Despite Dr. Alois Alzheimer describing cerebrovascular changes in his initial case report,1 the role of vascular contributions to the disease have largely taken a backseat to their two more famous neuropathological cousins, amyloid and tau. However, over a century later, it has become increasingly clear that mixed dementia – instances where classic Alzheimer’s disease (AD) pathology and cerebrovascular abnormalities co-exist – likely account for a majority of late onset AD (LOAD) cases.2
Interesting then, that the strongest and most replicated genetic risk factor for LOAD – possession of the ε4 allele (E4) of apolipoprotein E (APOE) – is one with a long history in vascular disease.3 Prior to its link to AD, E4 was primarily studied in regards to its association with increased low density lipoprotein (LDL) cholesterol levels and increased risk of cardiovascular disease.4–5 While this critical protein has been implicated in a variety of peripheral vascular processes, its function is not limited to below the neck. In fact, vascular dysfunction may serve as an initiating event leading to neurodegeneration,6 and multiple studies suggest E4 accelerates loss of cerebrovascular integrity,7–11 and impairs cerebral blood flow.12–14
Central to cerebrovascular function (or dysfunction as may be the case in AD) is the functional unit of the blood brain barrier (BBB) composed of endothelial cells, pericytes and astrocyte end-feet. What role might apoE-expressing pericytes have to play in the processes described above? Pericyte degeneration has been described in AD,15–16 and several studies have laid a framework to understand the role of apoE in a potential pericyte-endothelial cell-extracellular matrix (ECM) axis. This includes a description of accelerated degeneration of pericytes in E4+ AD brains relative to E3 carriers with AD and non-demented controls,10 and the observation that E4 fails to suppress the CypA-MMP-9 pathway in pericytes leading to degradation of BBB tight junctions.17
In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Yamazaki et al18 highlight a new and exciting role for apoE in pericytes, directly linking these rare but critical cells to BBB integrity by demonstrating that pericyte expression of apoE4 significantly diminishes induction of endothelial cell ECM production and barrier formation. The authors employ several creative approaches to detail the role of pericyte derived E4 on endothelial function and ECM composition, including in vitro BBB models and 3D vasculogenesis assays.
First, the study describes differential gene expression in E3 vs E4 pericytes (which produce a significant amount of lipidated apoE protein) cultured in isolation, highlighting angiogenesis as a primary pathway of interest. They then co-cultured E3 and E4 pericytes with human umbilical vein endothelial cells (HUVECs) either with or without direct contact, and measured the induction of several important ECM coding genes, showing that E3 pericytes induced higher levels of COL4A1 and COL4A2 compared to E4 pericytes. Interestingly, when endothelial cells were co-cultured in a non-contact trans-well system, there was no difference in ECM gene expression between those cultured with E3 or E4 pericytes. This implies that the apoE-isoform differences in ECM expression is mediated via direct contact with the endothelial cells, rather than exclusively through secreted factors (including the apoE-containing lipoproteins secreted by the pericytes)
By using a 3D co-culture, the authors were then able to assess endothelial cell-pericyte interactions in a physiological system and showed that E4 expressing pericytes were less able to stimulate tube-like structure formation, as evidenced by branching and network density analyses. Next, endothelial barrier function was assessed in an in vitro BBB model by measuring trans-endothelial electrical resistance (TEER). By comparing and contrasting clever combinations of E3, E4 and Apoe knockout pericytes, astrocytes and endothelial cells, the authors show that expression of apoE4 in pericytes, but not endothelial cells, reduces barrier formation. Together, these experiments describe decrements in ECM protein induction, barrier formation, and the creation of tube-like structures by endothelial cells cultured in tandem with E4 expressing pericytes.
Finally, the authors looked to the brains of the E3 and E4 mice to confirm these E4-associated deficiencies in vascular-related molecules in vivo. Compared to those with E3, E4 expressing mice had lower expressing of collagen IV, a critical basement membrane component in cerebral capillaries and cortical tissue, but no significant differences in expression of endothelial tight junction proteins or endothelial coverage by pericytes or astrocytic end-feet. The lower cortical collagen IV levels in E4 mice negatively correlated with the volume of leaked plasma proteins, suggesting that E4 decreases BBB integrity by modulating ECM proteins at the basement membrane.
Altogether, this exciting new work by Yamazaki et al adds compelling evidence to the case that impaired pericyte function contributes to the detrimental cerebrovascular effects associated with E4, further highlighting a new cellular player – and potential new therapeutic targets – on the growing list of E4-associated deficiencies.
Figure 1.
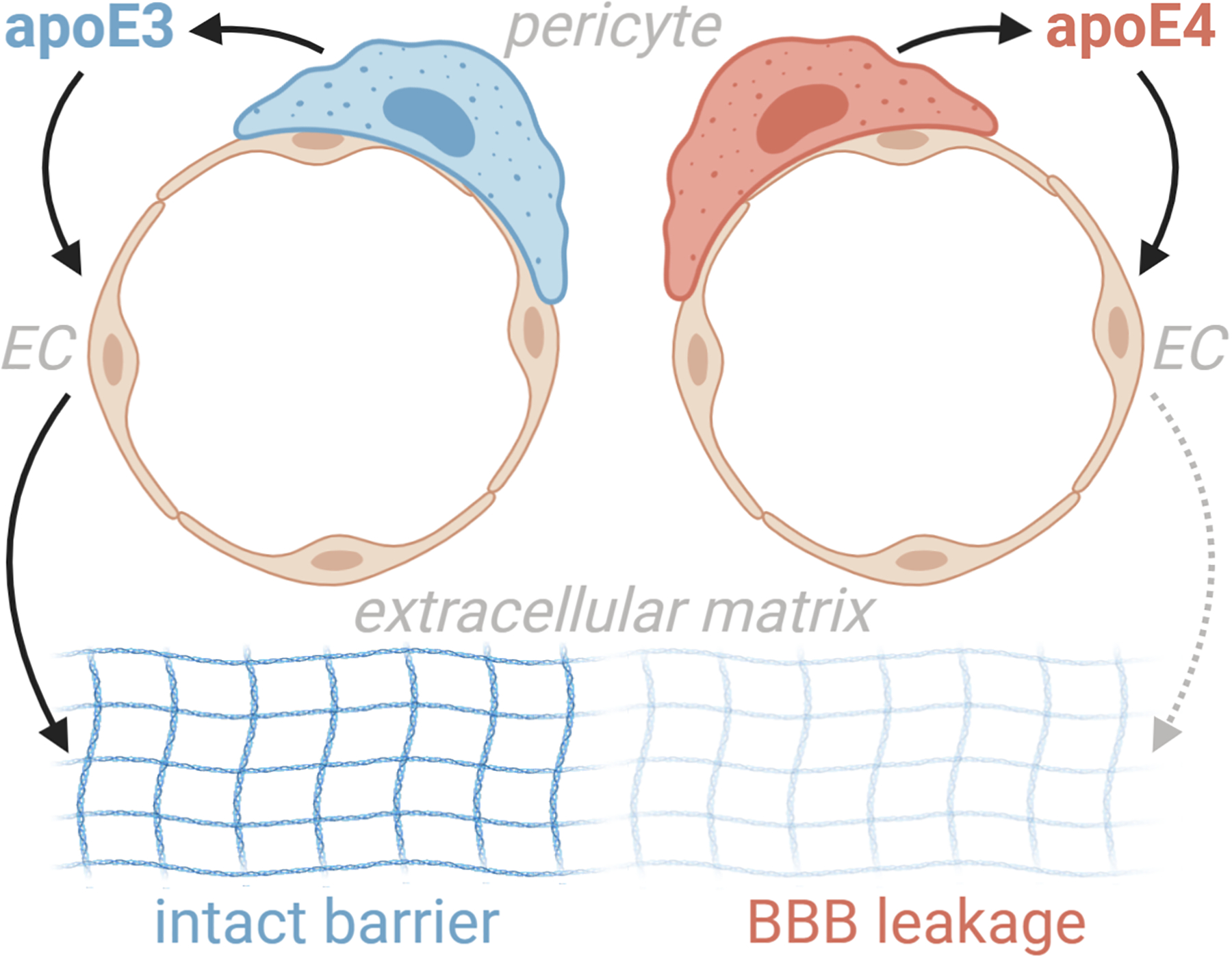
Yamazaki et al18 show that pericytes modulate endothelial cell function in an apoE isoform-specific manner. Endothelial cells cultured in direct contact with apoE4 expressing pericytes had lower induction of extracellular matrix proteins, less tube-like structure genesis, and decreased barrier formation. Compared to apoE3, apoE4 mice had less extracellular matrix protein expression, which correlated with increases in plasma protein leakage.
References
- 1.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8(6):429–31. [DOI] [PubMed] [Google Scholar]
- 2.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 2015; 11: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016. July;94(7):739–46. doi: 10.1007/s00109-016-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson PWF, Schaefer EJ, Larson MG, et al. Apolipoprotein E alleles and risk of coronary disease: a meta-analysis. Arterioscler Thromb Vasc Biol 1996; 16: 1250–1255. [DOI] [PubMed] [Google Scholar]
- 5.Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 2007; 298: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 6.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol 2014; 24: 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salloway S, Gur T, Berzin T, Tavares R, Zipser B, Correia S, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J Neurol Sci 2002; 203–204: 1837. [DOI] [PubMed] [Google Scholar]
- 8.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging 2007; 28: 977–986. [DOI] [PubMed] [Google Scholar]
- 9.Hultman K, Strickland S, Norris EH. The APOE ɛ4/ɛ4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab 2013; 33: 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab. 2016. January;36(1):216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 2015; 35: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippini N, Ebmeier KP, MacIntosh BJ, et al. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 2011; 54: 602–610. [DOI] [PubMed] [Google Scholar]
- 13.Thambisetty M, Beason-Held L, An Y, et al. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010; 67: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierenga CE, Clark LR, Dev SI, et al. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimer’s Dis 2013; 34: 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 2001; 64: 575–611. [DOI] [PubMed] [Google Scholar]
- 16.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 2013; 23: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ApoE (Apolipoprotein E) in Brain Pericytes Regulates Endothelial Function in an Isoform-Dependent Manner by Modulating Basement Membrane Components. [DOI] [PMC free article] [PubMed]


