Abstract
Objective:
To evaluate the oncologic outcomes and histologic concordance of post-chemotherapy residual liver mass resection with post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND).
Methods:
Retrospective review of our prospectively maintained germ cell tumor (GCT) surgical database identified patients with non-seminomatous GCT (NSGCT) who underwent both post-chemotherapy residual liver mass resection and PC-RPLND between 1990 and 2015.
Results:
A total of 36 patients were identified, of whom 29 (81%) presented with a liver mass at initial diagnosis and 17 (47%) received second-line chemotherapy prior to liver resection. Teratoma was found in 8 (22%) and 5 (14%) of PC-RPLND and liver resection specimens, respectively. Viable GCT was found in 5 (14%) and 4 (11%) of PC-RPLND and liver resection specimens, respectively. Histologic discordance was observed in 4 of 19 (21%; 95% CI 6.1–46%) patients; in all cases, liver resection specimens contained teratoma or viable GCT while PC-RPLND revealed only fibrosis/necrosis. At 3 years after surgical intervention, the Kaplan-Meier estimated probability of cancer-specific survival was 75% (95% CI 5585%) and the probability of progression-free survival was 75% (95% CI 56–87%).
Conclusion:
In this contemporary cohort, clinically significant discordance was observed between the histology of metastatic liver masses and that of retroperitoneal lymph nodes. The benefit of post-chemotherapy liver mass resection for patients with advanced NSGCT is supported by favorable survival outcomes. Until more reliable predictors of post-chemotherapy histology exist, complete surgical resection of all sites of residual disease should be performed whenever feasible.
Keywords: Non-seminomatous germ cell tumor, lymph node dissection, liver resection, second-line chemotherapy, histologic concordance
Introduction
Following first-line chemotherapy, viable germ cell tumor (GCT) and teratoma can be found in up to 15% and 45% of retroperitoneal lymph node dissections (RPLND), respectively [1–3]. The rates of chemotherapy-refractory GCT are even higher after second-line chemotherapy[4]. Clinical outcomes for patients with viable GCT or teratoma after chemotherapy are directly related to the completeness of surgical resection[5, 6]. Thus, post-chemotherapy surgery is integral to the effective management of NSGCT[2, 7, 8].
Unfortunately, clinical staging remains a significant challenge despite advances in radiographic imaging. Prediction of negative histology in post-chemotherapy residual masses in the retroperitoneum is inaccurate in about 20%−30% of cases [2, 9, 10]. Furthermore, multiple studies have shown that rates of histologic discordance between different sites of residual disease can range from 20–46%[11, 12], so necrosis/fibrosis in one site does not indicate that all residual disease is benign. Accordingly, our institution advocates complete surgical resection for all sites of residual disease.
Approximately 30–40% of patients with NSGCT will present with extra-retroperitoneal metastases, affecting the liver in 2–15% of cases[6, 12–14]. Patients presenting with liver metastases are considered to have International Germ Cell Consensus Classification Group (IGCCCG) poor risk disease and require appropriate management to optimize outcomes[14]. However, there is a paucity of contemporary data on the outcomes of post-chemotherapy residual liver mass resection, including survival and histological concordance between retroperitoneal lymph node and hepatic specimens.
Thus, we sought to characterize the clinical and histologic outcomes for patients undergoing post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND) and liver resection.
Methods
After obtaining Institutional Review Board approval, we reviewed our institution’s prospectively maintained database of NSGCT patients and identified all of those (n=36) with metastatic disease who underwent PC-RPLND and post-chemotherapy residual liver mass resection between June 1990 and March 2015. For patients not undergoing simultaneous RPLND and liver resection, only patients who had no intervening treatment between planned staged operations were included. Patients were treated with either first line or second-line chemotherapy before resection. First line chemotherapy was predominately bleomycin, etoposide, cisplatin (BEP) and etoposide plus ifosfamide and cisplatin (VIP). Paclitaxel, ifosfamide, and cisplatin (TIP) was given to 3 patients in the first line setting. Second line therapy was TIP or high-dose chemotherapy using mobilizing paclitaxel plus ifosfamide followed by high-dose carboplatin and etoposide (TI-CE).
Our aim was to assess the histologic concordance between specimens recovered by PC-RPLND and those from liver resection. To determine the predictive value of PC-RPLND for the histology of liver resection masses, we compared the number of patients with teratomas or viable GCTs detected on PC-RPLND to the number detected on liver resection and conversely, the number of patients with teratomas or viable GCTs in the liver that would have been missed if patients with necrosis/fibrosis on PC-RPLND had not undergone liver resection. We also evaluated the progression-free and cancer-specific survival in this select cohort of patients. Progression-free survival was determined from the date of PC-RPLND or liver resection (whichever was later) until the date of progression, death, or until the most recent patient contact. Cancer-specific survival was determined as death from testis cancer, death from other cause, or until the most recent patient contact. All analyses were conducted using Stata 13 (Stata Corp., College Station, TX).
Results
Patient characteristics are displayed in Table 1. The median age at diagnosis was 26 (interquartile range (IQR), 22 to 34 years); 15 patients (42%) had teratomous elements on their orchiectomy specimen; 29 patients (81%) presented with liver metastasis prior to surgical intervention, and 17 patients (47%) underwent second-line chemotherapy, including 13 patients treated with high-dose chemotherapy. Although 47% of patients had either teratoma or viable GCT on RPLND, 75% of patients had necrosis/fibrosis on liver resection. PC-RPLND and liver resection were simultaneous in 28 patients (78%), while 8 (22%) had staged surgical interventions with no systemic therapy given between surgeries. Liver resection was by wedge resection in 21 and right or left hepatectomy in 15 patients. Complete pre-and post-chemotherapy liver mass size data was available for 29 patients, which demonstrated a median pre-chemotherapy liver metastasis size of 5.0 cm (IQR: 2.5, 6.8) and post-chemotherapy size of 1.6 cm (IQR: 0.9, 3.0).
Table 1: Patient characteristics.
Values are displayed as median (interquartile range) or frequency (percentage).
| N=36 | |
|---|---|
| Age at diagnosis | 26 (22–34) |
| Laterality orchiectomy | |
| Left | 19 (53%) |
| Right | 17 (47%) |
| Teratoma in primary tumor | 15 (42%) |
| AJCC stage at presentation | |
| IB | 1 (2.8%) |
| IIA | 1 (2.8%) |
| IIC | 3 (8.3%) |
| IIIA | 3 (8.3%) |
| IIIC | 28 (78%) |
| IGCCC risk group prior to initial chemotherapy | |
| Good | 5 (14%) |
| Intermediate | 1 (2.8%) |
| Poor | 29 (81%) |
| N/A | 1 (2.8%) |
| Decrease in liver mass size post-chemo (%) (N=28) | 59 (33–79) |
| Decrease in RP mass size post-chemo (%) (N=29) | 38 (16–54) |
| Decrease in AFP post-chemo (%) (N=19) | 82 (−5–100) |
| Decrease in HCG post-chemo (%) (N=13) | 100 (100–100) |
| Second-line chemo before liver resection | 17 (47%) |
| RPLND histology | |
| Fibrosis/necrosis | 19 (53%) |
| Teratoma | 8 (22%) |
| Teratoma and viable GCT | 4 (11%) |
| Viable GCT | 5 (14%) |
| Hepatic procedure | |
| Partial hepatectomy | 15 (42%) |
| Wedge resection | 21 (58%) |
| Hepatic histology | |
| Fibrosis/necrosis | 27 (75%) |
| Teratoma | 5 (14%) |
| Viable GCT | 4 (11%) |
AJCC, American Joint Committee on Cancer; IGCCC, International Germ Cell Consensus Classification; RP, retroperitoneal; AFP, alpha fetoprotein; HCG, human chorionic gonadotropin; RPLND, retroperitoneal lymph node dissection; GCT, germ cell tumor
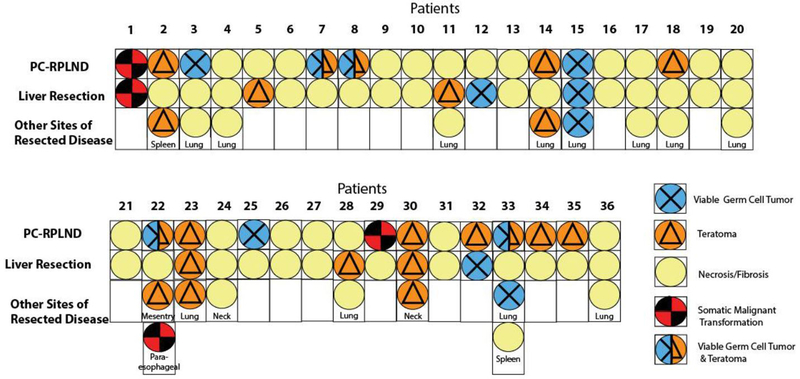
The histologies of PC-RPLND and liver resection specimens are compared in Table 2; for each histological type (fibrosis/necrosis, teratoma, viable GCT, or both teratoma and viable GCT) on PC-RPLND, the proportion of patients with each type of histology on liver resection is given. Four patients had necrosis/fibrosis on PC-RPLND but teratoma or viable GCT on liver resection (21%; 95% CI 6.0–46%). Among patients who had teratoma on PC-RPLND, 25% (95% CI 3.2–65%) had teratoma and 13% (0.32–53%) had viable GCT on liver resection. Among patients who had viable GCT on PC-RPLND, 40% (95% CI 5.3–85%) had viable GCT on liver resection. Somatic-type malignant transformation was found in 2 patients, one of whom had neuroectodermal tumor in both PC-RPLND and liver resections after second-line chemotherapy; the other had adenocarcinoma after first-line chemotherapy in PC-RPLND but fibrosis/necrosis in the liver resection. Figure 1 shows the histologic discordance between PC-RPLND, liver resection, and other extra-retroperitoneal resection sites for each patient. Of the 17 patients who received second-line chemotherapy prior to liver resection, liver histology revealed teratoma in 1 patient, somatic malignancy transformation in 1, viable GCT in 3, and the rest had fibrosis/necrosis.
Table 2.
Proportion of patients with various tumor histologies on liver resection according to RPLND histology.
| Liver resection histology (95% confidence interval) | |||
|---|---|---|---|
| RPLND histology | Necrosis/fibrosis (n=27) | Viable GCT (n=4) | Teratoma (n=5) |
| Fibrosis/necrosis (n=19) | 79% (54–94%) | 5.3% (0.13–26%) | 16% (3.4–40%) |
| Viable GCT (n=5) | 60% (15–95%) | 40% (5.3–85%) | 0% (0–52%)* |
| Teratoma (n=8) | 63% (24–91%) | 13% (0.32–53%) | 25% (3.2–65%) |
| Teratoma and viable GCT (n=4) | 100% (40–100%) | 0% (0–60%)* | 0% (0–60%)* |
One-sided, 97.5% confidence interval
Figure 1.
Histologic findings upon PC-RPLND, liver resection, and resections at other extra-retroperitoneal sites for each patient.
Table 3 presents the number of patients with teratoma or viable GCT on liver resection that would have been left unresected by a PC-RPLND histology-defined resection scheme, per 100 men. Restricting the number of resections performed to only those with teratoma and/or viable GCT on PC-RPLND would reduce the number of resections conducted by nearly half, 53 per 100 men, but would lead to a clinically relevant proportion of patients whose cancer would be missed, 8 per 100 men with teratoma and 3 per 100 men with viable GCT in their residual liver mass.
Table 3.
Comparison of various surgical selection strategies considering RPLND histology as the indication for liver resection in terms of the number of patients resected, the number whose liver teratoma or viable GCT would be removed by resection, and the number whose tumor would be missed per 100 men.
| Teratoma on liver resection | ||||
| RPLND histology | Number resected | Resections avoided | Number caught | Number missed |
| Resect all | 100 | 0 | 14 | 0 |
| Teratoma or viable GCT | 47 | 53 | 6 | 8 |
| Viable GCT | 14 | 86 | 0 | 14 |
| Teratoma and viable GCT | 11 | 89 | 0 | 14 |
| Teratoma | 22 | 78 | 6 | 8 |
| Viable GCT on liver resection | ||||
| RPLND Histology | Number resected | Resections avoided | Number caught | Number missed |
| Resect all | 100 | 0 | 11 | 0 |
| Teratoma or viable GCT | 47 | 53 | 8 | 3 |
| Viable GCT | 14 | 86 | 6 | 6 |
| Teratoma and viable GCT | 11 | 89 | 0 | 11 |
| Teratoma | 22 | 78 | 3 | 8 |
Among the 12 patients who died, 10 died of disease. The median follow-up time among those still living was 8.5 years (IQR, 4.6–15). At 3 years, the Kaplan-Meier estimated probability of cancer-specific survival for the entire cohort was 75% (95% CI, 55–85%) and the probability of progression-free survival was 75% (95% CI, 56–87%).
Discussion
In our contemporary series of 36 patients with advanced NSGCT, the histology of metastatic liver masses and that of post-chemotherapy retroperitoneal lymph nodes was significantly discordant. Specifically, among patients whose PC-RPLND revealed only necrosis or fibrosis, 21% had tumors in the liver, with a 95% confidence interval upper bound as high as 46%. This discordance rate is very similar to that found when comparing PC-RPLND histology to metastases in other extra-retroperitoneal locations, including lung, neck, and mediastinum[11, 12].
When Jacobsen et al updated the Indiana University’s post-chemotherapy hepatic resection experience from 1976 – 2006, they found necrosis in 74% (43/59) of hepatic resections, which is similar to our study’s rate of 75% [15, 16]. However, for their patients with necrosis only in their PC-RPLND specimen, they found teratoma in only one patient and no viable GCT in their liver resection for a histologic discordance rate of only 6% (1/18)[15]. Based on this small number, they argued that residual liver mass resection could potentially be avoided for patients with necrosis at PC-RPLND[15]. However, this 6% rate of histologic discordance is in marked contrast with the 21% seen in our study, as well as other reported experiences. For example, in a study from the Mayo clinic, among 15 patients undergoing hepatic resection for metastatic GCT [17], hepatic histology revealed teratoma or viable GCT in 38% (3/8) of patients with necrosis at extra-hepatic sites[17]. Furthermore, the German Testicular Cancer Study Group also reported a 39% discordance rate between hepatic and extrahepatic sites in 43 patients treated between 1990–1999[18]. In that study, patients with necrosis at extra-hepatic sites were found to have teratoma or viable GCT was seen in 42% (5/12) of liver resections. In another study of 14 patients with necrosis only at extra-hepatic resection sites, the histologic discordance rate was 29%; 3 patients had viable GCT and one had teratoma on liver resection[19]. These findings collectively argue against the suggestion by Jacobsen et al. that residual liver mass resection could be avoided for patients with necrosis at PC-RPLND[15].
Although several studies have found that PC-RPLND histology predicts extra-retroperitoneal histology better than other clinical variables, it does not overcome the substantial histologic discordance between different sites of residual disease[11, 12, 15, 20]. Since PC-RPLND histology cannot accurately predict the histology of other sites, it should not dictate the decision to resect residual hepatic masses, as unresected residual disease places patients at unnecessary risk for devastating late relapse[21, 22].
The small number of patients in this cohort limits our ability to determine predictive factors associated with histologic discordance. Furthermore, patients who progress with liver metastases may be a distinct clinical entity from those who initially present with liver disease, however we lack sufficient numbers for comparisons. The rates of viable GCT after second-line chemotherapy in our cohort were relatively low compared to other reported series[4]. This results in part from selection bias; only patients able to undergo post-chemotherapy surgery were included, as patients not responding to second-line chemotherapy with multiple sites of refractory disease may not be candidates for combined PC-RPLND and liver resection. Additionally, the lower rates of viable GCT may also be influenced by the improved efficacy of taxane-based regimens used at our center [4].
Regardless, in patients where surgery is feasible, aggressive management appears justified, as our patients experienced a favorable 3-year estimated cancer-specific survival of 75%, despite liver metastasis usually being associated with a poor prognosis. Since our survival rate is similar to the reported experiences from other centers, our data further supports the potential for favorable survival outcomes after hepatic resection[16–19].
Conclusion
In our contemporary series of patients undergoing PC-RPLND and liver resection, patients with necrosis only in their PC-RPLND specimen still had a substantial rate of teratoma or viable GCT in the liver. This histologic discordance rate is similar to the majority of other reported experiences and is in line with known histologic discordance at other extra-retroperitoneal sites. Our data supports post-chemotherapy hepatic resections in patients who are good candidates for surgery, given the favorable survival rates and known histologic discordance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shayegan B, et al. , Clinical outcome following post-chemotherapy retroperitoneal lymph node dissection in men with intermediate- and poor-risk nonseminomatous germ cell tumour. BJU Int, 2007. 99(5): p. 993–7. [DOI] [PubMed] [Google Scholar]
- 2.Daneshmand S, et al. , The management of subcentimeter residual mass in NSGCT: pcRPLND vs. observation. Urol Oncol, 2011. 29(6): p. 842–7. [DOI] [PubMed] [Google Scholar]
- 3.Tarin T, Carver B, and Sheinfeld J, The role of lymphadenectomy for testicular cancer: indications, controversies, and complications. Urol Clin North Am, 2011. 38(4): p. 439–49, vi. [DOI] [PubMed] [Google Scholar]
- 4.Eggener SE, et al. , Pathologic findings and clinical outcome of patients undergoing retroperitoneal lymph node dissection after multiple chemotherapy regimens for metastatic testicular germ cell tumors. Cancer, 2007. 109(3): p. 528–35. [DOI] [PubMed] [Google Scholar]
- 5.Winter C, et al. , Retroperitoneal lymph node dissection after chemotherapy. BJU Int, 2009. 104(9 Pt B): p. 1404–12. [DOI] [PubMed] [Google Scholar]
- 6.Fizazi K, et al. , Viable malignant cells after primary chemotherapy for disseminated nonseminomatous germ cell tumors: prognostic factors and role of postsurgery chemotherapy--results from an international study group. J Clin Oncol, 2001. 19(10): p. 2647–57. [DOI] [PubMed] [Google Scholar]
- 7.Masterson TA, et al. , Outcomes in patients with clinical stage III NSGCT who achieve complete clinical response to chemotherapy at extraretroperitoneal disease site. Urology, 2012. 79(5): p. 1079–84. [DOI] [PubMed] [Google Scholar]
- 8.Carver BS, et al. , The total number of retroperitoneal lymph nodes resected impacts clinical outcome after chemotherapy for metastatic testicular cancer. Urology, 2010. 75(6): p. 1431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carver BS, et al. , Long-term clinical outcome after postchemotherapy retroperitoneal lymph node dissection in men with residual teratoma. J Clin Oncol, 2007. 25(9): p. 1033–7. [DOI] [PubMed] [Google Scholar]
- 10.Steyerberg EW, et al. , Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol, 1995. 13(5): p. 1177–87. [DOI] [PubMed] [Google Scholar]
- 11.Masterson TA, et al. , Clinical impact of residual extraretroperitoneal masses in patients with advanced nonseminomatous germ cell testicular cancer. Urology, 2012. 79(1): p. 156–9. [DOI] [PubMed] [Google Scholar]
- 12.Carver BS and Sheinfeld J, Management of post-chemotherapy extra-retroperitoneal residual masses. World J Urol, 2009. 27(4): p. 489–92. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann JT, et al. , Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumours. Eur J Cancer, 1997. 33(6): p. 843–7. [DOI] [PubMed] [Google Scholar]
- 14.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol, 1997. 15(2): p. 594–603. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen NE, et al. , Is retroperitoneal histology predictive of liver histology at concurrent post chemotherapy retroperitoneal lymph node dissection and hepatic resection? J Urol, 2010. 184(3): p. 949–53. [DOI] [PubMed] [Google Scholar]
- 16.Maluccio M, Einhorn LH, and Goulet RJ, Surgical therapy for testicular cancer metastatic to the liver. HPB (Oxford), 2007. 9(3): p. 199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You YN, Leibovitch BC, and Que FG, Hepatic metastasectomy for testicular germ cell tumors: is it worth it? J Gastrointest Surg, 2009. 13(4): p. 595–601. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann JT, et al. , Role of postchemotherapy surgery in the management of patients with liver metastases from germ cell tumors. Ann Surg, 2005. 242(2): p. 260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivoire M, et al. , Multimodality treatment of patients with liver metastases from germ cell tumors: the role of surgery. Cancer, 2001. 92(3): p. 578–87. [DOI] [PubMed] [Google Scholar]
- 20.Steyerberg EW, et al. , Residual masses after chemotherapy for metastatic testicular cancer: the clinical implications of the association between retroperitoneal and pulmonary histology. Re-analysis of Histology in Testicular Cancer (ReHiT) Study Group. J Urol, 1997. 158(2): p. 474–8. [DOI] [PubMed] [Google Scholar]
- 21.Sharp DS, et al. , Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol, 2008. 26(34): p. 5524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheinfeld J, Risks of the uncontrolled retroperitoneum. Ann Surg Oncol, 2003. 10(2): p. 100–1. [DOI] [PubMed] [Google Scholar]