Abstract
Objective
The objective of this study was to improve the patient comfort and safety during procedures done under anaesthesia and sedation. The analgesia nociception index (ANI) noninvasively provides information on the nociception-antinociception balance, and it can be used to assess analgesia objectively. We aimed to compare the effects of analgesia management with conventional methods and with ANI monitoring on total opioid consumption, sedation and analgesia levels in patients who underwent colonoscopy using sedo-analgesia.
Methods
Adult patients (n=102), scheduled for procedural sedation, were prospectively analysed. After the induction with propofol and ketamine, infusions of propofol (2 mg kg−1 h−1) and remifentanil (0.05 mcg kg−1 min−1) were started. In Group A, remifentanil infusions were titrated to maintain the ANI value between 50 and 70, whereas in Group C, analgesic requirements were met according to the attending anaesthetist’s intention. The heart rate, blood pressure, respiratory rate, SpO2, BIS, Numeric Rating Scale (NRS) and Ramsay Sedation Scale were monitored. Complications, analgesics consumption, duration of the procedure, demographic information, NRS and the Modified Aldrete Score were evaluated.
Results
A total remifentanil amount used in Group A was 66.51±47.87 mcg and 90.15±58.17 mcg in Group C (p=0.011); there was no difference in total amounts of ketamine and propofol given. There was a negative correlation between ANI and NRS scores of Group A patients at Minute 0 at the level of 0.402, which was significant statistically (p=0.003).
Conclusion
Opioid consumption was diminished when ANI monitoring was used, and thus the patient safety was improved. Further studies with longer procedure times and with a greater number of patients are required to demonstrate whether there is a difference in side effects and recovery times.
Keywords: Analgesia nociception index, colonoscopy, sedation, sedo-analgesia
Introduction
The interventions done under anaesthesia and sedation outside the operating room are getting increasingly common. Patient safety must be ensured during procedures done under sedo-analgesia to enhance the patient tolerance and quality of the procedure (1, 2).
Research on rational drug use, of which the principles have been set, has been ongoing for many years to prevent increases in costs, morbidity and mortality due to erroneous and unnecessary use of drugs (3). When anaesthetics are used in appropriate doses according to the procedure, it is predicted that the drug side effects and the complications will decrease. The anaesthesia level should also be set to optimum; deep or superficial levels of anaesthesia and analgesia may lead to serious complications. To deliver medicine at appropriate doses, monitoring haemodynamic responses is required. In recent years, in line with the objective of the needs of analgesia monitoring, various devices and methods have been developed. One of them is the analgesia nociception index (ANI), which measures the parasympathetic tone by analysing the heart rate variability (HRV) continuously and non-invasively in a range of 0–100 to provide information on the nociception-antinociception balance (4).
The HRV analysis reveals information on the autonomic nervous system’s cardiac control. High-frequency changes above 0.15 Hz in HRV are specific to parasympathetic system dominance. In the presence of an unpleasant or painful stimulus, parasympathetic tonus is decreased. Logier et al. (5) based on these data, defined an algorithm employing the HRV analysis to measure the nociception-antinociception balance and developed ANI as a monitoring system. ANI measures the vagal tonus, and its computation is based on the respiratory cycle’s effect on the respiratory rate (RR) interval derived from the electrocardiogram. ANI is expressed by a number between 0 and 100. Current studies demonstrate the ideal interval between 50 and 70 for adequate analgesia against painful stimuli (6–8).
In this study, the aim was to compare the effects of analgesia management by conventional methods and by ANI monitoring on total opioid consumption, sedation and analgesia levels, respiratory and haemodynamic parameters, complications and recovery from anaesthesia in adult patients, who undergo colonoscopy with sedo-analgesia.
Methods
Inclusion and exclusion criteria
The study was confirmed and approved by the Acibadem University Clinical Research Ethics Committee (2015–16/15), and written informed consent was obtained from all participants. A total of 102 patients between ages 18 and 70 in ASA I-II, and who were to undergo elective colonoscopy under sedo-analgesia were included. Patients with cardiac rhythm disorder, autonomic nervous system disease, neuropsychiatric disease, medication which might affect cardiac autonomic regulation usage, known allergies against drugs that were to be applied, a body mass index >30, and patients who did not accept to participate in the study were excluded.
Parameters
No premedication was administered to patients before the procedure. The heart rate (HR), mean blood pressure (MBP), respiratory rate (RR) and peripheral oxygen saturation (SpO2) were recorded before the procedure (baseline values, Time 0), at the start of the procedure (Minute 0=Time 1) after induction, and every 3 minutes during the procedure. Simultaneously BIS monitoring was performed using a BIS Vista monitor (Medtronic, USA). The sedation level and pain level were assessed using the Ramsey Sedation Scale (RSS) and Numeric Rating Scale (NRS) scores, respectively.
Drug infusions were discontinued by the removal of the colonoscope. The duration of the procedure and a total amount of ketamine, propofol and remifentanil given to the patients was recorded. HR, MBP, RR, SpO2, NRS and the Modified Aldrete Score (MAS) were monitored in all patients every 3 minutes after the termination of the procedure to assess the level of recovery from anaesthesia. The time to reach a MAS >8 was recorded as recovery time. Complications (allergies, nausea, vomiting, hallucinations etc.), which occurred at any time point during the procedure and the recovery period, were recorded. The patients were discharged when their recovery period was over and when they felt ready to go.
In this prospective cohort study, patients were divided into two groups randomly. Randomisation was done using the sealed envelope method.
Groups
In Group A, ANI (ANI MetroDoloris, France) monitoring was performed by placing ANI electrodes on the sternum and the region of the V5 chest lead. Both instantaneous and 4-minute average values were monitored, but only the former one was recorded, as our study’s procedures are relatively shorter than in the other studies. Monitored values were recorded, and the patient was then placed in the left lateral decubitus position. In Group C, ANI monitoring was not applied. Ketamine, propofol and remifentanil were administered intravenously to all patients to provide sedation and analgesia. Ketamine (Ketalar, Pfizer, Turkey) 1 mg kg and propofol (Propofol, Fresenius Kabi, Austria) 0.5 mg kg−1 were used for induction. Propofol 2 mg kg−1 h−1 and remifentanil (Ultiva, GlaxoSmithKline, Italy) 0.05 mcg kg−1 min−1 were used for maintenance. Remifentanil titration was provided in Group A by changing the infusion rate to maintain the ANI scores in the range of 50–70 and in Group C by assessing the analgesia level by the attending anaesthetist with conventional methods; signs of insufficient analgesia are a facial grimace, movement, spontaneous complaint of pain, ±20% change in the HR and MBP from basal values, and the depth and rate of breathing. BIS scores were aimed to be in the range between 60 and 80 in both groups, although ketamine affects BIS scores as all patients received ketamine, we assumed it would not affect the analysis.
Patients with RSS equal to 6 at any time point were accepted to have no pain, and patients with a RSS 4–5 during the recovery period were investigated for their pain scores during the procedure. All patients received 3 L min−1 oxygen via face mask during the procedure. Patients’ spontaneous respiratory efforts were monitored. Rectification was provided by tactile stimuli or by airway repositioning when an upper airway obstruction was observed or when a SpO2 decline compared to the baseline values occurred. A balloon-valve mask system was kept at hand to provide manual ventilation in case of apnoea or when a SpO2 level below 90% would be observed. It was decided beforehand that a HR decrease <50 beats min−1 would be accepted as bradycardia to be treated with atropine 0.5 mg intravenously, a decrease of the MBP greater than 20% of the baseline value would be accepted as hypotension to be treated with 5 mg ephedrine intravenously and that all patients who received atropine or ephedrine would be excluded from the study.
Statistical analysis
Power analysis
In a previous study (9), the response within each subject Group was normally distributed with a standard deviation of 79.38. If the true difference in the experimental and control means is 46.07, we will need to study 48 experimental subjects and 48 control subjects to be able to reject the null hypothesis that the population means of the experimental and control groups are equal to probability (power) 0.8. The Type I error probability associated with this test of the null hypothesis is 0.05.
For the statistical analysis R version, the 2.15.3 programme was used (10). In addition to the descriptive statistics (mean, standard deviation, median, frequency and percentage), the Shapiro-Wilk test and graphical examinations were used to investigate the alignment of the quantitative data with the normal distribution. To compare the two groups with a normal distribution of variables, independent groups t-test was used. The Mann-Whitney U test was used to compare the two groups with variables that were not normally distributed. For paired-sample assessments within the groups, a dependent t-test was used. The Pearson correlation analysis was used to determine the correlation levels of quantitative data. Pearson’s chi-squared and Fisher’s exact tests examined the correlation of the qualitative data. A p-value ≤0.05 was accepted as statistically significant.
Results
From October 2015 to June 2016, a total of 102 patients were included in the study. The characteristics of the patients and the results of the colonoscopies of the study cohort are summarised in Tables 1 and 2, respectively. There were no significant differences between the groups in terms of total amounts of ketamine and propofol given (p>0.05) (Table 2), but the remifentanil amount applied in Group A was statistically significantly lower than that applied in Group C (p=0.011) (Figure 1).
Table 1.
Demographic data of the patients
| Group C Mean±SD |
Group A Mean±SD |
p | ||
|---|---|---|---|---|
|
| ||||
| Age [years] | 45.96±11.05 | 43.41±10.99 | a0.246 | |
| Weight [kg] | 71.18±10.49 | 70.88±11.65 | a0.894 | |
| n | % | |||
| Gender | Female | 34 [66.7] | 34 [66.7] | b0.999 |
| Male | 17 [33.3] | 17 [33.3] | ||
| ASA | I | 21 [41.2] | 24 [47.1] | b0.550 |
| II | 30 [58.8] | 27 [52.9] | ||
independent groups t-test;
Pearson chi-square test
Table 2.
Comparisons of duration of the procedure, recovery time, total amount of medications used, and complication rates
| Group C Mean±SD [Median] |
Group A Mean±SD [Median] |
p | ||
|---|---|---|---|---|
|
| ||||
| Duration of the procedure [min] | 15.31±6.61 [15] | 16.57±6.92 [14] | c0.421 | |
| Recovery time [min] | 4.82±1.9 [6] | 4.47±1.93 [3] | c0.289 | |
| Total amount of ketamine used [mg] | 71.08±10.41 [70] | 70.98±11.49 [70] | c0.932 | |
| Total amount of propofol used [mg] | 71.79±20.87 [70,5] | 74.19±20.41 [71,5] | c0.572 | |
| Total amount of remifentanil used [mcg] | 90.15±58.17 [75] | 66.51±47.87 [60] | c0.011* | |
| n | % | |||
| Complication | No | 48 [94.1] | 46 [90.2] | d0.715 |
| Yes | 3 [5.9] | 5 [9.8] | ||
Mann-Whitney U test,
Fisher’s exact test,
p<0.05
Figure 1.
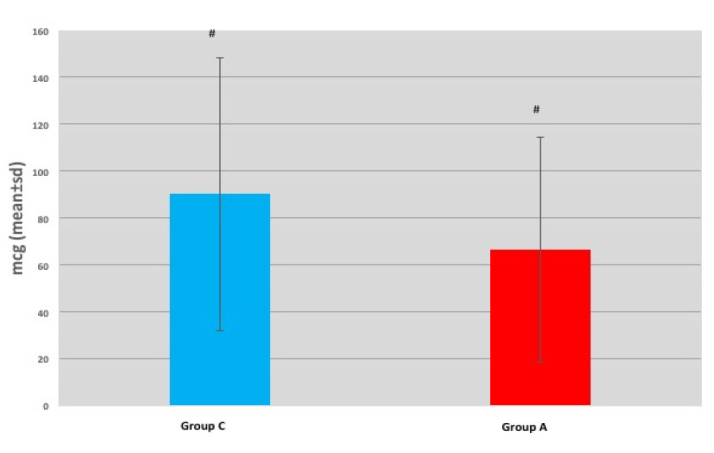
Total amount of remifentanil used
Values are the mean±standard error of the mean of each Group
#P value 0.011 versus control Group.
There were no statistically significant differences between the groups in terms of HR and RR before or during the procedure (p>0.05) (Figures 2, 3). The MBP level of Group A at Minute 9 was statistically significantly lower than the one of Group C (p=0.049). However, there were no statistically significant differences at other time points (p>0.05) (Figure 4).
Figure 2.
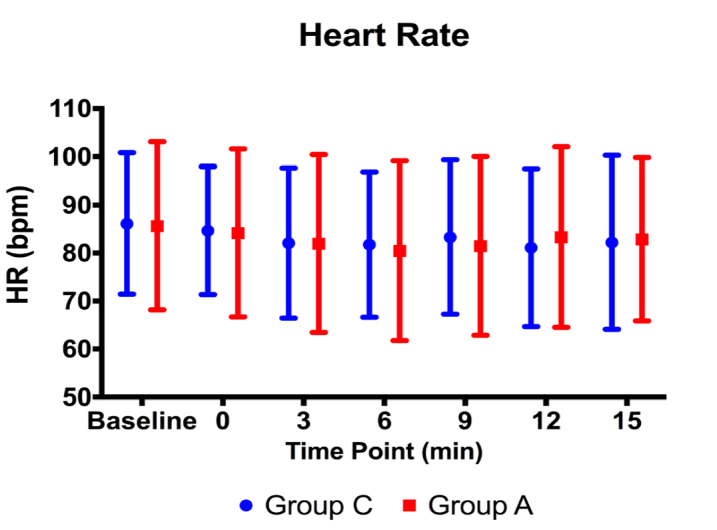
Heart rate values before and during the procedure
Figure 3.
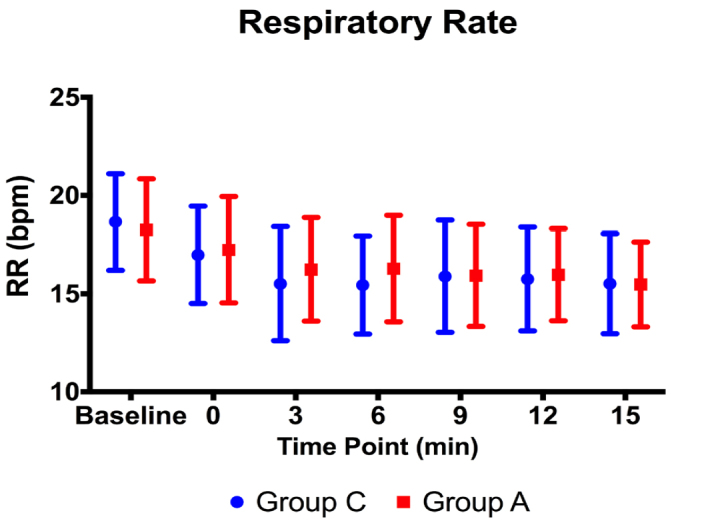
Respiratory rate values before and during the procedure
Figure 4.
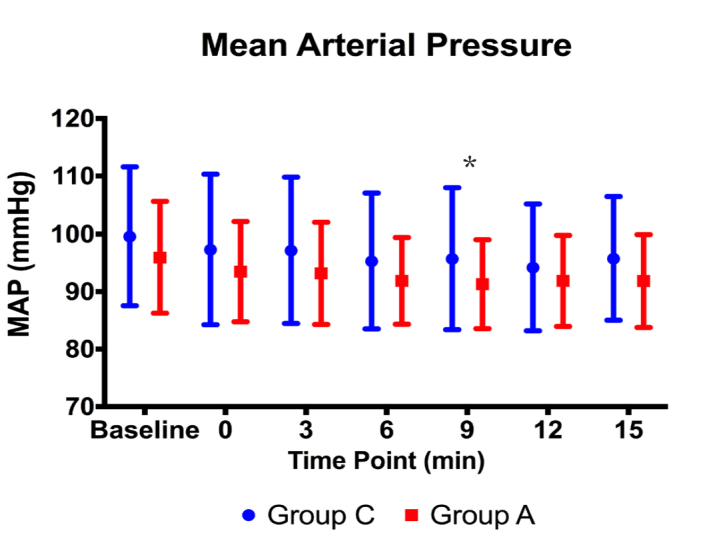
Mean blood pressure values before and during the procedure (*: p<0.05)
There was no statistically significant difference between the groups in terms of SpO2 levels before the procedure (p>0.05). The SpO2 levels of Group A were found to be statistically significantly higher than the other Group at minutes 0 and 3 (p=0.039, p=0.028, respectively). There were no statistically significant differences between the groups at other time points (p>0.05) (Figure 5). None of the patients needed manual ventilation.
Figure 5.
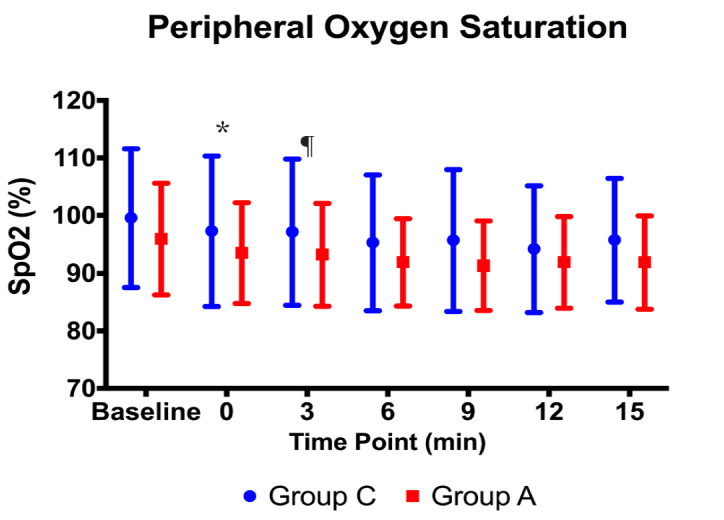
SpO2 values before and during the procedure (*,¶: p<0.05)
BIS scores of Group A were statistically significantly higher than those of Group C before the procedure (p<0.001). During the procedure, there were no statistically significant differences between the groups in terms of BIS scores (p>0.05). However, the BIS scores after Minute 3 were demonstrated to be statistically significantly lower in Group A as compared to those of Group C (Figure 6).
Figure 6.
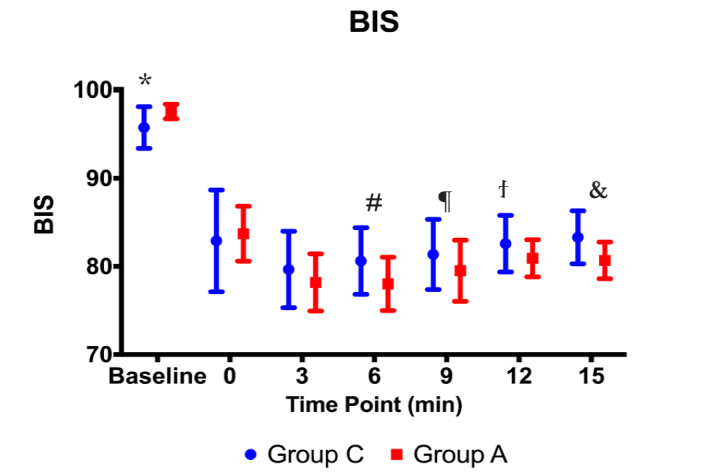
BIS values before and during the procedure (*, #, ¶, ., &: p<0.01)
There were no statistically significant differences between the groups in terms of the RSS scores at Minute 0 (p>0.05). It was demonstrated that the RSS scores of Group A were statistically significantly higher at minutes 3 and 6 than those of Group C (p<0.001, p=0.005, respectively). There were no statistically significant differences between the groups in terms of their RSS scores (p>0.05). It was determined that the RSS scores of Group A were statistically significantly higher than those of Group C before the procedure (p<0.001) (Figure 7).
Figure 7.
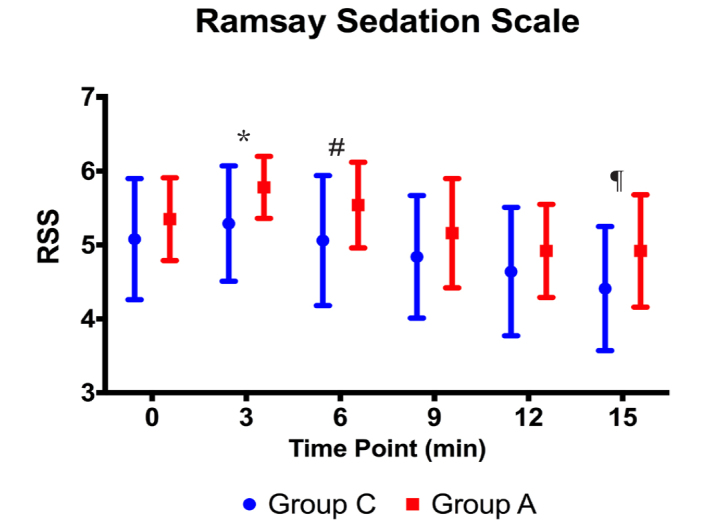
RSS values during the procedure (*, #: p<0,01, ¶: p<0.05)
There was no statistically significant difference between the groups in NRS scores during the procedure (p>0.05) (Figure 8).
Figure 8.
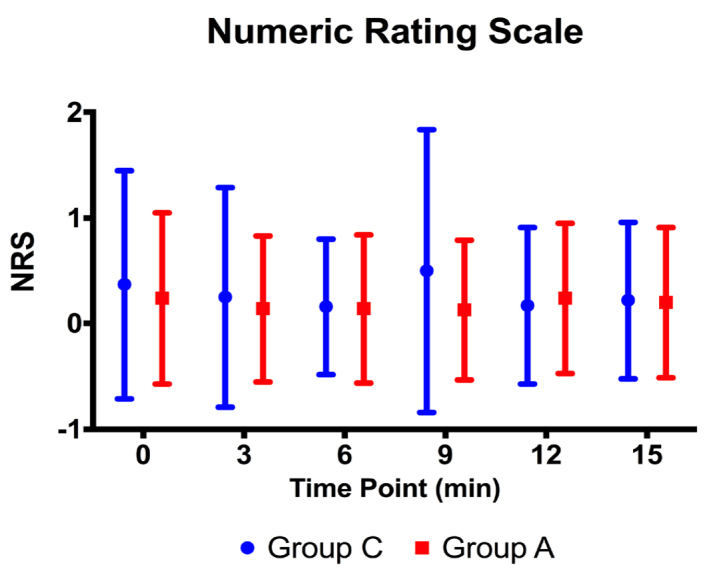
NRS values during the procedure at Minute 0
In Group A patients, ANI scores statistically significantly declined at Minute 3 and Minute 6, as compared to the baseline values (p<0.05); however, at minutes 0, 9, 12 and 15, it was not significant (p>0.05) (Figure 9).
Figure 9.
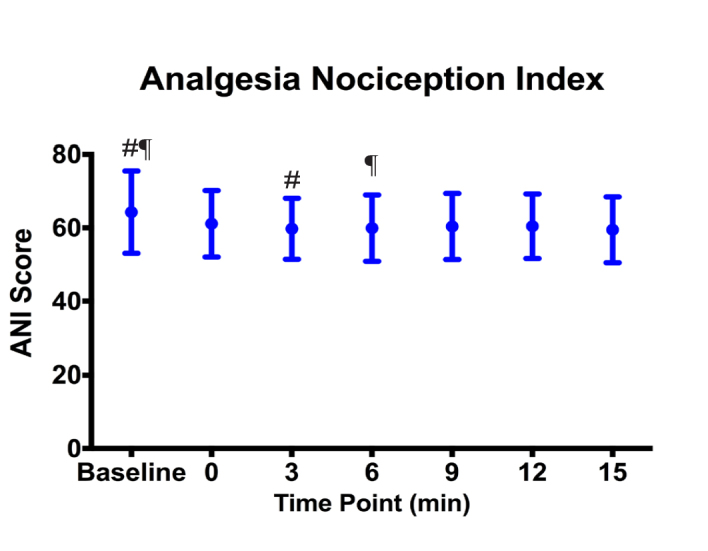
ANI values in Group A (#: p=0,021, ¶: p=0.049)
It was demonstrated that there was a negative correlation between the ANI and NRS scores of Group A patients at Minute 0 at the level of 0.402, which was statistically significant (p=0.003). There were no statistically significant correlations between the ANI and NRS scores at other time points (p>0.05) (Figure 10).
Figure 10.
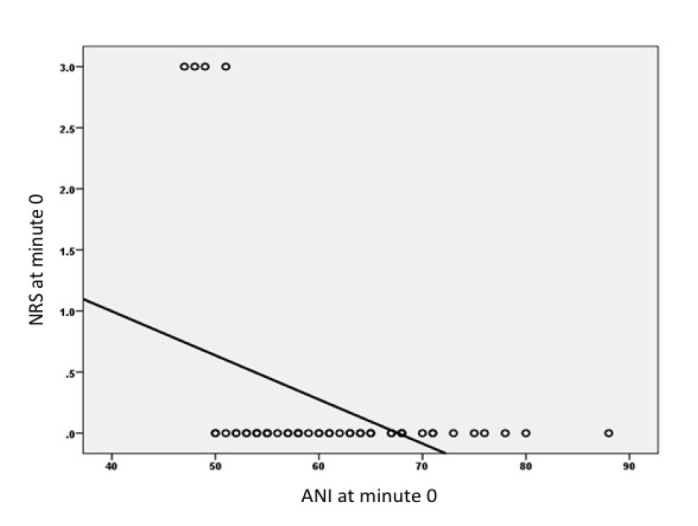
The correlation between NRS and ANI values
There were no statistically significant differences in HR, MBP, RR and SpO2 levels and the MAS and NRS scores (Figure 8) between the groups during recovery (p>0.05).
Discussion
The HRV-based ANI has been proposed to reflect different levels of acute pain. In our study, the ANI monitoring usage in adults undergoing colonoscopy with sedo-analgesia resulted in diminished opioid consumption and lower NRS scores, which were desired effects as patient safety was enhanced.
An anaesthesia technique in procedural sedation should guarantee patient safety and comfort and provide fast recovery and discharge. As there is no one drug to provide sedation, amnesia and analgesia, combinations of drugs are used. Mainly respiratory and haemodynamic instability threaten patient safety during sedo-analgesia (11).
There are publications investigating ANI’s efficacy in the intra-operative and post-operative period in patients administered general anaesthesia. We have not encountered any studies on opioid titration guided by ANI in patients who were applied sedo-analgesia.
Jeanne et al. (12, 13) demonstrated that ANI is more sensitive in detecting pain compared to the elevations of HR and blood pressure in patients who received propofol and opioid (remifentanil, sufentanil) combinations. Similar results were obtained with inhalation anaesthetic and opioid combinations as well (14, 15).
There was only one study in the literature where administering sedo-analgesia with ANI monitoring found that ANI levels <50 detected haemodynamic reactivity in advance (6). In our study, no haemodynamic reactivity was observed as the design of the study entailed to maintain an ANI score between 50 and 70, while providing remifentanil titration for analgesia.
At the recovery period from anaesthesia, sedation can be observed due to the residual drug effects. Ledowski et al. (16) reported a low sensitivity and specificity of ANI due to a statistically significant but low negative correlation between ANI and NRS during sevoflurane and fentanyl anaesthesia. Boselli et al. (17) demonstrated a negative linear correlation between NRS and ANI in patients during the early post-operative period after general anaesthesia and that ANI detected pain intensity with high sensitivity and specificity.
Le Guen et al. (18) detected a high level of negative linear correlation between ANI and the visual pain scale in parturients with epidural analgesia for vaginal delivery. However, Jess et al. (19) demonstrated that there was no negative linear correlation between ANI and NRS in experimentally induced pain.
In our study, there was a negative correlation between ANI and NRS only at Minute 0. We suggest that, as the drugs did not reach effective concentrations at Minute 0, the NRS scores were detected as high, and the ANI scores were detected as low. At the same time, there were no significant differences between the groups in terms of the NRS scores.
Mental stress causes a reduction in the cardiac vagal control and the high-frequency component of HRV (20, 21). In our study, ANI scores demonstrated a statistically significant reduction. We suggest that fear and anxiety due to colonoscopy might have contributed to this reduction.
In the literature, there is a limited number of studies on analgesic drug management by ANI. Szental et al. (22) administered morphine and fentanyl as entailed by a standard protocol and according to ANI scores during general anaesthesia, and they reported that ANI monitoring had no advantages over standard care in terms of intra-operative opioid consumption, post-operative pain intensity and on the reduction in the need for analgesic drugs. It can be predicted that remifentanil, which has a faster pharmacokinetic profile than morphine and fentanyl can be easier to administer during monitoring by ANI. Daccache et al. (23) targeted spectral entropy in the range of 40–60 for the depth of the anaesthesia level in patients undergoing elective vascular surgery under total intravenous general anaesthesia provided by target-controlled propofol and remifentanil infusions; titrated remifentanil to maintain ANI scores between 50–70 and reported it as a safe method.
In our study, the consumption of remifentanil, BIS and RSS in Group A was detected to be lower, and it was suggested that ANI monitoring could successfully lead to a reduced opioid consumption. Total remifentanil administered was lower in Group A, and we associated the occurrence of deeper sedation levels in this Group with obtaining of a stable nociception-antinociception balance. When propofol and ketamine consumption were same in both groups, no additional propofol dose had to be administered as BIS remained within desired limits.
Ketamine use during subanaesthetic doses may cause dissociation accompanied by psychotic symptoms (24). This situation may lead to erroneous sedation and analgesia level assumptions in patients who cannot express themselves, and it increases the need for objective assessment methods. In our study, when the ANI levels were within the targeted range, patients exhibited behaviour similar to the one at painful states. After the recovery when these patients were interviewed again, it was revealed that none of them felt pain, and it was suggested that these behaviours were associated with ketamine. Also, ketamine is known to cause sympathetic discharge (25). Sympathomimetic drugs lead to artefacts in ANI computations, but this is not the case for ketamine. Bollag et al. (26) demonstrated that the administration of ketamine at a single dose of 0.5 mg kg−1 to patients undergoing abdominal hysterectomy under general anaesthesia caused no statistical or clinically significant differences.
In our study, no statistically significant differences were detected on haemodynamic and respiratory parameters between groups in terms of NRS and MAS scores at the recovery period. The recovery durations of groups were equivalent. These results mean that the opioid titration in line with ANI monitoring during the procedure reflected equally effective with the conventional method on the recovery period.
As the costs of health care increase, process efficiency gets more important. Efficiency in medical services, patient safety and cost evaluations drew attention. In June 2010, EBA and ESA accepted the Helsinki Declaration on patient safety in anaesthesiology (27). Objective monitoring methods are suggested to be used to avoid possible untoward effects of anaesthesia medication and to apply sufficient doses tailored for individual patients. ANI monitoring can be one of these methods.
The most important limitation of our study was that it was conducted in one patient Group before and during the procedure. Recording the data of the patients in the control Group and recording the ANI scores at the recovery period could have provided a better investigation of the efficacy of ANI monitoring. The other limitations in our study were the short duration of colonoscopy procedure leading to a short period of sedo-analgesia administration and the limitation of patient number to 102. It would be adequate to assess the efficacy of ANI monitoring during various interventions under sedo-analgesia and by the inclusion of more patients. The increase in the number and complexity of interventions conducted out-of-operating room and the eventual increase in demand to anaesthesia administrations lead to anaesthetists to spend more time out of the operating room day by day.
Conclusion
In our study, an opioid titration was performed in adult patients undergoing colonoscopy under sedo-analgesia by conventional monitoring in one Group and by ANI monitoring in the other. Opioid consumption was observed to decrease in the Group where ANI monitoring was performed, and we suggest that this contributed to enhancing the patient safety. The reason of finding no differences between the groups in terms of side effects, complications and the duration of recovery may be associated with a short duration of the procedure or with the patient number’s being lower. In future studies, the efficacy of ANI monitoring should be assessed during procedures with a longer duration and a larger sample size to be able to detect any differences in these parameters.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Acibadem University Clinical Research Ethics Committee (2015–16/15).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.S., Z.A.; Design – M.S., G.T.A.; Supervision – Z.A., M.K.A.; Resources – M.S., G.T.A., P.Ç.D., M.K.A.; Materials – G.T.A., P.Ç.D., M.K.A.; Data Collection and/or Processing – M.S., G.T.A., P.Ç.D.; Analysis and/or Interpretation – M.K.A., G.T.A., P.Ç.D.; Literature Search – M.S., P.Ç.D., G.T.A.; Writing Manuscript – M.S., P.Ç.D., G.T.A., M.K.A.; Critical Review – M.S., G.T.A., P.Ç.D., M.K.A., Z.A.; Other – M.S., G.T.A., P.Ç.D., M.K.A., Z.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The study was funded by BAPKO (Bilimsel Araştırma Projeleri Birimi) Scientific Research Projects Department of Marmara University.
References
- 1.Van De Velde M, Kuypers M, Teunkens A, Devroe S. Risk and safety of anesthesia outside the operating room. Minerva Anestesiologica. 2009;75:345–8. [PubMed] [Google Scholar]
- 2.Chelazzi C, Consales G, Boninsegni P, Bonanomi GA, Castiglione G, De Gaudio AR. Propofol sedation in a colorectal cancer screening outpatient cohort. Minerva Anestesiologica. 2009;75:677–83. [PubMed] [Google Scholar]
- 3.World Health Organization. Promoting rational use of medicines: core components. WHO Policy Perspectives on Medicines. No: 005. Sep, 2002. Available from: http://apps.who.int/medicinedocs/pdf/h3011e/h3011e.pdf.
- 4.Gruenewald M, Ilies C. Monitoring the nociception-anti-nociception balance. Best Practice & Research Clinical Anaesthesiology. 2013;27:235–47. doi: 10.1016/j.bpa.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Logier R, Jeanne M, De Jonckheere J, Dassonneville A, Delecroix M, Tavernier B. PhysioDoloris: a monitoring device for analgesia / nociception balance evaluation using heart rate variability analysis. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference; 2010 31 August-4 Semptember; Buenos Aires: Argentina. pp. 1194–7. [DOI] [PubMed] [Google Scholar]
- 6.Boselli E, Bouvet L, Begou G, Torkmani S, Allaouchiche B. Prediction of hemodynamic reactivity during total intravenous anesthesia for suspension laryngoscopy using analgesia/nociception index (ANI): a prospective observational study. Minerva Anestesiologica. 2015;81:288–97. [PubMed] [Google Scholar]
- 7.Boselli E, Daniela-Ionescu M, Begou G, Dabouz R, Davidson J, Deloste JY, et al. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI) Br J Anaest. 2013;111:453–9. doi: 10.1093/bja/aet110. [DOI] [PubMed] [Google Scholar]
- 8.Jeanne M, Logier R, De Jonckheere J, Tavernier B. Heart rate variability during total intravenous anesthesia: effects of nociception and analgesia. Auton Neurosci. 2009;147:91–6. doi: 10.1016/j.autneu.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Rudner R, Jalowiecki P, Kawecki P, Gonciarz M, Mularczyk A, Petelenz M. Conscious analgesia/sedation with remifentanil and propofol versus total intravenous anesthesia with fentanyl, midazolam, and propofol for outpatient colonoscopy. Gastrointest Endosc. 2003;57:657–63. doi: 10.1067/mge.2003.207. [DOI] [PubMed] [Google Scholar]
- 10.Core Team R. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org. [Google Scholar]
- 11.Bellolio MF, Gilani WI, Barrionuevo P, Murad MH, Erwin PJ, Anderson JR, et al. Incidence of adverse events in adults undergoing procedural sedation in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:119–34. doi: 10.1111/acem.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeanne M, Clement C, De Jonckheere J, Logier R, Tavernier B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. 2012;26:289–94. doi: 10.1007/s10877-012-9354-0. [DOI] [PubMed] [Google Scholar]
- 13.Jeanne M, Delecroix M, De Jonckheere J, Keribedj A, Logier R, Tavernier B. Variations of the analgesia nociception index during propofol anesthesia for total knee replacement. Clin J Pain. 2014;30:1084–8. doi: 10.1097/AJP.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 14.Gruenewald M, Herz J, Schoenherr T, Thee C, Steinfath M, Bein B. Measurement of the nociceptive balance by Analgesia Nociception Index and Surgical Pleth Index during sevoflurane-remifentanil anesthesia. Minerva Anestesiol. 2015;81:480–9. [PubMed] [Google Scholar]
- 15.Ledowski T, Averhoff L, Tiong WS, Lee C. Analgesia Nociception Index (ANI) to predict intraoperative haemodynamic changes: results of a pilot investigation. Acta Anaesthesiol Scand. 2014;58:74–9. doi: 10.1111/aas.12216. [DOI] [PubMed] [Google Scholar]
- 16.Ledowski T, Tiong WS, Lee C, Wong B, Fiori T, Parker N. Analgesia nociception index: evaluation as a new parameter for acute postoperative pain. Br J Anaesth. 2013;111:627–9. doi: 10.1093/bja/aet111. [DOI] [PubMed] [Google Scholar]
- 17.Boselli E, Bouvet L, Begou G, Dabouz R, Davidson J, Deloste JY, et al. Prediction of immediate postoperative pain using the analgesia/nociception index: a prospective observational study. Br J Anaesth. 2014;112:715–21. doi: 10.1093/bja/aet407. [DOI] [PubMed] [Google Scholar]
- 18.Le Guen M, Jeanne M, Sievert K, Al Moubarik M, Chazot T, Laloë PA, et al. The Analgesia Nociception Index: a pilot study to evaluation of a new pain parameter during labor. Int J Obstet Anesth. 2012;21:146–51. doi: 10.1016/j.ijoa.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Jess G, Pogatzki-Zahn EM, Zahn PK, Meyer-Friessem CH. Monitoring heart rate variability to assess experimentally induced pain using the analgesia nociception index: A randomised volunteer study. Eur J Anaesthesiol. 2016;33:118–25. doi: 10.1097/EJA.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 20.Miu AC, Heilman RM, Miclea M. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Auton Neurosci. 2009;145:99–103. doi: 10.1016/j.autneu.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 21.De Jonckheere J, Rommel D, Nandrino JL, Jeanne M, Logier R. Heart rate variability analysis as an index of emotion regulation processes: interest of the analgesia nociception index (ANI). Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference; 2102 28 August-1 September; San Diego, USA. pp. 3432–5. [DOI] [PubMed] [Google Scholar]
- 22.Szental JA, Webb A, Weeraratne C, Campbell A, Sivakumar H, Leong S. Postoperative pain after laparoscopic cholecystectomy is not reduced by intraoperative analgesia guided by analgesia nociception index (ANI) monitoring: a randomized clinical trial. Br J Anaesth. 2015;114:640–5. doi: 10.1093/bja/aeu411. [DOI] [PubMed] [Google Scholar]
- 23.Daccache G, Caspersen E, Pegoix M, Monthé-Sagan K, Berger L, Fletcher D, et al. A targeted remifentanil administration protocol based on the analgesia nociception index during vascular surgery. Anaesth Crit Care Pain Med. 2017;36:229–32. doi: 10.1016/j.accpm.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacologica Sinica. 2016;37:865–72. doi: 10.1038/aps.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas DA, Harper DG. Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Bollag L, Ortner CM, Jelacic S, Rivat C, Landau R, Richebe P. The effects of low-dose ketamine on the analgesia nociception index (ANI) measured with the novel PhysioDoloris analgesia monitor: a pilot study. J Clin Monitoring and Computing. 2015;29:291–5. doi: 10.1007/s10877-014-9600-8. [DOI] [PubMed] [Google Scholar]
- 27.Mellin-Olsen J, Staender S, Whitaker DK, Smith AF. The Helsinki declaration on patient safety in anaesthesiology. Eur J Anaesthesiol. 2010;27:592–7. doi: 10.1097/EJA.0b013e32833b1adf. [DOI] [PubMed] [Google Scholar]


