Abstract
We report a case of spinal cord injury following an attempted epidural in a conscious woman for pain management in acute pancreatitis. The epidural needle was inserted at the T11–T12 interspace. On the second attempt, dural puncture occurred. The patient did not complain of pain or discomfort during the procedure. Thirty-two hours after the attempted epidural, the patient was found to have motor deficit on her right lower limb. Magnetic resonance imaging showed a spinal haematoma with direct spinal cord injury. Post-laminectomy neurological recovery was slow but progressive. The possible causes for spinal cord injury and spinal haematoma without pain or paraesthesia during the procedure are discussed.
Keywords: Direct spinal injury, epidural anaesthesia, spinal haematoma
Introduction
The use of thoracic epidural analgesia was shown to improve the outcome in patients with acute pancreatitis (1). Although it has a good safety profile, epidural analgesia has some potentially severe complications including infection, nerve damage and epidural or subdural haematoma (2). Direct spinal cord injury and subdural haematoma may occur during placement of the epidural catheter. It is an extremely rare complication with serious consequences. Several cases have been described in the literature with most of them not only in anaesthetised patients but also in conscious patients (3–5).
We report a case of spinal cord injury and subdural haematoma following two attempts of inserting a thoracic epidural in a conscious woman for pain management in acute pancreatitis.
Case Presentation
A 73-year-old woman (height 160 cm, weight 75 kg) with post-endoscopic retrograde cholangiopancreatography pancreatitis is referred to the analgesia team for intense abdominal pain. She had a history of hypertension, and her baseline arterial blood pressure was 160/80 mmHg. She received a multimodal treatment for pain consisting of nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol and opioids. The use of a thoracic epidural for pain relief was suggested and discussed with the patient because her abdominal pain persisted despite high doses of opioids. Laboratory evaluation showed normal coagulation and platelets, anaemia (haemoglobin 9.5 mg dL−1), leucocytosis (white blood cell 16,000/μL), serum lipase increased (150 U L−1), normal aspartate aminotransferase and alanine aminotransferase and normal blood urea nitrogen and creatinine. In addition, she was not taking any antithrombotic or anticoagulant therapy.
She was brought to the operating theatre where her vital signs were checked (heart rate 90 beats/min, blood pressure 165/74 mmHg), she was placed in a sitting position, and her T11–T12 space was identified. Prior to epidural analgesia, the skin where the epidural needle was to enter was infiltrated with 2 mL of 2% lignocaine with aseptic precautions. A median approach was performed using an 18-gauge Tuohy needle with a loss of resistance to saline. The first attempt did not identify the epidural space. A second attempt was made one interspace lower. Dural puncture occurred on the second attempt as free-flowing cerebrospinal fluid, and blood was seen in the syringe so the procedure was abandoned. The patient did not complain of pain or discomfort during the procedure. Throughout the procedure, the patient was monitored using a 3-lead electrocardiogram, pulse oximetry and non-invasive blood pressure recordings.
After the procedure, the patient complained of numbness in her right foot, but she could move it freely, and bladder and bowel functions were normal. She was transferred to the ward where she was prescribed three doses of 8 mg dexamethasone. She continued to receive AINS and opioids for abdominal pain. On the next day, the patient continued to complain of intense abdominal pain so she continued to receive high-dose opioids. On physical examination, 32 h after the attempted epidural, the patient was found to have motor deficit only without sensory deficit on her right lower limb with exception of the foot which she could move. Motor function and sensation were intact on her left leg, and she was not complaining of head or back pain.
An urgent spinal magnetic resonance (MR) scan was performed, together with a neurosurgical examination. MR imaging (MRI) showed a heterogeneous high signal (T2) mass compressing the cord from T5 to T11 and from T12 to L5. The appearances were felt to be consistent with a spinal haematoma. In addition, MRI showed spinal cord oedema and a high T2 signal intensity compatible with direct trauma at the T11–T12 interspace.
The neurosurgical team was consulted and subsequently performed an emergency laminectomy and evacuation of the haematoma. A puncture wound was seen in the dura at T11–T12, and the dural sac was severely distended. The dura was incised, revealing a haemorrhagic puncture wound. The clots and blood were removed, and the dura was repaired. On direct surgical examination, the haematoma appears to cause significant compression (Figure 1, 2).
Figure 1.
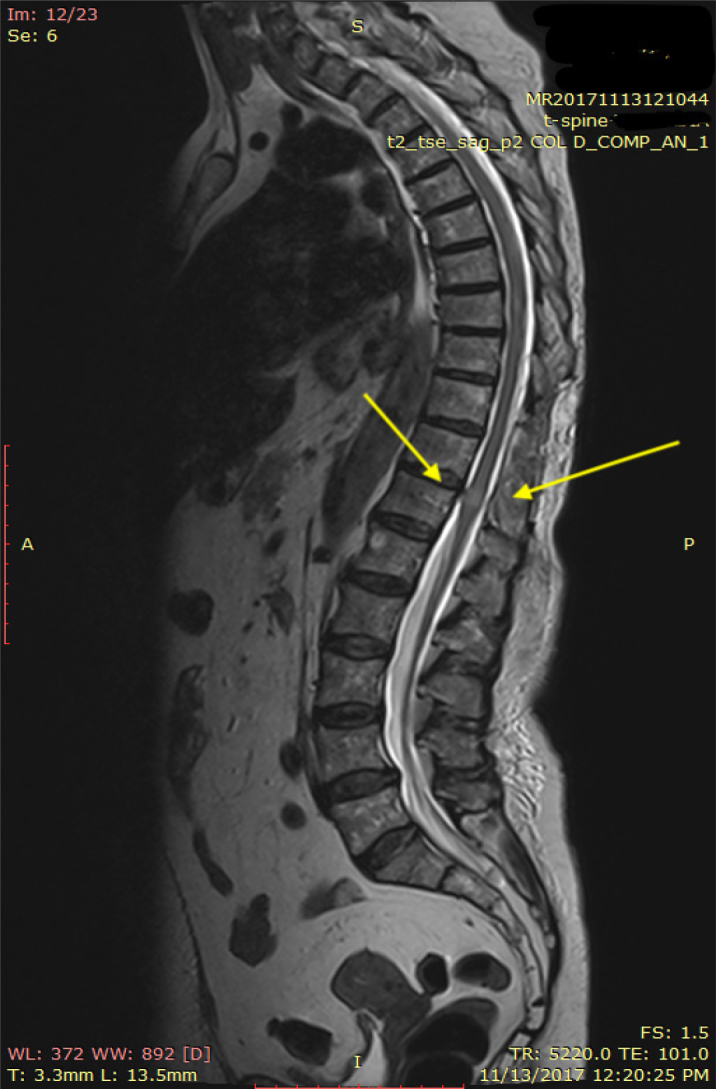
Sagittal view of magnetic resonance imaging with high T2 signal intensity within the spinal cord
Figure 2.
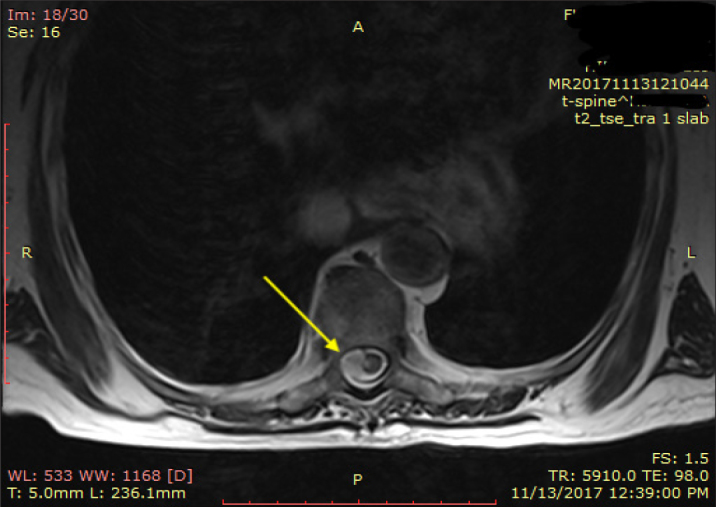
Axial T2-weighted magnetic resonance imaging image. Hyperintense signal intensity suggestive of a cord contusion
On post-laminectomy, motor function on the right leg was still profoundly depressed with proprioceptive and exteroceptive hypoesthesia on the right side with a T4 level. The left leg had normal motor and sensory functions. Micturition and defaecation were normal, but she complained of dizziness and backache on mobilisation.
She received neurological treatment with vitamin B complexes, alpha-lipoic acid, cyanocobalamin, steroids and physical therapy. Neurological recovery was slow but progressive through the next weeks and was assessed regularly by a neurologist with improvement in motor function of the right leg from 0/5 to 1/5 at 14 days post-laminectomy. She developed an ischaemic cerebellar stroke with worsening of the neurological status on postoperative day 16. The patient died 4 weeks later from complications due to ischaemic stroke. Informed consent for this case report was obtained from the family.
Discussion
Spinal cord damage can result from needle or catheter trauma, local anaesthetic toxicity, epidural or subdural haematoma, ischaemia from an arterial injury, or severe hypotension (5, 6).
In our case, the spinal cord damage could be resulted either from the subdural haematoma which produced significant compression of the spinal cord, a fact confirmed at laminectomy, or from an unrecognised spinal cord puncture during the epidural needle placement, a fact confirmed at MRI or a combination of those two.
Previously, many case reports of spinal cord injury following regional block in conscious patients mentioned that the patient complained of paraesthesia as a result of injury to the spinal cord. Paraesthesia associated with spinal cord injury can occur at the time of needle placement or injection. It is unusual that our patient never complained of localised radiating pain or paraesthesia during the procedure, as the patient was conscious (7). Tsui et al. (8) described a similar case of spinal cord injury in which the patient did not complain of pain despite a clinically obvious dural puncture.
The fact that our patient did not complain of paraesthesia can be explained by three mechanisms. First, as Tsui showed in his study, pain is more common in extra-axial lesions affecting the nerve roots or blood vessels that are innervated by sensory neurones mediating pain. In contrast, because there is no pain receptors within the spinal cord, intra-axial lesions may be painless, and pain reported from dural puncture is rare in clinical practice (8). Second, there is a possibility that the local anaesthetic, which was delivered via subcutaneous injection, was accidently injected into the subarachnoid space before epidural needle insertion, thereby leading to sensory loss. The distance from the skin to the epidural space is between 4 and 6 cm in the majority of patients. This distance can be <3 cm in thin patients (9). Although our patient was not thin, the distance between the skin and the subdural space was approximately 4 cm, as can be seen on MRI images. Third, this may be due to high doses of opioids administered to the patient prior to the procedure for controlling her pancreatitis abdominal pain. These high doses of opioids could have reduced the pain threshold, making the patient feel no pain during the procedure (10).
Another important aspect of this case consists of neurological damage. In spite of spinal cord injury at the lower thoracic level, lesion that we think was produced by a combination of direct needle injury and subsequent haematoma, with visualisation of intraoperative compression ischaemia at this level, the neurological deficit was limited to the lower right limb motor function, with minimal sensory deficit, without impairment of the opposite lower limb and without the patient eliciting back pain. The association between opioids, AINS and dexamethasone could explain the lack of back pain following the spinal cord injury. Dexamethasone is a potent anti-inflammatory glucocorticoid often used after injury to reduce oedema in neurological tissue and can act synergic with other anti-inflammatory drugs and opioids to reduce pain (11). Furthermore, it is able to down-regulate prostaglandin synthesis, contributing to analgesia peripherally and at spinal cord level by limiting sensitisation of nociceptive and inflammatory pathways (12). This could have contributed in part to the delay in neurological diagnosis. Although neurological diagnosis was delayed mainly due to the lack of vigorous neurological monitoring, the neurological outcome of the patient was partially favourable. The neurosurgical and neurological consultations estimated partial recovery of motor function. Unfortunately, the ischaemic cerebellar stroke affected the patient’s overall outcome leading to her death.
Conclusion
This case reminds us that accidental puncture of the cord does not always elicit severe pain and reflex movement in conscious patients. Any patient in whom there is a suspicion of direct trauma to the spinal cord during an attempted epidural catheterisation should undergo detailed neurological assessment and prompt treatment. In addition, the use of ultrasonography for neuraxial blocks can be used to limit this complication and improve clinical outcome (13).
Footnotes
Informed Consent: Written informed consent was obtained from the family who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – C.M.B., A.B., C.I.M.; Design – A.B., C.M.B.; Supervision – C.I.M., D.B.; Resources – C.M.B.; Materials – A.B., D.B.; Data Collection and/or Processing – C.M.B., C.I.M.; Analysis and/or Interpretation – C.M.B., A.B., D.B. C.I.M.; Literature Search – C.M.B.; Writing Manuscript – C.M.B., A.B.; Critical Review – C.I.M.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Jabaudon M, Belhadj-Tahar N, Rimmelé T, Joannes-Boyau O, Bulyez S, Lefrant JY, et al. Thoracic epidural analgesia and mortality in acute pancreatitis: a multicenter propensity analysis. Crit Care Med. 2018;46:198–205. doi: 10.1097/CCM.0000000000002874. [DOI] [PubMed] [Google Scholar]
- 2.Windisch O, Heidegger C, Giraud R, Morel P, Bühle L. Thoracic epidural analgesia: A new approach for the treatment of acute pancreatitis? Crit Care. 2016;20:116. doi: 10.1186/s13054-016-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson PA, Valentine A, Gibbs JM. Intrinsic spinal cord lesions complicating epidural anaesthesia and analgesia: report of three cases. J Neurol Neurosurg Psychiatry. 2002;72:537–9. doi: 10.1136/jnnp.72.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose JB. Spinal Cord Injury in a Child After Single-Shot Epidural Anesthesia. Anesth Analg. 2003;96:3–6. doi: 10.1213/00000539-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Kao MC, Tsai SK, Tsou MY, Lee HK, Guo WY, Hu JS. Paraplegia after delayed detection of inadvertent spinal cord injury during thoracic epidural catheterization in an anesthetized elderly patient. Anesth Analg. 2004;99:580–3. doi: 10.1213/01.ANE.0000130391.62612.3E. [DOI] [PubMed] [Google Scholar]
- 6.Fabio C, Romualdo DB, Eugenio AF, Vittoradolfo T, Massimiliano VA, Giovanna R. Thoracic unilateral spinal cord injury after spinal anaesthesia for total hip replacement: fate or mistake? Turk J Anaesthesiol Reanim. 2017;45:116–8. doi: 10.5152/TJAR.2016.32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer MJ, Krane EJ, Goldschneider KR, Klein NJ. Neurological complications associated with epidural analgesia in children: a report of 4 cases of ambiguous etiologies. Anesth Analg. 2012;115:1365–70. doi: 10.1213/ANE.0b013e31826918b6. [DOI] [PubMed] [Google Scholar]
- 8.Tsui BC, Armstrong K. Can direct spinal cord injury occur without paresthesia? A report of delayed spinal cord injury after epidural placement in an awake patient. Anesth Analg. 2005;101:1212–4. doi: 10.1213/01.ANE.0000175764.16650.85. [DOI] [PubMed] [Google Scholar]
- 9.Absalom AR, Martinelli G, Scott NB. Spinal cord injury caused by direct damage by local anaesthetic infiltration needle. Br J Anaesth. 2001;87:512–5. doi: 10.1093/bja/87.3.512. [DOI] [PubMed] [Google Scholar]
- 10.Kandel ER. The perception of pain. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of neural science. New York: McGraw-Hill Health Professions Division; 2000. pp. 472–91. [DOI] [Google Scholar]
- 11.Feng X, Yuan W. Dexamethasone enhanced functional recovery after sciatic nerve crush injury in rats. Biomed Res Int. 2015;2015 doi: 10.1155/2015/627923. 627923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon KG, Choi S, Rodseth RN. The role of dexamethasone in peripheral and neuraxial nerve blocks for the management of acute pain. SAJAA. 2016;22:163–9. doi: 10.1080/22201181.2016.1251063. [DOI] [Google Scholar]
- 13.Şahin T, Balaban O. Lumbar ultrasonography for obstetric neuraxial blocks: sonoanatomy and literature review. Turk J Anaesthesiol Reanim. 2018;46:257–67. doi: 10.5152/TJAR.2018.90277. [DOI] [PMC free article] [PubMed] [Google Scholar]


