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adipose Tissue Distribution and Cardiovascular Disease Risk Among Breast Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Wendy Y. Chen
Research Funding: Bayer AG (Inst)
Carla M. Prado
Honoraria: Abbott Nutrition
Consulting or Advisory Role: Abbott Nutrition
Research Funding: Almased, MyViva
Travel, Accommodations, Expenses: Abbott Nutrition
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society: Breast cancer facts & figures 2017–2018 2017 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf
- 2.Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardia A, Arieas ET, Zhang Z, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012;131:907–914. doi: 10.1007/s10549-011-1843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw PT, Stevens J, Khankari N, et al. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27:6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hooning MJ, Aleman BM, van Rosmalen AJ, et al: Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys 64:1081-1091, 2006. [DOI] [PubMed]
- 6.Mongraw-Chaffin M, Allison MA, Burke GL, et al. CT-derived body fat distribution and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2017;102:4173–4183. doi: 10.1210/jc.2017-01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicklas BJ, Penninx BW, Cesari M, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: The Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 8.Kouli GM, Panagiotakos DB, Kyrou I, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study. Nutr Metab Cardiovasc Dis. 2017;27:881–889. doi: 10.1016/j.numecd.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa AL, Takx RAP, MacNabb MH, et al. Relationship between measures of adiposity, arterial inflammation, and subsequent cardiovascular events. Circ Cardiovasc Imaging. 2016;9:e004043. doi: 10.1161/CIRCIMAGING.115.004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britton KA, Massaro JM, Murabito JM, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 13.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 16.Abraham TM, Pedley A, Massaro JM, et al. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: Associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves KW, Faulkner K, Modugno F, et al. Body mass index and mortality among older breast cancer survivors in the Study of Osteoporotic Fractures. Cancer Epidemiol Biomarkers Prev. 2007;16:1468–1473. doi: 10.1158/1055-9965.EPI-07-0051. [DOI] [PubMed] [Google Scholar]
- 19.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleeper LA, Harrington DP. Regression splines in the Cox model with application to covariate effects in liver disease. J Am Stat Assoc. 1990;85:941–949. [Google Scholar]
- 22.Clark TG, Bradburn MJ, Love SB, et al. Survival analysis part IV: Further concepts and methods in survival analysis. Br J Cancer. 2003;89:781–786. doi: 10.1038/sj.bjc.6601117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24. Lin G, So Y, Johnston G: Analyzing survival data with competing risks using SAS® software. Presented at SAS Global Forum. https://support.sas.com/resources/papers/proceedings12/344-2012.pdf. [Google Scholar]
- 25.Adams TD, Heath EM, LaMonte MJ, et al. The relationship between body mass index and per cent body fat in the severely obese. Diabetes Obes Metab. 2007;9:498–505. doi: 10.1111/j.1463-1326.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 27.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 28.Gurrici S, Hartriyanti Y, Hautvast JGAJ, et al. Relationship between body fat and body mass index: Differences between Indonesians and Dutch Caucasians. Eur J Clin Nutr. 1998;52:779–783. doi: 10.1038/sj.ejcn.1600637. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Nuñez C, Testolin C, et al. Anthropometry and methods of body composition measurement for research and field application in the elderly. Eur J Clin Nutr. 2000;54:S26–S32. doi: 10.1038/sj.ejcn.1601022. [DOI] [PubMed] [Google Scholar]
- 30.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28:2087–2093. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 31.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Gallagher D, Thornton JC, et al. Regional body volumes, BMI, waist circumference, and percentage fat in severely obese adults. Obesity (Silver Spring) 2007;15:2688–2698. doi: 10.1038/oby.2007.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heymsfield SB, Cefalu WT. Does body mass index adequately convey a patient’s mortality risk? JAMA. 2013;309:87–88. doi: 10.1001/jama.2012.185445. [DOI] [PubMed] [Google Scholar]
- 34.Heymsfield SB, Peterson CM, Thomas DM, et al. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17:262–275. doi: 10.1111/obr.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prado CM, Cushen SJ, Orsso CE, et al. Sarcopenia and cachexia in the era of obesity: Clinical and nutritional impact. Proc Nutr Soc. 2016;75:188–198. doi: 10.1017/S0029665115004279. [DOI] [PubMed] [Google Scholar]
- 36.Shah RV, Murthy VL, Abbasi SA, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: Implications for total and cardiovascular mortality. Ann Intern Med. 2015;163:827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez MC, Correia MITD, Heymsfield SB. A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care. 2017;20:314–321. doi: 10.1097/MCO.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez MC, Pastore CA, Orlandi SP, et al. Obesity paradox in cancer: New insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, Sugimoto M, Psutka SP, et al. Validation study of a new semi-automated software program for CT body composition analysis. Abdom Radiol (NY) 2017;42:2369–2375. doi: 10.1007/s00261-017-1123-6. [DOI] [PubMed] [Google Scholar]
- 41.Popuri K, Cobzas D, Esfandiari N, et al. Body composition assessment in axial CT images using FEM-based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging. 2016;35:512–520. doi: 10.1109/TMI.2015.2479252. [DOI] [PubMed] [Google Scholar]
- 42.Kim YJ, Park JW, Kim JW, et al. Computerized automated quantification of subcutaneous and visceral adipose tissue from computed tomography scans: Development and validation study. JMIR Med Inform. 2016;4:e2. doi: 10.2196/medinform.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bays H. Central obesity as a clinical marker of adiposopathy; Increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–351. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beasley LE, Koster A, Newman AB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aleman BM, Moser EC, Nuver J, et al. Cardiovascular disease after cancer therapy. EJC Suppl. 2014;12:18–28. doi: 10.1016/j.ejcsup.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rochette L, Guenancia C, Gudjoncik A, et al. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015;36:326–348. doi: 10.1016/j.tips.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005;93:S23–S27. doi: 10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwan ML, Sternfeld B, Ergas IJ, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat. 2012;131:679–690. doi: 10.1007/s10549-011-1788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw CS, Clark J, Wagenmakers AJM. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. 2010;30:13–34. doi: 10.1146/annurev.nutr.012809.104817. [DOI] [PubMed] [Google Scholar]
- 51.Cheema BS, Kilbreath SL, Fahey PP, et al. Safety and efficacy of progressive resistance training in breast cancer: A systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148:249–268. doi: 10.1007/s10549-014-3162-9. [DOI] [PubMed] [Google Scholar]
- 52.Sturgeon KM, Ky B, Libonati JR, et al. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res Treat. 2014;143:219–226. doi: 10.1007/s10549-013-2808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 54.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101:1712–1719. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 55.Sabatino SA, Coates RJ, Uhler RJ, et al. Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol. 2007;25:2100–2106. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]